Abstract
Background
The Tokyo Guidelines have greatly impacted the management of ascending cholangitis. Though ERCP is the favored modality for biliary decompression, no evidence exists for the timing of ERCP. The DEIM-I study set out to determine if the time from patient presentation to biliary decompression impacted in hospital all cause mortality in ascending cholangitis.
Method
DEIM-I cohort study was a single-blinded and consisted of 250 subjects with moderate to severe ascending cholangitis who underwent ERCP/PBD. Subjects were randomized into quartiles based upon time from presentation until ERCP/PBD. The primary outcome utilized logistic regression to estimate relative risk (RR) of all cause, in hospital mortality with time to procedure as the predictive covariate. Secondary outcomes were analyzed using multivariate logistic regression and included; multiple organ failure (MOF), sepsis, systemic inflammatory response syndrome (SIRS), surgical incidence, hospital readmission and length of stay (LOS).
Results
The risk for hospital mortality was significantly less when biliary drainage was performed within 11 h, compared to >42 h (RR 0.34, 95%CI 0.12 to 0.99, p=0.049). Hospital readmission was lower in subjects who underwent biliary decompression less than 11 h, when compared to those greater than 22 h. Subjects who underwent biliary decompression within 21 h had significant higher risk for surgery compared to those 22–42 h.
Conclusion
The relative risk of all cause in hospital mortality was lower in subjects who underwent biliary decompression in under 11 h compared to greater than 42 h.
Key words: ERCP, cholangitis, PBD, biliary, mortality
Introduction
The American College of Cardiology Foundation has examined whether the time from patient presentation until percutaneous coronary intervention (PCI) impacts all cause mortality for patients with acute coronary syndrome.1 Similarly, the Surviving Sepsis Campaign has demonstrated a mortality benefit for patient's that presented with septic shock and undergo source control within the first 6 h from presentation.2 It is well known in the literature that ERCP is presently the standard therapy for obtaining source control in patients with ascending cholangitis.3–6 The Tokyo Guidelines voted that ERCP be performed following conservative treatment and not emergently or within 12 h in mild cases of cholangitis.7 This voting was an expert panel and did not site specific trial data for this component of the discussion.
As timing of source control is a vital component of determining survival for patients with sepsis, and ERCP is the method of source control for ascending cholangitis, it was our hope to determine if the time from patient presentation until biliary compression impacts hospital all cause mortality.
Methods
Study Design
DEIM-I was a retrospective cohort study performed at Cooper University Hospital in Camden, NJ. Data retrieval and analysis occurred at our institution only was approved by our institutions Investigational Review Board in accordance with the guidelines set forth in the Declaration of Helsinki. This study had no needed funding and the authors of this trial guarantee the legitimacy of this article's content.
Study Population
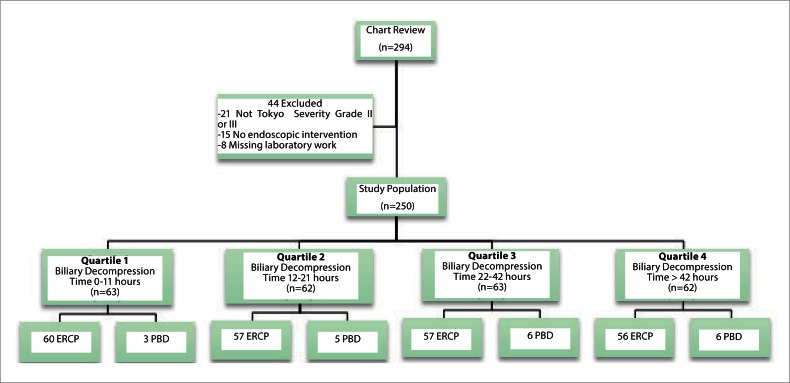
Eligibility criterion included subjects admitted to Cooper University Hospital from 1/1/2003 to 12/31/10, with a diagnosis of Ascending Cholangitis and subsequently have undergone either ERCP or PBD. Admissions were graded on severity of disease as defined by the Tokyo Guidelines, with only moderate to severe cholangitis admissions included for analysis (Appendix 1).8
The analytical sample was described in terms of age, sex, weight, co-morbid conditions, smoking history, presence or absence of right upper quadrant pain, jaundice, mental status on admission, temperature, blood pressure, heart rate, Fraction of Inspired Oxygen (FiO2), respiratory rate, central venous pressure (CVP), fluid in and out during hospital stay, white blood cell count, neutrophil count, hemoglobin, hematocrit, platelet count, creatinine, Prothrombin Time (PT), albumin, total bilirubin, direct bilirubin on admission, imaging performed (Ultrasound, Magnetic resonance cholangiopancreatography (MRCP), etc.) and results, presence/absence of common bile duct dilatation on size, ERCP/PBD and date/time of procedure, procedure related complications, date and time of antibiotic usage, pressors used with date and time of initiation, transfusion requirements (units), bacterial culture results.
Subjects were excluded if they had any of the following; no diagnosis of ascending cholangitis, a diagnosis of mild (grade I) ascending cholangitis, no ERCP or PBD during hospital stay and age < 18 years.
Interventions
All subjects underwent either ERCP or PBD. Interventions were randomized through judgment of either the Interventional Radiologist or Interventional Gastroenterologist under the standards of practice for each procedure.3–6,9
Among the study population, 22 underwent PBD and 228 underwent ERCP. Following the procedure, the subject's were examined for complications, need for additional intervention (ERCP/PBD) along with the primary end points.
End points
The primary end point of this study was all cause hospital mortality. Additional outcomes examined included; multiple organ failure (MOF), sepsis, systemic inflammatory response syndrome (SIRS), surgical incidence, hospital readmission and hospital length of stay (LOS).
MOF was diagnosed based upon the presence of altered organ function in acutely ill patients such that homeostasis cannot be maintained without intervention and had to involve two or more organ systems.10 SIRS was defined as any two out of the following; body temperature less than 36 degrees Celsius or greater than 38 degrees Celsius, heart rate greater than 90 beats per minute, respiratory rate greater than 20 breaths per minute or an arterial partial pressure of oxygen greater than 32 millimeters of mercury (mmHg), White blood cell count less than 4,000 cells/millimeter cubed (mm3)(4 ×109 cells/Liter), or greater than 9,000 cells/mm3, or greater than 10% immature neutrophils (band forms).10 Finally Sepsis was defined as a subject meeting the necessary criterion for SIRS, but also having a significant body fluid culture positive for bacteria.
Surgery was defined as major procedures performed excluding endoscopic intervention, percutaneous intervention. Length of stay was determined based upon time from presentation to time of discharge with one day as equivalent to 24 h.
Study analysis
Logistic regression was used to estimate the relative risk of all cause, in hospital mortality with time to procedure as the predictive covariate. A sample 250 provides 80% power to detect an odds ratio of 1.5, with time to procedure measured as a continuous variable and assuming as low a mortality rate as 27% (approximately 68 deaths) in the sample.
Multivariate logistic regression was used to examine the influence of additional risk factors, e.g. of age, sex, weight, co-morbid conditions, smoking history, presence or absence of right upper quadrant pain, jaundice, mental status on admission, temperature, blood pressure, heart rate, FiO2, respiratory rate, central venous pressure (if available)(CVP), fluid in and out during hospital stay, white blood cell count, neutrophil count, hemoglobin, hematocrit, platelet count, creatinine, PT, albumin, total bilirubin, direct bilirubin on admission, imaging performed (ultrasound, magnetic resonance cholangiopancreatography (MRCP), etc.) and results, presence/absence of common bile duct dilatation on size, ERCP/PBD and date/time of procedure, procedure related complications, date and time of antibiotic usage, pressors used with date and time of initiation, transfusion requirements (units), bacterial culture results.
The same methodology was applied to examine secondary binary outcomes; multiple organ failure (MOF), septic complications, systemic infection, surgical incidence. Ordinary least squares (OLS) regression was used for continuous secondary outcomes; hospital length of stay. Post hoc power was calculated for all non significant results.
Results
Study population
From January, 2003 through December, 2010, 294 subjects were examined. Two hundred and fifty subjects were separated in quartiles based upon the time from door until biliary intervention with 62 subjects in quartiles one and three, 63 subjects in quartiles two and four. Baseline characteristics were matched between the quartiles and can be summarized in table 1.
Table 1.
Composition of Study Population for Quartiles
| Intervention 0–11 h | Intervention 12–21 h | Intervention 22–42 h | Intervention > 42 h | p value1 | |
| Demographics | |||||
| Male Sex | 28 | 34 | 38 | 37 | 0.10 |
| Female Sex | 34 | 29 | 24 | 26 | 0.10 |
| Mean Age | 63.60 | 67.23 | 65.76 | 67.55 | 0.11 |
| Mean Weight (kg)a | 77.69 | 73.32 | 74.73 | 80.64 | 0.86 |
| Tobacco Use | 16 | 29 | 21 | 26 | 0.90 |
| APACHE II Score | 26 | 26 | 26 | 26 | 1.00 |
| Mean Tokyo Guidelines Severity | |||||
| Grade | 2.4 | 2.5 | 2.4 | 2.6 | 0.80 |
| Symptoms | |||||
| Right Upper Quadrant Pain | 50 | 45 | 54 | 43 | 0.88 |
| Jaundice | 52 | 38 | 41 | 42 | 0.67 |
| Vital Signs | |||||
| Mean Glasgow Coma Scaleb | 5.38 | 7.86 | 7.21 | 7.88 | 0.55 |
| Temperature (F)c | 99.26 | 99.41 | 99.27 | 99.63 | 0.15 |
| Heart Rate (bpm)d | 96.67 | 95.86 | 94.26 | 89.21 | 0.22 |
| Respiratory Rate (rpm)e | 20.60 | 19.57 | 19.78 | 18.67 | 0.37 |
| FiO2 (%)f | 31.19 | 32.40 | 30.55 | 29.49 | 0.16 |
| CVPg | 7.73 | 8.16 | 7.80 | 8.00 | 0.87 |
| Mean Fluid Intake (mL)h | 1984.30 | 3085.40 | 2960.69 | 2433.17 | 0.82 |
| Mean Fluid Output (mL) | 1014.72 | 1203.71 | 1568.27 | 1003.48 | 0.61 |
| Laboratory Values | |||||
| Mean WBC Count (×103/uL)i | 16.52 | 12.54 | 15.70 | 14.29 | 0.85 |
| Mean Neutrophil Count (%) | 75.38 | 75.55 | 71.48 | 75.71 | 0.38 |
| Hemoglobin (g/dL)j | 11.33 | 11.50 | 11.80 | 11.64 | 0.12 |
| Hematocrit (%) | 33.30 | 34.15 | 34.78 | 34.63 | 0.70 |
| Platelet (k/uL)k | 285.14 | 216.64 | 259.02 | 234.45 | 0.10 |
| Creatinine (mg/dL)l | 1.08 | 0.95 | 1.34 | 1.21 | 0.55 |
| Prothrombin Time (seconds) | 15.59 | 15.80 | 18.67 | 18.66 | 0.97 |
| Albumin (g/dL) | 2.94 | 3.20 | 3.15 | 3.20 | 0.28 |
| Total Bilirubin (mg/dL) | 7.90 | 6.71 | 7.65 | 6.48 | 0.80 |
| Direct Bilirubin (mg/dL) | 5.56 | 4.80 | 5.37 | 4.72 | 0.81 |
| Mean Common Bile Duct Size (mm)m | 11.75 | 11.64 | 12.50 | 12.42 | 0.88 |
(kg)=kilograms,
Glasgow Coma Scale refer to Appendix X,
(F)=Degrees Fahrenheit,
bpm= beats per minute,
rpm= respirations per minute,
%=percent,
CVP=Central Venous Pressure in millimeters of mercury(mmHg),
mL=milliliters,
x103/uL= thousand per microliter,
g/dL= grams per deciliter,
k/uL= thousand per microliter,
mg/dL=milligrams per deciliter,
mm=millimeter.
Multivariate logistic regression.
Quartile one was defined as door to ERCP/PBD times of zero to eleven hours. Quartiles 2,3,4 had times of 12 to 21 h, 22 to 42 h and >42 h respectively.
Efficacy outcomes
Of the 250 subjects, 20 (8%) underwent primary PBD, when compared to ERCP. Of the remaining subjects who underwent ERCP, 2 underwent PBD and one exploratory laparotomy due to failure of source control.
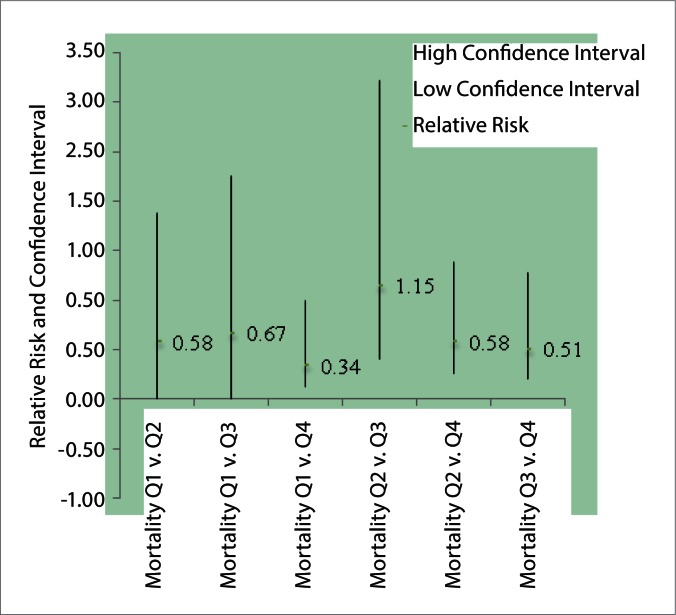
The all cause in hospital mortality for these quartiles can be summarized in table 2. Among the four quartiles, the risk for hospital mortality was significantly less when biliary drainage was performed within 11 h when compared to >42 h (RR 0.34, 95%CI 0.12 to 0.99, p=0.049). No additional differences were found between the quartiles for mortality (Fig. 2).
Table 2.
End point occurrences within quartiles
| End Point | Intervention 0–11 h | Intervention 12–21 h | Intervention 22–42 h | Intervention > 42 h | Total |
| Hospital All Cause Mortality | 4 | 7 | 6 | 12 | 29 |
| Multiple Organ Failure | 4 | 6 | 7 | 4 | 21 |
| Systemic Inflammatory Response Syndrome | 47 | 41 | 43 | 39 | 170 |
| Sepsis | 34 | 38 | 33 | 29 | 134 |
| Surgery | 13 | 15 | 3 | 9 | 40 |
| Readmission | 6 | 13 | 22 | 16 | 57 |
| Mean Hospital Length of Stay (days) | 11.88 | 8.30 | 8.28 | 13.33 |
Figure 2.
Relative risk of mortality between quartiles
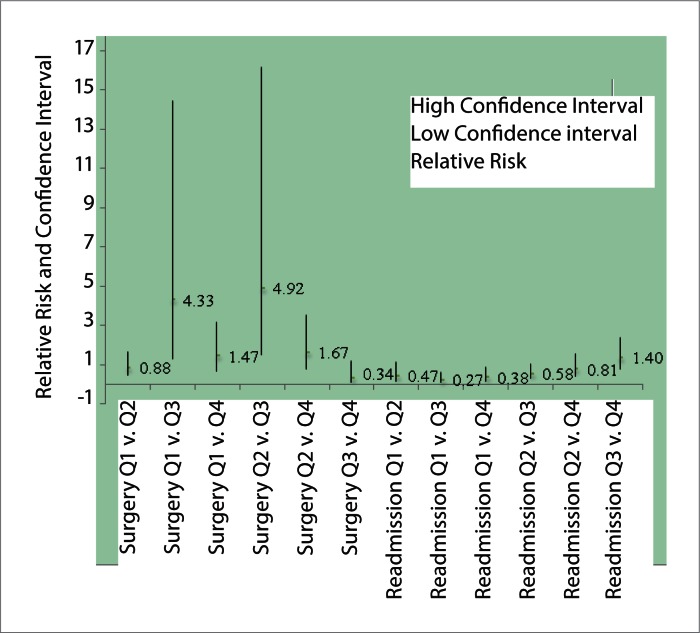
Furthermore, the risk of hospital readmission was lower in subjects who underwent biliary decompression less than 11 h, when compared to those who underwent PBD/ERCP greater than 22 h (Q1 v. 3: RR 0.27, 95%CI 0.12 to 0.62, p=0.002, Q1 v. Q4: 0.38, 95%CI 0.16 to 0.91, p=0.030 and versus Q4: RR 0.82, 95%CI 0.67 to 1.00)(Fig. 3). Subjects who underwent biliary decompression within 21 h had significant higher risk for surgery when compared to subjects who underwent ERCP/PBD 22-42 hours (Q1 v. Q3: RR 4.33, 95 % CI 1.30 to 14.46, p=0.0171 and Q2 v. Q3: RR 4.92, 95%CI 1.50 to 16.16, p=0.0086, respectively)(Fig. 3).
Figure 3.
Relative risk of surgery and readmission between quartiles
Of the patient who needed surgery, most underwent cholecystecomy prior to discharge only one patient underwent exploratory laparotomy due to gastrointestinal perforation. There were no differences in the quartiles when examining the incidence of MOF, SIRS and sepsis (Table 3). Finally, the mean LOS was not statistically different when comparing the quartiles (Table 4).
Table 3.
The relative risk and confidence intervals for the study end points
| Relative Risk | High Confidence Interval | Low Confidence Interval | |
| Mortality Q1v.Q2 | 0.58 | 1.88 | 0.1789 |
| Mortality Q1v.Q3 | 0.67 | 2.25 | 0.1978 |
| Mortality Q1v.Q4 | 0.34 | 0.99 | 0.12 |
| Mortality Q2v.Q3 | 1.15 | 3.22 | 0.41 |
| Mortality Q2v.Q4 | 0.58 | 1.38 | 0.25 |
| Mortality Q3v.Q4 | 0.51 | 1.27 | 0.20 |
| MOF1 Q1v.Q2 | 0.68 | 2.28 | 0.20 |
| MOF Q1v.Q3 | 0.57 | 1.85 | 0.18 |
| MOF Q1v.Q4 | 1.02 | 3.89 | 0.27 |
| MOF Q2v.Q3 | 0.84 | 2.37 | 0.30 |
| MOF Q2v.Q4 | 1.50 | 5.06 | 0.44 |
| MOF Q3v.Q4 | 1.78 | 5.77 | 0.55 |
| Sepsis Q1v.Q2 | 0.91 | 1.23 | 0.67 |
| Sepsis Q1v.Q3 | 1.03 | 1.43 | 0.74 |
| Sepsis Q1v.Q4 | 1.19 | 1.69 | 0.84 |
| Sepsis Q2v.Q3 | 1.13 | 1.54 | 0.83 |
| Sepsis Q2v.Q4 | 1.31 | 1.83 | 0.94 |
| Sepsis Q3v.Q4 | 1.16 | 1.65 | 0.81 |
| SIRS2 Q1v.Q2 | 1.16 | 1.45 | 0.93 |
| SIRS Q1v.Q3 | 1.09 | 1.36 | 0.88 |
| SIRS Q1v.Q4 | 1.22 | 1.56 | 0.96 |
| SIRS Q2v.Q3 | 0.94 | 1.2 | 0.73 |
| SIRS Q2v.Q4 | 1.05 | 1.37 | 0.81 |
| SIRS Q3v.Q4 | 1.12 | 1.45 | 0.87 |
| Surgery Q1v.Q2 | 0.88 | 1.69 | 0.46 |
| Surgery Q1v.Q3 | 4.33 | 14.46 | 1.30 |
| Surgery Q1v.Q4 | 1.47 | 3.18 | 0.68 |
| Surgery Q2v.Q3 | 4.92 | 16.16 | 1.5 |
| Surgery Q2v.Q4 | 1.67 | 3.52 | 0.79 |
| Surgery Q3v.Q4 | 0.34 | 1.19 | 0.1 |
| Readmission Q1v.Q2 | 0.47 | 1.16 | 0.19 |
| Readmission Q1v.Q3 | 0.27 | 0.62 | 0.12 |
| Readmission Q1v.Q4 | 0.38 | 0.91 | 0.16 |
| Readmission Q2v.Q3 | 0.58 | 1.05 | 0.32 |
| Readmission Q2v.Q4 | 0.81 | 1.55 | 0.43 |
| Readmission Q3v.Q4 | 1.40 | 2.4 | 0.81 |
Quartile (Q),
Multiple Organ Failure (MOF),
Systemic Inflammatory Response Syndrome (SIRS).
Table 4.
Comparison of Hospital Length of Stay between Quartiles
| Quartile Comparison | p value (95% Confidence Interval) |
| 0–11 h versus 12–21 h | p=0.11 (−0.85 to 8.01) |
| 0–11 h versus 22–42 h | p=0.09 (−0.62 to 7.82) |
| 0–11 h versus > 42 h | p=0.67 (−8.19 to 5.29) |
| 12–21 h versus 22–42 h | p=0.99 (−2.68 to 2.72) |
| 12–21 h versus > 42 h | p=0.09 (−10.91 to 0.85) |
| 22–42 h versus > 42 h | p=0.08 (−10.80 to 0.70) |
Adverse events
Among the sample population 15 adverse events were recorded as summarized in table 5. Among the ten subjects that required secondary intervention, 2 underwent primary ERCP and had to undergo subsequent PBD. This can be compared to the remaining 8 subjects who underwent PBD and required secondary ERCP. Pancreatitis occurred in 2 subjects one in quartile one and the other in quartile two. Two subjects had extensive bleeding both in quartiles three and four. Only one subject experienced hypotension after the procedure, likely due to anesthetic. There were similar incidences of secondary interventions and adverse events within each quartile.
Table 5.
Adverse events caused by biliary decompression within quartiles
| Adverse Event | Intervention 0–11 h | Intervention 12–21 h | Intervention 22–42 h | Intervention > 42 h | Total |
| Secondary Intervention Needed | 2 | 4 | 3 | 1 | 10 |
| Pancreatitis | 1 | 1 | 0 | 0 | 2 |
| Bleeding | 0 | 0 | 1 | 1 | 2 |
| Hypotension | 0 | 1 | 0 | 0 | 1 |
Discussion
Several studies have demonstrated mortality and morbidity benefit of ERCP when compared to other modalities for biliary decompression.3–6,11,12 With a clear benefit of ERCP in ascending cholangitis, the Tokyo guidelines stated that ERCP should occur following conservative treatment for patients with moderate to severe ascending cholangitis, but may occur electively in subjects with mild disease.7 Additional trials have investigated whether clinicians can predict which patients must undergo emergency versus elective ERCP, with some promising results.13,14 The culmination of this data alluded to the selection of subjects and its impact on the timing of ERCP. For this reason, we selected for subjects with moderate to severe ascending cholangitis.
In the DEIM-I study, we examined 250 subjects who presented to our hospital with moderate to severe ascending cholangitis and underwent biliary decompression by either ERCP or PBD. Our primary end point was the impact of time on all cause in hospital mortality. In our analysis, we demonstrated a lower relative risk of mortality in subjects who underwent biliary decompression under 11 hours, when compared to those who underwent the procedure in greater than 42 h (RR=0.34, 95%CI 0.12 to 0.99, p<0.049). It is our belief that this result cannot be explained by differences in the sample population including age, sex, etc (Table 1). This difference can also not be explained by variation the endoscopist, as the same Interventional Gastroenterologist performed all ERCPs. Additionally, the data gatherers were blinded as to the primary endpoints of this study, which eliminated potential bias in the analysis. Furthermore, as source control impacts mortality in sepsis, it is our belief that ERCP serving as source control for this infectious process is the reason for our discovered mortality benefit in sepsis.2
The Surviving Sepsis Campaign recommended that source control occur within 6 hours of presentation for subjects with sepsis, if an anatomic site can be identified. These guidelines specifically allude to endoscopic intervention in biliary infections. Jimenez, et al discussed the complicated process of infection in the critically ill patient and the alterations in a patient's pathyophysiology if intervened upon.15 We believe that timely source control may alter the process of endotoxemia and subsequent end organ dysfunction that may be seen in ascending cholangitis.
To prevent the reoccurrence of ascending cholangitis, cholecystectomy is commonly performed and has been shown to reduce mortality.16,17 With this in mind, the relative risk of surgery in the DIEM-I study was found to be higher when biliary decompression occurred under 21 h when compared to 22–42 h (Table 3). It is important to note that all surgeries, but one performed were cholecystectomys. Though expressed as relative risk, the authors of this study believed this outcome to be a marker of successful biliary decompression and standard of care. Additionally, this finding indicated that subjects were hemodynamically stable enough, after biliary decompression, to undergo surgery.
Other secondary outcomes investigated showed no difference in MOF, sepsis, SIRS and mean LOS, likely due to subjects randomization and similarities in disease severity between the quartiles. However, the relative risk for readmission was found to be lower in subjects who underwent biliary decompression in under 11 h when compared to those who underwent decompression greater than 22 h (Fig. 3). Again, this outcome is likely due to expedited source control and perhaps due to cholecystectomy preventing disease reoccurrence.
Potential limitations of early biliary decompression included the incidence of adverse events. Additional studies have examined the adverse outcomes of subjects undergoing emergent versus elective ERCP, showing some increased incidence of pancreatitis in the emergent group.18 Our study did demonstrate 15 complications of biliary decompression including summarized in table 5. There were no differences seen in complications between the quartiles. Further limitations of this study include the retrospective nature, yet it is our hope to obtain a prospective trial examining similar results (DEIM-II). We also would like to perform a study that examined only ERCP and not PBD.
Conclusions
The relative risk of all cause in hospital mortality was lower in subjects who underwent biliary decompression in under 11 h when compared to subjects who underwent the procedure in greater than 42 h.
The relative risk of readmission was also lower in subjects who underwent biliary decompression in under 11 h when compared to those who underwent decompression in over 22 h. Finally the risk of surgery, mostly cholecystectomy, was higher in subjects who underwent biliary decompression in under 21 h when compared to 22 to 42 h.
Figure 1.
Study review
Acknowledgements
SM created the concept, IRB proposal for this study and wrote majority of this scientific manuscript. CM, MD, PH and PS were main researchers for this study. TJ was the faculty advisor and BM was the statistician.
The writers of this paper would like to acknowledge the members of the medical records department at Cooper University Hospital and the Physicians and staff of Cooper University Hospital at Rowan University.
Abbreviations
- PCI
percutaneous coronary intervention
- CVP
central venous pressure
- MRCP
magnetic resonance cholangiopancreatography
- MOF
multiple organ failure
- SIRS
systemic inflammatory response syndrome
- LOS
length of stay
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
Disclosures
The authors of this paper required no funding for the creation of this study. Additionally, the authors have no financial disclosures to report. This article has not been submitted to any other journal and its text original.
References
- 1.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, author. 2011 ACCF/AHA Focused Update of the Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction (Updating the 2007 Guideline) J Am Coll Cardiol. 2011;57:1920–1959. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. International Surviving Sepsis Campaign Guidelines Committee; Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 3.Kimura Y, Takada T, Kawarada Y, Nimura Y, Hirata K, Sekimoto M, et al. Definitions, pathophysiology, and epidemiology of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:15–26. doi: 10.1007/s00534-006-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, et al. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;24:1582–1586. doi: 10.1056/NEJM199206113262401. [DOI] [PubMed] [Google Scholar]
- 5.Miura F, Takada T, Kawarada Y, Nimura Y, Wada K, Hirota M, et al. Flowcharts for the diagnosis and treatment of acute cholangitis and cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:27–34. doi: 10.1007/s00534-006-1153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuyuguchi T, Takada T, Kawarada Y, Nimura Y, Wada K, Nagino M, et al. Techniques of biliary drainage for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14(1):35–45. doi: 10.1007/s00534-006-1154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagino M, Takada T, Kawarada Y, Nimura Y, Yamashita Y, Tsuyuguchi T, et al. Methods and timing of biliary drainage for acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:68–77. doi: 10.1007/s00534-006-1158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wada K, Takada T, Kawarada Y, Nimura Y, Miura F, Yoshida M, et al. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:52–58. doi: 10.1007/s00534-006-1156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens CA, Funaki BS, Ray CE., Jr Expert Panel on Interventional Radiology. Radiologic management of benign and malignant biliary obstruction. American College of Radiology Appropriateness Criteria. 2008:1–7. doi: 10.1016/j.jacr.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Irwin RS, Rippe JM. Irwin and Rippe's Intensive Care Medicine. 6edition. San Diego, Ca: Lippincott, Williams and Wilkins; 2008. [Google Scholar]
- 11.Qureshi WA. Approach to the Patient Who Has Suspected Acute Bacterial Cholangitis. Gastroenterol Clin N Am. 2006;35:409–423. doi: 10.1016/j.gtc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Leese T, Neoptolemos JP, Baker AR, Carr-Locke DL. Management of acute cholangitis and the impact of endoscopic sphincterotomy. Br J Surg. 1986;73:988–992. doi: 10.1002/bjs.1800731214. [DOI] [PubMed] [Google Scholar]
- 13.Hui CK, Lai KC, Yuen MF, Ng M, Lai CL, Lam SK. Acute cholangitis predictive factors for emergency ERCP. Aliment Pharmacol Ther. 2001;15:1633–1637. doi: 10.1046/j.1365-2036.2001.01071.x. [DOI] [PubMed] [Google Scholar]
- 14.Pang YY, Wai Chun YA. Predictors for emergency biliary decompression in acute cholangitis. Eur J Gastroenterol Hepatol. 2006;18:727–731. doi: 10.1097/01.meg.0000219105.48058.df. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez MF, Marshall JC. International Sepsis Forum. Source control in the management of sepsis. Intensive Care Med. 2001;27:S49–S62. doi: 10.1007/pl00003797. [DOI] [PubMed] [Google Scholar]
- 16.Kinney TP. Management of ascending cholangitis. Gastrointest Endosc Clin N Am. 2007;17(2):289–306. doi: 10.1016/j.giec.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.McAlister VC, Davenport E, Renouf E. Cholecystectomy deferral in patients with endoscopic sphincterotomy. Cochrane Database Syst Rev. 2007;4:CD006233. doi: 10.1002/14651858.CD006233.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueki T, Otani K, Fujimura N. Comparison between emergency and elective endoscopic sphincterotomy in patients with acute cholangitis due to choledocholithiasis: is emergency endoscopic sphincterotomy safe? J Gastroenterol. 2009;44:1080–1088. doi: 10.1007/s00535-009-0100-4. [DOI] [PubMed] [Google Scholar]