Abstract
Background and Objectives
Adequate colon cleansing is an important factor in performing quality colonoscopy. Split dose Polyethylene Glycol (PEG) solutions have been shown to improve colon cleansing, but the effectiveness in a large clinical practice of elderly co-morbid patients has not been demonstrated. The aim of this study was to assess the efficacy of a simplified split PEG bowel prep in Veterans Health Administration (VHA) patients.
Methods
Prospective pre-post study design of VHA patients undergoing routine colonoscopy. Bowel prep quality was assessed using a standardized semi-quantitative 5-point scale. “Standard” 4L PEG prep was consumed once the evening before the procedure. “Split” prep was consumed half in the early evening and half in the late evening or early morning depending on procedure time.
Results
Right colon preps were Excellent/Good in 81.4% of split preps (n=199) vs. 63% of standard preps (n=447, p<0.001). Left colon preps were Excellent/Good in 85.9% of split preps vs. 71.6% of standard preps (p<0.001). Diabetics (n=133) had significantly more right colon preps rated fair or worse compared to non-diabetics irrespective of prep (39.9% vs. 29.0%, p=0.02). Split prep in diabetics resulted in fewer right colon preps rated fair or worse compared to diabetics using standard prep (28.3% vs. 45.9%, p=0.049). Average adenomas detected per colonoscopy were 1.04 for split prep vs. 0.85 for standard prep (p=NS). Patient satisfaction was higher for split preps.
Conclusion
System-wide implementation of a split PEG prep resulted in significantly improved bowel cleansing in VHA patients, particularly in the right colon. Improved bowel cleansing with split preps was associated with higher patient satisfaction.
Key words: colonoscopy, colorectal cancer screening, colorectal neoplasm, polyethylene glycol, quality improvement
Introduction
Colon cancer is the second leading cause of cancer death in the United States after lung cancer and is often treatable if identified at an early stage.1 Colon cancer screening is a cost effective, potentially life-saving measure particularly given the long asymptomatic phase of colon cancer.2 Current screening guidelines recommend colon cancer screening beginning at age 50 for all adults at average risk for colon cancer, and Medicare enacted coverage of screening colonoscopy in beneficiaries at average risk in 2001.1,3 Despite these guidelines, less than half of those who should be screened actually undergo colon cancer screening.2 In addition, recent case control studies indicate that colonoscopy is much less effective for reducing the incidence or mortality related to right-sided colon cancers.4–7 The reasons for the non-adherence to screening guidelines and success of screening are multi-factorial and likely includes failure to refer patients, patient refusal, avoidance of colonoscopy and/or intolerance of the bowel preparation, as well as perceived ineffectiveness of the screening methods themselves. Factors responsible for the reduced effectiveness of colonoscopy for prevention of right sided colon cancers include the higher rate of “flat” polyps in the right colon,8 the use of poor colonoscopy technique,9 and the presence of poor bowel cleansing. Kazarian et al. demonstrated the rate of inadequate bowel preparation to be 30.2% and the rate of poor bowel preparation that absolutely precluded an exam to be 9.9% in a large community based health system in Denver.10 Randomized controlled trials have also shown a high level of inadequate bowel preps ranging from 10% to 75%.11 The authors reported that procedures in poorly prepared patients were longer, more difficult, and more often incomplete. Additionally, the detection of polyps depended on cleansing quality with an OR 1.73 for intermediate-quality compared with low-quality preparation. The authors also presented data suggesting bowel preparation was poorer in elderly patients and patients with more comorbid conditions.11
Ideally the preparation used should empty the colon of all fecal material and have little impact on the visual or microscopic appearance of the mucosa. Additionally, it should cause little patient discomfort and have relatively few side effects such as fluid-electrolyte shifts to prevent adverse events and increase adherence. Colon cleansing is typically performed with polyethylene glycol (PEG). As we have increasingly identified the importance of the bowel preparation in performing quality colonoscopy, multiple new approaches have been studied to improve our commonly used bowel preparation regimens.12,13 Splitting the standard 4L PEG preparation has shown to be a superior methodology in a recent meta-analysis of prospective, randomized studies.14 Traditionally, the PEG preparation was prescribed to be taken over a 4 hour period the evening before the scheduled colonoscopy. Splitting the PEG preparation involves dividing the administration of the prep into two periods: half the prep is taken after dinner the evening prior to the procedure and the other half is taken in the early morning prior to the procedure. The theory is that reducing the volume of the preparation taken in one sitting will improve patient tolerability and therefore compliance. Additionally, Siddiqui et al. have shown that completion of the bowel preparation closer to the start time of the colonoscopy results in significantly higher quality bowel preparations.15 However, there is concern about patient compliance if they are expected to awaken in the middle of the night to take their preparation.
VHA patients represent a unique population who have been shown to be in poorer health than the general population,16 making bowel preparation prior to colonoscopy more challenging. Additionally, it has been shown that screening colonoscopy rates in VHA patients are lower than the general population with more VHA patients going for diagnostic exams after a less invasive screening test. The potentially increased likelihood of finding lesions in this population makes it of paramount importance to provide the highest quality colonoscopy possible. While “split dose” PEG protocols have been shown to be effective, they have not been studied in a real world practice involving non-selected VHA patient populations. The purpose of this study was to compare the effectiveness of a “Split” PEG bowel preparation with a standard PEG bowel preparation in a non-selected VHA patients. We used a pre-post study design with prospectively collected data evaluating bowel preparation scores. If “Split” PEG bowel preparations are feasible and effective, they may represent a low cost and safe solution for high quality colonoscopy in a real world practice situation.
Patients and Methods
Study Design
This was a prospective pre-post study design evaluating all colonoscopies before and after an institutional policy change in the method of bowel preparation for colonoscopy.
Participants
The participants in this prospective study involved consecutive patients undergoing a colonoscopy for any indication at the Veterans Affairs San Diego Healthcare System between March 26, 2009 and October 2, 2009. Both inpatients and outpatients were included in the analysis. Patients who did not use a standard PEG (Golytely) bowel preparation or a split PEG bowel preparation were excluded. This included a minority of patients that were unable to tolerate the PEG solution, and were generally prepped using magnesium citrate and bisacodyl. The study was approved by the Institution Review Board and the Research and Development Committee of the University of California, San Diego, and the VA San Diego Healthcare System, respectively.
Bowel Preparation Protocol
Standard Preparation Prior to July 1, 2009 all patients referred for colonoscopy underwent a standard bowel preparation before their procedure. As part of the standard bowel preparation all outpatients were instructed to adhere to a clear liquid diet starting 48 h prior to the colonoscopy. This clear liquid diet included water, tea, coffee (no milk), clear broth, juice with low pulp, Gatorade, soda, popsicles, kool-aid and jello (no red jello or kool-aid). Inpatients were either NPO or on a clear liquid diet the day prior to the procedure. All patients receiving a standard preparation were instructed to ingest all of the 4L PEG solution (Golytely, Braintree Laboratories, Braintree, MA) between 5:00 PM and 10:00 PM the evening prior to the colonoscopy. Patients were permitted to add sugar free lemonade (crystal light) instead of water to improve the taste.
Split Preparation
Beginning July 1, 2009 all patients referred for colonoscopy were instructed to take a split PEG bowel preparation before their procedure. Similar to the standard preparation, all outpatients were instructed to adhere to a clear liquid diet starting 48 h prior to the colonoscopy. Patients with a procedure start time earlier than 10 AM were instructed to drink 2L of PEG between 5 PM and 6 PM and another 2 L between 10 PM and 12 AM the evening prior to the procedure. Patients with a procedure start time later than 10 AM were instructed to drink 2L of PEG at 5–6 PM the evening prior to the procedure and a second 2L of PEG between 4 AM and 6 AM the day of the procedure. Patients were again permitted to add sugar free lemonade (crystal light) instead of water to improve the taste. This regimen was considered “patient friendly”, because it did not require them to awaken in the very early morning hours if they had a morning procedure scheduled.
Evaluation of Bowel Preparation
A demographic sheet was completed by the nurse prior to the procedure. Information collected included age, gender, current use of narcotics (yes or no), diabetes using an oral agent or insulin (yes or no), and amount of prep completed (<25%, 25–50%, 51–75%, 76–100%).
All colonoscopies were performed by an experienced attending gastroenterologist or by a gastroenterology fellow under the direct supervision of an experienced gastroenterologist using standard video colonoscopes. The bowel preparation in the left colon (rectum, sigmoid, descending) and right colon (transverse, ascending, cecum) was rated by the endoscopist (attending or fellow) using a semi-quantitative 5-point rating scale (Table 2). Bowel prep scores were reported as a left colon score, right colon score, and total score (left + right). The lower the score, the better the preparation. All staff were trained in the use of the scoring system by the use of standardized photos. The scoring system was validated using videotaped colonoscopies (n=10 right colon, n=10 left colon) with ratings by three independent colonoscopists. Inter-rater reliability was evaluated using intraclass correlation (ICC) coefficients.27 The number of polyps removed during each procedure was recorded. Pathology was then reviewed to calculate adenoma detection rates and number of adenomas detected per procedure in the left, right, and total colon. Adenomatous polyps included polyps categorized as tubular adenoma, serrated adenoma, tubulovillous adenoma, polyps with high grade dysplasia, or polyps with carcinoma in situ.
Table 2.
Bowel Prep Rating Scale
| Colon Prep Score | Description |
| (1) Excellent | Mucosal detail easily seen. Requires minimal washing |
| (2) Good | Liquid stool easily washed/suctioned |
| (3) Fair | Chunks of stool or lots of liquid; requires extensive washing/suctioning |
| (4) Poor | Unable to really see most flat lesions, despite washing |
| (5) Impossible | Unable to complete procedure or rule out obstructing lesions due to prep |
Patient Satisfaction
A subgroup of patients were administered a patient satisfaction questionnaire immediately before their procedure. Patients with a history of prior colonoscopies were asked to compare their experience with the split prep vs. the standard prep taken at one time the evening before the procedure.
Statistical Analysis
Statistical analysis of baseline characteristics and outcomes was carried out using statistical software (SPSS 18.0 for Mac; SPSS, Inc.). The proportions in 2 × 2 contingency tables were compared by using the chi-square test with the Yates correction for continuity. Means and continuous variables were compared by using the Student t-test of independent groups. P values of 0.05 or less were considered to be statistically significant.
The results for colon preparation were dichotomized to “adequate” preparation (excellent and good results pooled) and “inadequate” (fair, poor, and impossible results pooled). In addition to evaluating the overall colon preparation, preparations in the left and right colon were evaluated separately. Variables found to be significant predictors of an adequate preparation at a p < 0.10 in univariate analysis were assessed in multivariate logistic regression modeling. Multivariate models were obtained by running both forward and backward stepwise logistic regression. Variables significant at p < 0.05 were included in the final model.
Similarly, colonoscopies were dichotomized into adenoma detected and adenoma not detected. Variables found to be significant predictors of detecting an adenoma at a p < 0.10 in univariate analysis were assessed in multivariate logistic regression modeling. Multivariate models were obtained by running both forward and backward stepwise logistic regression. Variables significant at p< 0.05 were included in the final model.
Results
Demographics
Data was available for 653 colonoscopies performed at the Veterans Affairs San Diego Healthcare System between March 26, 2009 and October 2, 2009. 200 patients used a “Split” PEG preparation prior to their colonoscopy and 453 patients used a “Standard” PEG preparation. The two groups were similar with regards to gender, age, number of diabetic patients, number of patients using chronic narcotic medications, and procedures performed by a fellow with an attending (Table 1).
Table 1.
Demographic Information of “Split” PEG Prep and “Standard” PEG Prep Groups
| Split Prep | Standard Prep | |
| Total Cases | 200 | 453 |
| Gender, N (%) | ||
| Male | 185 (92.5) | 414 (91.4) |
| Female | 15 (7.5) | 38 (8.4) |
| Age (yr) | ||
| Mean ± SD | 59.8 ± 12.4 | 59.5 ± 11.3 |
| Range | 24–89 | 20–86 |
| Diabetic on medication, N (%) | 47 (23.5) | 85 (18.8) |
| Current Narcotic Use, N (%) | 22 (11) | 79 (17.4) |
| Fellow Involved in Case, N (%) | 123 (61.5) | 296 (65.3) |
PEG=Polyethylene Glycol.
Bowel Preparation Quality
The bowel preparation in the left colon and right colon was prospectively rated by the endoscopist using a semi-quantitative 5-point rating scale (Table 2) Inter-rater reliability of the three independent ratings of the bowel prep rating scale was found to be high (ICC = 0.921 and 0.893 for the right and left colon, respectively). Colon prep ratings for the left colon (85.9% vs. 71.6%, p<0.001), right colon (81.4% vs. 63.0%, p<0.001), and overall colon (81.3% vs. 61.6%, p<0.001) were significantly more often adequate (excellent or good) when using a “Split” PEG prep compared to a “Standard” PEG prep (Fig. 1).
Figure 1.
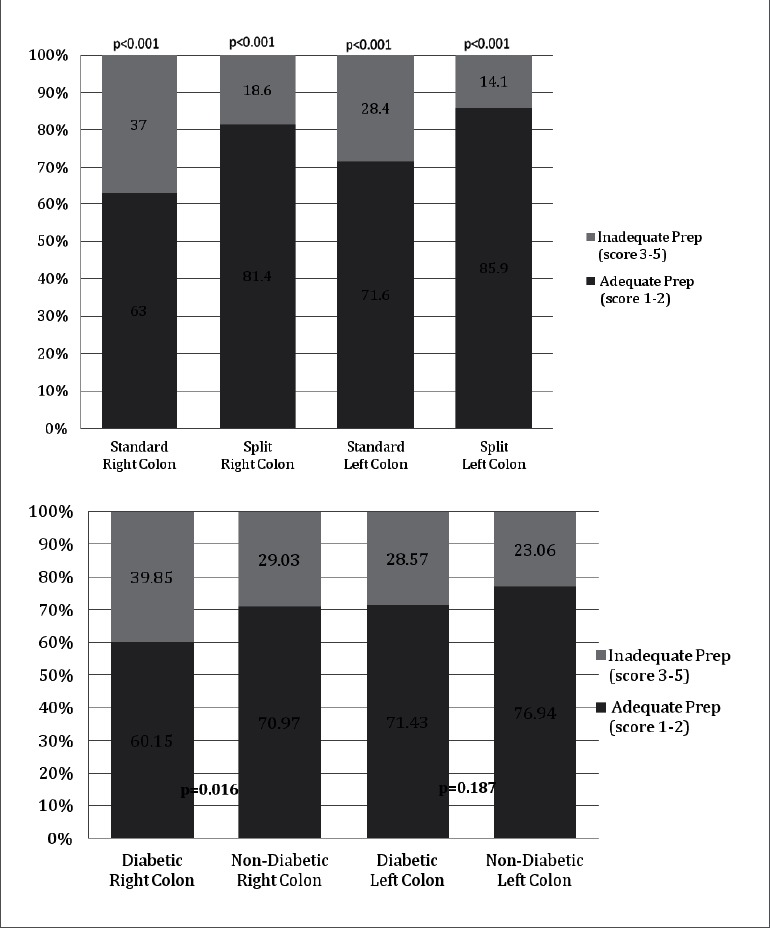
A, Percentage of procedures with adequate and inadequate prep for “standard” vs. “split” prep; B, Percentage of procedures with adequate and inadequate prep for diabetic vs. non-diabetic patients.
Predictor of Adequate Bowel Prep
“Split” PEG prep (p<0.001) and not using narcotic medications (p<0.003) were both univariate predictors of an adequate bowel prep score in the left colon. Gender, age, and diabetes were not significant predictors of an adequate bowel prep score in the left colon. In multivariate logistic regression, both not using narcotic medications (OR 1.93, p=0.006) and “Split” PEG prep (OR 2.46, p<0.001) were predictors of an adequate bowel prep. Univariate predictors of an adequate bowel prep in the right colon included not being a diabetic (p=0.022), not using narcotic medications (p=0.015), and “Split” PEG prep (p<0.001). Age and gender were not significant predictors of adequate prep in univariate analysis. However, gender was included in the multivariate analysis of the right colon prep because it hada p < 0.1. The final model in multivariate logistic regression yielded not using narcotics, not being a diabetic, and “Split” PEG prep as significant predictors of an adequate bowel prep score in the right colon. Univariate predictors of an adequate overall prep score were not using narcotics (p=0.001) and “Split” PEG prep (p<0.001). Diabetes (p=0.096), gender (p=0.065), and age (0.094) were not significant in univariate analysis, but all were included in the multivariate analysis because each had p<0.1. Multivariate logistic regression analysis yielded a final model with not using narcotics (OR 2.08, p=0.001) and “Split” PEG prep (OR 2.87, p=0.002) as significant predictors of adequate bowel prep. Results of the multivariate logistic regression are summarized in table 3.
Table 3.
Multivariate Predictors of Adequate Bowel Prep
| Predictors | Odds Ratio | 95% CI | p |
| Left Colon | |||
| No Narcotic Med | 1.93 | (1.21, 3.07) | 0.006 |
| “Split” PEG Prep | 2.46 | (1.55, 3.92) | 0.001 |
| Right Colon | |||
| No Narcotic Med | 1.71 | (1.08, 2.71) | 0.023 |
| Not Diabetic | 1.64 | (1.08, 2.50) | 0.020 |
| “Split” PEG Prep | 2.79 | (1.82, 4.28) | 0.001 |
| Overall Colon | |||
| No Narcotic Med | 2.08 | (1.32, 3.28) | 0.001 |
| “Split” PEG Prep | 2.87 | (1.88, 4.40) | 0.002 |
PEG=Polyethylene Glycol.
Adenoma Detection
Adenoma detection rates were calculated for the “Split” PEG prep group as well as the “Regular” PEG prep group. There was no significant difference in the adenoma detection rate between the “Split” PEG prep group and the “Regular” PEG prep group (38% vs 39.5%, p=0.715). However, there was a trend for a greater number of adenomas detected per colonoscopy in the “Split” PEG prep group compared with the “Regular” PEG prep group (1.04 vs. 0.85), however this did not reach statistical significance (p=0.221). Logistic regression was used to determine univariate predictors of detecting at least one adenoma. Significant predictors in univariate analysis included age (p<0.001), gender (p=0.005), not using narcotics (p=0.037), and being a diabetic (p=0.009). “Split” Peg Prep (p=0.715) and a fellow performing the case with an attending (p=0.721) were not significant univariate predictors of detecting at least one adenoma. In multivariate logistic regression, age (OR 1.039 [1.023, 1.055], p<0.001), not using narcotics (OR 1.626 [1.008, 2.622], p=0.046), and being diabetic (OR 1.505 [1.013, 2.234], p=0.043) remained significant predictors of detecting at least one adenoma.
Patient Satisfaction
A subgroup of patients (n=24) who had undergone at least one colonoscopy in the past received a patient satisfaction survey just prior to their procedure. The majority (60%) of these patients rated the “Split” prep as “easier” than the standard prep, while 28% reported the preps to be the “same” difficulty to complete and 12% reported the “Split” prep to be “more difficult or inconvenient.”
Discussion
The Veterans Health Administration (VHA) is the largest integrated healthcare provider in the United States; serving almost 5.5 million patients in 2008.17 Colon cancer is a common cancer among VHA patients with 3746 cases of colorectal cancer diagnosed and/or treated in the VHA in 2005.17 While previous studies have shown a national trend toward an increase in the use of colonoscopy compared to fecal occult blood testing (FOBT) for colon cancer screening,18 FOBT has remained the dominant mode of colon cancer screening in the VHA.19 This suggests a potentially higher pre-test probability of finding a lesion on colonoscopies performed in the VHA population compared to other patient populations in the United States. Further complicating the screening of VHA patients for colon cancer is the fact that VHA patients have been shown to be in poorer health than the general population (OR 14.7; 95% CI[10.7–20.2]).16 Hence colonoscopic screening plays an important role in the VHA and efforts need to be made to improve the quality of these procedures nationwide.
This study is the first to describe the quality of bowel preparation in a large non-selected VHA patient population. These data indicated that use of a simplified “patient friendly” split PEG bowel prep regimen resulted in significantly improved bowel cleansing in VHA patients, particularly in the right colon. Diabetic patients demonstrated worse bowel preps than non-diabetic patients, and had significantly improved right colon bowel preps using a split PEG regimen. In addition, narcotic use was a significant independent predictor of inadequate bowel prep. The prep is considered “patient friendly” in that it divided the ingestion of the prep into two parts and did not require patients to awaken in the very early morning hours. Patient satisfaction with the split prep regimen used in this study was higher than the standard PEG prep. These data demonstrate that this split prep protocol is effective and beneficial for patients in VHA medical centers, which represent represent a generally older population with multiple co-morbidities.16
To date, one meta-analysis has been published that included 9 high-quality prospective, randomized trials of the standard 4L PEG split dose without other adjuvants vs. other bowel preparation methods. They concluded that that the PEG split dose methodology was superior to other preparations in 7 of 9 studies, and that split dose PEG was superior to 4L single dose PEG preparations in 4 of 5 studies.14 However, all studies that specifically examined split vs full dose PEG preparations were from outside the United States. The purpose of this current study was not primarily to prove the effectiveness of a split prep per se, as the principle has been proven in prior randomized trials noted above. The purpose was to prove that the split prep could be applied in routine clinical practice of elderly patients with multiple co-morbidities in the United States. We chose a pre-post study design for this comparative effectiveness trial, but acknowledege the well known limitations of this design and therefore the results should be interpreted with this in mind. Recently, Samarasena, et al, published a randomized trial of split-dose vs single dosed 4L PEG, and split dose vs single dosed MiraLAX, in a highly select group of VHA patients. These data indicated that for both preparations, the split dosing of both preparations resulted in significantly better bowel cleansing scores compared with single dose preparations.20 This study excluded patients with underlying cardiac or renal conditions, did not analyze diabetic patients, and did not examine polyp detection rates. Additionally, the study did not include inpatients who have been shown to have significantly worse bowel preps than outpatients presenting for colonoscopy.21,22 These data combined with the current study in non-selected VHA patients clearly indicate that split dosing of PEG bowel preparations is effective and feasible.
Improved bowel cleansing with split PEG preps was associated with a trend for increased adenomas detected per patient. We note that none of the prior randomized trials of split dose PEG preparations include adenoma detection as an outcome and did not report adenoma detection rates.14,20 Since adequate bowel preparation is considered a requirement for improved adenoma detection,23 studies to date in general do not indicate the actual effect of bowel cleanliness on clinical adenoma detection. One exception is a recent retrospective pre-post study in a single center that showed an increase in polyp and adenoma detection after implementation of a split dose PEG protocol. Although retrospective and non-randomized, this study had a large sample size of 5175 patients.24 Additional future studies with adequate power will likely confirm this finding of a link between bowel preparation and adenoma or polyp detection. In addition, studies that include bowel cleansing methods during colonoscopy, such as the water-exchange method, have the potential for further improvements in colonoscopy quality and polyp detection.25,26
In summary, we have demonstrated system-wide implementation of a “patient friendly” split dose PEG preparation is feasible and effective in a non-selected cohort of VHA patients. Patients with diabetes are particularly prone to worse bowel cleanliness in the right colon, and the split dose PEG preparation is effective in these patients. These data combined with previously published data indicating the superiority of split dose PEG preparations compared to standard PEG preparations in other clinical settings justify the wide spread adoption of split dose PEG within the VHA and other hospital systems in order to improve colonoscopy quality.
Acknowledgements
The study is supported by the Research Service, Department of Veterans Affairs, VA San Diego Healthcare System, and NIH center grant DK080506 and NIH T32 grant of the University of California, San Diego.
Abbreviations
- PEG
polyethylene glycol
- VHA
Veterans Health Administration
Footnotes
Previously published online: www.landesbioscience.com/journals/jig
Conflict of Interests
None of the authors have a financial conflict of interest concerning this manuscript.
References
- 1.Levi B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Maciosek MV, Solberg LI, Coffield AB, Edwards NM, Goodman MJ. Colorectal cancer screening: health impact and cost effectiveness. Am J Prev Med. 2006;31:80–89. doi: 10.1016/j.amepre.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Screening for colorectal cancer, author. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 4.Mulder SA, van Soest EM, Dieleman JP, van Rossum LG, Ouwendijk RJ, van Leerdam ME, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol. 2010;22:437–443. doi: 10.1097/MEG.0b013e328333fc6a. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Chang-Claude J, Seiler CM, Sturmer T, Hoffmeister M. Potential for colorectal cancer prevention of sigmoidoscopy versus colonoscopy: population-based case control study. Cancer Epidemiol Biomarkers Prev. 2007;16:494–499. doi: 10.1158/1055-9965.EPI-06-0460. [DOI] [PubMed] [Google Scholar]
- 6.Baxter NN, Rabeneck L. Is the effectiveness of colonoscopy “good enough” for population-based screening? J Natl Cancer Inst. 2010;102:70–71. doi: 10.1093/jnci/djp469. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 8.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 9.Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointest Endosc. 2010;74:128–134. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Kazarian ES, Carreira FS, Toribara NW, Denberg TD. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol. 2008;6:438–442. doi: 10.1016/j.cgh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 12.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. 2006;8:247–258. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 13.Juluri R, Eckert G, Imperiale TF. Polyethylene glycol vs. sodium phosphate for bowel preparation: a treatment arm meta-analysis of randomized controlled trials. BMC Gastroenterol. 2011;11:38. doi: 10.1186/1471-230X-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enestvedt BK, Tofani C, Laine L, Tierney A, Fennerty MB. 4 L split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2012;10:1225–1231. doi: 10.1016/j.cgh.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69:700–706. doi: 10.1016/j.gie.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 16.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 17.Chao HH, Schwartz AR, Hersh J, Hunnibell L, Jackson GL, Provenzale DT, et al. Improving colorectal cancer screening and care in the Veterans Affairs Healthcare system. Clin Colorectal Cancer. 2009;8:22–28. doi: 10.3816/CCC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 18.Harewood GC, Lieberman DA. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin Gastroenterol Hepatol. 2004;2:72–77. doi: 10.1016/s1542-3565(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB, Petersen L, Hampel H, Richardson P, Cooper G. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch Intern Med. 2006;166:2202–2208. doi: 10.1001/archinte.166.20.2202. [DOI] [PubMed] [Google Scholar]
- 20.Samarasena JB, Muthusamy VR, Jamal MM. Split-dosed MiraLAX/Gatorade is an effective, safe, and tolerable option for bowel preparation in low-risk patients: a randomized controlled study. Am J Gastroenterol. 2012;107:1036–1042. doi: 10.1038/ajg.2012.115. [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014–2020. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 22.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 24.Gurudu SR, Ramirez FC, Harrison ME, Leighton JA, Crowell MD. Increased adenoma detection rate with system-wide implementation of a split-dose preparation for colonoscopy. Gastrointest Endosc. 2012;76:603–608. doi: 10.1016/j.gie.2012.04.456. e1. [DOI] [PubMed] [Google Scholar]
- 25.Leung FW, Leung JW, Siao-Salera RM, Mann SK, Jackson G. The water method significantly enhances detection of diminutive lesions (adenoma and hyperplastic polyp combined) in the proximal colon in screening colonoscopy - data derived from two RCT in US veterans. J Interv Gastroenterol. 2011;1:48–52. doi: 10.4161/jig.1.2.16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung FW, Amato A, Ell C, Friedland S, Harker JO, Hsieh YH, et al. Water-aided colonoscopy: a systematic review. Gastrointest Endosc. 2012;76:657–666. doi: 10.1016/j.gie.2012.04.467. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]


