Abstract
Background:
The TMPRSS2-ERG gene fusion resulting in ERG overexpression has been found in around 50% of prostate cancers (PCa) and is a very early event in tumorigenesis. Most studies have reported on selected surgical cohorts with inconsistent results. We hypothesized that ERG gene rearrangements impact tumor development and investigated the frequency of ERG overexpression in the context of clinicopathological tumor characteristics.
Methods:
ERG overexpression (ERG+ or ERG-) was determined by immunohistochemistry (IHC) in 1039 radical prostatectomy (RP) tumors and association with PSA, D'Amico risk score, histopathology, biochemical recurrence, body mass index and age of PCa cases was analyzed.
Results:
ERG+ was associated with younger age at diagnosis (P<0.0001), lower serum PSA (P=0.002) and lower prostate volume (PV) (P=0.001). It was most frequent in the youngest age quartile (⩽55 years, 63.9% ERG+) and decreased constantly with increasing age to 40.8% in the oldest age quartile (⩾67 years, P<0.0001). In the PSA range <4 ng ml−1 the frequency of ERG positivity was 60.2% compared with 47.5 and 49.1% in the PSA ranges 4–10 and ⩾10 ng ml−1, respectively. In the first age quartile, ERG+ patients had lower median serum PSA and fPSA% and smaller PV. In the highest age quartile tumor volume (TV) was increased. Similar differences were observed in the low PSA range. Multivariate analysis identified the first age quartile as a predictor for ERG status (odds ratios (OR) 2.05, P=0.007). No association was found with the D'Amico progression risk score and with biochemical tumor recurrence.
Conclusions:
ERG+ tumors manifest clinically at lower PSA levels and their prevalence is age dependent. This suggests acceleration of tumor development by ERG overexpression that results in earlier tumor detection in young patients. Long-term results are warranted to determine the impact of ERG overexpression on disease outcome.
Keywords: age, ERG frequency distribution, ERG overexpression, early-onset prostate cancer, PSA screening
Introduction
Prostate cancer (PCa) is the most common solid neoplasm in the developed countries accounting for almost 30% of cancer incident cases in men. It is the third leading cause of male cancer deaths in Europe1, 2 and second in the USA although an estimated 90% of newly diagnosed cases are local or regional with a 5-year relative survival approaching almost 100%.3 A frequently occurring genetic alteration of prostate tumors is a gene rearrangement involving transcription factors of the E26 transformation specific (ETS) family and an androgen-regulated gene. The most common event found in 40–60% of PCa cases results in the fusion of the transcription factor ERG and the androgen regulated transmembrane serine protease 2 (TMPRSS2) gene and leads to androgen-stimulated overexpression of ERG.4 Less common are rearrangements involving the ETS transcription factors ETV1, 4 and 5 or other androgen regulated 5′ partners such as SLC45A3.5
An ERG rearrangement regardless of the 5′ partner is highly specific for PCa and only found in tumor cells or a subset of high-grade prostatic intraepithelial neoplasia lesions.6, 7 Therefore, analysis of an ERG rearrangement is beginning to have a role in routine pathology.8, 9 The fusion status can reliably be determined using fluorescence in situ hybridization detecting the gene rearrangement,4, 10 by PCR measuring expression of a fusion transcript4 or using IHC detecting the overexpressed ERG protein.11 Positive immunohistochemical staining highly correlates with the ERG gene rearrangement status determined by fluorescence in situ hybridization or mRNA analyses.11, 12
The ERG rearrangement occurs early in prostate carcinogenesis7 and is then present at around the same frequency through all tumor stages up to metastatic, therapy-resistant disease.13 Despite numerous studies the implication of this common genetic alteration on tumor progression and consequences for the management and treatment of PCa have yet to be defined. This may in part be due to relative few studies that have focused on large well-characterized patient populations. The majority of studies reported no association between biochemical recurrence and ERG rearrangement status.14, 15 Conversely, population-based Watchful Waiting studies have found associations with PCa specific death.16, 17 Recent investigations suggested that gene-fusion driven ERG overexpression increases self-renewal and stimulates epithelial to mesenchymal transition.18, 19
We hypothesized that ERG overexpression is an early driver of tumor development and investigated the frequency of ERG overexpression in dependence on patient age and clinicopathological characteristics. The prevalence of ERG overexpression was investigated retrospectively in a large cohort of the Tyrolean PCa patients, the majority of whom have been diagnosed in an age-adjusted PSA-based screening program for early detection and treatment of PCa.20, 21 We observed an increased frequency of ERG overexpression in younger PCa patients and association with lower serum PSA.
Materials and methods
Study population
The analysis involved 1039 PCa patients (selected by the availability of archived tissue) who underwent radical prostatectomy (RP) in the Department of Urology of the University Hospital Innsbruck between 6/1993 and 4/2012). The majority of patients had been diagnosed in the PSA screening program employing age-adjusted PSA serum level cutoffs (1.25 (up to 49 year), 1.75 (50–59 year), 2.25 (60–69 year) and 3.75 ng ml−1 (70–75 year)) in combination with fPSA%.20, 21, 22 The prostate specimens underwent routine histopathological processing and analysis.23 Tumor volume (TV) was assessed using computerized morphometric analysis24 in patients operated after 2009. Serum PSA and fPSA% values leading to PCa positive biopsy, PSA density, D'Amico progression risk group, Gleason score (GS) of the RP tumor, prostate volume (PV), TV, percent TV of PV (%TV), pathological stage (pTNM), prostate specimen margin status (R), time to PSA recurrence or follow-up time after RP, age at the time of prostatectomy and patients body mass indexes were retrieved for analysis. The D'Amico low, intermediate and high-risk group classification was based on PSA values at diagnosis, biopsy GS and clinical stage assessed by digital rectal examination.25 PSA recurrence was defined as two consecutive serum PSA values ⩾0.2 ng ml−1, follow-up time in patients without PSA recurrence as time of RP to the most recent serum PSA measurement. Patients gave their informed consent and the study was approved by the local ethics committee.
Evaluation of ERG protein expression by IHC
ERG protein expression was assessed by IHC in RP specimens. Standard 0.5-μm sections were prepared and immunohistochemical staining was applied using a commercially available antibody for ERG (EPR3864, dilution 1:100, Ventana Medical Systems, Tucson, AZ, USA) on the Discovery XT biomarker platform (Ventana). In RP specimens with multifocal tumors, only the index tumor was analyzed. It was defined as the dominant and usually the largest, tumor with the highest GS.26 Antigen recovery was conducted by heat retrieval (CC1) pretreatment. For 143 archived cases, frozen tumor sections were stained after formalin fixation (10% neutral buffered formalin, 3 min) using the same protocol without CC1 pretreatment. Staining specificity was controlled using the internal controls benign tissue (negative) and small vessels (positive). Study pathologists performed semi-quantitative evaluation of nuclear ERG expression using a four-tier grading system: negative (0), weakly (1+), moderately (2+) and strongly (3+) positive. Any positive staining with 2+ or 3+ intensity of >5% of total cells was used as a cutoff for each area assessed. This was shown to be corresponding with positive ERG gene rearrangements with TMPRSS2, NDRG1 and SLC45A3 assessed by fluorescence in situ hybridization.11
Statistical analysis
In order to identify age and PSA dependent associations, patients were classified into age quartiles (35–55, 56–61, 62–66, 67–82 years) and PSA ranges (<4, 4–10, ⩾10 ng ml−1). Associations of clinicopathological parameters and ERG status as dependent variables were determined using logistic regression analysis. Multivariate logistic regression analysis was performed by stepwise backward elimination. For frequency comparisons χ2 tests, for non-normal distributed variables in group comparisons Mann–Whitney U or Wilcoxon tests were applied. A two-tailed significance level of 0.05 was considered as statistically significant. Disease-free lifetime was calculated using the Kaplan–Meier method, the Cox regression model was used to identify associated risk factors. All calculations were carried out with IBM-SPSS 20.0 (IBM Corporation, New York, NY, USA).
Results
ERG protein expression was analyzed by IHC in 1039 patients who underwent RP at the University Hospital of Innsbruck between 1993 and 2012 and ERG positivity was correlated to PSA, D'Amico progression risk groups, clinical and histological parameters and to biochemical recurrence (Table 1). The majority of the cohort patients were diagnosed in the Tyrolean PSA screening program. Patients' median age was 61 years (range 35–82), PR tumor GS's were <7 in 341 (32.8%), 7 in 571 (55.0%) and >7 in 120 (11.5%) cases. In total 757 (73.1%) tumors were organ-confined (pT2) and 276 (26.6%) patients showed extraprostatic extension (pT3/4). Surgical margins were positive in 283 (27.2%) cases. Nodal invasion was diagnosed in eight (0.8%) cases. Tumors of 492 patients (47.4%) were ERG- and of 547 (52.6%) were ERG+. Patients with ERG+PCa were significantly younger than those harboring ERG- tumors (median age 60.0 vs 63.0, P<0.0001), and median PSA values were significantly lower in ERG+ compared with ERG- PCa patients (4.7 vs 5.5 ng ml−1, P=0.002). PV in individuals with ERG+ cancer was significantly smaller with a median volume of 35.0 ml compared with 40.0 ml in ERG- cases (P=0.001) and ERG+ tumors were more frequent in GS=7 cancers (P=0.036). There was no significant relation between ERG status and body mass index, fPSA%, PSA density, TV, pT stage, surgical margins, D'Amico progression risk groups or frequency of biochemical recurrence.
Table 1. Clinicopathological characteristics of the study cohort in relation to ERG status.
| Characteristics | ERG negative | ERG positive | Total | P-value |
|---|---|---|---|---|
| (n=492) | (n=547) | (n=1039) | ||
| Age at RP, year | <0.0001* | |||
| Median | 63.0 | 60.0 | 61.0 | |
| s.d. | 7.2 | 7.5 | 7.5 | |
| 95% CI | 61.4–62.7 | 59.0–60.2 | 60.3–61.2 | |
| Range | 35.0–82.0 | 41.0–78.0 | 35.0–82.0 | |
| Age quartiles, year | <0.0001* | |||
| 1 (35–55) | 90 | 159 | 249 | |
| 2 (56–61) | 125 | 156 | 281 | |
| 3 (62–66) | 122 | 125 | 247 | |
| 4 (67–82) | 155 | 107 | 262 | |
| BMI kg m−2 | 0.407 | |||
| Median | 26.0 | 26.0 | 26.0 | |
| s.d. | 3.7 | 3.2 | 3.5 | |
| 95% CI | 26.5–27.3 | 26.2–26.9 | 26.5–30.0 | |
| Range | 19.0–41.0 | 18–42 | 18–42 | |
| PSA, indication to biopsy, ng ml−1 | 0.002* | |||
| Median | 5.5 | 4.7 | 5.1 | |
| s.d. | 5.5 | 5.1 | 5.3 | |
| 95% CI | 6.3–7.4 | 5.7–6.6 | 6.1–6.8 | |
| Range | 1.2–57.9 | 1.35–54.7 | 1.2–57.9 | |
| PSA ranges, ng ml−1 | 0.004* | |||
| <4 | 109 | 165 | 274 | |
| 4–10 | 221 | 200 | 421 | |
| >10 | 58 | 56 | 114 | |
| Missing | 104 | 126 | 130 | |
| fPSA%, indication to biopsy,% | 0.073 | |||
| Mean | 13.9 | 13.0 | 13.3 | |
| s.d. | 6.4 | 6.4 | 6.4 | |
| 95% CI | 14.0–15.4 | 13.1–14.4 | 13.7–14.7 | |
| Range | 3.9–45.4 | 1.02–40.0 | 1.0–45.4 | |
| PSA density, ng ml−2 | 0.409 | |||
| Mean | 0.13 | 0.12 | 0.12 | |
| s.d. | 0.21 | 0.14 | 0.18 | |
| 95% CI | 0.16–0.20 | 0.15–0.18 | 0.16–0.19 | |
| Range | 0.03–2.31 | 0.02–1.05 | 0.02–2.31 | |
| D'Amico score | 0.390* | |||
| Low | 173 | 214 | 387 | |
| Intermediate | 118 | 139 | 257 | |
| High | 39 | 34 | 73 | |
| Missing | 162 | 160 | 322 | |
| PV, ml | 0.001* | |||
| Mean | 40.0 | 35.0 | 39.0 | |
| s.d. | 15.8 | 15.0 | 15.5 | |
| 95% CI | 41.2–44.5 | 41.2–44.5 | 39.9–42.2 | |
| Range | 20.0–110.0 | 20.0–110.0 | 20.0–110.0 | |
| TV, ml | 0.778 | |||
| Mean | 1.30 | 1.32 | 1.30 | |
| s.d. | 1.72 | 4.03 | 3.00 | |
| 95% CI | 1.57–2.05 | 1.83–3.09 | 1.79–2.41 | |
| Range | 0.01–10.85 | 0.05–38.06 | 0.01–38.06 | |
| Tumor volume % of PV (%TV), % | 0.404 | |||
| Mean | 3.04 | 3.05 | 3.04 | |
| s.d. | 3.13 | 7.51 | 5.89 | |
| 95% CI | 3.71–4.84 | 4.45–6.79 | 4.28–5.50 | |
| Range | 0.02–21.69 | 0.12–56.80 | 0.02–56.80 | |
| GS, n | 0.036* | |||
| <7 | 167 (33.9) | 174 (31.8) | 341 (32.8) | |
| 7 | 253 (51.4) | 318 (58.1) | 571 (55.0) | |
| >7 | 68 (13.8) | 52 (9.5) | 120 (11.5) | |
| Missing | 4 (0.8) | 3 (0.5) | 7 (0.7) | |
| pTNM, n | 0.697 | |||
| pT2 | 356 (72.8) | 401 (73.3) | 757 (73.1) | |
| pT3/4 | 132 (27.0) | 144 (26.3) | 276 (26.6) | |
| Missing | 1 (0.2) | 2 (0.4) | 3 (0.3) | |
| Nodal invasion, n | 0.032 | |||
| Yes | 3 (0.6) | 5 (0.9) | 8 (0.8) | |
| No | 216 (43.9) | 197 (36.0) | 413 (39.7) | |
| Missing | 273 (55.5) | 345 (63.1) | 618 (59.5) | |
| R, n | 0.454 | |||
| Negative | 331 (67.3) | 371 (67.8) | 702 (67.6) | |
| Positive | 126 (25.6) | 157 (28.7) | 283 (27.2) | |
| Missing | 35 (7.1) | 19 (3.5) | 54 (5.2) | |
| Biochemical recurrence | 0.994 | |||
| Yes, n | 53 (10.8) | 60 (11.0) | 113 (10.9) | |
| No, n | 378 (76.8) | 434 (79.3) | 812 (78.1) | |
| Unknown, n | 61 (12.4) | 53 (9.7) | 114 (11.0) | |
| Median time to recurrence, d | 667 | 998 | 832 | |
| Range, d | 0–5071 | 0–3676 | 0–5071 | |
| 95% CI | 686–1294 | 913–1439 | 982–1286 | |
| No biochemical recurrence | ||||
| Median follow-up, d | 614 | 941 | 768 | |
| Range | 27–6274 | 27–5657 | 27–6274 | |
| 95% CI | 685–1294 | 1248–1500 | 1184–1367 |
Abbreviations: BMI, body mass index; CI, confidence interval; GS, gleason score; pTNM, pathologic T stage; PV, prostate volume; RP, radical prostatectomy; R, margin status; TV, tumor volume.
*P<0.05.
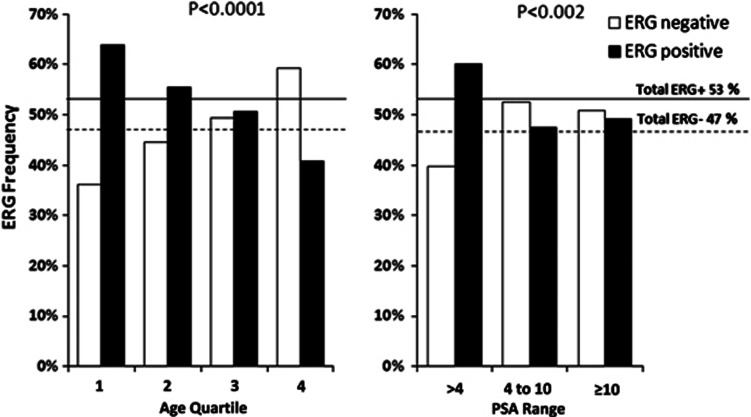
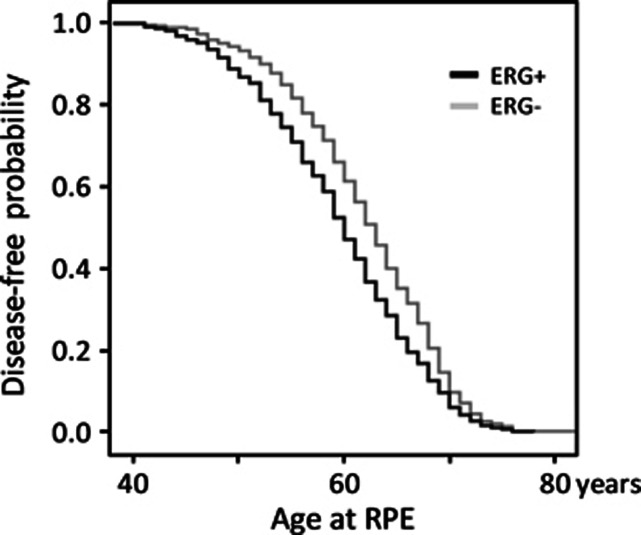
To further analyze ERG-status, age quartiles (35–55, 56–61, 62–66, 67–82 years) and serum PSA ranges (>4, 4–10, ⩾10) were considered. In the first age quartile 63.9% of the patients had ERG+ PCa. ERG+ status was significantly lower in the 2nd, 3rd and 4th quartile (55.5%, 50.6% and 40.8%, respectively, P<0.0001, Figure 1 and Table 2). ERG status and clinicopathological parameters in relation to quartiles showed statistically significant differences only in the first and last quartile. In the youngest age quartile PSA was significantly lower in ERG+ compared with ERG- patients (3.1 vs 4.1 ng ml−1, P=0.011, Table 3A), fPSA% (12.0% vs 14.4%, P=0.004) and the PV (34 vs 35 ml, P=0.026) were significantly reduced. These differences vanished with increasing age in all quartiles with no differences at all in the second and third. However, in the oldest quartile other differences emerged with significantly higher TV (1.6 vs 1.3 ml, P=0.042) and %TV (4.2% vs 2.9%, P=0.029) in ERG+ patients (Table 3A). Association between ERG status and age of diagnosis and disease-free lifetime is shown in Figure 2 as a Kaplan–Meier plot. The ERG+ cohort curve is significantly shifted to younger age (P=<0.001, log-rank test) with a median disease-free lifetime of 60 compared with 63 years in the ERG- patients. A Cox model analysis using age at diagnosis as the independent variable, revealed an increased risk factor for cancer diagnosis for ERG positivity (relative risk (RR)=1.254, P=0.001) and a slightly decreased risk for serum PSA (RR=0.956, P=<0.001) (Table 1).
Figure 1.
Distribution of ERG status within the age quartiles and the PSA ranges. Tumors from the study cohort were assigned to age quartiles of patients' age at RP and the frequencies of ERG+ and ERG- were calculated for each age quartile (a). Similarly, tumors of the study cohort were divided into three PSA ranges (<4, 4–10 and >10 ng ml−1) and ERG+ and ERG- frequencies were calculated (b). The distribution of ERG+ and ERG- tumors was significantly different in the four age quartiles (P<0.0001) and in the three PSA ranges (P=0.002).
Table 2. Distribution of ERG status in age quartiles and PSA ranges.
| ERG Status |
Age quartile (years) |
PSA range (mg ml−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. (35–55) | 2. (56–61) | 3. (62–66) | 4. (67–82) | Total | 1. (<4) | 2. (4–10) | 3. (⩾10) | Total | |
| Negative | 36.1 (90) | 44.5 (125) | 49.4 (122) | 59.2 (155) | 47.4 (492) | 39.8 (109) | 52.5 (221) | 50.9 (58) | 48.0 (388) |
| Positive % (n) | 63.9 (159) | 55.5 (156) | 50.6 (125) | 40.8 (107) | 52.6 (547) | 60.2 (165) | 47.5 (200) | 49.1 (56) | 52.0 (421) |
| Total | 100 (249) | 100 (281) | 100 (247) | 100 (262) | 100 (1039) | 100 (274) | 100 (421) | 100 (114) | 100 (809) |
Table 3. Age quartiles and PSA ranges in relation to ERG status and clinicopathological features.
| A. Age quartiles (years) |
1. (35–55) |
2. (56–61) |
3. (62–66) |
4. (67–82) |
1. (35–55) |
2. (56–61) |
3. (62–66) |
4. (67–82) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERG status | − | + | − | + | − | + | − | + | ||||
| Median |
% Differences (ERG+ to ERG−) |
|||||||||||
| %TV, % | 3.4 | 2.6 | 3.5 | 3.3 | 2.9 | 4.2 | 2.9 | 4.2 | −23 | −4 | 45 | 44* |
| TV, ml | 1.2 | 0.9 | 1.3 | 1.4 | 1.3 | 1.6 | 1.3 | 1.6 | −25 | 2 | 22 | 20* |
| PSA diagnostic for biopsy, ng ml−1 | 4.1 | 3.1 | 5.4 | 4.9 | 5.5 | 5.5 | 6.4 | 6.2 | −24* | −10 | 2 | −3 |
| fPSA%, % | 14 | 12 | 12 | 12 | 14 | 14 | 15 | 15 | −17* | 2 | −2 | 5 |
| PV, ml | 35 | 34 | 36 | 35 | 45 | 40 | 45 | 40 | −3* | −3 | −11 | −11 |
| PSA density, ng ml−2 | 0.11 | 0.09 | 0.14 | 0.13 | 0.11 | 0.12 | 0.14 | 0.15 | −18 | −9 | 13 | 7 |
| B. PSA ranges (ng ml−1) |
1 (<4) |
2 (4– 10) |
3 (⩾10) |
1 (<4) |
2 (4–10) |
3 (⩾10) |
|||
|---|---|---|---|---|---|---|---|---|---|
| ERG status | − | + | − | + | − | + | |||
| Median |
% Differences (ERG+ to ERG−) |
||||||||
| %TV, % | 2.3 | 2.3 | 3.0 | 3.1 | 6.7 | 18.5 | 0 | 3 | 176* |
| TV, ml | 0.9 | 0.8 | 1.3 | 1.4 | 2.4 | 9.3 | −11 | 8 | 288* |
| PSA diagnostic for biopsy, ng ml−1 | 3.0 | 2.8 | 6.0 | 5.8 | 13.7 | 13.9 | −7* | −3 | 1 |
| fPSA%, % | 16.0 | 12.6 | 14.0 | 12.7 | 9.9 | 16.2 | −21* | −9* | 64 |
| PV, ml | 40 | 35 | 50 | 43 | 35 | 50 | −13* | −14* | 43* |
| PSA density, ng ml−2 | 0.08 | 0.08 | 0.1 | 0.1 | 0.3 | 0.3 | 0 | 0 | 0 |
Abbreviations: PV, prostate volume; TV, tumor volume.
*P<0.05
Figure 2.
Kaplan–Meier plot of the probability of no PCa diagnosis vs age. The probability curve of ERG+ cases is significantly shifted to younger age compared with the cases with no ERG overexpression with a median age difference of 3.0 years.
As the patient enrollment protocol used age-adjusted serum PSA thresholds to trigger a biopsy for the majority of patients, we also investigated ERG association within discrete PSA ranges. In the low PSA range <4 ng ml−1 60.2% of the tumors were ERG+ whereas the frequency decreased to 47.5 and 49.1% in the intermediate (4–10 ng ml−1) and high (⩾10 ng ml−1) PSA ranges, respectively (P=0.002, Table 2). A computer generated schematic visualization of the observed PSA differences showed a clear shift of the frequency peak of ERG+ tumors to lower PSA levels compared with the peak of ERG- tumors for two sets of equal size (Figure 3). The selection of the distribution parameters was based on the experimental PSA level data from the validation cohort taken from Demichelis et al.27 Mean serum PSA was smaller in ERG+ compared with ERG- tumors within the low PSA range (2.8 vs 3.0 ng ml−1, P=0.022, Table 3B). Likewise mean PV and fPSA% were smaller (35 vs 40 ml, P=0.02; 12.6% vs 16.0%, P=0.004). On the other end, in the highest PSA range, remarkable differences of mean PV, TV and %TV (50 vs 35 ml, P=0.020; 9.3 vs 2.4 ml, P=0.003 and 18.5% vs 6.7%, P=0.002) were noticed (Table 3B).
Figure 3.
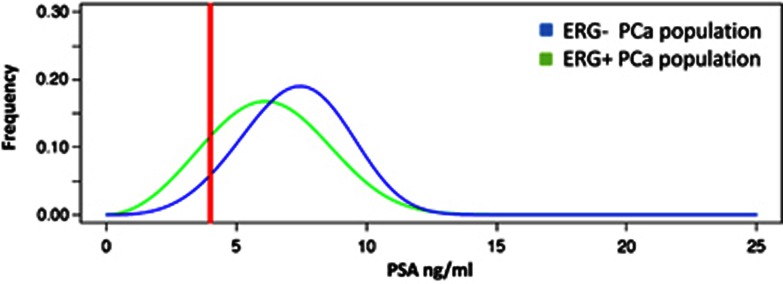
Computer simulated distribution of PSA levels in the screened population based upon ERG status. The two curves represent the distribution of an equal number of ERG+ and ERG- cases along the serum PSA levels for ERG+ and ERG- cases. A PSA cutoff of 4 ng ml−1 is indicated by the red line. The color reproduction of this figure is available on Prostate cancer and Prostatic Disease journal online.
Categorization into GS groups, low (GS<7), intermediate (GS=7) and high (GS>7), revealed a higher prevalence of GS=7 tumors in the ERG+ group (P=0.036, 51.4% ERG- and 58.1% ERG+). Categorization of patients into progression risk groups according to the criteria of D'Amico25 with 387 (54%) patients in the low, 257(35.8%) in the intermediate and 73 (10.2%) in the high-risk group showed no correlation to ERG overexpression. Biochemical progression after RP occurred in 113 (10.9%) patients. No significant difference was found in the two ERG categories (ERG- 10.8%, ERG+ 11.0%) and in median time to biochemical recurrence (ERG- 667d, ERG+ 998d) (Table 1).
Univariate logistic regression revealed the youngest age quartile, lowest PSA range and GS=7 group to increase the probability for an ERG+ tumor with OR of 1.81, 1.65 and 1.31, respectively, and oldest age quartile and PSA range 4–10 ng ml−1 to reduce the risk (OR=0.53 and 0.68, Table 4). To extend these findings we fitted clinical parameters available in routine diagnosis (age quartile, PSA range, GS group, PV, serum PSA and fPSA%) into a multivariate logistic regression model. For a positive ERG status we obtained a strong increased risk for the first age quartile (OR 2.05, P=0.007) and a marginally decreased risk for PV (OR 0.99, P=0.031).
Table 4. Logistic and Cox regression analysis for identification of factors associated with ERG+ status.
| Factor | OR | 95% CI | P-value |
|---|---|---|---|
| Logistic regression—univariate | |||
| Age quartile 1 | 1.81 | 1.35–2.44 | 0.000* |
| Age quartile 2 | 1.18 | 0.91–1.56 | 0.260 |
| Age quartile 3 | 0.90 | 0.71–1.20 | 0.460 |
| Age quartile 4 | 0.53 | 0.40–0.70 | 0.000* |
| PSA range <4 | 1.65 | 1.23–2.22 | 0.001* |
| PSA range 4–10 | 0.68 | 0.52–0.91 | 0.007* |
| PSA range ⩾10 | 0.87 | 0.59–1.30 | 0.501 |
| GS group <7 | 0.91 | 0.71–0.11 | 0.446 |
| GS group =7 | 1.31 | 1.02–1.69 | 0.030* |
| GS group >7 | 0.65 | 0.44–0.96 | 0.290 |
| Logistic regression—multivariate step 1 | |||
| GS group >7 | 1.00 | ------------- | 0.27 |
| GS group <7 | 1.13 | 0.62–2.07 | 0.69 |
| GS group =7 | 1.45 | 0.84–2.51 | 0.18 |
| PV, ml | 0.99 | 0.97–1.00 | 0.05 |
| PSA range ⩾10 | 1.00 | 0.58 | |
| PSA range <4 | 0.77 | 0.40–1.49 | 0.43 |
| PSA range 4–10 | 0.72 | 0.39–1.33 | 0.30 |
| Age quartile 4 | 1.00 | ------------- | 0.05 |
| Age quartile 1 | 2.03 | 1.17–3.50 | 0.01* |
| Age quartile 2 | 1.41 | 0.87–2.28 | 0.17 |
| Age quartile 3 | 1.07 | 0.66–1.75 | 0.78 |
| fPSA% | 0.99 | 0.96–1.02 | 0.65 |
| Logistic regression—multivariate final backward step 4 | |||
| Age quartile 4 | 1.00 | ------------- | 0.036* |
| Age quartile 1 | 2.05 | 1.22–3.44 | 0.007* |
| Age quartile 2 | 1.42 | 0.89–2.28 | 0.146 |
| Age quartile 3 | 1.01 | 0.67–1.76 | 0.673 |
| PV, ml | 0.99 | 0.99–1.00 | 0.031* |
| Cox regression (inclusion model) | |||
| RR | 95% CI | P-value | |
| ERG+ | 1.25 | 1.09–1.44 | 0.001* |
| PSA | 0.96 | 0.94–0.97 | <0.001* |
Abbreviations: CI, confidence interval; GS, gleason score; OR, odds ratio; PV, prostate volume.
*P<0.05.
Multivariate logistic regression was performed using a stepwise backward elimination model. OR values of 1.00 indicate reference values with no CI computable. Data of the first and the last steps are shown. For Cox regression the inclusion model was applied.
Discussion
In this study, we show for the first time significantly different frequencies of ERG positivity in early-onset PCa and association of ERG positivity with lower PSA and fPSA% in this age group. The analysis method used in our study determines the ERG protein level and was shown to cover different ERG gene rearrangements with 96% sensitivity and 97% specificity.11 A limitation to our study is the lack of detection of other ETS transcription factors such as ETV1 and ETV5. However, the frequency of gene fusions and overexpression of these ETS proteins is very low compared with ERG overexpression.28, 29
In the present cohort, comprised of 1039 Caucasian patients with the majority diagnosed in a PSA screening program, the frequency of ERG overexpression was 52.6%. Similar frequencies were found in RPs in two recently published prevalence studies.30, 31 In an Early Detection Research Network cohort study of prospectively collected PCa biopsies from PCa patients detected by PSA screening a frequency of 46% was reported32 whereas an investigation of PSA screening detected tumors from the European Randomized Study of Screening for Prostate Cancer found 65% ERG positivity.14 In that study 1+ ERG staining was considered positive in contrast to the current study where we considered 2+ and 3+ positive based on a prior validation.11 Additionally, differences in the selection criteria of the study groups with respect to PSA cutoffs could account for the reported frequency differences.
We found a 3-year median decrease of age at diagnosis in ERG+ PCa patients and a predominance of ERG+ PCa in the first age quartile (<56 years, 63.9% ERG+) in contrast to a predominance of ERG- tumors in the last quartile (>66 years, 59.2% ERG-). Similarly, ERG+ tumors were over-represented in the low PSA range group (60.2%). The Cox regression model identified ERG+ as a significant risk factor for shorter disease-free lifetime. In the logistic regression model, age remained the strongest valid factor predicting a positive ERG status. Up to now only a few studies have addressed associations of ERG status and age at tumor diagnosis. In a Japanese study of 194 RPs with 28% ERG+ patients, ERG rearrangement frequency was strongly reduced in patients younger than 59 and older than 71 years.33 In another study of 178 hormonally treated patients with 34% of ERG positivity, age was in mean 2.4 years lower in ERG+ patients.34 The median ages in different study populations reflect a high variability of patient selection14, 30, 32, 35 and makes a direct comparison difficult.
Concerning associations between ERG rearrangement and serum PSA levels, published data are ambiguous. On the one hand no association of ERG status and preoperative PSA in patients treated with a RP was found,31 but on the other hand a recent PSA screening study14 reported a higher frequency of ERG+ cancer in low PSA patients, however, ‘low PSA' was defined as <10 ng ml−1. In the study of Rice et al.36 evaluating ERG mRNA in urine for the detection of PCa, the mRNA score performed particularly well in Caucasian patients with serum PSA of 4 ng ml−1 or lower. Our data can explain these findings, as ERG overexpression is most frequent in this subgroup of patients and support a rationale that in men suspected of having PCa, lower levels of PSA might trigger an ETS specific assay such as a urine TMPRSS2-ERG mRNA assay.37, 38 Mosquera et al.32 reported low PSA density to be one of the best predictors for a positive TMPRSS2-ERG fusion status. In our cohort we could not confirm this conclusion.
One potential limitation in our current study is an inability to separate age and PSA entirely. Age adjusted PSA cutpoints ensure that a young man with a lower PSA is more likely to get a biopsy than an older man with the same PSA level. The reasoning has to do with the elevations of PSA due to prostate gland enlargement with age. To adequately address this issue, one would need to examine a cohort of men where biopsies were performed at low PSA levels regardless of age-adjustment. Therefore, currently we cannot entirely exclude, that ERG+ cancers can be detected at lower PSA levels independent of age.
An association of a positive ERG status with larger tumors was previously reported in a series of some 100 single-focus peripheral zone tumors.39 Furthermore, a positive TMPRSS2-ERG urine test result correlated with TV.40 In our study ERG+ PCa patients also show a significantly higher TV, but only in the oldest quartile. On one hand ERG rearrangements seems to occur more frequently in tumors emerging at a younger age. In a PSA screening program using age-dependent PSA cutoffs these tumors are detected early despite their lower serum PSA level. On the other hand ERG rearrangements are less frequent in tumors diagnosed in older age and lead to larger tumors probably by providing a growth advantage under this circumstance.17 Higher PSA thresholds applied in older patients might allow ERG+ tumors to grow to larger sizes before they reach a PSA level triggering a biopsy. Differences of tumor size associations between the first and the last age quartile suggest interaction of ERG overexpression with conditions that change with age such as the hormonal environment.41 An interaction with the hormonal status is supported by the fact that the fusion genes are all positively regulated by androgens42 and ERG and androgen receptor signaling interact at the gene transcription level.43, 44
Association of ERG+ with younger age at diagnosis suggests a speed-up of progression of early cancer lesions to a clinically relevant tumor. At the current time, there is strong emerging genomics data that ETS+ and ETS- PCas are different. ERG and AR have overlapping DNA binding sites45 and DNA rearrangement breakpoints were enriched near open chromatin, AR and ERG DNA binding sites in ETS+ but not ETS- tumors.46, 47 Taken together mounting data would support ERG+ PCa as a distinct molecular subclass of PCa.
Information on progression risk based on PSA values at diagnosis, biopsy GS and clinical stage assessed by digital rectal examination were available for 71.4% of the patients. However, no correlation of the D'Amico progression risk classification and ERG overexpression was found. About every tenth men of our PCa cohort progressed after RP with rising serum PSA. Neither the number of progressing patients nor the median disease-free survival times were statistically different in the two ERG categories. These results are in agreement with previous studies and meta-analysis.14, 15, 48 On the other hand association of an ERG rearrangement with cancer specific death was reported for a watchful waiting cohort.17 This calls for more long-term outcome results to establish the impact of ERG overexpression on the course and death of PCa.
Conclusions
ERG overexpression is significantly more frequent in tumors detected at a younger age and is associated with lower PSA levels in this age group. In tumors detected at an older age, ERG overexpression is significantly associated with a higher TV but not with differences in PSA. ERG overexpression seems to accelerate carcinogenesis and drive prostate tumors to early clinical manifestation and detection but have no effect on tumor progression.
Acknowledgments
The study was supported by Oncotyrol Center for Personalized Cancer Medicine within the scope of the Austrian COMET program through BMVIT, BMWFJ and the Standortagentur Tirol (Project 1.3.1 to HK), the University Hospital Innsbruck, DOD (PC094516 to FD), Fondazione Trentina per la Ricerca sui Tumori (to FD) and NCI U01 CA111275 from the Early Detection Research Network of the National Cancer Institute (JMM, FD, MAR).
JMM has a sponsored research agreement with Ventana Medical Systems (Development of tissue-based biomarkers in PCa); MAR receives consultation fees from Ventana Medical Systems (Development of tissue-based biomarkers in PCa).
References
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 23:1044–1052. doi: 10.1093/annonc/mds024. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- Esgueva R, Perner S, J LaFargue C, Scheble V, Stephan C, Lein M, et al. Prevalence of TMPRSS2-ERG and SLC45A3-ERG gene fusions in a large prostatectomy cohort. Mod Pathol. 2010;23:539–546. doi: 10.1038/modpathol.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquera J-M, Perner S, Genega EM, Sanda M, Hofer MD, Mertz KD, et al. Characterization of TMPRSS2-ERG fusion high-grade prostatic intraepithelial neoplasia and potential clinical implications. Clin Cancer Res. 2008;14:3380–3385. doi: 10.1158/1078-0432.CCR-07-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner S, Mosquera J-M, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Sur Pathol. 2007;31:882–888. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- He H, Magi-Galluzzi C, Li J, Carver P, Falzarano S, Smith K, et al. The diagnostic utility of novel immunohistochemical marker ERG in the workup of prostate biopsies with ‘atypical glands suspicious for cancer'. Am J Sur Pathol. 2011;35:608–614. doi: 10.1097/PAS.0b013e31820bcd2d. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Palanisamy N, Siddiqui J, Chinnaiyan AM, Kunju LP. Antibody-based detection of erg rearrangements in prostate core biopsies, including diagnostically challenging cases: ERG staining in prostate core biopsies. Arch Pathol Lab Med. 2012;136:935–946. doi: 10.5858/arpa.2011-0424-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera J-M, Setlur S, et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res. 2006;66:8337–8341. doi: 10.1158/0008-5472.CAN-06-1482. [DOI] [PubMed] [Google Scholar]
- Park K, Tomlins SA, Mudaliar KM, Chiu Y-L, Esgueva R, Mehra R, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12:590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13:228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner S, Svensson MA, Hossain RR, Day JR, Groskopf J, Slaughter RC, et al. ERG rearrangement metastasis patterns in locally advanced prostate cancer. Urology. 2010;75:762–767. doi: 10.1016/j.urology.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland AM, Jenster G, van Weerden WM, Trapman J, van der Kwast T, Roobol MJ, et al. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Mod Pathol. 2012;25:471–479. doi: 10.1038/modpathol.2011.176. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Graff RE, Bauer SR, Pitt MJ, Lis RT, Stack EC, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarker Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–4599. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- Casey OM, Fang L, Hynes PG, Abou-Kheir WG, Martin PL, Tillman HS, et al. TMPRSS2- driven ERG expression in vivo increases self-renewal and maintains expression in a castration resistant subpopulation. PloS One. 2012;7:e41668. doi: 10.1371/journal.pone.0041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem O, Madar S, Kogan-Sakin I, Kamer I, Goldstein I, Brosh R, et al. TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model. PloS One. 2011;6:e21650. doi: 10.1371/journal.pone.0021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch G, Horninger W, Klocker H, Pelzer A, Bektic J, Oberaigner W, et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008;101:809–816. doi: 10.1111/j.1464-410X.2008.07502.x. [DOI] [PubMed] [Google Scholar]
- Oberaigner W, Siebert U, Horninger W, Klocker H, Bektic J, Schafer G, et al. Prostate-specific antigen testing in Tyrol, Austria: prostate cancer mortality reduction was supported by an update with mortality data up to 2008. Int J Public Health. 2012;57:57–62. doi: 10.1007/s00038-011-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberaigner W, Horninger W, Klocker H, Schonitzer D, Stuhlinger W, Bartsch G. Reduction of prostate cancer mortality in Tyrol, Austria, after introduction of prostate-specific antigen testing. Am J Epidemiol. 2006;164:376–384. doi: 10.1093/aje/kwj213. [DOI] [PubMed] [Google Scholar]
- Epstein JI, Allsbrook WC, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Sur Pathol. 2005;29:1228–1242. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RCN, Hoedemaeker RF, van Leenders GJLH, et al. Should pathologists routinely report prostate tumour volume? The prognostic value of tumour volume in prostate cancer. Eur Urol. 2010;57:821–829. doi: 10.1016/j.eururo.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Lowrance WT, Scardino PT. Predictive models for newly diagnosed prostate cancer patients. Rev Urol. 2009;11:117–126. [PMC free article] [PubMed] [Google Scholar]
- Furusato B, Gao CL, Ravindranath L, Chen Y, Cullen J, McLeod DG, et al. Mapping of TMPRSS2-ERG fusions in the context of multi-focal prostate cancer. Mod Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- Demichelis F, Setlur SR, Banerjee S, Chakravarty D, Chen JY, Chen CX, et al. Identification of functionally active, low frequency copy number variants at 15q21.3 and 12q21.31 associated with prostate cancer risk. Proc Natl Acad Sci USA. 2012;109:6686–6691. doi: 10.1073/pnas.1117405109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhibrahim Z, Braun M, Nikolov P, Boehm D, Scheble V, Menon R, et al. Rearrangement of the ETS genes ETV-1, ETV-4, ETV-5, and ELK-4 is a clonal event during prostate cancer progression. Hum Pathol. 2012;43:1910–1916. doi: 10.1016/j.humpath.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Svensson MA, LaFargue CJ, MacDonald TY, Pflueger D, Kitabayashi N, Santa-Cruz AM, et al. Testing mutual exclusivity of ETS rearranged prostate cancer. Lab Invest. 2011;91:404–412. doi: 10.1038/labinvest.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, Esgueva R, et al. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate. 2011;71:489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- Rubio-Briones J, Fernandez-Serra A, Calatrava A, Garcia-Casado Z, Rubio L, Bonillo MA, et al. Clinical implications of TMPRSS2-ERG gene fusion expression in patients with prostate cancer treated with radical prostatectomy. J Urol. 2010;183:2054–2061. doi: 10.1016/j.juro.2009.12.096. [DOI] [PubMed] [Google Scholar]
- Mosquera J-M, Mehra R, Regan MM, Perner S, Genega EM, Bueti G, et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res. 2009;15:4706–4711. doi: 10.1158/1078-0432.CCR-08-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi Y, Sasaki T, Fujinami K, Sano J, Senga Y, Miura T, et al. ETS family-associated gene fusions in Japanese prostate cancer: analysis of 194 radical prostatectomy samples. Mod Pathol. 2010;23:1492–1498. doi: 10.1038/modpathol.2010.149. [DOI] [PubMed] [Google Scholar]
- Leinonen KA, Tolonen TT, Bracken H, Stenman U-H, Tammela TLJ, Saramaki OR, et al. Association of SPINK1 expression and TMPRSS2:ERG fusion with prognosis in endocrine-treated prostate cancer. Clin Cancer Res. 2010;16:2845–2851. doi: 10.1158/1078-0432.CCR-09-2505. [DOI] [PubMed] [Google Scholar]
- Falzarano SM, Zhou M, Hernandez AV, Klein EA, Rubin MA, Magi-Galluzzi C. Single focus prostate cancer: pathological features and ERG fusion status. J Urol. 2011;185:489–494. doi: 10.1016/j.juro.2010.09.093. [DOI] [PubMed] [Google Scholar]
- Rice KR, Chen Y, Ali A, Whitman EJ, Blase A, Ibrahim M, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–1576. doi: 10.1158/1078-0432.CCR-09-2191. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG Gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2012;S0302-2838:01345–0. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Falzarano SM, Zhou M, Carver P, Tsuzuki T, Simmerman K, He H, et al. ERG gene rearrangement status in prostate cancer detected by immunohistochemistry. Virchows Archiv. 2011;459:441–447. doi: 10.1007/s00428-011-1128-4. [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Aubin SMJ, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Science Tranl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Wormstall H, Doller PC. Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab. 2005;51:625–632. [PubMed] [Google Scholar]
- Gasi D, Trapman J. Androgen regulation of ETS gene fusion transcripts in prostate cancer. Methods Mol Biol. 2011;776:335–348. doi: 10.1007/978-1-61779-243-4_19. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sawyers CL. Coordinate transcriptional regulation by ERG and androgen receptor in fusion-positive prostate cancers. Cancer Cell. 2010;17:415–416. doi: 10.1016/j.ccr.2010.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman DS, Chen YB, Banerjee S, Pan Y, Yu J, Vuong T, et al. ERG cooperates with androgen receptor in regulating trefoil factor 3 in prostate cancer disease progression. Neoplasia. 2010;12:1031–1040. doi: 10.1593/neo.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickman DS, Soong TD, Moss B, Mosquera JM, Dlabal J, Terry S, et al. Oncogene-mediated alterations in chromatin conformation. Proc Natl Acad Sci USA. 2012;109:9083–9088. doi: 10.1073/pnas.1112570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minner S, Enodien M, Sirma H, Luebke AM, Krohn A, Mayer PS, et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin Cancer Res. 2011;17:5878–5888. doi: 10.1158/1078-0432.CCR-11-1251. [DOI] [PubMed] [Google Scholar]