Abstract
Objective
An ex vivo study found a copper-containing intrauterine device (IUD) to be safe for women undergoing an MRI examination at a 3.0-T field. No significant artifacts caused by the metallic implant were detected. However, there are still no in vivo data about these concerns. The aim of this study was to evaluate 3.0-T magnetic field interactions of copper-containing IUDs in vivo.
Materials and Methods
Magnetic field interactions and potential adverse events were evaluated in 33 women using a questionnaire-based telephone survey. Two experienced radiologists performed artifact evaluation on MR images of the pelvis.
Results
Eighteen patients were eligible for the survey. One patient reported a dislocation of the IUD after the MR examination. All other patients had no signs of field interactions. No IUD-related artifacts were found.
Conclusion
MRI at 3.0-T is possible for women with copper-containing IUDs. However, consulting a gynecologist to check the correct position of the IUD and exclude complications after an MR examination is highly recommended. High-quality clinical imaging of the female pelvis can be performed without a loss in image quality.
Keywords: Intrauterine device, Copper, Safety, Magnetic resonance, 3.0T
INTRODUCTION
Long-acting reversible contraception methods, such as intrauterine devices (IUDs), provide the highest contraceptive efficacy and rates of satisfaction and continuation compared to other contraceptive methods (1-3). Copper-containing IUDs (e.g., Cu 380A) have shown excellent contraceptive effectiveness (3, 4), with a rare rate of dislocation (5). IUDs have been recommended as the first-line contraceptive methods offered (1), particularly for women with contraindications to different pharmaceuticals (6) and for cancer survivors (7). Diagnostic imaging with several different modalities in these patients with IUDs is possible (8), including magnetic resonance imaging (MRI).
Magnetic resonance imaging is an increasingly powerful imaging method. The new generation of 3.0-T scanners has been used more often in the clinical routine for several years now, and these scanners offer various advantages due to high spatial resolution and improved signal-to-noise ratio, leading to an improvement in the diagnostic yield (9-14). However, safety concerns about imaging metallic implants, such as copper-containing IUDs, have arisen with the higher field strengths, and devices proven to be safe at 1.5-T need to be evaluated again in a 3.0-T magnetic field environment. Dislocation, torsion, and perforation of the uterine wall, leading to unintended pregnancies, bleeding, and pelvic inflammatory disease (PID), may be caused by field interactions. The copper utilized in these devices might also lead to susceptibility artifacts and interfere with image quality, resulting in less optimal diagnostic efficacy.
Several publications indicate that copper-containing IUDs are safe for women scanned on a 1.5-T system (15-18). Zieman and Kanal (19) evaluated the Copper T 380A IUD in a 3.0-T system ex vivo. No magnetic field interactions and no risk for dislocation or migration were found. The temperature elevation of the IUD was reported to be minimal. Furthermore, artifact testing showed only small artifacts. However, there are still no data in vivo with regard to safety conditions and the presence and impact of artifacts with these IUDs. Therefore, the aim of this study was to evaluate the magnetic field interactions of copper-containing IUDs in vivo in patients examined at a 3.0-T field strength.
MATERIALS AND METHODS
Subjects
Our case series consisted of 33 women (mean age, 41.2 years; range, 33-59 years), who underwent abdominal, musculoskeletal, or neuro imaging on a 3.0-T MR unit (Trio Tim, Siemens, Erlangen, Germany). The patients were examined because of different clinical indications. A potential dislocation of the IUD was not suspected in any of the patients; none of the patients was referred for MRI to search for a dislocated/migrated IUD. During the obligatory pre-examination interview immediately before the MRI examination, individual risks and contraindications for MRI were assessed. Every patient was asked about the presence of metallic implants, particularly a copper-containing IUD. With regard to contraception purposes with a copper-containing IUD, the women were informed about the benefits of a 3.0-T examination and the fact that, in experimental studies about the safety conditions of these devices, no significant temperature elevations or potentially harmful magnetic field interactions were found. However, they were also informed that there are no published in vivo data about the potential risk of a dislocation or even migration of the IUD. All women were examined according to the guidelines and recommendations of the database "MRI-safety" (20).
The patients were asked to indicate discomfort during the exam, such as sensations of heat or pain, and advised to have the correct position of the IUD checked by a gynecologist after the MRI exam to avoid an infection or a potential unintended pregnancy due to dislocation. Every woman gave written, informed consent for the MRI examination.
Methods
The study protocol was reviewed and approved by our Institutional Review Board. Written informed consent was given from every participant. In a period of one to 36 months after the MRI examination, all patients were contacted by mail, e-mail, or telephone. If the patients wanted to participate in the study, they received the same questionnaire, including subjective parameters (sensations of heat or pain during examination) and objective parameters (dislocation or partial/complete perforation of the IUD into the uterine wall, PID, bleeding, or undesired pregnancy) (Table 1). A correct position of the device was defined as a location in the cavum uteri, with the top of the IUD close to the fundus. The IUD was dislocated if any part of the device was located in the cervix uteri or outside the cavum uteri (endo-, myometrium, parametrium, cavum douglasi, urinary bladder).
Table 1.
Questionnaire to Evaluate Subjective and Objective Parameters
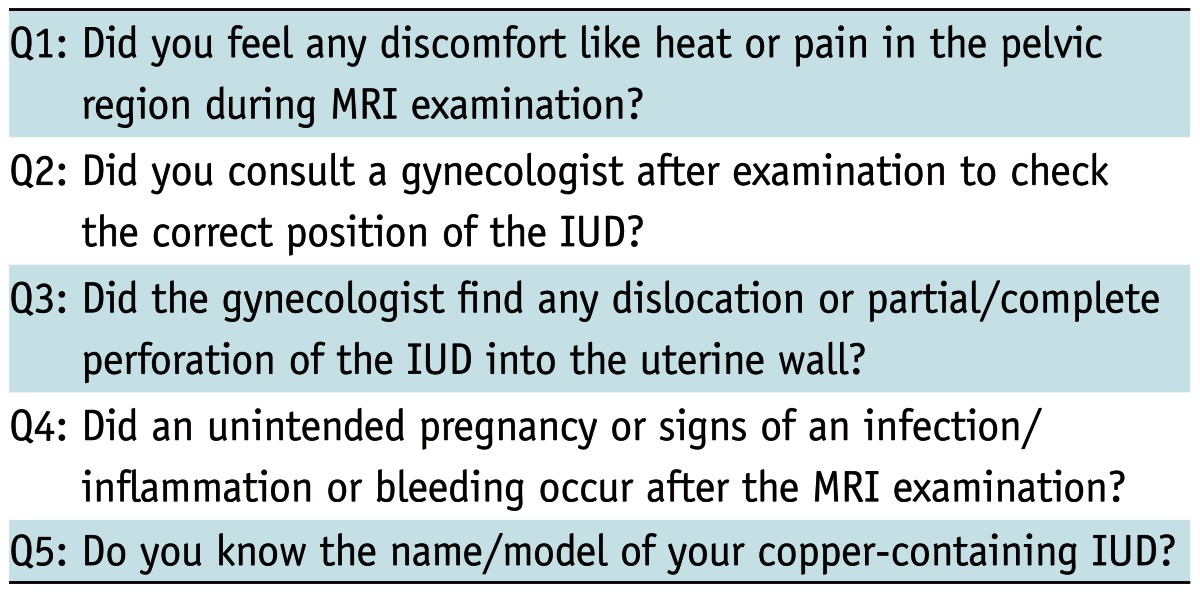
Note.- IUD = intrauterine device
Artifact Evaluation
In case of a pelvic MRI, the presence of significant artifacts was evaluated. Details about the aquisition protocol and the MRI parameters are summarized in Table 2.
Table 2.
Pelvic MRI Imaging Parameters
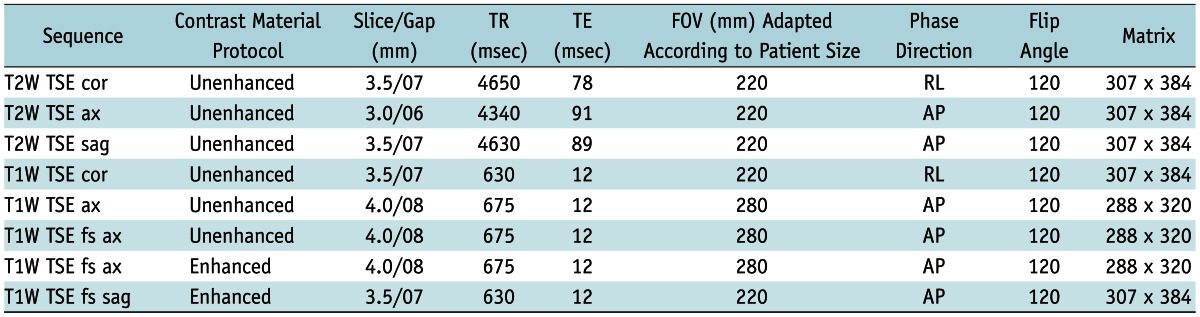
Note.- T2W = T2-weighted, T1W = T1-weighted, TR = repetition time, TE = echo time, FOV = field of view, TSE = Turbo Spin Echo, RL = right-left, AP = anterior-posterior
Two experienced radiologists independently rated axial and coronal T1-weighted (T1W) and T2-weighted (T2W) images, as well as fat saturated images (fs) and contrast-enhanced T1-weighted images on the picture archiving and communication system (PACS, AGFA-Healthcare, Mortsel, Belgium, version 5.2), assisted by the tools of the PACS, such as the zoom. The raters were asked to record the presence of susceptibility artifacts according to a grading system (Table 3).
Table 3.
Presence of Susceptibility Artifacts and Interference with Imaging in Different Sequences in Patients with MRI of Pelvis, Rated by Two Radiologists (a/b)
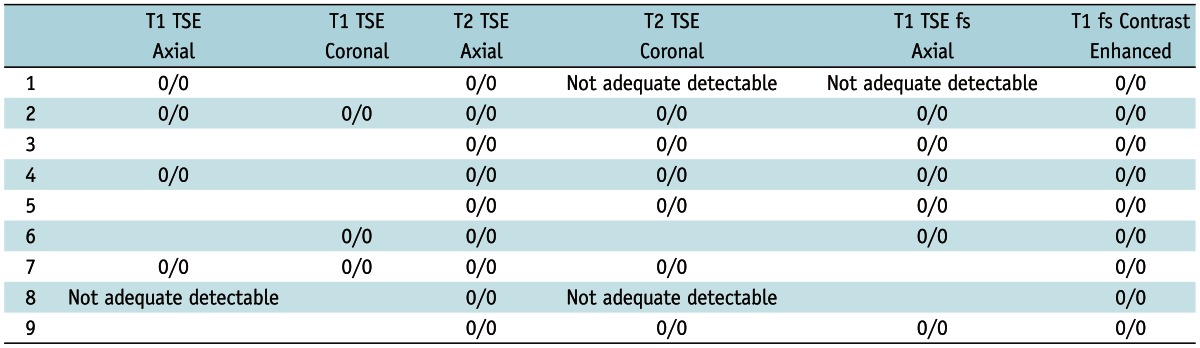
Note.- Definition of grading system for susceptibility artifact evaluation: 0 = No artifact detectable; 1 = Minimal artifact detectable, endometrium is visible, image quality sufficient for clinical routine; 2 = Artifact detectable with impaired image quality and signal loss in endometrium/myometrium. TSE = Turbo Spin Echo
Our MRI aquisition protocol improved in recent years and is adapted to the clinical indication and organ of interest. The protocols additionally included sagittal T1-weighted and T2-weighted sequences. However, we did not include sagittal images in the artifact evaluation because in only three patients the organ of interest was the uterus. In these cases the sagittal plane was adjusted to the axis of the uterus. In all other cases the IUD was not optimally visualized in this plane.
Statistical Analysis
A descriptive statistical analysis giving the absolute and relative frequencies and an evaluation of the inter-reader agreement using kappa (k) statistics was performed using SPSS 17.0.1 (SPSS Inc., Chicago, IL, USA).
RESULTS
Survey
Of the 33 patients who underwent 3.0-T MRI, 18 patients were eligible for the survey (flow chart) (Fig. 1). The results of the survey and the regions of examination are summarized in Table 4.
Fig. 1.
Patient inclusion chart. IUD = intrauterine device
Table 4.
Results of Survey, including Region of Exam
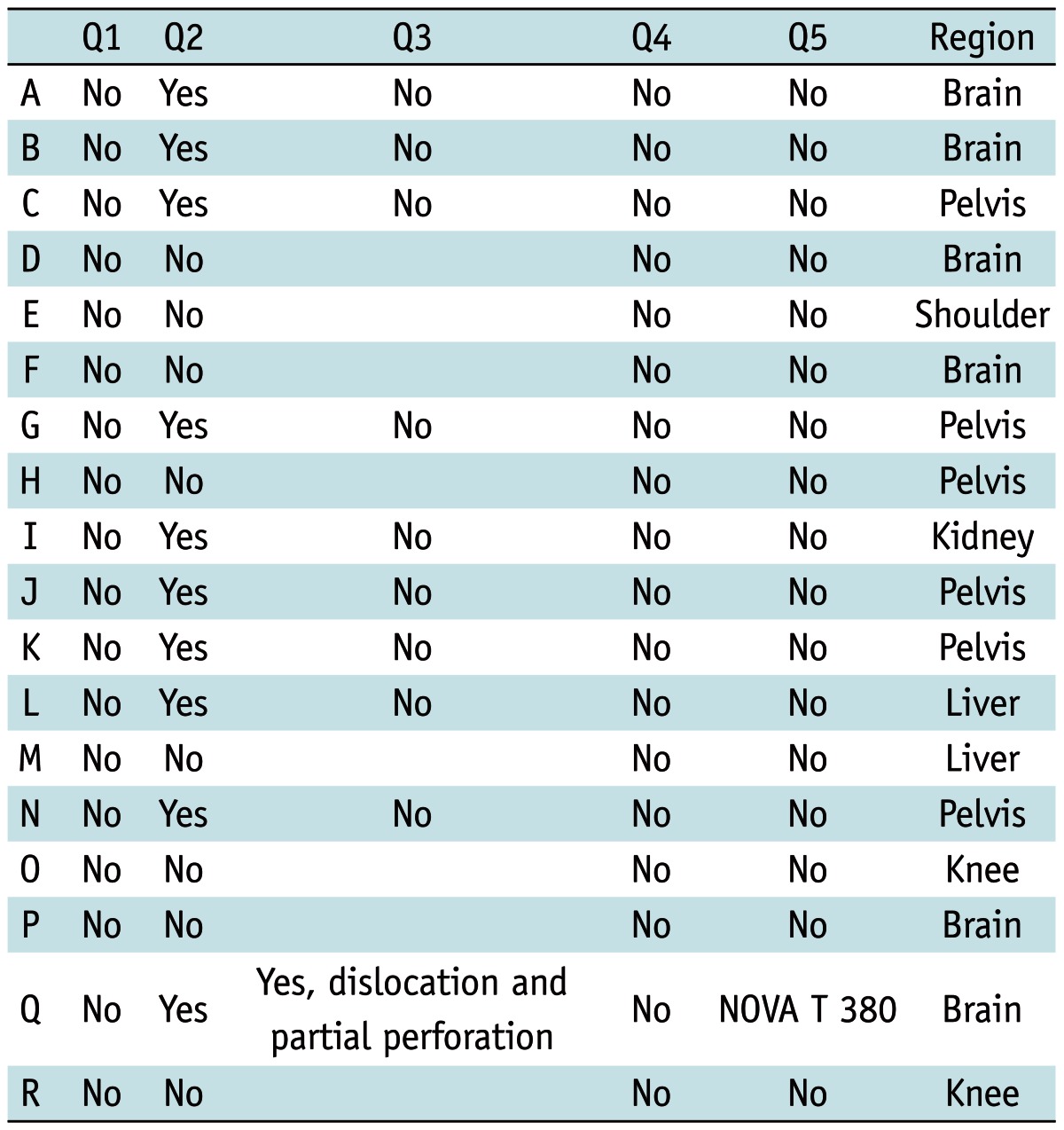
With a gynecological check-up, the position of the IUD was checked by intravaginal ultrasound. One patient reported an adverse event. During the check-up, the gynecologist detected a dislocation and partial perforation of the IUD (NOVA T 380) into the fundal myometrium without any signs of PID. The patient was asymptomatic; no surgical manipulation was reported. The IUD was removed and a new device (NOVA T 380) was implanted. This patient had two more 3-T MRI examinations at intervals of six months after the event. The following gynecological check-ups revealed no complications and no signs of dislocation.
Artifact Evaluation
Overall, 9/33 patients underwent MRI of the pelvis. The IUD was best detectable on T1W images after the administration of contrast agent, followed by native T1W and T2W images (Fig. 2). The performed sequences and the results of the artifact evaluation are shown in Table 3. In one patient (No. 8), the IUD was only adequately visible on T2W and on contrast-enhanced images. This woman had a small uterus after extirpation of a cervical carcinoma. Another woman underwent surgical therapy because of a vaginal carcinoma (No. 1). In two women, we detected motion artifacts caused by breathing and intestinal motion; however, there were no IUD-related susceptibility artifacts. There was a perfect agreement between the two readers (k = 1).
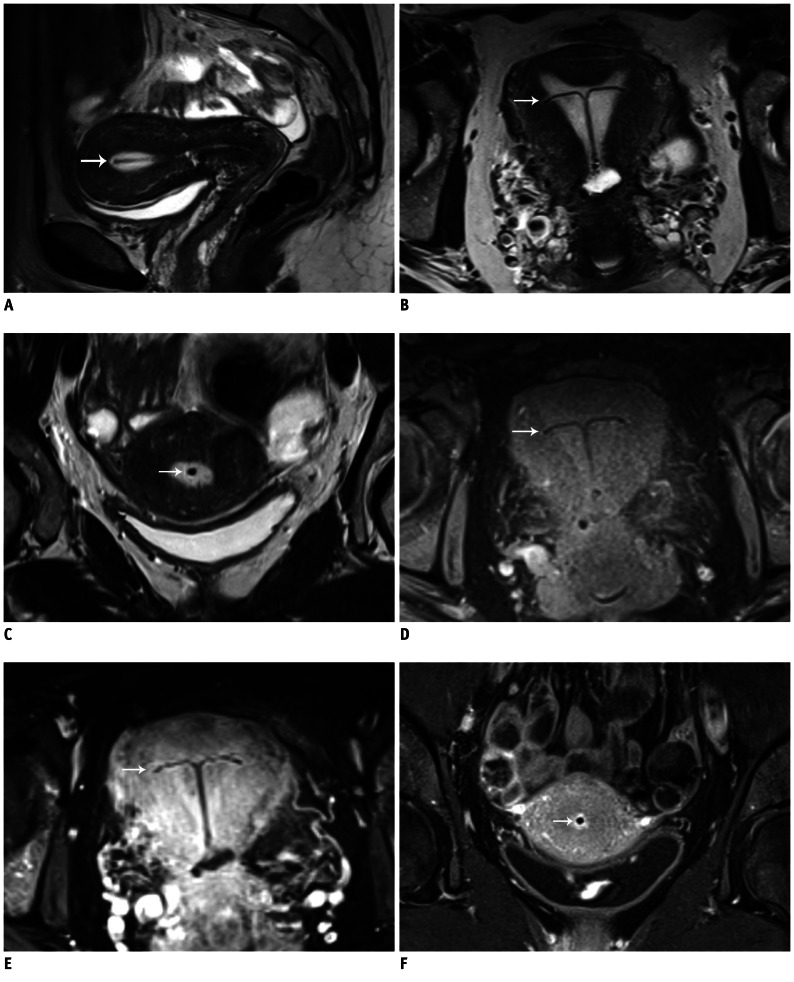
Fig. 2.
MR imaging of IUD.
A-C. Images of uterus of 42-year-old woman with undefined abdominal inflammatory disease. Sagittal (A), axial (B), and coronal (C) T2W images. IUD in fluid-filled hyperintense cavum uteri is clearly detectable without susceptibility artifacts. D. Axial T1W image. Contrast between fluid-filled cavum uteri, endo-/myometrium, and IUD is not as pronounced as in T2W images; however, IUD is clearly definable. E, F. Axial (E) and coronal (F) fat-saturated T1W images with CA (gadolinium). High contrast between hyperintense cavum uteri and endo-/myometrium and hypointense IUD. White arrow is pointing to copper-containing IUD located in cavum uteri. IUD = intrauterine device, T1W = T1-weighted, T2W = T2-weighted, CA = contrast agent
DISCUSSION
The results of our in vivo study suggest that MR imaging of women with a copper-containing IUD at a 3.0-T field can be performed safely. Nevertheless, this study did not allow us to exclude any complications due to field interactions.
That only half of the patients consulted a gynecologist to check the correct position of the IUD demonstrated the patients' confidence in modern imaging techniques and in the ex vivo data about the safety of metallic implants. Of the patients who did not consult a gynecologist, none reported any subjective signs of complications, such as bleeding, an infection, or an unintended pregnancy.
One patient was referred for an adverse event after the MRI examination. The gynecological check-up revealed a dislocation and partial perforation of the IUD into the fundal myometrium. However, it is unclear whether this complication was induced by magnetic field interactions. Complications like bleeding, PID, and dislocation of IUDs have been described in the literature (2, 5, 21), with higher IUD-associated complication rates in adolescents than in adults (22). A perforation through the uterine wall into the abdominal cavity is rare and may be the result of mechanical force applied during the insertion procedure of the IUD; this event also may be generated by uterine contractions or surgical intervention after insertion (23, 24), and has been reported in several case reports and case series (24-27). The incidence of perforation ranges from 1.3 to 2.2 of 1000 insertions of IUDs (24, 28, 29). We cannot be certain that, in our one case of dislocation, the device was actually in situ before the MRI examination. Considering the literature and the fact that this patient had another two 3.0-T MRI examinations at intervals of six months after the initial MR exam, with no dislocation of the device in the gynecological check-up, we assume that this complication was probably not induced by magnetic field interactions. However, this cannot be proven by the recent data and field interactions are possible, regardless of which part of the body needs to be examined. A clinical study with a larger study population that can avoid limiting factors, such as a lack of information about the implanted copper-containing devices, and about the correct position of the device before the MRI examination, is needed, before all safety concerns about copper IUDs in 3.0-T MRI are resolved.
International databases (20, 30) have been established to provide required information about the MRI safety of medical devices at different magnetic field strengths. Four different copper-containing IUDs were rated as "MR-safe" at a 1.5-T field (20), including the NOVA T 380. Ex vivo data about the safety of the copper IUD T 380A (19) led to the status "Conditional 5" at a 3.0-T field in non-clinical testing. "Conditional 5" means that the advice is acceptable for women undergoing MRI if specific guidelines or recommendations, given at the website of MR-safety and by the manufacturer, are followed (20). All women were examined according to these guidelines, even if the name and the manufacturer of the device were not known, resulting in adequate safety conditions and an excellent image quality.
Zieman and Kanal (19) described ex vivo small-sized, image-distorting susceptibility artifacts and suggested that these artifacts would cause no significant impairment of clinical image quality. However, there were no in vivo data about these artifacts and it was not known whether the artifacts would lead to a signal loss in the nearby endometrium in clinical imaging (19). The recent in vivo data confirmed that suggestion by Zieman and Kanal: no susceptibility artifacts were detected on routine clinical images. High-quality MR images of the female pelvis, particularly of the endometrium and myometrium, can be performed safely at 3.0-T without obscuring any relevant anatomical or pathological information.
Conclusion
Due to the increasing use of 3.0-T in the daily clinical routine, and based on the clinical indication for imaging of women with a copper-containing IUDs, we found that 3.0-T MRI is not only possible, but also may be advantageous. However, consulting a gynecologist to check the correct position of the IUD and exclude complications after the MRI examination is highly recommended. High-quality clinical imaging of the female pelvis can be performed with no loss in image quality due to the absence of susceptibility artifacts caused by copper-containing IUDs.
References
- 1.Peipert JF, Zhao Q, Allsworth JE, Petrosky E, Madden T, Eisenberg D, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105–1113. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Araujo FF, Barbieri M, Guazzelli CA, Lindsey PC. The T 380A intrauterine device: a retrospective 5-year evaluation. Contraception. 2008;78:474–478. doi: 10.1016/j.contraception.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008;198:248–253. doi: 10.1016/j.ajog.2007.10.787. [DOI] [PubMed] [Google Scholar]
- 4.Farr G, Amatya R, Doh A, Ekwempu CC, Toppozada M, Ruminjo J. An evaluation of the copper-T 380A IUD's safety and efficacy at three African centers. Contraception. 1996;53:293–298. [PubMed] [Google Scholar]
- 5.Reinprayoon D, Gilmore C, Farr G, Amatya R. Twelve-month comparative multicenter study of the TCu 380A and ML 250 intrauterine devices in Bangkok, Thailand. Contraception. 1998;58:201–206. doi: 10.1016/s0010-7824(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 6.Suri V, Aggarwal N, Kaur R, Chaudhary N, Ray P, Grover A. Safety of intrauterine contraceptive device (copper T 200 B) in women with cardiac disease. Contraception. 2008;78:315–318. doi: 10.1016/j.contraception.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz EB, Hess R, Trussell J. Contraception for cancer survivors. J Gen Intern Med. 2009;24(Suppl 2):S401–S406. doi: 10.1007/s11606-009-1023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007;26:1389–1401. doi: 10.7863/jum.2007.26.10.1389. [DOI] [PubMed] [Google Scholar]
- 9.Craven I, Griffiths PD, Hoggard N. Magnetic resonance imaging of epilepsy at 3 Tesla. Clin Radiol. 2011;66:278–286. doi: 10.1016/j.crad.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Stankiewicz JM, Glanz BI, Healy BC, Arora A, Neema M, Benedict RH, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. 2011;21:e50–e56. doi: 10.1111/j.1552-6569.2009.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arizono S, Isoda H, Maetani YS, Hirokawa Y, Shimada K, Nakamoto Y, et al. High spatial resolution 3D MR cholangiography with high sampling efficiency technique (SPACE): comparison of 3T vs. 1.5T. Eur J Radiol. 2010;73:114–118. doi: 10.1016/j.ejrad.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka M, Kido A, Koyama T, Isoda H, Umeoka S, Tamai K, et al. MRI of the female pelvis at 3T compared to 1.5T: evaluation on high-resolution T2-weighted and HASTE images. J Magn Reson Imaging. 2007;25:527–534. doi: 10.1002/jmri.20842. [DOI] [PubMed] [Google Scholar]
- 13.Michaely HJ, Attenberger UI, Kramer H, Nael K, Reiser MF, Schoenberg SO. Abdominal and pelvic MR angiography. Magn Reson Imaging Clin N Am. 2007;15:301–314. v–vi. doi: 10.1016/j.mric.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Bauer JS, Monetti R, Krug R, Matsuura M, Mueller D, Eckstein F, et al. Advances of 3T MR imaging in visualizing trabecular bone structure of the calcaneus are partially SNR-independent: analysis using simulated noise in relation to micro-CT, 1.5T MRI, and biomechanical strength. J Magn Reson Imaging. 2009;29:132–140. doi: 10.1002/jmri.21625. [DOI] [PubMed] [Google Scholar]
- 15.Pasquale SA, Russer TJ, Foldesy R, Mezrich RS. Lack of interaction between magnetic resonance imaging and the copper-T380A IUD. Contraception. 1997;55:169–173. doi: 10.1016/s0010-7824(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 16.Hess T, Stepanow B, Knopp MV. Safety of intrauterine contraceptive devices during MR imaging. Eur Radiol. 1996;6:66–68. doi: 10.1007/BF00619957. [DOI] [PubMed] [Google Scholar]
- 17.Kido A, Togashi K, Kataoka ML, Nakai A, Koyama T, Fujii S. Intrauterine devices and uterine peristalsis: evaluation with MRI. Magn Reson Imaging. 2008;26:54–58. doi: 10.1016/j.mri.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Mühler M, Taupitz M. [How safe is magnetic resonance imaging in patients with contraceptive implants?] Radiologe. 2006;46:574–578. doi: 10.1007/s00117-005-1212-3. [DOI] [PubMed] [Google Scholar]
- 19.Zieman M, Kanal E. Copper T 380A IUD and magnetic resonance imaging. Contraception. 2007;75:93–95. doi: 10.1016/j.contraception.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Shellock FG. MRIsafety.com. [Accessed August 29, 2012]. http://www.mrisafety.com/list.asp/
- 21.O'Brien PA, Kulier R, Helmerhorst FM, Usher-Patel M, d'Arcangues C. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception. 2008;77:318–327. doi: 10.1016/j.contraception.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Rasheed SM, Abdelmonem AM. Complications among adolescents using copper intrauterine contraceptive devices. Int J Gynaecol Obstet. 2011;115:269–272. doi: 10.1016/j.ijgo.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Glass T, Baker T, Kauffman RP. Migration of an intrauterine contraceptive device during the course of pregnancy: a case report. J Minim Invasive Gynecol. 2009;16:81–83. doi: 10.1016/j.jmig.2008.09.579. [DOI] [PubMed] [Google Scholar]
- 24.Andersson K, Ryde-Blomqvist E, Lindell K, Odlind V, Milsom I. Perforations with intrauterine devices. Report from a Swedish survey. Contraception. 1998;57:251–255. doi: 10.1016/s0010-7824(98)00029-8. [DOI] [PubMed] [Google Scholar]
- 25.Markovitch O, Klein Z, Gidoni Y, Holzinger M, Beyth Y. Extrauterine mislocated IUD: is surgical removal mandatory? Contraception. 2002;66:105–108. doi: 10.1016/s0010-7824(02)00327-x. [DOI] [PubMed] [Google Scholar]
- 26.Turok DK, Gurtcheff SE, Gibson K, Handley E, Simonsen S, Murphy PA. Operative management of intrauterine device complications: a case series report. Contraception. 2010;82:354–357. doi: 10.1016/j.contraception.2010.04.152. [DOI] [PubMed] [Google Scholar]
- 27.Taras AR, Kaufman JA. Laparoscopic retrieval of intrauterine device perforating the sigmoid colon. JSLS. 2010;14:453–455. doi: 10.4293/108680810X12924466006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caliskan E, Oztürk N, Dilbaz BO, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003;8:150–155. [PubMed] [Google Scholar]
- 29.Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53–56. doi: 10.1016/s0010-7824(02)00417-1. [DOI] [PubMed] [Google Scholar]
- 30.MagResource - MRI safety worldwide. [Accessed August 29, 2012]. http://www.magresource.com/