Abstract
Purpose
Chromoendoscopy with dye spray and the water method both increase adenoma detection.
Hypothesis
Adding indigocarmine to the water method will enhance further the effectiveness of the latter in adenoma detection.
Methods
Screening colonoscopy was performed with the water method (control) or with 0.008% indigocarmine added (study) by two endoscopists. Randomization was based on computer-generated codes contained in blocks of pre-arranged opaque sealed envelopes. High resolution colonoscopes were used. Upon insertion into the rectum, air was suctioned. With the air pump turned off, water was infused using a blunt needle adaptor connected to the scope channel and a foot pump to facilitate scope insertion until the cecum was reached. Residual stool causing cloudiness was suctioned followed by infusion of clear or colored water (water exchange) to facilitate scope passage with minimal distention of the colonic lumen. Upon seeing the appendix opening under water, water was suctioned and air was insufflated to facilitate inspection on scope withdrawal.
Statistics
Sample size calculation revealed 168 patients (84/group) needed to be randomized. Study was IRB-approved and registered (NCT01383265).
Results
There were no significant differences in mean age, gender distribution, BMI, and family history of colon cancer. Cecal intubation success rate was 100% in both groups. The overall adenoma detection rate was 44% (water only) versus 62% (water with indigocarmine), respectively (p=0.03). One cancer was detected in each group.
Conclusion
In a RCT, indigocarmine at 0.008% concentration, added to the water method, significantly enhanced further the effectiveness of the latter in detecting adenomas.
Key words: colon cancer screening, water method, indigocarmine, adenoma detection
Introduction
Chromoendoscopy with target dye spraying has been used to outline the extent of mucosal lesions and in defining the possible underlying pathology of colonic lesions. In cases of underlying polyps, chromoendoscopy has been used to identify potential neoplastic lesions. However, it is a cumbersome technique as it uses a concentrated dye solution with subsequent washing before examination and biopsy. As a result, it has not become a popular or commonly practiced procedure except for research purposes.1 The water method (with water exchange) has been shown to be superior to the conventional air method in screening colonoscopy. Patients were able to complete the colonoscopy with less sedative medication,2–5 a higher proportion of patients were able to complete the procedure without medication when offered the option of o-demand sedation.6 A review of published data on prospective RCTs involving water colonoscopy showed that water method (with exchange) may be superior to water immersion in adenoma detection rate (ADR).7 Our pilot retrospective study suggested that use of water method appeared to increase adenoma detection.8–9 The addition of indigocarmine to the water method showed a significantly higher ADR compared to the water method using plain water and the conventional air method.10 In this study, we performed a RCT to evaluate the potential benefits of adding indigocarmine to the water method compared to the standard water method in adenoma detection in patients undergoing screening colonoscopy. We test the hypothesis that with dye added to the water used in the water method, ADR can be enhanced. The study was approved by the Sacramento VA IRB and registered with ClnicalTrial.gov (NCT01383265).
Methods
As reported in our previous study,10 plain water was used as control and the combined dye and water (study) method utilized a 0.008% indigocarmine solution (by adding 10 ml 0.8% indigocarmine to a liter of water) used with the standard water method.
Patients received usual bowel preparation including a low-residue diet for 2 days before and 2 tablets of bisacodyl plus 1 gallon of Colyte (polyethylene glycol-electrolyte) on the day before colonoscopy given in two divided doses.2–4 Informed consent was obtained from the patient after full discussion of the risk and benefits of the study. Patients who signed the consent were randomized according to a computer generated code contained in a series of sealed opaque envelopes. Premedication included a combination of intravenous Diphenhydramine, Fentanyl and Midazolam.2–4 High resolution colonoscopes from Olympus were used.
Water method
The air pump on the light source generator (CLV 180; Olympus, Tokyo, Japan) was turned off prior to insertion of the colonoscope. Warm water (at 37 °C) was infused using a peristaltic pump (Endolav EL-100C, Cooper Surgical, Trumbull, CT, USA) with a blunt needle adaptor inserted through the biopsy channel of the colonoscope. Upon scope insertion into the rectum, air was suctioned and water was infused using the needle adaptor and a paddle pump to facilitate the passage of the colonoscope with minimal distension of the colonic lumen. When air pockets were encountered, the air was suctioned to reduce looping and straightened the colon at the flexures, before further infusion of water. Water was suctioned during scope passage to keep a minimum amount of water in the colon upon scope insertion. When cloudiness of water secondary to residual stool was seen, the water was suctioned to remove the residual feces before water (clear or colored) was infused to identify the lumen to facilitate scope passage. The colonoscope was advanced by a series of to and fro, back and forth, or repeated insertion and withdrawal motions of the shaft of the colonoscope with a torque in the direction of the expected lumen, using intermittent water infusion. Water exchange with suction and infusion in rapid sequence was performed to facilitate clear visualization of the lumen. To minimize suction of the mucosa into the endoscope channel the water infusion was started first followed by application of suction. The volume of water needed to clear the view (200 to 2000 ml) was kept to a minimum, but not restricted. The collapsed colonic lumen allowed the water to more adequately soak the colonic surfaces and remove the adherent stool from the colonic mucosa. The turbulence set up by the sequential infusion and suction of water in the collapsed lumen dislodged the residual feces from the surrounding mucosa in close proximity to the tip of the colonoscope. This maneuver made removal of the residual feces “easier” than washing with a single water jet in a dilated air filled colon. Most of the infused water was aspirated into the suction bottle rapidly instead of being left in the colon, and over-distension of the colon was obviated. If advancement failed, the assistant provided abdominal compression followed by changing the patient position if necessary. If the advancement was uninterrupted, no abdominal pressure or change in patient position was used. For the combined dye and water method, residual stool in the proximal colon (especially in patients with inadequate bowel preparation) changed the indigocarmine solution to a greenish color. Continuous exchange of the dirty water was performed until a clear blue color was seen. If the appendix opening was seen under water or when the cecum was thought to be reached by external finger palpation, the air button was turned on to confirm the location. If the cecum had not been reached, failed intubation was recorded based on intent-to-treat (ITT) but colonoscopy was continued. Cecal intubation was defined as described above. Any residual water was suctioned on scope withdrawal to facilitate examination.
Intermittent air insufflation (air pump set at high flow) was used to distend the colon on scope withdrawal for inspection, biopsy and polypectomy. Washing of any residual stool covered mucosa was performed by water or in the combined method with diluted indigocarmine irrigation. Inspection of the mucosa including examination behind folds was performed systematically as needed. After turn around in the rectum, residual air in the colon was removed by suction.
Statistics
The ADR (defined as proportion of subjects with at least one adenoma of any size) in the study group (combined indigocarmine and water method) was compared with the water method using plain water. Power calculation indicated that 168 patients (84/group) were needed to be randomized. The tabulated data were analyzed using t-test and Fisher's exact test. A p value of <0.05 was considered to be significant.
Results
The results are presented in the tables. A total of 168 patients (84 in each group) were randomized. There were no significant differences in the mean age, gender distribution, BMI, and family history of colon cancer (Table 1). The cecal intubation success rate was 100% in both groups. The overall ADR was 37/84 (44%)(control with water only) versus 52/84 (62%)(study or combined dye plus water), respectively (p=0.03)(Table 2). One cancer was detected in each group.
Table 1.
Patient demographic variables
| Water Method (n=84) | Indigocarmine added to Water Method (n=84) | p | |
| Mean Age | 58 | 58 | ns* |
| Male/Female | 81/3 | 76/8 | 0.2108** |
| BMI | 28.9 | 29 | ns* |
| Smoker | 29 (35%) | 38 (45%) | 0.2073** |
| Family history of colon cancer | 5 | 7 | ns* |
t-test;
Fisher's exact test.
Table 2.
Procedure-rated outcomes
| Water Method | Indigocarmine added to Water Method | p | |
| Number of patients | 84 | 84 | |
| Cecal intubation success | 100% | 100% | ns* |
| Adenoma detection rate (ADR) | 37/84 (44%) | 52/84 (62%) | 0.0302** |
| Cancer | 1 | 1 | ns* |
| Number of patients with normal biopsies | 12 | 18 | 0.3139** |
t-test;
Fisher's exact test.
Discussions
Canadian and German data indicated that colonoscopy failed to reduce incident cancers and cancer mortality in the right colon.11–13 More recent US data have confirmed the same.14,15 A plausible explanation is missed adenomas in the proximal colon during screening colonoscopy. Small lesions are more easily missed especially in the proximal colon. Different reasons may account for the high proximal missed rates. These include poor bowel preparation and failed cecal intubation. Despite an improvement in the overall bowel preparation score, split-dose bowel preparation did not improve overall adenoma detection. Adenoma detection rate, but not cecal intubation rate was an independent predictor of the risk of interval colorectal cancer after screening colonoscopy.16 The impact of recent development in technology and techniques on ADR has been highly variable and inconclusive. These include colonoscopy performed with high-definition, wide-angle endoscope;17–21 narrow band imaging;22–25 transparent hood attached to the tip of the colonoscope23,26,27 withdrawal time >6 min28,29 or modified (e.g. split-dose) bowel preparation.30,31 An optimal “colonoscopist-controlled” technique to enhance ADR and minimize “missed” right-sided lesions is desirable. The water method (with water exchange) has been shown to increase overall ADR compared to the conventional air method and water immersion method where a combination of air and water was used during the examination.7 The increased ADR may be a result of higher cecal intubation success rate as compared to the air method with a combined effect of warm water that reduces colon spasm and facilitates examination.
A major limitation of the conventional dye-spray chromoendoscopy method is poor bowel preparation. In these studies, proportion of patient excluded varied from 4%,32 5%,33, 8%34 to 9%.17 Intent-to-treat analysis would have dictated an adjustment (decrease) of the ADR reported in each of these studies by the proportion of patients excluded due to poor bowel preparation (see values in [ ] in Table 3). The results of the current report indicate that the water method with dye in the form of 0.008% indigocarmine added to the water method is feasible. A high overall ADR of 62% was achieved. The salvage cleansing effect of the water exchange, which is an integral part of the water method,5,9 obviated the need to exclude any patients because of poor bowel preparation even when indigocarmine was added to the water. The water (with or without indigocarmine) could provide cleansing of the colon with suboptimal bowel preparation. The water exchange reduces the amount of residual water and allows undistracted examination on scope withdrawal. Coupled with the dye serving as a surface contrasting agent, the diluted indigocarmine highlighted the surface irregularities and outline of mucosal lesions including polyps and enhanced the detection of adenomas.
Table 3.
Comparison of ADR in the current study and those reported in the literature
| ADR water method | 44% | Current report |
| ADR water method with indigocarmine added | 62% | |
| Representative published overall ADR | ADR | Literature Reference |
| Narrow band imaging | 23% | 24 |
| High definition colonoscopies | 24.7% | 20 |
| Withdrawal time >6 min | 28.3% | 29 |
| High definition colonoscopies | 28.8% | 19 |
| Chromoendoscopy and standard colonoscope (after excluding 4% with poor bowel preparation) | 33.6% [29.6%] | 32 |
| Chromoendoscopy and standard colonoscope (after excluding 5% with poor bowel preparation) | 35.4% [30.4%] | 33 |
| High resolution colonoscope | 42 | 37 |
| White light and high definition colonoscope | 41–57% | 17 |
| Narrow band imaging | 51% | 21 |
| Chromoendoscopy and high definition colonoscope (after excluding 9% with poor bowel preparation) | 55.5% [46.5%] | 17 |
| Narrow band imaging | 57.3% | 25 |
| White light | 58.3% | 25 |
| High definition colonoscope | 60.4% | 18 |
| Chromoendoscopy (after excluding 8% with poor bowel preparation) | 66.2% [58.2%] | 34 |
Values in [ ] indicate results based on intent-to-treat analysis without excluding the patients with suboptimal bowel preparation.
Recent published rates of adenoma and cancer detection are summarized in tables 3. The ADR of 62% using the combined dye plus water method was similar to our previous reported pilot study and this was higher than those derived from studies using other modalities, even those obtained with high definition colonoscopes,17,18–20 narrow band imaging (NBI)21,24,25 or dye spray chromoendoscopy.32–34
Like the dye spray method, a similar limitation of the current study is inadequate bowel preparation as the residual stool turned the color of the blue water green. The presence of residual stool especially in the proximal colon required additional water irrigation and suction in order to maintain the blue color of the indigocarmine solution. The finding of more adenoma obviously will require additional time in performing biopsies or removal of the lesions found. However, the dye plus water method did not appear to complicate the simple and easy approach of the water method. The diluted solution of indigocarmine did not interfere with examination under water. This combined method is compatible with conventional colonoscope (not requiring high definition or NBI) and minimizes set up cost.
Prevention of colorectal cancer by detection and removal of adenomas has been the recommended practice in the US for almost 20 years.35 National guideline has recommended that endoscopists performing screening colonoscopy should detect adenomas in at least 25% of men and 15% of women age 50 years or older.36 However, variability exists in the detection of adenomas by endoscopists (Table 3). Various methods have been proposed to improve detection of neoplastic lesions including taking adequate time for examination on scope withdrawal.29 However, adherence to mandated withdrawal time of >7 min did not increase ADR.28 The use of chromoendoscopy with dye-spraying has been shown to improve ADR. Soetikno et al.37 reported a neoplastic polyp detection rate of 42% using a targeted dye-spray technique with traditional air insufflation colonoscopy. Chromoendoscopy is particularly useful in the detection of no-polypoid colorectal adenomas.37
Targeted or pa-colonic dye staining during colonoscopy is cumbersome and requires the use of spray catheters1 or injection of the dye and air into the working channel of endoscope to apply a uniform mist of the staining agent onto the mucosa1. In addition, the delivery of dyes via capsule and enemas has been described.38,39 Implementation of chromoendoscopy, however, has not been adopted for routine use in screening and surveillance colonoscopy.1,40 The combination of indigocarmine-the active component of dye spray chromoendoscopy with the water method further enhanced the benefits of the water method. The ADR of 62%, is much higher than the plain water method alone. This prospective RCT confirmed our pilot observation of the beneficial role of this combined method in adenoma detection. The provocative finding indicates a head-to-head comparison of air insufflation, water exchange and water exchange plus indigocarmine to evaluate the hypothesis that the combination of chromoendoscopy and water exchange yields the highest ADR deserves to be performed.
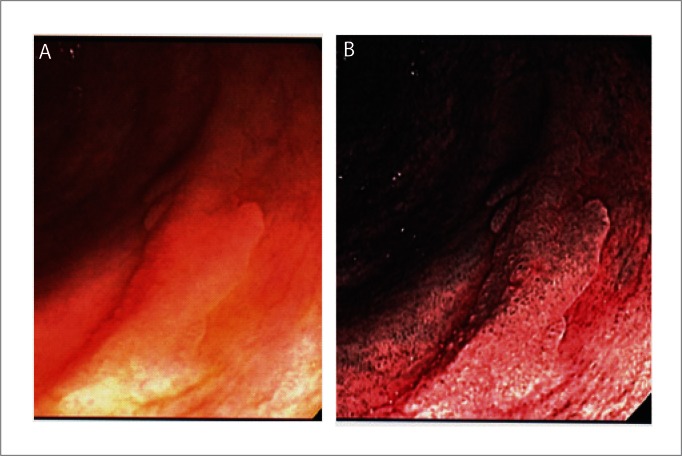
Figure 1.
Polyp seen with water method. A: Flat polyp (adenoma) in proximal colon; B: NBI view of same polyp.
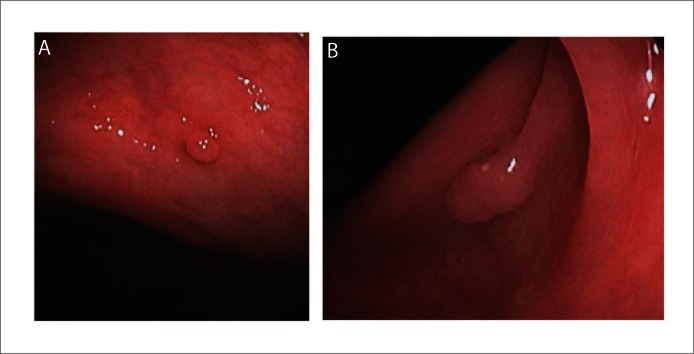
Figure 2.
Polyps seen with water method. A: Small cecal polyp; B: Sessile polyp in ascending colon.
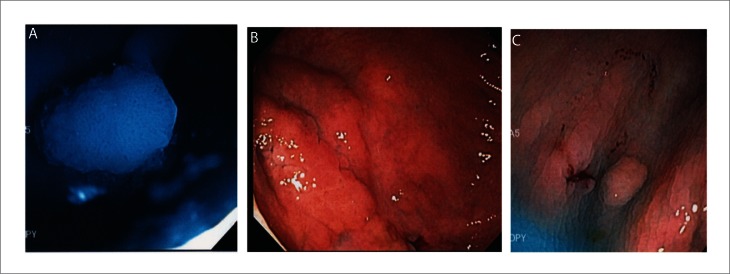
Figure 3.
Polyp seen with dye plus water method. A: Polyp seen under blue (indigocarmine) water; B: Large flat adenoma in proximal colon; C: Small polyp in ascending colon. Note the bluish color fluid filling the surface irregularities and outline of the polyps.
Acknowledgement
The study is support in part by an ASGE and ACG Clinical Research Awards Grant, and by the C.W. Law Research Fund and the Research and Medical Services of the VANCHCS and VAGLAHS.
Abbreviations
- ADR
adenoma detection rate
- NBI
narrow band imaging
- SD
standard deviations
- US
United States
- VA
Veterans Affairs
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
Disclosure
The data was reported as an oral presentation at the ACG Annual Meeting in Washington DC in October 2011; and presented at the Third Colorectal Cancer Screening Symposium, Sacramento VAMC, Mather, CA on March 17, 2012.
JWL received research and educational support from Olympus. The other authors have no conflict of interests relevant to this study to disclose.
References
- 1.ASGE Technology Committee. Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, et al. Chromoendoscopy. Gastrointest Endosc. 2007;66:639–649. doi: 10.1016/j.gie.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Leung JW, Mann S, Leung FW. Option for screening colonoscopy without sedatio- a pilot study in United States veterans. Alimen Pharmacol Thera. 2007;26:627–631. doi: 10.1111/j.1365-2036.2007.03404.x. [DOI] [PubMed] [Google Scholar]
- 3.Leung JW, Salera R, Toomsen L, Mann S, Leung FW. A pilot feasibility study of the method of water infusion without air insufflation in sedated colonoscopy. Dig Dis Sci. 2009;54:1997–2001. doi: 10.1007/s10620-008-0576-4. [DOI] [PubMed] [Google Scholar]
- 4.Leung JW, Mann SK, Siao-Salera R, Ransibrahmanakul K, Lim B, Cabrera H, et al. A randomized, controlled comparison of warm water infusion in lieu of air insufflation versus air insufflation for aiding colonoscopy insertion in sedated patients undergoing colorectal cancer screening and surveillance. Gastrointest Endosc. 2009;70:505–510. doi: 10.1016/j.gie.2008.12.253. [DOI] [PubMed] [Google Scholar]
- 5.Leung FW, Aharonian HS, Leung JW, Guth PH, Jackson G. Impact of a novel water method on scheduled unsedated colonoscopy in U.S. veterans. Gastrointest Endosc. 2009;69:546–550. doi: 10.1016/j.gie.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Leung FW, Harker JO, Jackson G, Okamoto KE, Behbahani OM, Jamgotchian NJ, et al. A proof-of-principle, prospective, randomized controlled trial (RCT) demonstrating improved outcomes in scheduled unsedated colonoscopy by the water method. Gastrointest Endosc. 2010;72:693–700. doi: 10.1016/j.gie.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Leung FW, Harker JO, Leung JW, Siao-Salera RM, Mann SK, Ramirez FC, et al. Removal of infused water predominantly during insertion (water exchange) is consistently associated with an increase in adenoma detection rate — review of data in randomized controlled trials (RCTs) of water-related methods. J Interv Gastroenterol. 2011;1:121–126. doi: 10.4161/jig.1.3.18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung JW, Do L, Siao-Salera RM, Parikh DA, Mann SK, Leung FW. Retrospective analysis showing the water method increased adenoma detection rate-a hypothesis generating observation. J Interv Gastroenterol. 2011;1:3–7. doi: 10.4161/jig.1.1.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung FW, Leung JW, Siao-Salera RM, Mann SK. The water method significantly enhances proximal diminutive adenoma detection rate in unsedated patients. J Interv Gastroenterol. 2011;1:8–13. doi: 10.4161/jig.1.1.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung JW, Ransibrahmanakul K, Toomsen L, Mann SK, Siao-Salera RM, Leung FW. The water method combined with chromoendoscopy enhances adenoma detection. J Interv Gastroenterol. 2011;1:53–58. doi: 10.4161/jig.1.2.16827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bressler B. Colonoscopic miss rates for right-sided colon cancer: A populatio-based analysis. Gastroenterology. 2004;127:452–456. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer: a populatio-based, case-control study. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: populatio-based study. J Natl Can Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 14.Stock C, Pulte D, Haug U, Brenne H. Subsite-specific colorectal cancer risk in the colorectal endoscopy era. Gastrointest Endosc. 2012;75:621–630. doi: 10.1016/j.gie.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in Medicare beneficiaries. Cancer. 2012;118:3044–3052. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. New Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 17.Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am J Gastroenterol. 2010;105:1301–1307. doi: 10.1038/ajg.2010.51. [DOI] [PubMed] [Google Scholar]
- 18.Beduya D, Oliner C, Lee Y. Adenoma detection rate using high-definition white-light colonoscopy versus standard colonoscopy: Abstract318.
- 19.Buchner AM, Shahid MW, Heckman MG, McNeil RB, Cleveland P, Gill KR, et al. High-definition colonoscopy detects colorectal polyps at a higher rate than standard white-light colonoscopy. Clin Gastroenterol Hepatol. 2010;8:318–321. doi: 10.1016/j.cgh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Burke CA, Choure AG, Sanaka MR, Lopez R. A comparison of high-definition versus conventional colonoscopes for polyp detection. Dig Dis Sci. 2010;55:1716–1720. doi: 10.1007/s10620-009-0941-y. [DOI] [PubMed] [Google Scholar]
- 21.Pellise M, Fernandez-Esparrach G, Cardenas A, Cárdenas A, Sendino O, Ricart E, et al. Impact of wide-angle, high-definition endoscopy in the diagnosis of colorectal neoplasia: a randomized controlled trial. Gastroenterology. 2008;135:1062–1068. doi: 10.1053/j.gastro.2008.06.090. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, et al. Comparative study of conventional colonoscopy and pa-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43:45–50. doi: 10.1007/s00535-007-2125-x. [DOI] [PubMed] [Google Scholar]
- 23.Horiuchi A, Nakayama Y, Kato N, Ichise Y, Kajiyama M, Tanaka N. Hood-assisted colonoscopy is more effective in detection of colorectal adenomas than narrow-band imaging. Clin Gastroenterol Hepatol. 2010;8:379–383. doi: 10.1016/j.cgh.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Adler A, Aschenbeck J, Yenerim T, Mayr M, Aminalai A, Drossel R, et al. Narrow-band versus white-light high definition television endoscopic imaging for screening colonoscopy: a prospective randomized trial. Gastroenterology. 2009;136:410–416. doi: 10.1053/j.gastro.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Paggi S, Radaelli F, Amato A, Meucci G, Mandelli G, Imperiali G, et al. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049–1054. doi: 10.1016/j.cgh.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Harada Y, Hirasawa D, Fujita N, Noda Y, Kobayashi G, Ishida K, et al. Impact of a transparent hood on the performance of total colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2009;69:637–644. doi: 10.1016/j.gie.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, et al. Efficacy of cap-assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2009;104:41–46. doi: 10.1038/ajg.2008.56. [DOI] [PubMed] [Google Scholar]
- 28.Sawhney MS, Cury MS, Neeman N, Ngo LH, Lewis JM, Chuttani R, et al. Effect of institutio-wide policy of colonoscopy withdrawal time ≥7 minutes on polyp detection. Gastroenterology. 2008;135:1892–1898. doi: 10.1053/j.gastro.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. New Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 30.Matro R, Shnitser A, Spodik M, Daskalakis C, Katz L, Murtha A, et al. Efficacy of morning-only compared with split-dose polyethylene glycol electrolyte solution for afternoon colonoscopy: a randomized controlled single-blind study. Am J Gastroenterol. 2010;105:1954–1961. doi: 10.1038/ajg.2010.160. [DOI] [PubMed] [Google Scholar]
- 31.Parra-Blanco A, Nicolás-Pérez D, Gimeno-García A, Grosso B, Jimenez A, Ortega J, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: A randomized study. World J Gastroenterol. 2006;12:6161–6166. doi: 10.3748/wjg.v12.i38.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SY, Lee SK, Kim BC, Han J, Kim JH, Cheon JH, et al. Efficacy of chromoendoscopy with indigocarmine for the detection of ascending colon and cecum lesions. Scand J Gastroenterol. 2008;43:878–885. doi: 10.1080/00365520801935442. [DOI] [PubMed] [Google Scholar]
- 33.Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73–78. doi: 10.1136/gut.2008.153601. [DOI] [PubMed] [Google Scholar]
- 34.Bustamante-Balen M, Bernet L, Cano R, Pertejo V, Ponce J. Prevalence of nonpolypoid colorectal neoplasms in symptomatic patients scheduled for colonoscopy: a study with total colonic chromoscopy. J Clin Gastroenterol. 2010;44:280–285. doi: 10.1097/MCG.0b013e3181aed327. [DOI] [PubMed] [Google Scholar]
- 35.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 36.Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 37.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 38.Mitooka H, Fujimori T, Maeda S, Nagasako K. Minute flat depressed neoplastic lesions of the colon detected by contrast chromoscopy using an indigo carmine capsule. Gastrointest Endosc. 1995;4:453–459. doi: 10.1016/s0016-5107(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 39.Carroll RE. Colon preparation for magnification endoscopy: a rapid novel approach. Endoscopy. 2004;36:609–611. doi: 10.1055/s-2004-814516. [DOI] [PubMed] [Google Scholar]
- 40.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]