Abstract
Objective
To assess the feasibility and safety of magnetic-controlled capsule endoscopy (MCE) system for examination of human stomach.
Methods
This pilot study enrolled 34 healthy volunteers. All subjects swallowed the MCE and gas-producing powder for gastric distention. An external robot was used to generate magnetic field to manipulate MCE inside the stomach. The primary measurements included safety, gastric preparation, maneuverability and visualization of gastric mucosa.
Results
Gastric preparation and examination was well accepted by subjects and there were no adverse events. The examination in the stomach takes 43.8±10.0min (27–60). The cleanliness was evaluated as good in the 30 (88.2%) subjects and as moderate in 4 (11.8%) subjects. The distention of gastric cavity was evaluated as good in the 29 (85.3%) subjects and moderate in 5 (14.7%) subjects. Maneuverability of the MCE to movements of the guidance magnet robot was graded as good in 29 (85.3%) subjects and moderate in 5 (14.7%) subjects. More than 75% gastric mucosa was visualized in 27 (79.4%) subjects and 50% to 75% in 7 (20.6%) subjects. Visualization of the gastric cardia, fundus, body, angulus, antrum and pylorus was subjectively assessed as complete in 82.4%, 85.3%, 100.0%, 100.0%, 100.0% and 100.0%, respectively. Polyp and erosive lesions were found in 7 subjects.
Conclusion
Magnetic-controlled capsule endoscopy used for examination of the human stomach is feasible and safe.
Key words: capsule endoscopy, magnetic-controlled, robot, feasibility studies
Introduction
Gastric diseases are great burden worldwide, especially in East Asia.1–5 Esophagogastroduodenoscopy (EGD) is recognized as the essential method in screening, diagnosis and management of gastric diseases.6–8 However, the standard EGD requires flexible tube carrying the light guide and equipment channel and this design always makes the patients discomfort and lowers the compliance.9 Although conscious sedation in endoscopy can improve these drawbacks, the potential drug-related side effects limit its use in certain population and the increased cost also limits its use in whole population.10
Capsule endoscopy (CE) has been widely used in clinical practice for more than a decade and its use in small bowel has been proved with high detectability and good safety.11,12 Moreover, CEs specially designed for esophagus and colon have been developed, with good outcome and acceptance.13–16 The capsule endoscopy for stomach also advances dramatically and several kinds of magnetically guided capsules have been reported and showed promising benefits.17–19 However, these systems still have some limitations. The magnetic force generated by handheld external magnet appeared to be insufficient to prevent accidental emptying of the capsule from strong retraction of pylori.17 The equipment derived from CT and magnetic resonance imaging procedures provided adequate force and acceptable performance but indicated possibly fairly high cost.18 Robotic control on magnetic capsule endoscopy (MCE) based on industry robot may provide a much more cost effective solution. The report from Ciuti et al. demonstrated that robotic control on magnetic steering capsule was more precise and reliable than manual operation,19 but the effectiveness of this technique in human body was not reported.
We have developed a novel magnetic-controlled capsule endoscopy (MCE) system with magnetic field generated by an external industry robot, which has the advantage of adequate magnetic force at potential cost effectiveness as low as tens of thousands US dollars in the future. Herein we report the pilot study of this system in healthy volunteers to assess the feasibility and safety of this system in the examination of human stomach.
Methods
MCE system
The MCE system consisted of capsule endoscopy, a guidance magnet robot, a data recorder and a computer workstation with software for real-time view and control, all provided by ANKON Technologies Co. LTD.
The capsule endoscopy had been proved effective in small bowel in another study (in press) and the examination in stomach was performed well and safe in simulator model and porcine model (See Supplementary Video 1). The capsule has a size of 28×12mm with a permanent magnet inside the dome (Fig. 1). The guidance magnet robot provides five degrees of control freedom: two rotational and three translational. The capture rate of MCE is two frames per second from a single CMOS sensor. It transmits images to the data recorder via a set of sensors placed on the patient's skin. The images are viewed in real time on monitor and stored into workstation simultaneously.
Figure 1.
The AKE-1 magnetic-controlled capsule endoscopy. A. The prototype of AKE-1. B. The top view of AKE-1. C. The size of AKE-1 is 28×12mm. D. Data recorder and sensors placed on the patient's skin.
The guidance magnet robot is of C-arm type with five degrees of freedom. The complete working area on the MCE is more than 50×50×50cm3. The magnetic field generated by guidance robot system can be adjusted during the examination and reach 200mT at maximum, which is much less than that from standard 1.5T MRI. Theoretically the maximum pressure of the controlled capsule against the wall of gastrointestinal tract due to the magnetic force is less than 10kPa, which was considered safe from the previous study.17 Actual strength of magnetic field used to control the navigation of MCE is about 5mT to 30mT which is 60 to 300 times greater than the Earth's magnetic field and generates magnetic force in the order of the capsule's weight. With permanent magnet the guidance magnet robot runs very quiet and consumes low electric power requiring no cooling system at all.
During examination the investigator sits in front of the workstation with dual monitors (Fig. 2). The left monitor displays the real-time view of stomach from the capsule and the view of patients from cameras. The right monitor is the operating interface collecting the information about strength of magnetic field, attitude of capsule, and so on. The attitude information of capsule is obtained through simulation on the basis of the magnetic field generated by the guidance system. MCE can be controlled by the magnet guidance robot through a joystick or automatic mode by which the MCE can make linear movement or rotation without manual control.
Figure 2.
The front view of the magnetic-controlled capsule system.
Subjects
All subjects were selected from healthy volunteers aged 18–60, without significant abdominal symptom or medication history during last 3 months, and without abdominal surgery history.
Procedure
The subjects arrived at the hospital between 8:00am and 10:00am after fasting overnight (>8 h). All subjects drank 500 ml of clear water about 1 h before capsule ingestion, another 500 ml clear water 15 min before ingestion, and 6 g air-producing power (Tianzhili Biological Technology, Fuzhou, China) with 5 ml water 5 minutes before ingestion. The air-producing powder served to distend the stomach via releasing about 540 ml CO2 every 6 g. After swallowing the MCE together with 5 ml water, the subjects immediately lie down on the bed attached to the guidance robot. The position of the bed was adjustable for optimal gastric imaging and maximal magnetic force for capsule navigation.
During the examination, the subjects remained supine position and kept minimum movement. After the MCE reached stomach, the investigator guided the capsule based on the real-time images and parameters displayed on the operating interface. The investigator performed following steps: lifting the capsule away from the posterior wall, rotating and advancing the capsule to the fundus and cardiac region, then rotating capsule to observe stomach body, and finally observing the angulus, antrum and pylorus. If the distension was insufficient, ingestion of additional air-producing powder or water was repeated.
The examination duration lasted for as long as 60min. Afterward, the maneuverability of MCE was recorded and graded by the investigators. The images recorded were compiled and reviewed for evaluation of gastric preparation and visualization of the gastric mucosa. Subjects completed a questionnaire about the discomforts and the acceptance of the gastric preparation and MCE examination process. Acceptance was assessed in 5 grades: very good, good, fair, poor and very poor. After the procedures no subject was allowed to leave before determined well. Within 14 days, subjects were contacted via telephone to confirm capsule excretion and adverse event if there was any.
Study end points
Safety was assessed according to occurrence of adverse events and tolerability of subjects through the questionnaire.
Gastric preparation included the degree of cleanliness and distention. Cleanliness was evaluated by 3 grades: good (the fluid was transparent and <5% of gastric mucosa was obscured by stomach contents), moderate (the fluid was a little opaque or 5% to 10% of gastric mucosa was obscured by stomach contents) and poor (the fluid was opaque or >10% of gastric mucosa was obscured by stomach contents). Distention was evaluated by 3 grades: good (existence of a small amount of gastric folds), moderate (existence of significant amount of gastric folds and gastric cavity was smaller than expected) and poor (gastric cavity was not inflated).
Feasibility was evaluated through two end points: (1) overall maneuverability of MCE (good, the MCE followed control and moved to targeted anatomical landmark precisely; moderate, the MCE followed control and moved towards the direction of anatomical landmark but did not reach target precisely; poor, the MCE did not follow control); and (2) visualization of gastric mucosa (good, >75% of mucosa was observed; moderate, 50% to 75% was observed; poor, <50% of the gastric mucosa was observed) and primary anatomical landmarks (cardia, fundus, body, angulus, antrum and pylorus of stomach).
The evaluation of gastric preparation, overall maneuverability and visualization of gastric mucosa were completed by two investigators with lots of experience in CE and the lower value was selected for further analysis.
Ethical considerations
The study was approved by the institutional review board of Shanghai Changhai Hospital, and informed consent was obtained from each subject before the procedure.
Results
From February to June, 2012, 34 healthy volunteers (14 women and 20 men) participated this study with the mean age as 41.3±13.1 years and the mean body mass index as 23.2±3.2 kg/m2.
Examination process
Gastric preparation and capsule swallowing were done well in all subjects. All subjects tolerated the standard volume of 1000ml water and 6g air-producing power well. Ten (29.4%) subjects drank additional water and ten ingested additional 2g (n=7) or 4g (n=3) air-producing powder, respectively.
All MCEs passed the esophagus smoothly and fell onto the upper part of posterior wall of gastric body at first, which was the lowest position in the stomach on supine position. Opaque fluid pool with mucus and food residue was observed in this location in 6 subjects. Additional water was ingested attempting to remove or dissolve the pool but no significant improvement was observed in 4 subjects. The cleanliness degree was evaluated as moderate in these 4 (11.8%) subjects and as good in the other 30 (88.2%) subjects. The distention of gastric cavity was evaluated as moderate in 5 (14.7%) subjects and good in the other 29 (85.3%) subjects.
The examinations were performed following the steps described in Method section. When the capsule was stuck between folds on the gastric wall or covered in mucus, the capsule could be grasped off the mucosa by magnetic control.
The average examination time was 43.8±10.0 min (27–60). In one subject (Subject 9) the investigator misidentified pylorus as cardia and the capsule quickly passed into duodenum due to strong pyloric contraction before the investigator took counter action. This incomplete examination took 27 minutes. In other 33 subjects, the investigator completed or terminated examinations within 60 min with average duration in the stomach of 44.3±9.6 min (34–60).
Feasibility
In 5 (14.7%) subjects the MCE followed guidance magnet robot but failed to reach cardia and fundus region, so the maneuverability was graded as moderate. One of these 5 subjects (Subject 3) had highest body mass index (33.2 kg/m2). The cleanliness and distention of stomach in 2 subjects was moderate. The reason for the other 2 subjects was not definitive.
In all other 29 (85.3%) subjects (including Subject 9), the MCE followed guidance magnet robot smoothly and the maneuverability was graded as good.
Visualization of the gastric mucosa was assessed as good (>75%) in 27 (79.4%) subjects and moderate (50% to 75%) in 7 (20.6%) subjects. The inability to visualize >75% of mucosa was due to difficulty for the MCE to reach all regions in 1 hour (n=5), significant opaque fluid (n=1) and early pyloric passage of the capsule (n=1). No entire gastric mucosa was observed in all subjects for three reasons: 1) small amounts of fluid blocked the view of the most apical parts of the fundus; 2) insufficiency of gastric distention; 3) difficult to guidance MCE in the cardiac region.
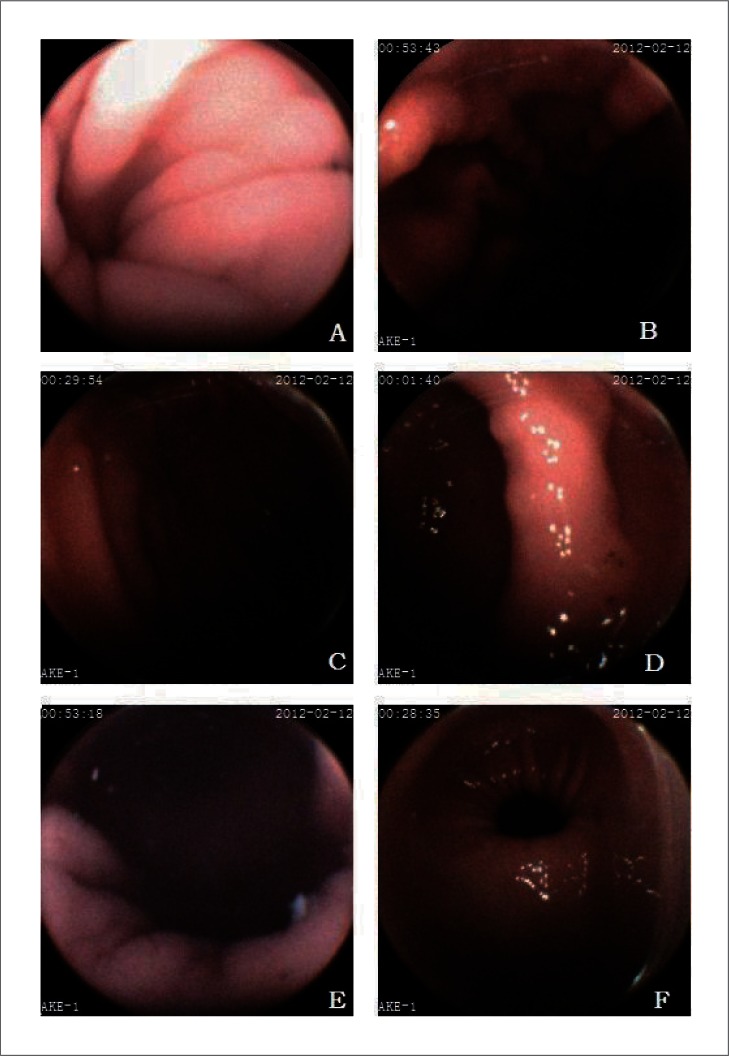
The anatomical landmarks of stomach are well visualized in most subjects (Fig. 3). In summary, visualization of the gastric cardia, fundus, body, angulus, antrum and pylorus was subjectively assessed as complete in 82.4%, 85.3%, 100.0%, 100.0%, 100.0% and 100.0% of subjects, respectively (Table 1).
Figure 3.
The images of primary landmarks in stomach. A. cardia, B. fundus, C. body, D. angulus, E. antrum, F. pylorus.
Table 1.
Results for complete visualization of the stomach in 34 healthy volunteers
| Gastric area | Complete visualization | |
| No. | % | |
| Cardia | 28 | 82.4 |
| Fundus | 29 | 85.3 |
| Body | 34 | 100.0 |
| Angulus | 34 | 100.0 |
| Antrum | 34 | 100.0 |
| Pylorus | 34 | 100.0 |
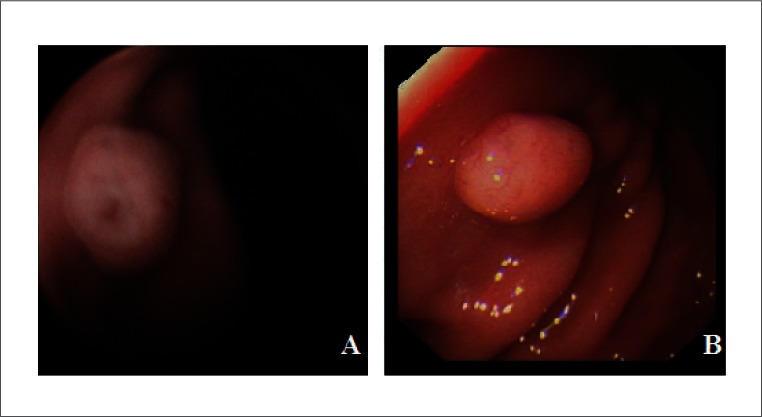
Regarding pathological findings, a single polyp located in greater curvature was found in one subject (Subject 1) and assessed in gastroscopy 1 week later (Fig. 4). Erosive lesions were found in 6 (17.6%) subjects.
Figure 4.
A gastric polyp was found during magnetic-controlled capsule examination (A) and confirmed by the following gastroscopy (B).
Safety
The gastric preparation and the examination of MCE were well tolerated and accepted. The acceptance about the gastric preparation was graded as very good in 15 (44.1%) subjects and as good in other 19 (55.9%) subjects. The acceptance about the MCE examination in stomach was graded as very good in all 34 (100.0%) subjects. Thirty-three (97.1%) subjects reported no discomfort during the examination and follow-up, and one (2.9%, Subject 3) reported slight abdominal distention after additional ingestion of 4g air-producing powder. All capsules were excreted spontaneously.
Discussion
This pilot study provided a preliminary assessment of the novel MCE system in healthy volunteers. In the present study, the examination process was highly accepted and no adverse event occurred. Maneuverability of the MCE was graded as good in 29 (85.3%) subjects and moderate in 5 (14.7%) subjects. Visualization of the gastric mucosa was assessed as good (>75%) in 27 (79.4%) subjects and moderate (50% to 75%) in 7 (20.6%) subjects. The visualization of gastric cardia, fundus, body, angulus, antrum and pylorus was completed in 79.4%, 85.3%, 100.0%, 100.0%, 100.0% and 100.0% of subjects, respectively. CE has been widely recognized as a revolutionary innovation over the decade and its prominent advantages includes noninvasiveness and convenience. Although CE plays more and more important role in the diagnosis and management of diseases through esophagus, small bowel and colon,11,13–16 its application has not yet been expanded to stomach. The anatomical and physiological characteristics of stomach demand traditional passive capsule to be actively controlled by endoscopists. Years of studies have suggested that steerable capsules with external magnetic field may be the most viable approaches for active control20 and several explorations have showed promising benefits.18,19,21,22 According to these previous studies and ours, three issues are required to be solved for the stomach capsule: maneuverability, location and gastric preparation.
Maneuverability, largely depending on control equipment and experiences of operator, is probably the most essential factor to realize the active control of CE in gastric cavity. Most of reported active CEs are guided by magnet.20 Theoretically our guidance magnet robot can generate maximum magnetic field of 200mT. In this pilot study we applied magnetic field of 5mT to 30mT, higher than that of previous reports.17,18 Moreover, we programmed automatic mode so the MCE could complete linear movement or rotation without manual control. In the present study, the maneuverability of MCE was graded as good in 29 (85.3%) subjects and moderate in 5 (14.7%), and MCE did not advance to the cardia and fundus in these 5 subjects. Cardia and fundus were observed to be the most difficult targets for active control in other reports.17,18,21 In our study, we observed same situation and attributed reasons to high body mass index and moderate cleanliness and distention in 3 subjects. In other 2 subjects, there was no definitive reason and we suspect that lack experience of controlling capsule backwards to these regions may be the possible reason. Sometimes, we could not recognize the cardia clearly and control the capsule to advance. According to Rey et al.,21 navigating ability to assess the fundus and cardia region could be improved as learning phase and we will focus on improving guidance software and operator experience to achieve better maneuverability in future.
Location of CE is critical information for efficient control on capsule in gastric cavity. Unlike the gastroscopy with its tube as a guide, the capsule may get lost in gastric lumen, especially when fluid blocks the view or gastric distention is inadequate. Even though our MCE system had an interface displaying capsule attitude and movement information, when the capsule got too close to the mucosa, the investigators could not determine the location of capsule precisely and had to drag the capsule backwards to get a broader view for location. Magnetic tracking algorithms compatible with external magnetic locomotion have been implemented to obtain capsule space information with the position errors less than 20 mm by other researchers.23 Integrating this technology into active CE may bring benefits in gastric examination.
Gastric preparation is also important to active capsule examination, as current CE cannot supply water or air as standard gastroscopy, nor has functions of suction. Ideal preparation not only removes the residue and mucus from the stomach, but also distends the gastric cavity. Several strategies were adopted by previous studies. The strategy of Keller et al. was fasting overnight, 500 ml water and 5.8 g sherbet powder,18 while Rey et al. adopted fasting overnight, 900 ml water and light exercise. In our study, subjects ingested 1000 ml water and 6 g powder producing about 540 ml CO2. All subjects tolerated our preparation strategy well except one reported slight abdominal distention after adding 4 g air-producing powder. However, the cleanliness degree in 4 (11.8%) subjects and the distention of gastric cavity in 5 (14.7%) subjects was not considered good enough. Fluid pooled in the upper part of posterior wall sometimes impaired visualization and ingesting more water did not bring improvement. Simethicone is used widely in bowel preparation of traditional CE to decrease bubbles and residue,24 but the suspension module lead to opaque fluid in Keller et al's report18 and our previous porcine study. Other premedication proven effective in gastroscopy such as pronase25 may be tested to improve gastric preparation for MCE.
We did not adopt the strategy in the previous studies that the subjects would change the position to assist more effective movement of CE. Instead we let subjects to stay supine position through entire examinations for following reasons: First we would like to evaluate the maneuverability of CE by guidance magnet robot itself; Secondly we consider that a convenient capsule examination shall require patients to do nothing but remain relax position, especially for special groups such as infant or elderly. However, two of ten subjects were graded as moderate especially in cardiac and fundus regions. Whether changing position would assist visualization of these regions requires further evaluation in our future studies.
Despite initial and encouraging advancement in various kinds of magnetic-controlled capsule endoscopy, there was concern in its practical value at present.26 The drawbacks of current magnetic-controlled capsule endoscopy comparing with the standard EGD are clear: complicated gastric preparation, lack of biopsy capacity and long examination time. However, in our view all these drawbacks may be solved in the future as the technology keeps advance. As pointed by Rey et al,18 after balancing the pros and cons of standard EGD and magnetic-controlled capsule endoscopy, the latter might be a more cost-effective use of medical and social resources. In the future, MCE may be adopted as the screening method for gastric disease, especially for the elderly with sedation contraindications.
There were several limitations in our study. First, we assessed the completeness of visualization after examination. Sometimes it is difficult to judge whether the visualization was the result of active guidance or random movement, and it is possible to magnify this result. Secondly, there is no objective standard to evaluate the cleanliness and distention of stomach and maneuverability of capsule. We used semi-quantitative grade and this is not perfect but suboptimal and feasible method. Thirdly, the evaluation of gastric distention in our study was conducted when the distention is at the largest degree during the examination. Gastric distention decreased significantly as the examination progressed. The subjects with long examination time required additional water and air-producing powder.
In conclusion, MCE system had good maneuverability and detectability, and the acceptance and tolerance were excellent. MCE system appeared to be clinically valuable and should be developed further.
Supplementary Material
Acknowledgements
This research was supported by grant from the Foundation for the Author of National Excellent Doctoral Dissertation of China (to Z. Liao, No. 201271).
Abbreviations
- EGD
esophagogastroduodenoscopy
- CE,
apsule endoscopy
- MCE
magnetic capsule endoscopy
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
References
- 1.Bai Y, Li ZS, Zou DW, Wu RP, Yao YZ, Jin ZD, et al. Alarm features and age for predicting upper gastrointestinal malignancy in Chinese patients with dyspepsia with high background prevalence of Helicobacter pylori infection and upper gastrointestinal malignancy: an endoscopic database review of 102,665 patients from 1996 to 2006. Gut. 2010;59:722–728. doi: 10.1136/gut.2009.192401. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology. 2009;136:376–386. doi: 10.1053/j.gastro.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, et al. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol. 2010;105:2570–2577. doi: 10.1038/ajg.2010.324. [DOI] [PubMed] [Google Scholar]
- 4.Kim JI, Kim SG, Kim N, Kim JG, Shin SJ, Kim SW, et al. Changing prevalence of upper gastrointestinal disease in 28 893 Koreans from 1995 to 2005. Eur J Gastroenterol Hepatol. 2009;21:787–793. doi: 10.1097/MEG.0b013e32830e285a. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, Sobue T, et al. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2012;42:139–147. doi: 10.1093/jjco/hyr184. [DOI] [PubMed] [Google Scholar]
- 6.Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 7.Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–2337. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 8.Lanza FL, Chan FK, Quigley EM. Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol. 2009;104:728–738. doi: 10.1038/ajg.2009.115. [DOI] [PubMed] [Google Scholar]
- 9.Abraham N, Barkun A, Larocque M, Fallone C, Mayrand S, Baffis V, et al. Predicting which patients can undergo upper endoscopy comfortably without conscious sedation. Gastrointest Endosc. 2002;56:180–189. doi: 10.1016/s0016-5107(02)70175-2. [DOI] [PubMed] [Google Scholar]
- 10.Vargo JJ, DeLegge MH, Feld AD, Gerstenberger PD, Kwo PY, Lightdale JR, et al. Multisociety Sedation Curriculum for Gastrointestinal Endoscopy. Am J Gastroenterol. 2012;76:e1–e25. [Google Scholar]
- 11.Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, et al. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220–227. doi: 10.1055/s-0029-1243968. [DOI] [PubMed] [Google Scholar]
- 13.Heresbach D, Leray E, d'Halluin PN, Cholet F, Lapalus MG, Gaudric M, et al. Diagnostic accuracy of esophageal capsule endoscopy versus conventional upper digestive endoscopy for suspected esophageal squamous cell carcinoma. Endoscopy. 2010;42:93–97. doi: 10.1055/s-0029-1243856. [DOI] [PubMed] [Google Scholar]
- 14.Liao Z, Gao R, Xu C, Xu DF, Li ZS. Sleeve string capsule endoscopy for real-time viewing of the esophagus: a pilot study (with video) Gastrointest Endosc. 2009;70:201–209. doi: 10.1016/j.gie.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez FC, Akins R, Shaukat M. Screening on Barrett's esophagus with string-capsule endoscopy: a prospective blinded study of 100 consecutive patients using histology as the criterion standard. Gastrointest Endosc. 2008;68:25–31. doi: 10.1016/j.gie.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Keller J, Fibbe C, Volke F, Gerber J, Mosse AC, Reimann-Zawadzki M, et al. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos) Gastrointest Endosc. 2011;73:22–28. doi: 10.1016/j.gie.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 18.Rey JF, Ogata H, Hosoe N, et al. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75:373–381. doi: 10.1016/j.gie.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, et al. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010;42:148–152. doi: 10.1055/s-0029-1243808. [DOI] [PubMed] [Google Scholar]
- 20.Ciuti G, Menciassi A, Dario P. Capsule endoscopy: from current achievements to open challenges. IEEE Rev Biomed Eng. 2011;4:59–72. doi: 10.1109/RBME.2011.2171182. [DOI] [PubMed] [Google Scholar]
- 21.Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, et al. Feasibility of stomach exploration with a guided capsule endoscope. Endoscopy. 2010;42:541–545. doi: 10.1055/s-0030-1255521. [DOI] [PubMed] [Google Scholar]
- 22.Morita E, Ohtsuka N, Shindo Y, Nouda S, Kuramoto T, Inoue T, et al. In vivo trial of a driving system for a self-propelling capsule endoscope using a magnetic field (with video) Gastrointest Endosc. 2010;72:836–840. doi: 10.1016/j.gie.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Salerno M, Ciuti G, Lucarini G, Rizzo R, Valdastri P, Menciassi A, et al. A discrete-time localization method for capsule endoscopy based on on-board magnetic sensing. Meas Sci Technol. 2012;23:015701. [Google Scholar]
- 24.Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB, Xiao SD. Purgative bowel cleansing combined with simethicone improves capsule endoscopy imaging. Am J Gastroenterol. 2008;103:77–82. doi: 10.1111/j.1572-0241.2007.01633.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujii T, Iishi H, Tatsuta M, Hirasawa R, Uedo N, Hifumi K, et al. Effectiveness of premedication with pronase for improving visibility during gastroendoscopy: a randomized controlled trial. Gastrointest Endosc. 1998;47:382–387. doi: 10.1016/s0016-5107(98)70223-8. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkman DJ. Maneuverable Videocapsule Gastroscopy: Not Ready for Prime Time. Journal Watch Gastroenterology. 2012 Mar 2; http://gastroenterology.jwatch.org/cgi/content/full/2012/302/5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.