Abstract
Background
Wound care is the cornerstone of treatment for patients with epidermolysis bullosa (EB); however, there are currently no guidelines to help practitioners care for these patients.
Objectives
The objective of this study was to generate a list of recommendations that will enable practitioners to better care for patients with EB.
Methods
An expert panel generated a list of recommendations based on the best evidence available. The recommendations were translated into a survey, and sent to other EB experts to generate consensus using an online-based modified Delphi method. The list was refined and grouped into themes and specific recommendations.
Results
There were15 respondents (45% response rate), with significant experience in the EB field (>10 years [67%]). Respondents included physicians (67%), nurses (17%), and allied health professionals (7%). There was more than 85% agreement for all the proposed items. These were further refined and grouped into 5 main themes (assessment and management of factors that impair healing, patient-centered concerns, local wound care, development of an individualized care plan, and organizational support) and 17 specific recommendations.
Limitations
There is a paucity of scientific evidence with most recommendations based on expert opinion.
Conclusions
These recommendations will provide practitioners with a framework for caring for these patients. Additional scientific research including effectiveness studies for everyday practice and expert consensus, may further refine these recommendations.
Keywords: consensus, epidermolysis bullosa, guidelines, wound care
Epidermolysis bullosa (EB) is a group of inherited diseases characterized by mechanical fragility of the skin and mucous membranes. There are 4 subtypes of EB resulting from structural protein gene mutations at the cutaneous basement membrane zone or the relatively rare, suprabasal cell-cell adhesion desmosomal proteins.1 The severity of mucocutaneous and other organ disease varies considerably between EB types, and is largely determined by the nature of mutations and the gene penetration resulting in different phenotypic expression.2,3 In the absence of a cure, supportive wound care and early recognition and treatment of complications are the mainstays of patient treatment.
Wound care in the EB population poses unique challenges: clinical variability requires an individualized management plan; availability of a myriad of wound care products complicates the decision process and there is a high overall cost to the family and health units.
To date, there are no specific wound care guidelines or any evidence that address the wound care challenges of the EB population. The objective of this study was to generate a list of recommendations that will allow practitioners to better manage the complex needs of this population.
Methods
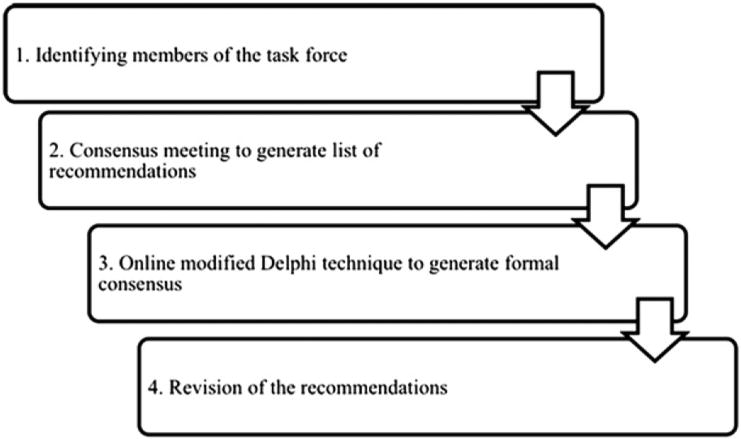
A group of international experts in the fields of EB, wound care, infectious diseases, and bone-marrow transplantation met for 3 days in Alton, Ontario, Canada, to address wound care in patients with EB (Fig 1). The 11 attendees (physicians and nurses) were selected based on their EB clinical and research expertise and background in wound care, wound-healing biology, infectious diseases, and bone-marrow transplantation. Attendees were asked to review all the literature that pertained to their area of expertise and present their findings during the meeting. A group discussion ensued that generated a list of recommendations considered essential for enhancing wound healing in patients with EB. Using an online-based modified Delphi method for generating consensus,4,5 the list was translated into a survey that was sent to 33 other international EB experts. The experts were asked to rate each recommendation on a 4-point Likert scale (strongly disagree, slightly disagree, slightly agree, strongly agree). At least an 80% agreement was required for each item to be adopted in the final list of recommendations.
Fig 1.
Methods diagram.
Results
Fourteen items (Table I) were generated through plenary discussion and sent for formal consensus. There were 15 respondents (45% response rate), with significant experience in the EB field (<1 year [13%]; 1-5 years [13%]; 6-10 years [7%]; 11-15 years [7%]; and >15 years [60%]). Most respondents were physicians (67%), while nursing (17%) and allied health professionals (7%) represented the difference. All items were retained because of a high degree of agreement with the original recommendations (Table I). The list was further refined by grouping items into main themes and specific recommendations (Table II).
Table I. Summary of formal consensus (modified Delphi technique).
| Recommendations | “Somewhat agree” response, % | “Strongly agree” response, % | Consensus, % |
|---|---|---|---|
| A. Treat cause | |||
| Assess patient ability to heal | 11.8 | 82.4 | 94.2 |
|
|||
| Develop individualized goals and plan of care | 0 | 94.1 | 94.1 |
|
|||
| B. Patient-centered concerns | |||
| Address and support management of patient-centered concerns to enable healing: pain | 13.3 | 86.7 | 100 |
| Address and support management of patient-centered concerns to enable healing: itch | 31.3 | 56.3 | 87.6 |
| Address and support management of patient-centered concerns to enable healing: activities of daily living | 13.3 | 80.0 | 93.3 |
| Provide education and support to patient/parent and circle of care to increase adherence (coherence) | 13.3 | 86.7 | 100 |
| C. Local wound care | |||
| Assess wound(s), location and characteristics | 0.0 | 100.0 | 100.0 |
| Gently cleanse wounds with low-toxicity solutions | 33.3 | 66.7 | 100.0 |
| Debridement | 0.0 | 92.9 | 92.9 |
| Assess and treat: | 7.1 | 92.9 | 100.0 |
|
|||
| Select dressing/topical therapy that is appropriate for needs of patient and | 13.3 | 73.3 | 86.6 |
| Evaluate expected rate of healing or reassess wound goals of care (including potential maintenance status) | 26.7 | 73.3 | 100 |
| Edge effect: if wound is stalled or edge/other area appear atypical, consider skin biopsy to rule out squamous cell carcinoma or other complications before considering active therapeutic options | 26.7 | 73.3 | 100.0 |
| D. Provide organizational support | |||
| Consider health care system support structure including specialized nurses, interprofessional clinics, and structured approach to new cases | 13.3 | 86.7 | 100.0 |
EB, Epidermolysis bullosa.
Table II. Wound care recommendations for persons with epidermolysis bullosa.
| Main themes | Specific recommendations |
|---|---|
| A. Treat cause (assessment and management of factors that impair healing) |
|
| B. Patient-centered concerns | 5. Evaluate and manage pain
|
6. Evaluate and manage of itch
|
|
7. Identify and address limitations in ADL
|
|
8. Provide education and support to patient/parent and circle of care to increase treatment adherence*
|
|
| C. Local wound care | 9. Assess wound(s) location and characteristics
|
10. Gently cleanse wounds with low-toxicity solutions
|
|
11. Debridement
|
|
12. Assess and treat
|
|
13. Select appropriate dressing/topical therapy based on EB subtype
|
|
| 14. Evaluate expected rate of healing or reassess wound goals of care | |
15. Evaluate edge effect
|
|
| D. Develop individualized goals and plan of care | 16. Develop/review periodically individualized plan tailored to:
|
| E. Provide organizational support | 17. Consider health care system support structure including specialized nurses, interprofessional clinics, and structured approach to new cases |
ADL, Activities of daily living; DEBRA, Dystrophic Epidermolysis Bullosa Research Association; EB, epidermolysis bullosa; PHMB, polyhexamethylene biguanide.
For professionals requiring further support contact DEBRA or other established EB centers.
If cellular therapy candidate (identify early, especially junctional EB): use filtered blood products; consider theoretical risk of HLA sensitization with any cellular products (eg, allogeneic skin grafting); optimization of vaccine strategies for potentially immunocompromised individuals.
Assess patient ability to heal and treat the cause
Recommendation 1: evaluate EB type–specific involvement
Patients with EB simplex (EBS) present predominantly with acral blisters exacerbated by heat and friction.6 Blistering can be more extensive in the generalized forms of EBS and recessive forms of EBS with suprabasal cleavage.6,7 Dowling-Meara form of EBS is characterized by grouped blisters that extend at the periphery resembling a string of pearls and acral blisters that lead to painful keratoderma. The Herlitz variant of junctional EB has a pathognomonic presentation with periorificial blistering, exuberant hypergranulation tissue, and periungual involvement with nail shedding. The diaper region is often particularly difficult to manage, as large denuded areas are difficult to protect from urine and feces. Scarring and milia formation are a hallmark of dystrophic EB (DEB). The location of the blisters is variable, but tends to affect trauma-prone areas. Patients with severe forms of recessive DEB (RDEB) commonly present with chronic wounds (lasting months, sometimes years) affecting large body surface areas.8 Kindler syndrome is a rare autosomal recessive genodermatosis in which skin fragility early in life is gradually replaced by poikiloderma, scarring, and photosensitivity of the skin9.
Before deciding on a wound care management strategy it is important to take an inventory of the body surface area affected, and the types of skin involvement (intact blisters, erosions, chronic wounds). Ideal methods of serial assessment of wounds in patients with EB are lacking. In addition, most patients are very reluctant to expose their entire skin surface at each visit. Often the care team needs to negotiate a rotating skin examination schedule that allows for the entire skin surface to be carefully inspected at least every 6 months. Signs of local infection such as increased redness, local pain, odor, and exudate should be documented for each problematic wound.
Recommendation 2: consider patient age
The patient age is an important consideration when developing a wound care plan. Infants typically require a lot more control of their immediate environment to prevent trauma.10 This includes, but is not limited to, techniques of gentle handling by the caregivers. Foam dressings are preferred for padding of bony prominences. The diaper area is particularly difficult to manage as it is prone to more physical and chemical trauma. The elastic bands of the diapers should be removed and cleansing wipes avoided. The diaper can be lined with a nonstick dressing or “buttered” with a thick layer of zinc oxide paste. As the child becomes more mobile, knee padding and soft special shoes are required to prevent blistering. Older patients tend to have more chronic ulcers that are critically colonized and infected and there is increased likelihood of colonization with antibiotic-resistant bacteria. Patients with more severe forms of EB are at an increased risk of squamous cell carcinoma (SCC). Although this is an unusual occurrence before the second or third decade of life,11 it has been described in a 6-year-old child12.
Recommendation 3: assess and manage poor nutritional status
Wound healing can be delayed or interrupted in persons with existing comorbidities. Malnutrition is very common in the severe types of EB, resulting from a combination of reduced intake and increased demands.13,14 Malnutrition leads to failure to thrive, delayed puberty, and anemia, a cascade of clinical and biological events, further affecting wound healing and increasing skin breakdown.15-17 Low protein intake or relative deficiency can prevent the production of granulation tissue contributing to “stalled” healing.18 Albumin levels, a gross indicator of long-term nutritional deficit, less than 2.0 to 3.0 g/dL (normal: 3.0-5.4 g/dL)19 are associated with impaired healing. Blood sampling can be difficult as a result of poor venous access, therefore a more practical approach for assessing the overall nutritional status is monitoring of the growth curves in pediatric patients and body mass index in adults with EB. Regular nutritional consults (including calorimetry) to evaluate caloric needs are recommended. To optimize nutritional status, patients with severe forms of EB may require a gastrostomy tube. Supplementation of identified deficiencies is commonly suggested by many EB centers and 6- to 12-month monitoring to identify them is endorsed.
Recommendation 4: monitor and maintain hemoglobin levels above 80 g/L
Anemia, likely multifactorial in nature,13 is a frequent and serious complication of the severe types of EB such as RDEB and junctional EB. Hemoglobin less than 100 g/L causes impaired wound healing in patients with venous ulcers as a result of decreased tissue oxy-genation.20 Low hemoglobin levels in patients with EB are one of the factors that may contribute to delayed healing. There is no ideal management strategy for dealing with anemia in patients with EB. A pathogenic-based approach is sensible, but not always possible. Adequate skin care and preventing/treating infection can minimize blood losses through the skin. Oral iron supplementation for correction of iron deficiency is widely used but its individual effectiveness varies. Moreover, gastrointestinal upset and constipation are reasons for non-adherence. Intravenous iron21 plus erythropoietin were beneficial in a small study of patients with RDEB.22 Blood transfusions should be considered for cases where hemoglobin levels are consistent below 80 g/L and/or for symptomatic patients who do not respond to other measures.
Patient-centered concerns
Recommendation 5: pain assessment and management
Pain is the most common symptom experienced by patients with EB, irrespective of subtype. The frequency and severity of pain is often proportional to disease severity, with up to 50% of patients with the most extensive type of EB (RDEB) experiencing daily pain greater than 5 (0-10 scale).23 Although the cause of pain in EB is multifactorial, the skin and related EB lesions are by far the most significant source of pain. The pain can occur at rest from blisters and denuded skin, secondary infection, friction, and shearing with physical movements.24 Pain can also be exacerbated during dressing changes, bathing, and other activities of daily living. Development of a pain management approach requires adequate documentation of pain levels before and after dressing changes, bathing, and other painful interventions.25 Pain assessments using age-appropriate tools also allow identification of a temporal pattern and aggravating factors.26,27 Other patient-related factors (anxiety, depression, past experiences) contributing to the pain experience should be recognized and treated. The approach to pain in a patient with EB includes preventative and therapeutic modalities (Table III). In general, wound-associated pain is both nociceptive, stimulus-dependent (gnawing, throbbing); and neuropathic, nonstimulus-dependent (burning, stinging, shooting, stabbing). Nociceptive pain is treated with the World Health Organization pain ladder medication (Table III).28 Short-acting agents are used to determine the dose of longer-acting agents and for breakthrough pain before painful procedures. Neuropathic pain often responds to tricyclic agents, particularly second-generation agents. For nonresponders, gabapentin,29 pregabalin, or other antiepileptics may be helpful. Procedural pain requires an interprofessional approach and communication to those within the circle of care. This is especially important for children with EB as they are often subjected to repeated procedures. Oral sucrose 24% is a useful, short-acting analgesic that is effective for children younger than 2 years of age.30 For older children and adults, acetaminophen or morphine administered 30 minutes before the procedure may be used. Ketamine is another alternative drug that can also be used for dressing changes.31 Nonpharmacological modalities (Table III) are also helpful in combination with the pharmacological measures listed above. Some potentially useful topical options include adding salt to the bathwater32 and dressings with analgesics (Biatain-IBU, Coloplast, Humlebaek, Denmark).
Table III. Pain management strategies.
| Pain management strategies | Goals/types | Actions |
|---|---|---|
| Preventative | Avoid trauma Avoid blister expansion Prevent local infection |
|
| Therapeutic | Pharmacological | Nociceptive:
|
| Nonpharmacological | Neuropathic:
|
NSAIDs, Nonsteroidal anti-inflammatory drugs.
Recommendation 6: control itch
Itch is a common symptom in the EB population, often poorly controlled, affecting quality of life. The exact mechanism is not known; abnormal persistent skin inflammation, overheating caused by dressings, local sensitizers,33 and systemic opioids are potential contributors.24 Management should start with a thorough history to identify the timing and exacerbating factors. Occasionally, changing the topical routine (discontinuation of dressings or topical antibiotics) may be sufficient. Itching at night may be related to body overheating and treated with sedating antihistamines (hydroxyzine) or a tricyclic with prominent H-1 antihistamine action (doxepin). Daytime pruritus may require a nonsedating antihistamine H-1 blocker (cetirizine, loratadine). Liquid preparations are always preferable as they have a shorter onset of action and are easier to swallow. There are anecdotal reports of successful use of ondansetron or low-dose gabapentin for persistent pruritus29.
Recommendation 7: recognize and address limitations in activities of daily living
Pain, odor, and mobility limitations have a significant impact on patients with EB and their daily living. The disease burden may include difficulties in performing personal care, engaging in school or employment activities, and increased financial bur-den.34 Depression and anxiety are also common35 and further contribute to social isolation. Fostering independence and safety during activities of daily living requires environmental modifications (special beds, seating in baths, wheelchairs, footwear). An early rehabilitation consult with frequent reevaluations is recommended.
Recommendation 8: provide education and support to the patient/parent and circle of care to increase treatment adherence
Development of a therapeutic relationship involves appropriate support and education. This occurs when trust, communication, and open dialogue allow patients and their caregivers to understand that each involved person has a meaningful contribution in the decision-making process. EB is a complex multisystem disease; therefore communication among various health care professionals is paramount. A centralized, interprofessional approach with care coordination including open communication with the general practitioner and home-care team is the most effective way of caring for these patients. The burden of caring for these patients is taxing for health teams. As not all patients can be looked after in specialized centers, non-EB practitioners should seek support from established EB centers, EB care network, or Dystrophic Epidermolysis Bullosa Research Association (DEBRA) foundations.
Local wound care
Recommendation 9: assess wound locations and characteristics
The first step is to create an inventory of the body surface area involved and the type of wounds (intact blisters, erosions/ulcers, chronic, exudative vs nonexudative wounds). There are very limited tools available for determining the extent of skin involvement. The palm method, used for patients with burn,36 is not always feasible. Digital photography may be helpful particularly for assessing and monitoring the progress of problematic lesions. Another objective method is the MEASURE37 paradigm used for assessment of chronic wounds (measure size; exudate [amount and characteristics]; appearance [base or granulation tissue]; suffering [pain]; undermining [depth measured in centimeters]; re-evaluate; and edge). We propose using this paradigm for patients with EB (by eliminating the undermining and allocating suffering SU rather than S) in nonhealing wounds for developing a wound care plan and monitoring the response over time. The wound care decision approach should consider the wound location, need for extra padding and protection, specialized dressings, and feasibility for everyday use (Tables IV to VI).
Table IV. Dressing choices according to indications/type of wounds.
| Type of wound/ indication | Primary dressing | Secondary dressing | Topical therapy |
|---|---|---|---|
| Protection | Foams Modified absorbent pads Lipidocolloid dressings Contact layers |
Burn net to keep in place (if feasible) | None |
| Open nonexudative | Foams Modified absorbent pads Lipidocolloid dressings Contact layers |
Burn net to keep in place (if feasible) | None |
| Exudative | Foams Lipidocolloid dressings Hydrofibers |
Burn net to keep in place (if feasible) | Topical antibiotics (avoid allergens) |
| Eschar | Hydrogels Biosynthetic cellulose Hydrocolloids |
Foams Modified absorbent pads |
None |
| Critically colonized or infected | Contact layer Hydrofibers Alginates Antimicrobials |
Foams Modified absorbent pads |
Topical antibiotics (avoid allergens) |
| Painful | Biosynthetic cellulose Hydrogel sheets |
Foams Modified absorbent pads |
Topical NSAIDs |
| Itchy | Biosynthetic cellulose Hydrogel sheets |
Foams Modified absorbent pads |
Short course of topical midpotency corticosteroids |
| Hypergranulation | Contact layer with antimicrobial, anti-inflammatory | Foams Modified absorbent pads |
Short course of topical potent corticosteroids, beware of infection |
NSAIDs, Nonsteroidal anti-inflammatory drugs.
Table VI. Dressing choices/topical therapy for special locations/indications.
| Location | Dressing/topical therapy | Properties | Expert comment (opinion) |
|---|---|---|---|
| Perianal area | Restore contact layer* | Autolytic debridement Provides moisture |
Difficult to keep in place Can be used to line diaper |
| Intrasite conformable† | Autolytic debridement Provides moisture |
Difficult to keep in place Can be used to line diaper |
|
| Bepanthen (ointment with pro-vitamin B5)‡ | Aids in moisture balance | ||
| Cavilon (liquid barrier film)§ | Creates breathable, transparent coating on skin | Does not sting Alcohol free |
|
| Emollin 50/50 emollient spray(white soft paraffin and liquid paraffin)║ | Water repellent Provides barrier protection |
Does not sting | |
| Oral mucosa | BioXtra (salivary substitute)¶ | Provides moisture | |
| Difflam spray (active ingredient is benzydamine hydrochloride, a NSAID)# | Reduces pain and inflammation Also acts as local anesthetic |
||
| Corsodyl (mouthwash containing chlorhexidine)# | Provides antiseptic and disinfectant properties | ||
| Gelclair (bioadherent oral gel)** | Creates barrier that protects nerve endings, reducing pain | Can be used before meals | |
| Feeding tube sites | AMD-PHMB foam fenestrated disc dressing (antimicrobial foam dressing)†† | Moisture balance Contains antiseptic (PHMB) (effective against MRSA, VRE, gram-positive and gram-negative bacteria, fungi, and yeast) | |
| 4% Sucralfate mixed with Cavilon§ | Protectant | ||
| PC/C lines | Mepitac*, Adaptic touch‡‡, Siltape§§, or Silflex§§ | Nonstick | |
| Adhesives | Medical adhesive remover Appeel║║ or Niltac¶¶ Adhesive remover spray*** | Adhesive remover is temporary These sprays are silicone based |
|
| Retention bandage | Acti-Wrap cohesive retention bandage††† | Secures dressings in place | Useful to fix small dressings |
AMD, Advanced micro devices; MRSA, methicillin-resistant Staphylococcus aureus; NSAID, nonsteroidal anti-inflammatory drug; PC/C, percutaneous catheter; PHMB, polyhexamethylene biguanide; VRE, vancomycin resistant enterococcus.
Molnlycke Health Care, Gothenburg, Sweden.
Smith and Nephew, London, UK.
Bayer Health Care, Wayne, NJ.
3M, St. Paul, MN.
CD Medical Ltd, Derbyshire, UK.
Lighthouse Health Products, Cambridge, Ontario, Canada.
Glaxosmithkline Consumer, Brentford, UK.
Helsinn Healthcare, SA, Pazzallo, Switzerland.
Kendall Company Ltd, Mansfield, MA.
Systagenix, Gatwick, West Sussex, UK.
W M Bamford Co & Ltd, Lower Hutt, New Zealand.
CliniMed Ltd, Bucks, UK.
Trio Health International Ltd, Great Missenden, Buckinghamshire, UK.
Humlebaek, Denmark.
Active Healthcare, Staffordshire, UK.
Recommendation 10: gently cleanse wounds with low-toxicity solutions
The standard of care for wound cleansing is to use solutions that are gentle and noncytotoxic.38 For patients with EB we recommend gentle cleansing with a saline solution, water, or dermol 500 (containing benzalkonium chloride 0.1%, chlorhexidine hydrochloride 0.1%). Avoidance/ short-term use of cytotoxic solutions (Dakin, Century Pharmaceuticals Inc, Indianapolis, IN, and povidone-iodine) is prudent because of skin fragility and pain associated with open wounds. Soaking of each individual wound for 5 to 10 minutes or removing the dressings in the bathtub may help reduce pain and trauma associated with dressing changes. A dilute acetic solution (5% white vinegar diluted to 0.25%-1.0%) or bleach (5-10 mL in 5L of water) may decrease the bacterial carriage.39 Bathing facilitates cleansing, nontraumatic dressing removal, and supplemental antibacterial control (using diluted acetic acid or bleach) and is better tolerated than showering32.
Recommendation 11: blister management and gentle debridement of eschar/slough
New blister formation is the hallmark of EB. To prevent blister extension we recommend puncturing it (at multiple sites to facilitate optimal drainage) with a sterile needle to release the inner fluid. The overlying skin is left in place, acting as a biological dressing, reducing pain, and minimizing infection risk. A firm dehydrated eschar or soft slough requires debridement to remove senescent cells that are deficient in cellular activities and biofilms that maintain the inflammatory process.40 Debridement in the EB population should, whenever possible, involve nonphysical methods (hydrogel, calcium alginate dressings).
Recommendation 12: assess and treat critical colonization, infection, and abnormal inflammation
Inflammation or infection impairs normal healing. The difference between colonization and infection is the interplay between the number and type of colonies and host resistance.41 In bacterial colonization, bacterial colonies do not interfere with healing. Critical colonization occurs when the bacterial proliferation causes local damage and wounds get “stuck” precluding healing. Surface critical colonization and deep and surrounding skin infection are clinical diagnoses. The mnemonics NERDS (nonhealing; increased exudate; red, friable tissue; debris, dead slough; smell) and STONEES (increased size; temperature >3°F warmer than contralateral skin; os, exposed/probing to bone; new areas of breakdown; erythema/edema of surrounding skin; increased exudate and smell)42,43 represent the two levels of superficial bacterial damage or deep and surrounding skin infection and have been validated for use in chronic wounds. Any 3 NERDS criteria are indicative of superficial critical colonization and require a topical antimicrobial. Three or more criteria from STONEES suggest deeper/surrounding skin infection and need for systemic therapy. Although these concepts need to be validated for EB, more than 3 criteria are useful to distinguish infection from persistent inflammation. The most common bacteria isolated from chronic and most likely EB wounds are gram-positive organisms (Staphylococcus aureus and Streptococci species), gram negatives (Pseudomonas aeruginosa), and anaerobes (R. G. Sibbald, MD, oral communication, July 2012). As such, documentation of critical colonization/infection in the EB population is rarely needed and skin swabs are indicated only to determine antibiotic selection in cases of multiresistant organisms or nonresponsive infection.
Critical colonization can be controlled with topical agents. The bacterial load may be reduced by bathing with diluted bleach, applying compresses, or using sprays with diluted vinegar.39 Lipid-stabilized hydrogen peroxide cream (Crystacide, DermaUK, Stotfold, UK) is well tolerated and effective when applied directly on the wound or contact dressing.39 Topical antibiotics/antimicrobials (eg, polymyxin B-gramicidin, fusidic acid, mupirocin, silver sulfadiazine) should be used only for short periods of time and rotated every 2 to 6 weeks to prevent resistance and sensitization.39 When using these agents, we recommend applying them on the dressing rather than directly on the skin to limit pain and trauma. Other options include dressings containing silver, honey, iodine, and polyhexamethylene biguanide (Table V).44 Silver has broad-spectrum antimicrobial activity and must be ionized to exert maximum effect. Ionized silver requires an aqueous or water environment.44 High serum levels of silver have been documented in patients with EB who use silver dressings (J. Mellerio, MD, oral communication, July 2011); therefore, their prolonged use over large surface areas should be discouraged. Medical-grade honey products (ointments, dressings) may provide short-term benefit, but their use can increase local pain and may temporarily increase exudate levels.39 The use of antimicrobial dressings should be reviewed at regular intervals, and discontinued if critical colonization has been corrected or if there is no beneficial effect.
Table V. Dressings categories, properties, indications.
| Dressing type | Commercial name | Proposed scientific mechanism of action/precautions | Expert comment (opinion) |
|---|---|---|---|
| Foams | Mepilex* Mepilex lite* Mepilex border* Mepilex border lite* PolyMem† |
Some contain silicone layer to make these nonadherent Generally made from hydrophilic polyurethane Nonocclusive Semipermeable surface allows exudate into dressing and foam traps moisture |
Allow large amounts of fluid and wound drainage to be absorbed Provide padding and protection to wounds Depending on amount of exudate, can be left in place up to 7 d Some require secondary dressing to hold in place Bordered dressing may sometimes be too sticky and should be used with caution |
| Hydrogels | Gels: Duoderm‡ Intrasite§ Sheets (cool dressings): ActiFoamCool‖ Intrasite Conformable§ |
Made of insoluble polymers that expand in water and hydrate wounds Provide autolytic debridement |
For wounds with minimal or no exudate Because of hydrating capacity, these offer cooling effect and may aid in relief of pain, itch, and discomfort |
| Alginates (calcium or calcium/sodium) | Kaltostat‡ | Made of nonwoven fibers derived from seaweed Turn into nonsticky gel when in contact with wound drainage |
Requires exudates Does not work on dry wounds or wounds with eschar Calcium alginate dressings release calcium ions that help stop bleeding |
| Hydrofibers | Aquacel‡ | Made of sodium carboxymethyl-cellulose that, when in contact with wound drainage, becomes gel and provides moist environment | More absorbent than alginates Consider in wounds with heavy drainage |
| Modified absorbent pads | Telfa¶ Restore# ETE* Mesorb* |
Thin layer of absorbent cotton fibers that are enclosed in sleeve of perforated polyethylene terephthalate and sealed along two edges Plastic film prevents dressing from adhering to wound surface and perforated surface allows passage of exudate into pad |
Relatively inexpensive and nonadherent If there is significant bleeding or exudate, dressing will adhere |
| Contact layers | Mepitel* Silflex** Mepitac* Adaptic touch†† Siltape or Silflex** |
Protective, inert material that allows nontraumatic removal | |
| Biosynthetic cellulose | Suprasorb X‖ | Dressing consisting of cellulose, water, and 0.085% chlorhexidine gluconate (preservative) that has ability to both absorb and donate moisture | Also considered cooling dressing, aids in pain reduction and adding moisture to wounds May also reduce itch |
| Lipidocolloid dressings | Urgotul‡‡ Restore# (North American equivalent to Urgotul) |
Composed of open-weave polyester mesh impregnated with hydrocolloid polymers dispersed within petrolatum When in contact with exudate, hydrocolloid polymers are hydrated and constitute with petrolatum lipidocolloid interface that provides nonadherent surface |
For wounds with exudate Also used for protection of vulnerable areas |
Molnlycke Health Care, Gothenburg, Sweden.
Ferris Manufacturing, Burr Ridge, IL.
ConvaTec, Skillman, NJ.
Smith and Nephew, London, UK.
Activa Healthcare, Staffordshire, UK.
Kendall Company Ltd, Mansfield, MA.
Hollister, Libertyville, IL.
Advancis Medical, Oxfordshire, UK.
Systagenix, Gatwick, West Sussex, UK.
Urgo, Shepshed, Longhborough, UK.
Signs of deep and surrounding tissue infection (lymphadenopathy, fever, and malaise) require systemic antimicrobial therapy. Empirical use of systemic antibiotics that cover common pathogens is recommended. The antibiotic choice can be further refined based on identified pathogenic organisms and their antimicrobial sensitivities. Streptococcus pyogenes presence requires treatment even in the absence of overt clinical infection because of risk of complications. For chronic nonhealing wounds, long-term, alternating, low-dose antibacterial agents (trimethoprim, macrolides, doxycycline) may be beneficial for their anti-inflammatory effects.
Recommendation 13: select a dressing/topical therapy that is appropriate for the needs of the patient and the caregiver based on the subtype of EB
Dressing choices should be individualized based on EB subtype, extent and wound location, dressing frequency, cost, and availability (Tables IV to VI). Wound healing requires an appropriate wound surface moisture balance. This is achieved by using dressings with occlusive, semi-occlusive, absorptive, hydrating, and hemostatic characteristics, depending on the wound characteristics and drainage (Tables IV to VI). Another consideration for the EB population is management of chronic wounds. Chronic wounds are often stalled in the inflammatory stage.44 These wounds demonstrate marked increased activity of inflammatory cells and associated mediators such as matrix metalloproteinase and elastase,44 responsible for degradation of extracellular matrix and growth factors that hinder progression toward re-epithelialization.45 Combined local and systemic intervention may be required to facilitate wound healing (recommendation 12).
Recommendation 14: evaluate the expected rate of healing or reassess wound goals of care (including potential maintenance status)
A wound size reduction of 20% to 40% in 2 and 4 weeks is quoted to be a reliable predictor of healing at 12 weeks.46-48 In addition, clinical observation of the edge of the wound is foretelling: nonhealing wounds often have a “cliff edge” instead of the purple “tapered sandy shore beach” of healable ones. If the wound edge is not advancing after appropriate wound-bed preparation, advanced therapies should be considered49 after all causes of delayed healing have been ruled out (SCC). Complete healing may not be an achievable goal in EB. Other wound-related outcomes such as pain reduction, decreased bacterial load, and need for dressing changes, or increased quality of life are more attainable.
Recommendation 15: edge effect–if a wound is stalled, the edge or other areas appear atypical; consider a skin biopsy to rule out SCC or other complications before considering active therapeutic options
The cumulative risk of developing SCC in severe generalized RDEB by age 55 years is 90%8 representing a major cause of morbidity and mortality.11 They tend to occur much earlier in the EB population, are multifocal, and more aggressive. As wound chronicity is the norm in many patients with EB, a high degree of suspicion is required. Biopsy of wounds that enlarge rapidly, have increased pain, change in appearance on serial photographic documentation, or “feel different” is recommended50.
Develop individualized goals and plan of care
Recommendation 16: develop and reassess tailored plan of care
A comprehensive assessment should result in an individualized wound care plan tailored to the individual, taking into consideration unique biopsychosocial needs (Table VII). Patient preference must be respected and reflected in the wound care plan.51 It is common for EB families to have time-tested routines that do not necessarily follow the currently accepted medical wisdom. A flexible approach will most likely increase adherence, increase satisfaction with care, and lead to improved outcomes. With the new, disease-modifying cellular therapies that are currently emerging,52 it is also important to maximize the chances of each patient being a potential candidate for these therapies (Table VIII). The wound care plan should be clearly outlined in a written document given to the family and copied to the family practitioner and home-care personnel. The care plan should also be evaluated and updated regularly.
Table VII. Considerations for addressing biopsychosocial needs.
| Biopsychosocial need | Considerations |
|---|---|
| Individual personal preferences |
|
| Risk factor Comorbidities |
|
| Quality-of-life issues |
|
| Support systems/ circle of care |
|
| Access to care |
|
EB, Epidermolysis bullosa.
Table VIII. Care principles for potential candidates to cellular therapies (eg, stem cell transplantation).
| Principles | Actions |
|---|---|
| Maintain overall health by preventing, recognizing, and treating disease-related complications |
|
| Minimize risks of exposure to antibodies |
|
| Optimize vaccination strategies for potentially immune-compromised individuals |
|
Provide organizational support
Recommendation 17: consider a health care system support structure including specialized nurses, interprofessional clinics, and a structured approach to new cases
EB is not just a skin disorder; therefore treating a patient with EB requires involvement of a dedicated team with expertise in all aspects of care. Over the past decade specialized EB clinics have opened in 16 countries worldwide, providing an interprofessional model of care with input from many allied health professionals (eg, nurses, physicians, surgeons, occupational therapists, physical therapists, social workers, dietitian, music therapists).53-67 Isolated cases can be overwhelming to health practitioners particularly when referral to an established EB center is not feasible. Access to international EB experts via http://www.internationalebforum.org is possible and has changed the fabric of pre-existing professional isolation. Other resources for patients and practitioners are DEBRA foundations that exist in many countries.
The birth of a child with EB is a traumatic event for a family. We have found that early education about the disease, determining the type/subtype of EB as soon as possible, and providing ongoing support from knowledgeable practitioners allows a family to regroup and focus on providing the best care to their baby.
Discussion
EB is one of the most complex diseases in medicine, with severe EB types having devastating effects on the quality of life and life span of affected patients. Until a cure is available, anticipatory guidance for possible disease-related complications and appropriate wound care are the cornerstones of EB management. EB is a prototype of an orphan disease. Although rare, its severity combined with little evidence for clinical practice leads to suboptimal patient care and practitioner isolation. Practice guidelines (systematic statements that assist physician in decision making)68 are increasingly recognized as tools that reduce inappropriate care, control geographic variation, and make use of best health care resources.69 To date there is no randomized controlled trial scientific evidence for any of the health care interventions that we use in these patients. To our knowledge, this is the first attempt to develop guidelines of care for the EB population that focus on wound care, with a holistic approach that takes into account other patient-related factors, patient preferences, and the immediate and extended care teams. We have brought together experts in the fields of EB, wound care biology, and clinical practice to provide the best available approaches to optimize wound care in patients with EB. The next step is to seek further consensus on specific statements that make up each recommendation. These guidelines need to be periodically renewed to reflect new scientific and clinical practice knowledge.
International experts
Edward Barrett (Canada), Anna Bruckner (United States), Maya El Hachem (Italy), Louise Fret-Lalonde (Canada), Gerry Kelly-Mancuso (United States), Michelle Lee (Canada), Andrew Lin (Canada), Anne Lucky (United States), Celia Moss (United Kingdom), Dedee Murrell (Australia), Annmarie Ormonde (Italy), Francis Pallison (Chile), Agnes Schwieger (Germany), Rosemarie Watson (Ireland), Karen Wiss (United States).
Acknowledgments
Supported in part by an unrestricted educational grant from Molnlycke Health Care.
Abbreviations used
- DEB
dystrophic epidermolysis bullosa
- DEBRA
Dystrophic Epidermolysis Bullosa Research Association
- EB
epidermolysis bullosa
- EBS
epidermolysis bullosa simplex
- RDEB
recessive dystrophic epidermolysis bullosa
- SCC
squamous cell carcinoma
Footnotes
Conflicts of interest: None declared.
References
- 1.Fine JD, Eady RA, Bauer EA, Bauer JW, Bruckner-Tuderman L, Heagerty A, et al. The classification of inherited epidermolysis bullosa (EB): report of the third international consensus meeting on diagnosis and classification of EB. J Am Acad Dermatol. 2008;58:931–50. doi: 10.1016/j.jaad.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa, part I: epithelial associated tissues. J Am Acad Dermatol. 2009;61:367–84. doi: 10.1016/j.jaad.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Fine JD, Mellerio JE. Extracutaneous manifestations and complications of inherited epidermolysis bullosa, part II: other organs. J Am Acad Dermatol. 2009;61:387–402. doi: 10.1016/j.jaad.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:i–iv. 1–88. [PubMed] [Google Scholar]
- 5.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311:376–80. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath JA, Mellerio JE. Ectodermal dysplasia—skin fragility syndrome. Dermatol Clin. 2010;28:125–9. doi: 10.1016/j.det.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 7.McGrath JA, Bolling MC, Jonkman MF. Lethal acantholytic epidermolysis bullosa. Dermatol Clin. 2010;28:131–5. doi: 10.1016/j.det.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the national EB registry experience, 1986-2006. J Am Acad Dermatol. 2009;60:203–11. doi: 10.1016/j.jaad.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Lai-Cheong JE, McGrath JA. Kindler syndrome. Dermatol Clin. 2010;28:119–24. doi: 10.1016/j.det.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Denyer JE. Wound management for children with epidermolysis bullosa. Dermatol Clin. 2010;28:257–64. viii–ix. doi: 10.1016/j.det.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Fine JD, Johnson LB, Weiner M, Suchindran C. Cause-specific risks of childhood death in inherited epidermolysis bullosa. J Pediatr. 2008;152:276–80. doi: 10.1016/j.jpeds.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Shivaswamy KN, Sumathy TK, Shyamprasad AL, Ranganathan C. Squamous cell carcinoma complicating epidermolysis bullosa in a 6-year-old girl. Int J Dermatol. 2009;48:731–3. doi: 10.1111/j.1365-4632.2009.03910.x. [DOI] [PubMed] [Google Scholar]
- 13.Mellerio JE, Weiner M, Denyer JE, Pillay EI, Lucky AW, Bruckner A, et al. Medical management of epidermolysis bullosa: proceedings of the IInd international symposium on epidermolysis bullosa, Santiago, Chile, 2005. Int J Dermatol. 2007;46:795–800. doi: 10.1111/j.1365-4632.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- 14.Allman S, Haynes L, MacKinnon P, Atherton DJ. Nutrition in dystrophic epidermolysis bullosa. Pediatr Dermatol. 1992;9:231–8. doi: 10.1111/j.1525-1470.1992.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard L, Haynes L, Sklar M, Martinez AE, Mellerio JE. The challenges of meeting nutritional requirements in children and adults with epidermolysis bullosa: proceedings of a multidisciplinary team study day. Clin Exp Dermatol. 2011;36:579–83. doi: 10.1111/j.1365-2230.2011.04091.x. [DOI] [PubMed] [Google Scholar]
- 16.Fine JD, Tamura T, Johnson L. Blood vitamin and trace metal levels in epidermolysis bullosa. Arch Dermatol. 1989;125:374–9. [PubMed] [Google Scholar]
- 17.Ingen-Housz-Oro S, Blanchet-Bardon C, Vrillat M, Dubertret L. Vitamin and trace metal levels in recessive dystrophic epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2004;18:649–53. doi: 10.1111/j.1468-3083.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 18.Zagoren AJ, Johnson DR, Amick N. Nutritional assessment and intervention in the adult with a chronic wound. Malvern (PA): HMP Communications; 2007. [Google Scholar]
- 19.Dugdale DC. Albumin-serum. [Accessed September 1, 2011];Medline Plus Encyclopedia. Available from: URL: http://www.nlm.nih.gov/medlineplus/ency/article/003480.htm.
- 20.Keast DH, Fraser C. Treatment of chronic skin ulcers in individuals with anemia of chronic disease using recombinant human erythropoietin (EPO): a review of four cases. Ostomy Wound Manage. 2004;50:64–70. [PubMed] [Google Scholar]
- 21.Atherton DJ, Cox I, Hann I. Intravenous iron (III) hydroxide-sucrose complex for anemia in epidermolysis bullosa. Br J Dermatol. 1999;140:773. [PubMed] [Google Scholar]
- 22.Fridge JL, Vichinsky EP. Correction of the anemia of epidermolysis bullosa with intravenous iron and erythropoietin. J Pediatr. 1998;132:871–3. doi: 10.1016/s0022-3476(98)70321-x. [DOI] [PubMed] [Google Scholar]
- 23.Fine JD, Johnson LB, Weiner M, Suchindran C. Assessment of mobility, activities and pain in different subtypes of epidermolysis bullosa. Clin Exp Dermatol. 2004;29:122–7. doi: 10.1111/j.1365-2230.2004.01428.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldschneider KR, Lucky AW. Pain management in epidermolysis bullosa. Dermatol Clin. 2010;28:273–82. ix. doi: 10.1016/j.det.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Woo K, Sibbald G, Fogh K, Glynn C, Krasner D, Leaper D, et al. Assessment and management of persistent (chronic) and total wound pain. Int Wound J. 2008;5:205–15. doi: 10.1111/j.1742-481X.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs M, Ferris FD, Glynn C, Harding K, Hofman D, Hollinworth H, et al. Assessing pain at wound dressing-related procedures. Nurs Times. 2004;100:56–7. [PubMed] [Google Scholar]
- 27.National Institute of Health Pain Consortium. Pain Intensity Scales. [Accessed June 1, 2011]; Available from: URL: http://painconsortium.nih.gov/pain_scales.index.html.
- 28.Price P, Fogh K, Glynn C, Krasner DL, Osterbrink J, Sibbald RG. Managing painful chronic wounds: the wound pain management model. Int Wound J. 2007;4(Suppl):4–15. doi: 10.1111/j.1742-481X.2007.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allegaert K, Naulaers G. Gabapentin as part of multimodal analgesia in a newborn with epidermolysis bullosa. Paediatr Anaesth. 2010;20:972–3. doi: 10.1111/j.1460-9592.2010.03396.x. [DOI] [PubMed] [Google Scholar]
- 30.Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155:173–80. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 31.Saroyan JM, Tresgallo ME, Farkouh C, Morel KD, Schechter WS. The use of oral ketamine for analgesia with dressing change in an infant with epidermolysis bullosa: report of a case. Pediatr Dermatol. 2009;26:764–6. doi: 10.1111/j.1525-1470.2009.01036.x. [DOI] [PubMed] [Google Scholar]
- 32.Arbuckle HA. Bathing for individuals with epidermolysis bullosa. Dermatol Clin. 2010;28:265–6. ix. doi: 10.1016/j.det.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Sibbald RG. Dermatological aspects of wound care. Chapter 30. In: Krasner DL, Sibbald RG, Rodeheaver GT, editors. A clinical source book for healthcare professionals. 3rd. Wayne, PA; HMP Communications: 2001. pp. 273–85. [Google Scholar]
- 34.Tabolli S, Pagliarello C, Uras C, Di Pietro C, Zambruno G, Castiglia D, et al. Family burden in epidermolysis bullosa is highly independent of disease type/subtype. Acta Derm Venereol. 2010;90:607–11. doi: 10.2340/00015555-0947. [DOI] [PubMed] [Google Scholar]
- 35.Tabolli S, Sampogna F, Di Pietro C, Paradisi A, Uras C, Zotti P, et al. Quality of life in patients with epidermolysis bullosa. Br J Dermatol. 2009;161:869–77. doi: 10.1111/j.1365-2133.2009.09306.x. [DOI] [PubMed] [Google Scholar]
- 36.Hettiaratchy S, Papini R. Initial management of a major burn, II: assessment and resuscitation. BMJ. 2004;329:101–3. doi: 10.1136/bmj.329.7457.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keast DH, Bowering CK, Evans AW, Mackean GL, Burrows C, D'Souza L. MEASURE: a proposed assessment framework for developing best practice recommendations for wound assessment. Wound Repair Regen. 2004;12(Suppl):S1–17. doi: 10.1111/j.1067-1927.2004.0123S1.x. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev. 2008;1:CD003861. doi: 10.1002/14651858.CD003861.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Mellerio JE. Infection and colonization in epidermolysis bullosa. Dermatol Clin. 2010;28:267–9. ix. doi: 10.1016/j.det.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Kirshen C, Woo K, Ayello EA, Sibbald RG. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care. 2006;19:506–17. doi: 10.1097/00129334-200611000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Sibbald G, Orsted H, Shultz GS, Coutts P, Keast D. Preparing the wound bed 2003: focus on infection and inflammation. Ostomy Wound Manage. 2003;49:23–51. [PubMed] [Google Scholar]
- 42.Sibbald G, Woo K, Ayello EA. Increased bacterial burden and infection: the story of the NERDS and STONEES. Adv Skin Wound Care. 2006;19:447–61. doi: 10.1097/00129334-200610000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Woo KY, Sibbald RG. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage. 2009;55:40–8. [PubMed] [Google Scholar]
- 44.Sibbald RG, Goodman L, Woo KY, Krasner DL, Smart H, Tariq G, et al. Special considerations in wound bed preparation 2011: an update. Adv Skin Wound Care. 2011;24:415–36. doi: 10.1097/01.ASW.0000405216.27050.97. [DOI] [PubMed] [Google Scholar]
- 45.Moore K, McCallion R, Searle RJ, Stacey MC, Harding KG. Prediction and monitoring the therapeutic response of chronic dermal wounds. Int Wound J. 2006;3:89–96. doi: 10.1111/j.1742-4801.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantor J, Margolis DJ. A multicenter study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol. 2000;142:960–4. doi: 10.1046/j.1365-2133.2000.03478.x. [DOI] [PubMed] [Google Scholar]
- 47.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12:163–8. doi: 10.1111/j.1067-1927.2004.012207.x. [DOI] [PubMed] [Google Scholar]
- 48.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–82. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 49.Woo K, Ayello EA, Sibbald RG. The edge effect: current therapeutic options to advance the wound edge. Adv Skin Wound Care. 2007;20:99–117. doi: 10.1097/00129334-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Venugopal SS, Murrell DF. Treatment of skin cancers in epidermolysis bullosa. Dermatol Clin. 2010;28:283–7. ix–x. doi: 10.1016/j.det.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB. Evidence-based medicine: how to practice and teach EBM. Edinburgh (Scotland): Churchill Livingstone; 2000. [Google Scholar]
- 52.Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–39. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arbuckle HA. Epidermolysis bullosa care in the United States. Dermatol Clin. 2010;28:387–9. xiii. doi: 10.1016/j.det.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Pope E. Epidermolysis bullosa care in Canada. Dermatol Clin. 2010;28:391–2. xiii. doi: 10.1016/j.det.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Liy-Wong C, Cepeda-Valdes R, Salas-Alanis JC. Epidermolysis bullosa care in Mexico. Dermatol Clin. 2010;28:393–4. xiii. doi: 10.1016/j.det.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Mellerio JE. Epidermolysis bullosa care in the United Kingdom. Dermatol Clin. 2010;28:395–6. xiv. doi: 10.1016/j.det.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 57.Watson R. Care of epidermolysis bullosa in Ireland. Dermatol Clin. 2010;28:397–9. xiv. doi: 10.1016/j.det.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 58.Bodemer C. Epidermolysis bullosa in France: management in the national reference center for genodermatosis. Dermatol Clin. 2010;28:401–3. xiv. doi: 10.1016/j.det.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Bruckner-Tuderman L. Epidermolysis bullosa care in Germany. Dermatol Clin. 2010;28:405–6. xiv. doi: 10.1016/j.det.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 60.Castiglia D, Zambruno G. Epidermolysis bullosa care in Italy. Dermatol Clin. 2010;28:407–9. xiv–xv. doi: 10.1016/j.det.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 61.Duipmans JC, Jonkman MF. Epidermolysis bullosa in the Netherlands. Dermatol Clin. 2010;28:411–3. xv. doi: 10.1016/j.det.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Pohla-Gubo G, Hintner H. Epidermolysis bullosa care in Austria and the Epidermolysis Bullosa House Austria. Dermatol Clin. 2010;28:415–20. xv. doi: 10.1016/j.det.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Medvecz M, Karpati S. Epidermolysis bullosa care in Hungary. Dermatol Clin. 2010;28:421–3. xv. doi: 10.1016/j.det.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Vahlquist A, Tasanen K. Epidermolysis bullosa care in Scandinavia. Dermatol Clin. 2010;28:425–7. xv. doi: 10.1016/j.det.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 65.Sprecher E. Epidermolysis bullosa care in Israel. Dermatol Clin. 2010;28:429–30. xv. doi: 10.1016/j.det.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Shinkuma S, Natsuga K, Nishie W, Shimizu H. Epidermolysis bullosa in Japan. Dermatol Clin. 2010;28:431–2. xvi. doi: 10.1016/j.det.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 67.Murrell DF. Epidermolysis bullosa in Australia and New Zealand. Dermatol Clin. 2010;28:433–8. xvi. doi: 10.1016/j.det.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342:1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 69.Woolf SH. Practice guidelines: a new reality in medicine, I: recent developments. Arch Intern Med. 1990;150:1811–8. [PubMed] [Google Scholar]