Abstract
A coordinated movement of the eyes and head in the head-unrestrained condition is often used to change orientation between targets. Under natural conditions, these gaze shifts are typically generated with the eyes roughly centered in the orbits. To achieve experimental control of eye and head positions, a miniature laser was mounted on the head implants of monkeys that were trained to point the head to one target and direct gaze to another before generating a head-unrestrained gaze shift to a third target (dissociation paradigm). For comparison, monkeys were also required to make gaze shifts between stimuli, without any constraints on eye and head positions (standard paradigm). Analyses indicated that movement parameters, limited to horizontal gaze shifts, were similar for both behavioral conditions. Thus, the proposed technique and behavioral paradigm, when used in conjunction with electrophysiological and pharmacological experiments, may facilitate the study of neural control of gaze.
Keywords: Gaze, Saccade, Laser, Head movement, Oculomotor
1. Introduction
In head-restrained animals, saccades accomplish a quick redirection of the visual axis. If the head is unrestrained a coordinated eye and head movement, referred to as gaze saccade or gaze shift, typically produces the reorientation of the line of sight.
Head-unrestrained gaze shifts generated under normal conditions are usually initiated with the eyes nearly centered in the head. The initial eye position in head (IEPh), or the eye position prior to onset of gaze shifts, is known to significantly modulate the eye and head contributions of gaze shifts (Tomlinson, 1990; Delreux, Abeele, Lefévre, & Roucoux, 1991; Becker & Jürgens, 1992; Volle & Guitton, 1993; Freedman & Sparks, 1997b). For example, for a constant amplitude gaze movement, the eye component increases and the head contribution decreases as the IEPh is increasingly contralateral to the direction of the movement. The effects of IEPh have also yielded insights into the neural control of head-unrestrained gaze shifts. For example, Freedman and Sparks (1997a) recorded activity of superior colliculus neurons as the monkey generated same amplitude gaze shifts for various IEPh and found that the discharge was better correlated with gaze amplitude than either eye or head component.
A limitation of previous studies examining the effects of eye position was a lack of experimental control of IEPh. As most natural gaze shifts are usually initiated with the eyes nearly centered in the head, many sessions and trials were required to collect data over an extended range of IEPh. With the added technical difficulty of maintaining neural isolation during head-unrestrained gaze shifts, a more efficient control of IEPh is required, particularly for neurophysiology experiments.
To systematically dissociate the head-in-space and eye-in-head positions prior to a head-unrestrained gaze shift, we placed a micro-miniature laser in an explant on the monkey’s skull and trained the animal to orient the head to a red light emitting diode (LED). Next, they were required to execute an ocular saccade to a green LED while maintaining head orientation toward the red LED. Finally, they were permitted to generate an eye–head coordinated gaze shift to a yellow LED. This dissociation paradigm, designed to extend the range of IEPh prior to gaze shifts, also possesses the potential of altering behavior because natural gaze shifts are typically initiated with the eyes centered in the orbits. For comparison, we also collected data during trials in which the monkey made gaze shifts from one yellow LED to another, without any constraints on IEPh.
In this paper, we describe the methodology used to systematically control the IEPh and then compare the results of horizontal gaze shifts produced during the dissociation and standard paradigms. In particular, we examined the relationships concerning the ratio of eye and head contributions, the main sequence properties of gaze shifts, and the timing of head onset relative to gaze onset. Our analyses determined that these parameters were similar for movements produced in the two behavioral conditions. We also studied the effect of head position on the relationship of eye and head components during horizontal gaze shifts. From our results, we conclude that the proposed dissociation paradigm does not alter the characteristics of rapid gaze shifts and that this method, in conjunction with electrophysiological and pharmacological experiments, may facilitate the study of the neural control of gaze.
2. Methods
Two juvenile, male, Macaca mulatta monkeys (BE and CH) were used for this study. All experimental protocols were approved by the Institute Animal Care and Use Committee at the Baylor College of Medicine and complied with the guidelines of the Public Health Service policy on Humane Care and Use of Laboratory Animals.
2.1. Surgical and experimental preparations
All surgical procedures were performed in an aseptic environment and under isofluorane anesthesia. A stainless steel post, secured by stainless steel screws and bone cement, was placed on the skull for immobilizing the head. Also, a Teflon-coated, scleral search coil was set under the conjunctiva of one eye (Fuchs & Robinson, 1966; Judge, Richmond, & Chu, 1980). Another coil of the Teflon-coated, stainless steel wire was implanted in the bone cement on the skull. The orientations of the two coils were similar when the animal directed its gaze and pointed its head in the straight-ahead direction.
The coils around the eyeball and on the head were used to measure gaze (eye-in-space) and head positions, respectively. Eye position relative to the head was computed as the difference between the gaze and head signals. A phase-angle detection system (CNC Engineering, Seattle, WA) was used to measure the position signals. Although this system is insensitive to translation and not adapted to measure torsion, it gauges rotation linearly to within 2% over 360° in azimuth, has absolute calibration and is void of non-linearities associated with large gaze and head rotations in the phase-locked amplitude technique (Robinson, 1963). The vertical component of the position signal, in contrast, was corrected off-line for the non-linearities inherent in the coil system.
Each monkey was first trained to perform various saccade paradigms in the head-restrained condition. After successfully learning these tasks, the animal was required to elicit gaze shifts in the head-unrestrained preparation. Initially, the training was limited to natural gaze shifts, which posed no constraints on the head or eye-in-head positions (see standard paradigm below). Upon mastery of this task, the animals were trained on the dissociation task (see below).
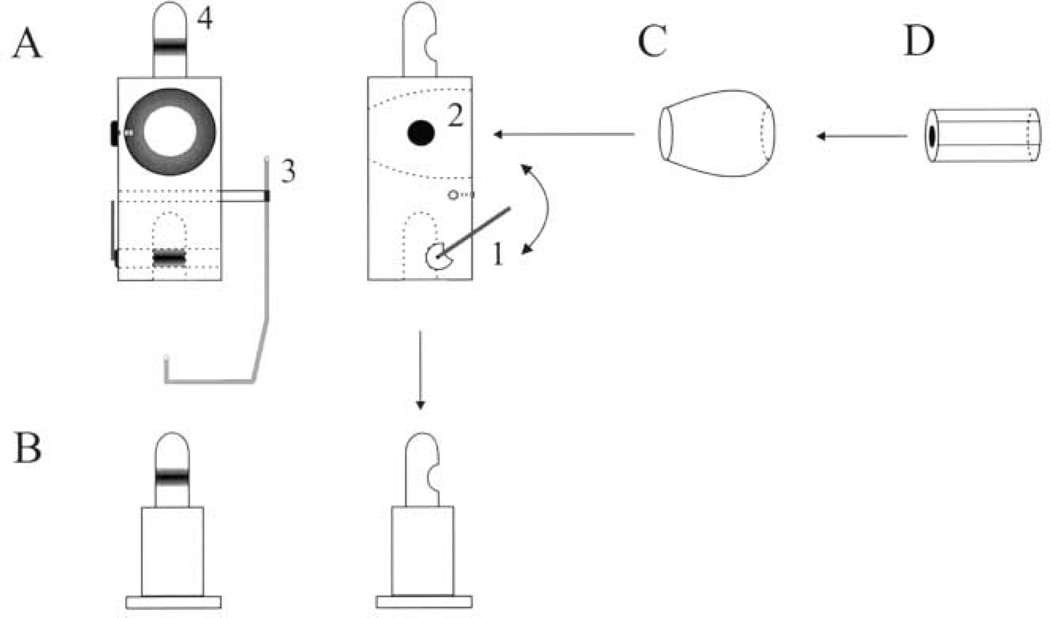
We used a micro-miniature laser (Edmund Scientific Inc., #52–263) to provide visual feedback for accurate pointing of the head. An apparatus was designed to secure the miniature laser on the animal’s head (Fig. 1). An addendum piece (Fig. 1A) was constructed to fit on the head post of the animal (Fig. 1B), and a swivel arm (Fig. 1A–1) locked the two pieces together. A cone-type hole (Fig. 1A–2) was drilled out of the addendum to secure a ball-socket (Fig. 1C). The micro-miniature laser (Fig. 1D) was then placed into the lumen of the ball-socket. Heat-shrink tubing was wrapped around the laser module for a tight fit into the ball-socket. The miniature laser, which emits a red beam (670 nm), was connected to a relay circuit that could be activated by a TTL pulse generated by the computer. In addition to the laser setup, a reward tube (Fig. 1A–3) was also secured to the extra piece. A copper tube, molded into a shape that minimized visual obstruction, delivered water or juice smoothly into the mouth. [In the second animal, the reward was delivered through a Tygon tube (Performance Plastics, Akron, OH) twisted around a durable plastic rod.]
Fig. 1.
A schematic diagram of the addendum designed for mounting a micro miniature laser on the monkey’s head. See text for details. The left and right panels in A and B are front and side views, respectively.
The addendum piece was designed to facilitate transition between head-restrained and -unrestrained preparations. Thus, this piece also has a head post design (Fig. 1A–4) to which a rigid bar can be attached and clamped to a primate chair. To provide strength to the apparatus, while minimizing the weight, the addendum piece was made from titanium, was 17 mm × 17 mm × 35 mm and weighed ~ 60 g.
2.2. Behavioral paradigms
An array of tri-state (red, green and yellow) light emitting diodes (LEDs) consisting of 42 rows of 49 lights, equally spaced at 2° intervals on a tangential panel at a distance of 57 cm, was used to display the visual targets. In principle, target displacements as large as 96° horizontal and 84° vertical could be tested. For large eccentricities away from the straight-ahead position, large discrepancies occur between the tangential display and rotational movement coordinates (Huebner, Paloski, Reschke, & Bloomberg, 1995). To adjust for the rotational movements of eyes and head during gaze shifts, the tangential display of the screen was converted online into circumferential coordinates using the following equations:
x′ = arctan(x/dpr) × dpr
y′ = arctan(y × cos(x/dpr)/dpr) × dpr,
where, (x, y) is the target location in degrees in tangential coordinates, (x′, y′) is the equivalent position in degrees in circumferential dimensions and dpr is degrees per radians (180/pi). Thus, the largest, horizontal target displacement in rotational coordinates was ~ 80°.
2.2.1. Dissociation paradigm
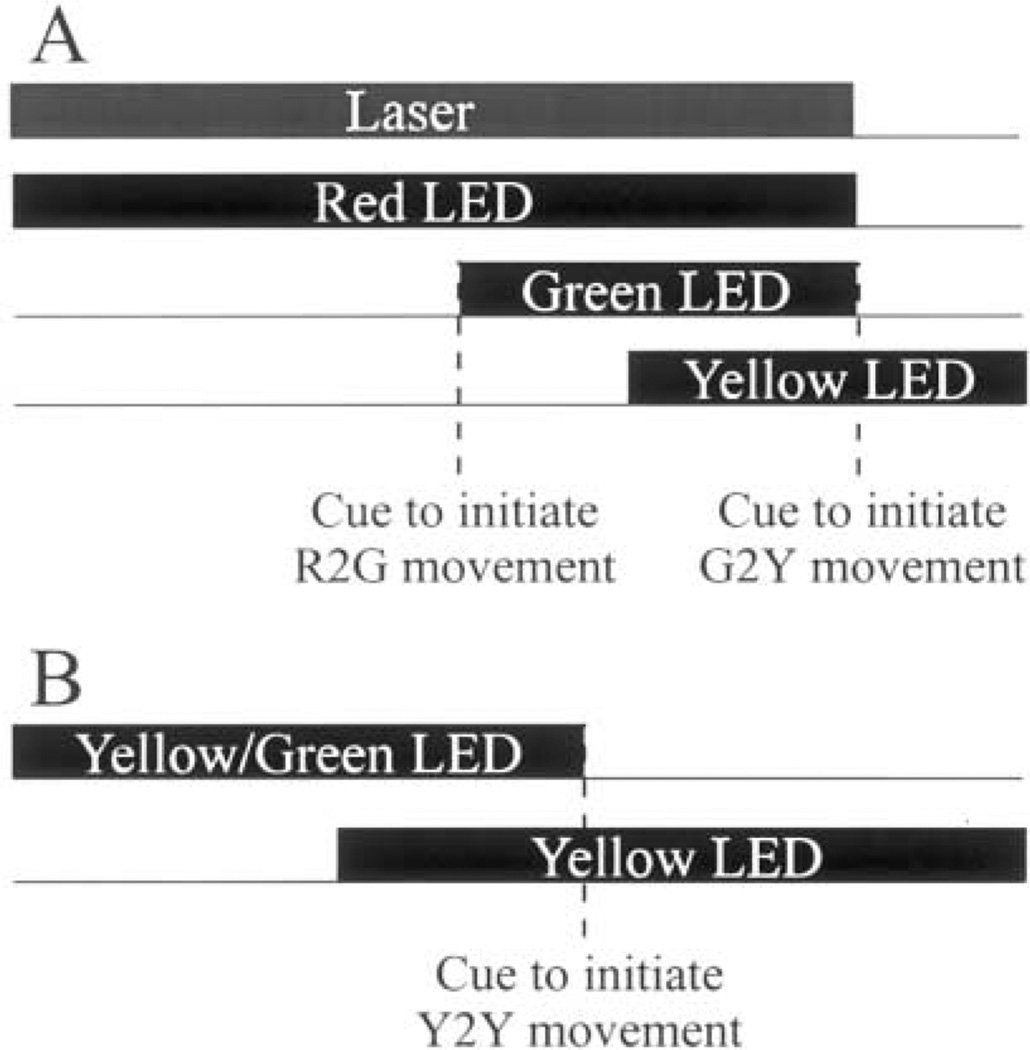
The task that systematically controlled the initial eye position in head prior to head-unrestrained gaze shifts is schematized in Fig. 2A. At trial onset, a red LED and the miniature laser were illuminated, and the monkey was required to direct the laser beam within 5° and align its gaze within 2° of the red LED. After 700–1000 ms of fixation, a green LED was presented within 30° of the red LED. The monkey was trained to direct gaze within 2–4° of the green target within 500 ms and without significantly changing the head position (R2G movement). During a subsequent fixation for 700–1000 ms, after which a yellow LED was illuminated, the head often drifted slowly toward the direction of gaze before stabilizing. For this epoch, the window monitoring head position around the red LED was increased to 8–10°. The extinction of red and green LEDs and the miniature laser another 300–500 ms later served as the cue to initiate the gaze shift (G2Y movement). Note that the monkey was not required to direct the laser beam or the head to the yellow LED to obtain a reward. Once consistent performance was reached, the dissociation paradigm was randomly interleaved with the standard task.
Fig. 2.
Schematic of the two behavioral paradigms. (A) In the dissociation task, the monkey first aligns the laser and gaze with the red LED. Next, the monkey is required to direct gaze from the red to green LED (R2G movement). This movement, cued by the onset of the green target, is to be accomplished primarily by the eyes. The head is required to stay aligned with the red LED, thus, dissociating the eye-in-head and head-in-space positions. The gaze shift from the green to yellow LED (G2Y movement), cued by the offset of the laser and both red and green targets, does not place any constraints on the head movement; (B) In the standard paradigm, the monkey is required to shift its gaze from a fixation LED, either yellow or green, to a yellow stimulus (Y2Y movement). This movement, cued by the offset of the fixation target, does not place any constraints on initial head position because the laser is not presented in this task.
2.2.2. Standard paradigm
To compare the results of the dissociation task with gaze shifts evoked under ‘natural’ conditions, we utilized the delayed gaze shift paradigm summarized in Fig. 2B (also see Freedman & Sparks, 1997b). The monkey initially achieved fixation within a 2° window around a yellow LED for 700–1000 ms. Next, another yellow, target stimulus was illuminated and overlapped with the fixation target for 500–1000 ms before the latter was extinguished, which cued the monkey to initiate the gaze shift (Y2Y movement). Note that laser addendum was placed on the skull but the miniature laser was never illuminated at any point during the trial and, therefore, no constraints on the eye-in-head and head-in-space positions were imposed.
In later experiments in monkey BE and for all experiments in monkey CH, the initial yellow LED used for fixation was changed to a green LED, to remain consistent with the latter part of the dissociation task. This modification may have led the monkeys to interpret the green LED as a cue for making an eye movement without moving the head, but such effects were not observed in any monkey’s behavior; gaze shift characteristics as well as IEPh distributions were similar and independent of the color of the fixation LED.
2.2.3. Head-restrained saccades
In one series of experiments, the animal’s head was restrained during the interleaved trials. Of course, the red LED was always presented at the straight-ahead position, and the other targets were presented within 30° from center. By definition, the gaze shift was completed by the eye movement. The kinematics of these saccades were analyzed and compared with gaze shifts elicited during the head-unrestrained condition.
2.3. Monkeys’ strategies during the training phase
The first aspect of dissociation task introduced to the monkeys was the alignment of laser beam with red LED. They initially attempted to perform the trial by horizontal and vertical translation of the body and neck. A chest plate, a back plate, and a seat platform with side barriers were placed in the primate chair to minimize body movements. During early training sessions, the monkeys also used head tilt to control the location of the head beam. However, for these movements the head position signal detected by the computer typically did not match the target position (because the experimental setup does not measure torsion), resulting in a low number of rewarded trials. Then, they tried rotating the head, which increased the consistency of rewards. Also, the experimenter manually rewarded the monkeys on trials in which they rotated the head. After extensive training, the monkeys predominantly used head rotation to align the laser with the red target. The remainder of the dissociation trial was then introduced step-by-step.
2.4. Data analysis
Data were analyzed off-line on the PC using in-house software. Velocity criteria were used to detect the onset and offset of gaze, eye and head movements. For gaze and eye, the onset and offset velocity thresholds were 80 and 60°/s, respectively. For head movements, the onset and offset velocity thresholds were 15 and 10°/s, respectively. Head contribution was defined as the change in head position during the gaze shift.
In our experimental apparatus, the ocular counter-rotation rarely commenced before gaze reached the desired target location. (but see, e.g. Phillips, Ling, Fuchs, Siebold, & Plorde, 1995; Cullen & Guitton, 1997). Thus, gaze duration typically equaled eye duration (also see Freedman & Sparks, 1997b). Consequently, eye contribution and eye component were similar and, therefore, referred to interchangeably.
3. Results
The data presented in this paper were collected over several days from each of the two monkeys once they had learned the dissociation task. The dissociation and standard paradigms were randomly interleaved during the data collection. This report describes the effects of systematically controlling eye and head positions prior to gaze shifts made to acquire targets presented along the horizontal meridian.
3.1. Distribution of IEPh
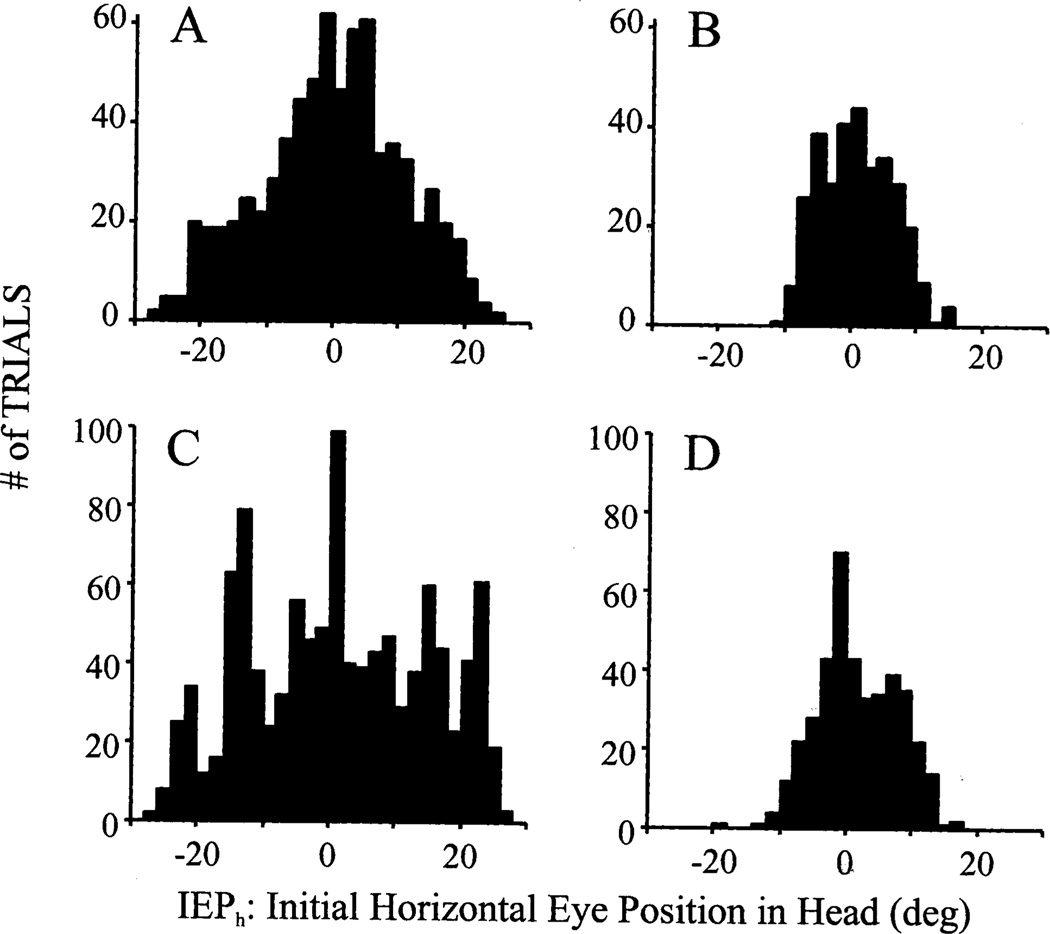
Fig. 3 plots for both monkeys the distributions of the initial eye position relative to the head (IEPh) prior to onset of G2Y and Y2Y movements in the dissociation and standard paradigms, respectively. For the G2Y trials, the red LED was illuminated at straight-ahead position and the green LED was presented within 30° of it. The key feature of the figure is to illustrate the range covered by the distributions of the two task conditions. The standard deviation of the IEPh distribution for the dissociation (Fig. 3A) and standard tasks (Fig. 3B) were 10.82° (n=728) and 5.40° (n=317), respectively, for monkey BE and 13.50° (n=1070; Fig. 3C) and 6.05° (n=404; Fig. 3D), respectively, for mon-key CH. For both monkeys, the variances of the dissociation and standard tasks were found to be significantly different (monkey BE: F=4.02, P < 0.01; monkey CH: F=4.98, P < 0.01). Thus, a larger range of IEPh can be achieved with the dissociation task. Note that the data illustrated in Fig. 3 were obtained from a single session to demonstrate that the laser method is effective in producing a range of IEPh during a time period similar to an electrophysiology recording session.
Fig. 3.
Distribution of initial eye position in head (IEPh) prior to onset of (A, C) G2Y movements in the dissociation paradigm and (B, D) Y2Y gaze shifts in the standard condition. For the dissociation task, the red LED was presented at the straight-ahead position. Data are from monkey BE (A, B) and monkey CH (C, D).
Data were also collected for other red LED positions in monkey CH (not shown), and these IEPh distributions were similar to that shown in Fig. 3C. For example, the standard deviation of IEPh for − 20° and 20° red LED locations, with the green LED illuminated within 30° from the head target, was 12.45° and 14.02°, respectively. Comparisons of the variances with the data collected for 0° placement of the red LED failed to show any statistical differences (F-test; P > 0.05).
3.2. Coordinated eye and head movements
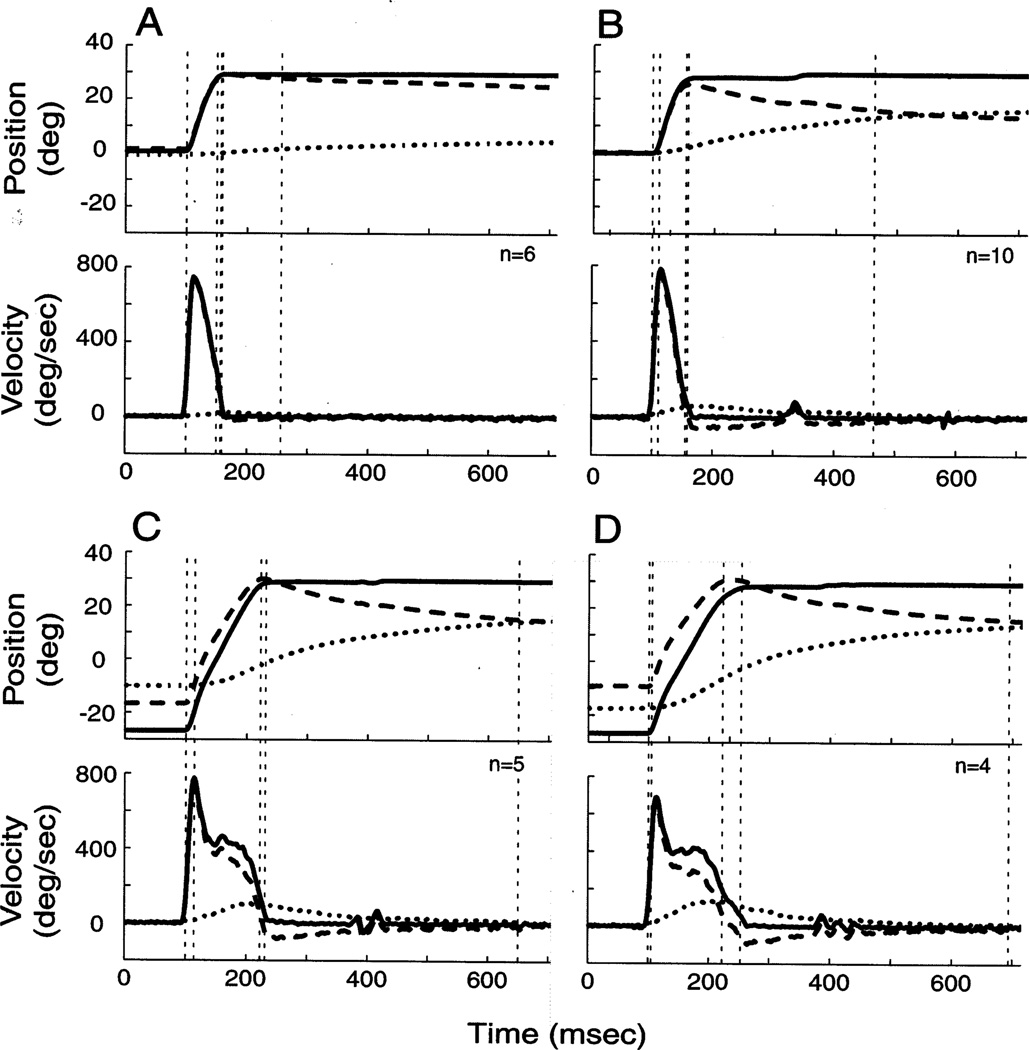
In attempting to dissociate the eye-in-head and head-in-space positions, does the laser technique compromise the coordination of eye and head movements during gaze shifts? For a qualitative assessment, we compared the temporal traces of R2G and G2Y movements with similar amplitude Y2Y gaze shifts. Movements to a 30° displacement from straight-ahead in the R2G condition (Fig. 4A) on average induced a smaller head amplitude than in the Y2Y paradigm (Fig. 4B). Thus, the monkey may have learned to associate the presence of the laser with minimizing or preventing head movements. Since the laser was not turned on during G2Y gaze shifts, we hypothesized that the coordination of eye and head movements would be comparable for G2Y and Y2Y movements. Fig. 4C shows that head movements contribute significantly during large G2Y gaze shifts, similar to Y2Y movements for the same target displacement (Fig. 4D).
Fig. 4.
Temporal traces of gaze shifts during (A) R2G; (C) G2Y; and (B, D) Y2Y movements. Each panel plots the average, horizontal position (top) and velocity (bottom) waveforms for gaze (solid curves), head-in-space (dotted curves) and eye-in-head (dashed curves) as a function of time. The target displacement, or the desired gaze shift, was 30° starting from center (A, B) and 60° starting from −30° (C, D). The traces are aligned on gaze onset (the first vertical, dashed line at 100 ms). The remaining four vertical lines, from left to right, mark head movement onset, eye offset, gaze offset and head offset. The velocity criteria used to detect these measures are discussed Section 2.4. For R2G movements (A), eye and gaze offset marks are nearly superimposed. The ripples apparent in the velocity traces around 400 ms for the G2Y and Y2Y conditions resulted from averaging unaligned corrective movements. Data are from monkey CH.
For the gaze shifts shown in Fig. 4C–D, the green and yellow LEDs were presented at − 30° and 30°, respectively. In the Y2Y paradigm (Fig. 4D), the monkey shifted the initial head position such that the IEPh was within 10° of the center of orbits. Because the R2G movement that preceded the G2Y gaze shift dissociated the eye-in-head and head-in-space positions, a larger range of IEPh were sampled with ease.
For head-unrestrained gaze shifts, eye amplitude saturates and both head contribution and head amplitude increase linearly with gaze amplitude (Morasso, Bizzi, & Dichgans, 1973; Guitton, Douglas, & Volle, 1984; Tomlinson & Bahra, 1986; Guitton & Volle, 1987; Guitton, Munoz, & Galiana, 1990; Volle & Guitton 1993; Phillips, Ling, Fuchs, Siebold, & Plorde, 1995; Freedman & Sparks, 1997b). Furthermore, this relation is influenced by the IEPh (Tomlinson, 1990; Delreux et al., 1991; Becker & Jürgens, 1992; Volle & Guitton; Freedman & Sparks). When the eyes are centered in the orbits, small gaze shifts (<20°) are usually completed without any head contributions. The head contribution increases linearly, while the eye component saturates asymptotically around 35° with further increases in gaze amplitude. When the eyes are deviated contralateral to the direction of gaze shifts, the ocular component increases and the head contribution decreases. Does the dissociation task, which routinely positioned the eyes away from the center of the head before the onset of G2Y movements, alter the relationship of eye and head contributions?
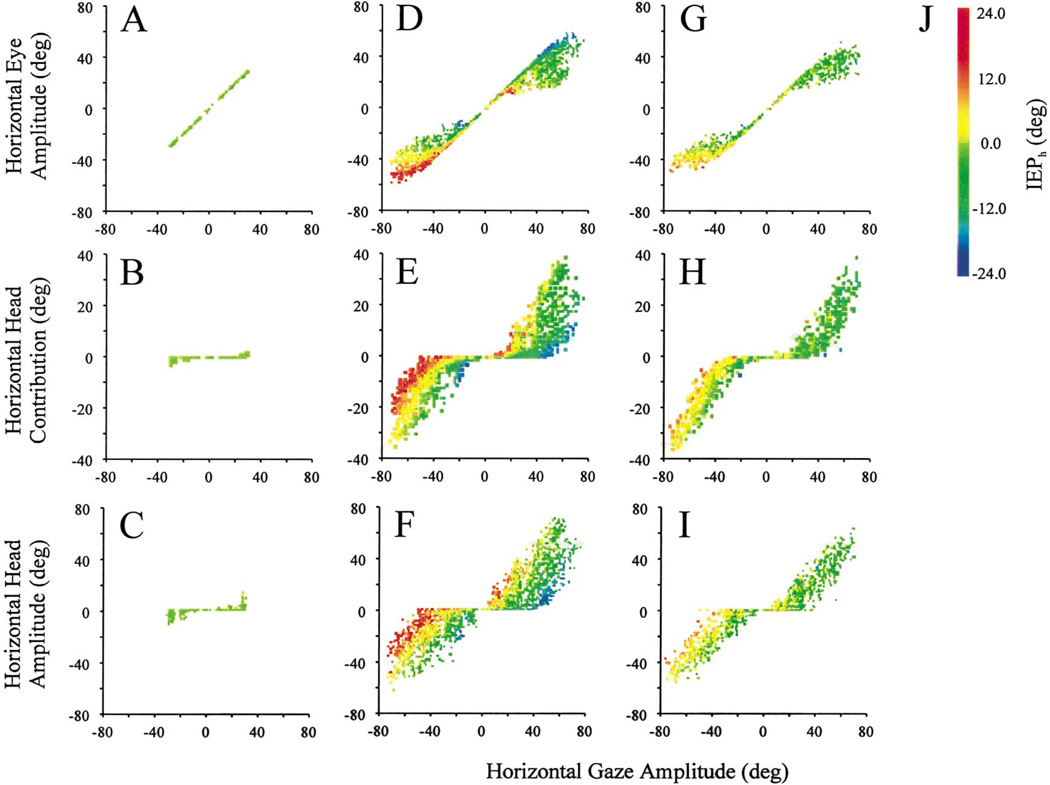
To answer this question, we analyzed and compared the horizontal eye amplitude, horizontal head contribution and horizontal head amplitude of gaze shifts executed during the dissociation and standard tasks. Fig. 5 plots these parameters as a function of horizontal gaze amplitude for R2G, G2Y, and Y2Y movements. For R2G condition, gaze amplitudes <30° were successfully completed by the eyes (Fig. 5A) with minimal contribution by the head (Fig. 5B). Because the head often drifted during fixation after the initial gaze shift, its amplitude may be slightly larger than its contribution (Fig. 5C). As these gaze shifts began with the visual axis and head direction approximately aligned, the eyes were typically centered in the orbits at onset of R2G movements, as noted by the same color symbols in Fig. 5A–C. These movements were designed to dissociate the eye position in head, such that a range of IEPh was available prior to the G2Y movement. This observation is illustrated by a full spectrum of color of the symbols in Fig. 5D–F.
Fig. 5.
Illustration of the eye–head lawful relationship for gaze shifts for R2G movements (A–C), G2Y movements (D–F), and Y2Y movements (G–I). The horizontal components of eye amplitude (A, D, G), head contribution (B, E, F) and total head amplitude (C, F, I) are plotted as a function of horizontal gaze amplitude for the different classes of gaze shifts. The colored symbols in these plots mark the initial eye position in head (IEPh), as shown by the spectrum in (J). The data presented here and in subsequent figures were pooled across 2 days sessions to obtain a definitive comparison of gaze shifts produced in the dissociation and standard tasks.
For G2Y movements <30°, eye amplitude increased with gaze amplitude. For larger gaze shifts, the ocular component saturated only when the eyes were centered in the orbits or were ipsilateral to the direction of movement. For IEPh contralateral to the direction of the movement, our data set typically did not show a saturation of the eye component. Similarly, the head contribution (Fig. 5E) and head amplitude (Fig. 5F) were minimal during small gaze shifts, but increased for larger movements. For a specific (large) gaze amplitude, both head contribution and amplitude increased as the IEPh was increasingly more ipsilateral to the direction of movement.
In contrast to the dissociation task, initial head or eye-in-head positions were not controlled in the standard task (Fig. 5G–I). Thus, most Y2Y movements began with the eyes within ±10° from the center of orbits. In this condition, the eye amplitude began to saturate with increasing gaze amplitude (Fig. 5G), whereas head contribution (Fig. 5H) and head amplitude (Fig. 5I) increased linearly.
A statistical comparison of the G2Y and Y2Y movements was performed to determine whether gaze shifts were quantitatively different in the two paradigms. Our analysis was limited to movements initiated when IEPh was ±10°, and data from both monkeys were combined. We found that for rightward gaze shifts larger than 40°, each of the two monkeys displayed small, but significant differences in the ratio of eye–head contributions in the two tasks (two-tailed t-test; P < 0.01). The magnitude (mean ±SD) of the difference was 6.6 ± 3.4° for eye amplitude, 5.7 ± 2.0° for head contribution, and 9.4 ± 3.4° for head amplitude. In addition, the direction of discrepancy was in opposite directions for the two monkeys — the head contribution, for example, was greater (smaller) during G2Y movements compared to Y2Y gaze shifts in monkey CH (monkey BE). In contrast, leftward gaze shifts larger than 40° were statistically not different between the two task conditions (two-tailed t-test; P > 0.2), and the mean magnitude of the difference was <1° for all three measures of interest.
The small differences, observed during rightward gaze shifts, do not distract from the utility of the method because the overall eye–head decomposition principles are similar for the two paradigms. This technique is able to dissociate eye and head positions in non-humans in a way that is not possible without using these behavioral methods. Hence, the goal of the design, to allow electrophysiology experiments during experimental control of eye-in-head and head-in-space positions, is fulfilled.
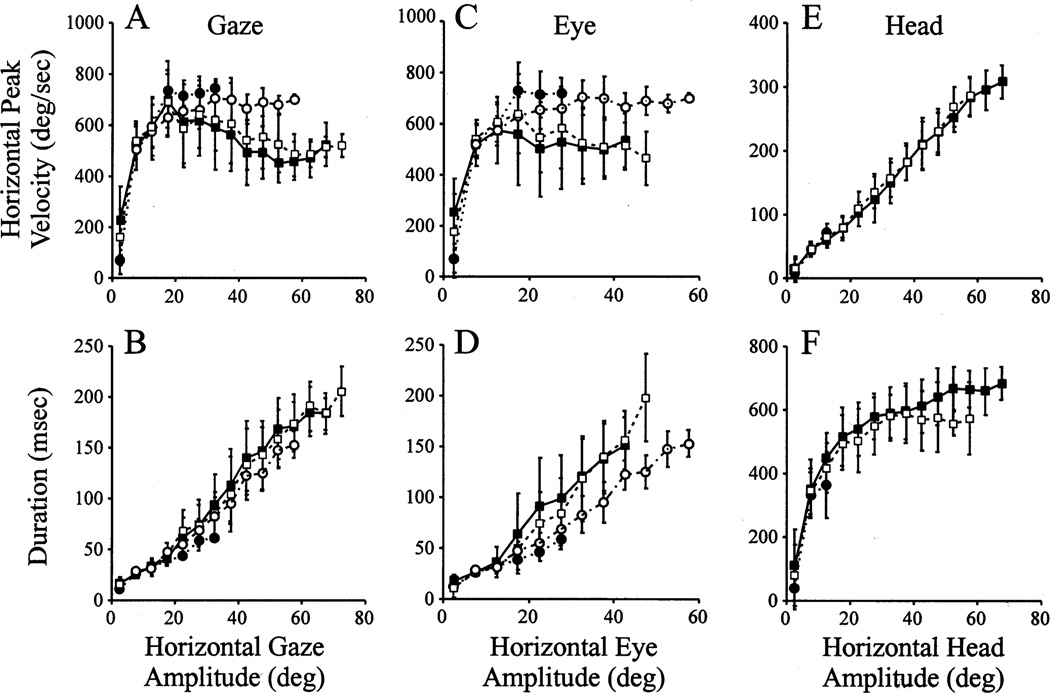
3.3. Main sequence properties
Since the relationship of eye and head contributions does not provide insight into the kinematics of the gaze shifts, we also examined their main sequence properties (Bahill, Clark, & Stark, 1975). Fig. 6 shows average plots of peak velocity- and duration-amplitude relationships for horizontal gaze, eye, and head movements of monkey CH. For the G2Y and Y2Y gaze shifts, the main sequence properties are remarkably similar, as noted by the overlap of the open and filled squares. The peak velocity of gaze and eye initially increased with gaze amplitude but then decreased for gaze shifts larger than approximately 20° (Fig. 6A). The saturation of peak velocity during large head-restrained saccades indicates that the peak eye velocity for eye amplitudes larger than 20° associated with head-unrestrained gaze shifts (Fig. 6C) did not saturate but instead declined (two-tailed t-test, P < 0.001). Thus, the decline in peak gaze velocity associated with large movements was concurrent with and due to the decrease in peak eye velocity.
Fig. 6.
Average plots of the main sequence properties of gaze, eye and head movements during head-unrestrained gaze shifts of monkey CH. (A) Horizontal gaze peak velocity vs. horizontal gaze amplitude; (B) Horizontal gaze duration vs. horizontal gaze amplitude; (C) Horizontal eye peak velocity vs. horizontal eye amplitude; (D) Horizontal eye duration vs. horizontal eye amplitude; (E) Horizontal head peak velocity vs. horizontal head amplitude; (F) Horizontal head duration vs. horizontal head amplitude. The filled squares joined by solid lines refer to G2Y movements in the dissociation paradigm. The open squares linked by dash lines quantify Y2Y movements in the standard task. The filled circles connected by dots represent R2G movements in the dissociation task. The open circles associated with the dot-dash lines correspond to saccades collected in the head-restrained condition. In all cases, the error bars mark one standard deviation. Only those movements with IEPh = ± 10° (of center) were included in the analysis. Leftward and rightward gaze shifts were combined. The window size to average the data was 5°.
The duration of both gaze (Fig. 6B) and eye (Fig. 6D) movements increased linearly with amplitude, independent of the task. For both dissociation and standard paradigms, head velocity increased linearly with head amplitude (Fig. 6E). Head duration initially increased with head amplitude but then gradually saturated for larger movements (Fig. 6F). For head movements larger than ~ 40°, the head duration was longer for the dissociation paradigm. However, only the mean duration corresponding to head amplitudes spanning 50–55° (2nd open square from the right in Fig. 6F) was statistically different for the two trial-types (two-tailed t-test, P < 0.01). All other duration and peak velocity comparisons for gaze, eye and head were not statistically different (two-tailed t-test, P > 0.05). Overall, the main sequence results for the G2Y and Y2Y movements resemble those reported previously (cat: Guitton et al., 1984; Guitton et al., 1990; humans: Guitton & Volle, 1987; Volle & Guitton 1993; monkeys: Morasso et al., 1973; Tomlinson & Bahra, 1986; Phillips et al., 1995; Freedman & Sparks, 1997b).
We also examined the main sequence properties of G2Y movements for IEPh outside the ±10° range (data not shown). The general finding was an increase (decrease) in peak gaze and eye velocity and duration for movements initiated with the eyes deviated contralateral (ipsilateral) to the direction of the gaze shift. Conversely, the head peak velocity and duration decreased (increased) for gaze shifts initiated with contralateral (ipsilateral) IEPh. This trend is expected given the effects of IEPh on eye and head contributions to a specific amplitude gaze shift (Fig. 5; Tomlinson, 1990; Delreux et al., 1991; Becker & Jürgens, 1992; Volle & Guitton, 1993; Freedman & Sparks, 1997b).
While the kinematics of G2Y and Y2Y gaze shifts were similar, those of R2G movements were notably different. The peak velocity of R2G movements and head-restrained saccades were compared for eye amplitudes greater than 15°, referring to the right three filled circles (and corresponding open circles) in Fig. 6A, C. For all three distributions, the mean peak velocities of R2G gaze shifts and head-restrained saccades were significantly different (two-tailed t-test, P < 0.01). Monkey BE also exhibited similar behavior (data not shown). Thus, both monkeys appeared to have developed a strategy to make faster ocular saccades in order to minimize their head movements, but only while shifting their line of sight from the red to green LED.
3.4. Head latency relative to gaze onset
Freedman and Sparks (1997b) showed that the onset of head movement typically followed the initiation of small gaze shifts, and that the lag decreased as gaze amplitude increased until the onset of both were nearly synchronous. This property, when pooled over all IEPh, was also observed in our data (not shown) for both paradigms. We next examined whether the timing of head movements was altered differentially by the experimental variation of eye position during G2Y movements (data not shown). For a given gaze amplitude, the timing of the head movement relative to gaze onset decreased as the IEPh became increasingly ipsilateral to the direction of movement. For a constant IEPh, head onset occurred increasingly closer to gaze onset as the gaze amplitude increased. For certain combinations of IEPh and gaze amplitude, such as IEPh ipsilateral to the direction of large gaze shifts, head movement onset consistently led gaze onset. These trends were present in the gaze shifts collected during both tasks and, in addition, were similar to previous observations (Becker & Jürgens, 1992; Volle & Guitton 1993; Fuller 1996; Freedman & Sparks).
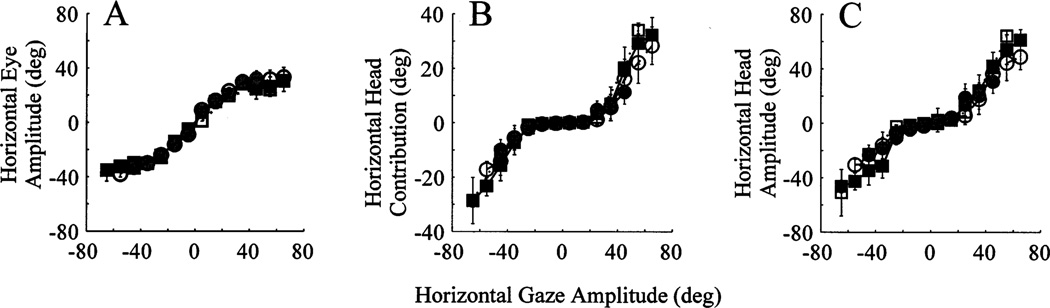
3.5. Effect of head position (eyes centered in orbits)
The miniature micro-laser method enables the experimenter to systematically control not only the eye position in head but also the head position in space. We examined whether the relationship of eye and head contributions changed for gaze shifts starting from different initial head position. Horizontal eye amplitude, horizontal head contribution and horizontal head amplitude are plotted as a function of horizontal gaze amplitude in Fig. 7 for four different ranges of initial head position. The analysis was limited to gaze shifts whose IEPh was within 10°. A pronounced overlap of the four curves emphasizes that the initial head position does not affect the eye and head contributions for gaze shifts initiated with the eyes roughly centered in orbits.
Fig. 7.
Effect of head position on the lawful relationship of eye and head contributions to head-unrestrained gaze shifts. The horizontal components of eye amplitude (A), head contribution (B) and total head amplitude (C) are plotted as a function of horizontal gaze amplitude for G2Y movements. Each curve represents a different initial head position range: filled circles, dot-dashed lines, initial head position within 10° of straight-ahead position; open circles, dashed lines, initial head position 10–20° of straight-ahead position; filled squares, solid lines, initial head position 20–30° of straight-ahead position; open squares, dotted lines, initial head position 30–40° of straight-ahead position. Only gaze shifts with IEPh within 10° were averaged in the plot. The window size to average the data was 5°.
4. Discussion
When the head is unrestrained, visual redirection between targets is typically achieved by a coordinated movement of the eyes and head, and their contributions depend on IEPh. Under natural conditions, these gaze shifts are usually initiated with the eyes nearly centered in the orbits, and many trials and sessions are required to collect data spanning an extended range of IEPh. In this paper, we have described a technique that allows the experimenter to control the eye-in-head and head-in-space positions and effectively collect the desired data in a single session. To demonstrate that this technique serves its purpose, the reader is referred to Fig. 3, which illustrates that, compared to the standard task, the dissociation paradigm can produce a larger range of IEPh in a limited number of trials. To test if the properties of head-unrestrained gaze shifts were altered by the dissociation paradigm, we examined the relationship of eye and head contributions, main sequence properties, the timing of head movement relative to gaze onset and the effects of head position. These parameters were similar to those quantified from the standard task and, in turn, verified previous reports (e.g. Morasso et al., 1973; Guitton et al., 1984; Tomlinson & Bahra, 1986; Guitton & Volle, 1987; Guitton et al., 1990; Tomlinson, 1990; Delreux et al., 1991; Becker & Jürgens, 1992; Volle & Guitton 1993; Phillips et al., 1995; Freedman & Sparks, 1997b). Therefore, we conclude that the miniature laser method in conjunction with the dissociation paradigm does not change the behavioral properties of gaze shifts.
4.1. Applications in neurophysiology experiments
One controversial issue in oculomotor neurophysiology has been the role of neurons in the primate pontomedullary reticular formation in generating gaze shifts (e.g. Whittington, Lestienne, & Bizzi, 1984; Cullen & Guitton, 1997; Ling, Fuchs, Phillips, & Freedman, 1999; Sparks, 1999; Cullen, Galiana, & Sylvestre, 2000). For the head-unrestrained condition, it is now debated whether the activity of these neurons, whose role during saccades in head-restrained condition had been dogma (see Moschovakis, Scudder, & Highstein, 1996 for a review), correlates better with the ocular component or the coordinated eye–head gaze shift. One potential approach that may elucidate whether neurons in these oculomotor structures issue gaze or eye related commands is to investigate the neural responses associated with gaze shifts whose magnitude remains relatively constant but the ocular component spans a large range of amplitudes. Such movements can be generated if the IEPh can be efficiently varied for the same amplitude gaze shift.
We have been using this methodology in conjunction with microstimulation experiments to investigate the role of pontine reticular formation and frontal cortex in controlling head-unrestrained gaze shifts (Chen & Sparks, 2000; Gandhi & Sparks, 2000; Sparks, Freedman, Chen, & Gandhi, 2001). We have found that varying the IEPh while stimulating, for example, the pontine burst neuron region (with constant stimulation parameters) changes the eye and head contributions while producing the same amplitude displacement in gaze. Thus, the techniques described in this paper enable neurophysiology experiments that require systematic control of IEPh.
4.2. Previous studies that controlled eye and head positions
Other studies have implemented various techniques to dissociate eye/gaze and head positions, both in monkeys and humans. To analyze the effects of head position on saccade-related activity of parietal neurons, Brotchie, Andersen, Snyder, and Goodman (1995) taught monkeys to associate different stimulus sizes as gaze and head targets. To examine potential violations of Donder’s law of the head, Ceylan, Henriques, Tweed, and Crawford (2000) asked human subjects to fixate a central target while aligning a laser, which was mounted on a helmet fastened on the head, with eccentric targets. To understand eye–head coordination rules under conditions that require sensorimotor transformations, Goossens and Van Opstal (1997) controlled eye-in-head and head positions in humans using different color targets. To investigate the role of frontal eye fields in the control of head-unrestrained gaze shifts, Tu and Keating (2000) required monkeys to align an onscreen cursor, which represented the monkey’s instantaneous head position, with a red target and then direct its gaze (eyes only) to a larger white target. To study head-unrestrained smooth pursuit, Wellenius and Cullen (2000) trained one monkey first to voluntarily align its head with a primary visual cue, next make an ocular saccade to a second target, and finally pursue the latter stimulus as it moved at constant velocity.
Insights on the behavioral effects of the dissociation paradigms can be gathered from some of these reports. Ceylan et al., (2000) found that Donder’s law breaks down when the subjects aligned the head-mounted laser with eccentric targets while holding gaze constant, implying that they had changed their strategy of accomplishing the task. As their study addressed different questions from our experiments, it is not clear whether the lawful relationship of eye and head contributions to gazes shifts and their kinematics would change in their experimental conditions. Wellenius and Cullen (2000) found the monkey became conscious of its head position and, consequently, made unnatural movements during head-unrestrained pursuit, leading them to exclude the animal’s data from their study. As these authors did not describe the procedures used to train their monkey, it remains unclear why their monkey’s head-unrestrained pursuit characteristics differed from normal behavior.
To date, we have trained a total of four monkeys using the head-mounted laser in conjunction with several variants of the dissociation paradigms; in all cases, the results are comparable to gaze shifts evoked during natural conditions. We have recently extended this paradigm to control eye-in-head and head-in-space positions in both horizontal and vertical domains. Chen, Gandhi, and Sparks (1999) has compared the properties of vertical gaze shifts in the dissociation and standard paradigms and found that head contribution and amplitude are significantly smaller than during amplitude matched horizontal gaze shifts (also see, Freedman & Sparks, 1997b).
4.3. Summary
In summary, we have developed a technique and behavioral paradigms that enable the experimenter to systematically control gaze and head positions in space. Our analyses demonstrate that the head-unrestrainedgaze shifts produced in this task are comparable to those observed during a standard paradigm; the relationship of eye and head contributions, the main sequence properties of the movements, and the timing of head onset relative to gaze onset are similar for both paradigms. In addition, we found that changing the initial head position of gaze shifts does not alter the eye–head coordination relationship. Thus, we conclude this behavioral method, in conjunction with electrophysiological and pharmacological experiments, may facilitate the study of the neural control of gaze.
Acknowledgements
We acknowledge Dr Longtang L. Chen for meaningful discussions and ideas on modifying the primate chair. We also thank Kathy Pearson for programming assistance and the anonymous referees for their critical reviews.
This study was supported by NIH Grants EY07009 (NJG), EY07001 (NJG) and EY01189 (DLS).
References
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Mathematical Biosciences. 1975;24:191–204. [Google Scholar]
- Becker W, Jürgens R. Gaze saccades to visual targets: does head movement change the metrics. In: Berthoz A, Graf W, Vidal PP, editors. The head–neck sensory motor system. New York: Oxford University Press; 1992. pp. 427–433. [Google Scholar]
- Brotchie PR, Andersen RA, Snyder LH, Goodman SJ. Head position signals used by parietal neurons to encode locations of visual stimuli. Nature. 1995;375:232–235. doi: 10.1038/375232a0. [DOI] [PubMed] [Google Scholar]
- Ceylan M, Henriques DY, Tweed DB, Crawford JD. Task-dependent constraints in motor control: pinhole goggles make the head move like an eye. Journal of Neuroscience. 2000;20:2719–2730. doi: 10.1523/JNEUROSCI.20-07-02719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Gandhi NJ, Sparks DL. Minimal head contribution to large vertical gaze shifts in rhesus monkeys. Society for Neurosciences Abstract. 1999;25:1649. Abstract. [Google Scholar]
- Chen LL, Sparks DL. Microstimulation of the frontal eye field primarily produces eye movements in head-unrestrained monkeys. Society for Neurosciences Abstract. 2000;26:6755. Abstract. [Google Scholar]
- Cullen KE, Galiana HL, Sylvestre PA. Comparing extraocular motoneuron discharges during head-restrained saccades and head-unrestrained gaze shifts. Journal of Neurophysiology. 2000;83:630–637. doi: 10.1152/jn.2000.83.1.630. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. II. Relationship to gaze, eye, and head movement dynamics during head-free gaze shifts. Journal of Neurophysiology. 1997;78:3283–3306. doi: 10.1152/jn.1997.78.6.3283. [DOI] [PubMed] [Google Scholar]
- Delreux V, Abeele SV, Lefévre P, Roucoux A. Eye–head coordination: influence of eye position on the control of head movement amplitude. In: Paillard J, editor. Brain and space. London: Oxford University Press; 1991. pp. 38–48. [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. Journal of Neurophysiology. 1997a;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eyehead coordination during head-unrestrained head coordination during head-unrestrained gaze shifts in rhesus monkeys. Journal of Neurophysiology. 1997b;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. Journal of Applied Physiology. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuller JH. Eye position and target amplitude effects on human visual saccadic latencies. Experimental Brain Research. 1996;109:457–466. doi: 10.1007/BF00229630. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Sparks DL. Microstimulation of the pontine reticular formation in monkey: effects on coordinated eye–head movements. Society for Neurosciences Abstract. 2000;26:3532. Abstract. [Google Scholar]
- Goossens HHLM, Van Opstal AJ. Human eye–head coordination in two dimensions under different sensorimotor conditions. Experimental Brain Research. 1997;114:542–560. doi: 10.1007/pl00005663. [DOI] [PubMed] [Google Scholar]
- Guitton D, Douglas RM, Volle M. Eye–head coordination in cats. Journal of Neurophysiology. 1984;52:1030–1050. doi: 10.1152/jn.1984.52.6.1030. [DOI] [PubMed] [Google Scholar]
- Guitton D, Munoz DP, Galiana HL. Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. Journal of Neurophysiology. 1990;64:509–531. doi: 10.1152/jn.1990.64.2.509. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye–head coordination during orienting movements to targets within and beyond the oculomotor range. Journal of Neurophysiology. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Huebner WP, Paloski WH, Reschke MF, Bloomberg JJ. Geometric adjustments to account for eye eccentricity in processing horizontal and vertical eye and head movement data. Journal of Vestibular Research. 1995;5:299–322. [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Research. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuchs AF, Phillips JO, Freedman EG. Apparent dissociation between saccadic eye movements and the firing patterns of premotor neurons and motoneurons. Journal of Neurophysiology. 1999;82:2808–2811. doi: 10.1152/jn.1999.82.5.2808. [DOI] [PubMed] [Google Scholar]
- Morasso P, Bizzi E, Dichgans J. Adjustment of saccade characteristics during head movements. Experimental Brain Research. 1973;16:492–500. doi: 10.1007/BF00234475. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Progress in Neurobiology. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Ling L, Fuchs AF, Siebold C, Plorde JJ. Rapid horizontal gaze movement in the monkey. Journal of Neurophysiology. 1995;73:1632–1652. doi: 10.1152/jn.1995.73.4.1632. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method for measuring eye movement using a scleral search coil in a magnetic field. IEEE Transactions in Bio-Medical Engineering, BME-10. 1963:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Current Opinion in Neurobiology. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Freedman EG, Chen LL, Gandhi NJ. Cortical and subcortical contributions to coordinated eye and head movements. Vision Research. 2001;41:3295–3305. doi: 10.1016/s0042-6989(01)00063-3. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD. Combined eye–head gaze shifts in the primate. III. Contributions to the accuracy of gaze saccades. Journal of Neurophysiology. 1990;64:1873–1891. doi: 10.1152/jn.1990.64.6.1873. [DOI] [PubMed] [Google Scholar]
- Tomlinson RD, Bahra PS. Combined eye–head gaze shifts in the primate. I. Metrics. Journal of Neurophysiology. 1986;56:1542–1557. doi: 10.1152/jn.1986.56.6.1542. [DOI] [PubMed] [Google Scholar]
- Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. Journal of Neurophysiology. 2000;84:1103–1106. doi: 10.1152/jn.2000.84.2.1103. [DOI] [PubMed] [Google Scholar]
- Volle M, Guitton D. Human gaze shifts in which head and eyes are not initially aligned. Experimental Brain Research. 1993;94:463–470. doi: 10.1007/BF00230204. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Cullen KE. A comparison of head-unrestrained and head-restrained pursuit: influence of eye position and target velocity on latency. Experimental Brain Research. 2000;133:139–155. doi: 10.1007/s002210000369. [DOI] [PubMed] [Google Scholar]
- Whittington DA, Lestienne F, Bizzi E. Behavior of preoculomotor burst neurons during eye–head coordination. Experimental Brain Research. 1984;55:215–222. doi: 10.1007/BF00237272. [DOI] [PubMed] [Google Scholar]