Abstract
Background
With standard miniature swine donors, survivals of only 3 days have been achieved in primate liver-transplant recipients. The recent production of alpha1,3-galactosyl transferase knockout (GalT-KO) miniature swine has made it possible to evaluate xenotransplantation of pig organs in clinically relevant pig-to-non-human primate models in the absence of the effects of natural anti-Gal antibodies. We are reporting our results using GalT-KO liver grafts.
Methods
We performed GalT-KO liver transplants in baboons using an immunosuppressive regimen previously used by our group in xeno heart and kidney transplantation. Post-operative liver function was assessed by laboratory function tests, coagulation parameters and histology.
Results
In two hepatectomized recipients of GalT-KO grafts, post-transplant liver function returned rapidly to normal. Over the first few days, the synthetic products of the donor swine graft appeared to replace those of the baboon. The first recipient survived for 6 days and showed no histopathological evidence of rejection at the time of death from uncontrolled bleeding, probably caused by transfusion-refractory thrombocytopenia. Amicar treatment of the second and third recipients led to maintenance of platelet counts of over 40 000 per μl throughout their 9- and 8-day survivals, which represents the longest reported survival of pig-to-primate liver transplants to date. Both of the last two animals nevertheless succumbed to bleeding and enterococcal infection, without evidence of rejection.
Conclusions
These observations suggest that thrombocytopenia after liver xenotransplantation may be overcome by Amicar therapy. The coagulopathy and sepsis that nevertheless occurred suggest that additional causes of coagulation disturbance must be addressed, along with better prevention of infection, to achieve long-term survival.
Keywords: baboon, liver, swine, xenotransplantation
Introduction
Despite recent progress in stem cell research and tissue engineering, xenotransplantation remains the best near-term hope for overcoming the two critical limitations imposed on the field of clinical liver transplantation by (i) the shortage of suitable human donor organs; and (ii) the frequent recurrence of underlying HCV in the allograft that occurs despite initially successful transplantation. Both of these obstacles would be removed if xenotransplantation were to become a reality, as there is evidence that human hepatoviral disease does not infect the porcine liver. The recent availability of alpha1,3-galactosyl transferase knockout (GalT-KO) miniature swine has made it possible to evaluate xenotransplantation of pig organs to baboons in the absence of the deleterious effects of natural anti-Gal antibodies [1]. Using GalT-KO donors, our laboratory has demonstrated marked improvement in survivals of both heterotopic heart [2] and orthotopic, life-supporting kidney [3] xenografts compared to those achieved in studies using standard miniature swine donors. Prior pig-to-baboon liver transplants, using standard miniature swine donors, achieved maximum survival of only 3 days [4]. We report here our initial experience using GalT-KO liver grafts.
Materials and methods
Animals
Recipient baboons (Papio hamadryus, n = 3, male, blood type B) were purchased from Mannheimer Foundation, Homestead, FL. Xenogeneic organs were obtained from GalT-KO miniature swine produced in our own swine facility from breeding stock derived by homologous recombination and nuclear transfer, as previously described [1]. The weights of the recipient baboons were in the 8–10 kg range, while the pig donors were 10–15% smaller.
All animals were cared for according to the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985). The experimental protocols were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC No. 2009N000004).
Surgery
Donor hepatectomy
Pigs were sedated with glycopyrrolate (0.01 mg/kg), xylazine (1 mg/kg) and Telazol (2 mg/kg), followed by intubation. Under Fluoroethane inhalation anaesthesia, a midline abdominal incision was made and the liver mobilized. The hilum was dissected next, skeletonizing the hepatic artery down to the celiac trunk and also the portal vein to the confluence of splenic vein and superior mesenteric vein. The bile duct was ligated distally and divided. Next the abdominal aorta was cannulated with a 16 Fr. cannula connected to a cysto-tubing system. After cross-clamping the aorta cephalad to the celiac trunk, the liver was flushed with 1 l of cold Lactated Ringer’s followed by 1 l of UW solution (Organ Recovery Systems, Chicago, IL, USA). The liver was then excised and placed into an intestinal bag surrounded by ice-slush until implantation. Back table biopsies were taken for light and electron microscopy; blood vessels were prepared for anastomosis and the gallbladder was removed.
Recipient procedures
Five days before the transplant procedure, a dual-lumen port-a-cath system was placed under general anaesthesia into the right internal jugular vein. The venous access chamber was placed subcutaneously between the scapulae. The animal was jacket-trained, and a tether (Lomir, Malone, NY, USA) was connected to the jacket. On the day of transplantation, the recipient animal was pre-medicated with Atropine (0.01 mg/kg) and Ketamine (10 mg/kg). After intubation and establishment of maintenance anaesthesia under Fluorothane inhalation, a femoral arterial line and a peripheral large-bore i.v. were placed. Via a midline abdominal incision recipient splenectomy was performed, followed by recipient hepatectomy. Our previous experience had established that veno-venous bypass was not needed in these primate recipients [4]. During the implantation of the donor graft, dopamine was infused as needed to maintain a mean arterial pressure of 50 mmHg. The suprahepatic vena caval anastomosis was fabricated first, followed by the portal vein anastomosis. The liver was then flushed with about 50 ml of blood via the unclamped portal vein. It exited via the still open infrahepatic vena cava. Following this flush the infrahepatic vena cava was clamped as the suprahepatic vena cava clamp was removed. This allowed completion of the infrahepatic vena cava anastomosis using 5-0 Prolene (Ethilon, Somerville, NJ, USA) as a continuous running suture during ongoing perfusion of the xenograft. At this point, the dopamine could be weaned. The arterial anastomosis was performed using a donor iliac artery jump graft from the recipient’s infrarenal aorta to the celiac trunk of the donor graft. The bile duct was reconstructed as a Roux-en-Y hepaticojejunostomy, using 6-0 PDS. One hour after reperfusion, another liver biopsy was obtained for light and electron microscopy. The animals were rewarmed to 36 °C by flushing the abdominal cavity with warm lactated Ringer’s solution. Then, the abdomen was closed in layers. Following initial recovery in the operating room, the animals were transported back to their cages. They were provided oral liquids 6 hours after surgery and soft food within 24 h.
Immunosuppression
The immunosuppressive regimen was based upon that previously described for xenogeneic heart and kidney transplants [2]. As we did not observe any signs of rejection in the first recipient, we modified the regimen to make it less toxic and more clinically applicable in the second recipient. The induction therapy was started with three doses of Thymoglobulin on day −3 in all cases, complemented by LoCd2b (rat anti-primate CD2 IgG2b; Immerge BioTherapeutics, Willington, DE, USA) to ensure T-cell depletion in B274. In the second and third animals LoCd2b was replaced by higher doses of Thymoglobulin. All animals received cobra-venom-factor (Quidel Corp., San Diego, CA, USA) to deplete complement factors (CH50) before surgery to below 5% of baseline levels, but none was given after transplantation. In addition, anti-CD154 (25 mg/kg) was given on days −1, 0 and 5, and Azathioprine (B291 only) on days −1 and 0. Maintenance therapy was started on day −1 with Tacrolimus (target serum levels 10–25 ng/ml) and a tapering schedule of methylprednisolone starting with 10 mg/kg on day 0.
Clinical monitoring
Daily blood samples were assayed for complete blood count (Hemavet 950 FS Drew Scientific Group; Waterbury, CT, USA), chemistry (Catalyst Dx; IDEXX, Holliston, MA, USA) and Tacrolimus serum levels (Architect i1000SR; Abbott diagnostics, Abbott Park, IL, USA). Clotting studies were performed in the clinical special coagulation laboratory at Massachusetts General Hospital.
Immunologic assays
Whole blood CD3 counts were obtained by fluorescent activated cell sorter (FACS) analyses. To measure anti-pig antibody levels, baboon blood was centrifuged at 3100 RPM for 10 min and the sera sterilely aliquoted and stored at −20 °C for later testing. 1 × 106 PBMCs from a GalT-KO pig were incubated with decomplemented baboon sera for 30 min at 4 °C in the dark. Cells were then washed twice with FACS media to remove any unbound antibody and incubated with fluorescein isothiocyanate-conjugated polyclonal rabbit antihuman IgG (F0058; Dako, Carpinteria, CA, USA) and IgM (Dako F0185) for 30 min at 4 °C in the dark. Cells were finally washed two times and acquired on a FACS calibur with PI gating to exclude dead cells. Mode fluorescence intensity of baboon antibody binding to pig lymphocytes was analysed using WinList mode analysis software (Verity Software House, Topsham, ME, USA).
Clotting studies
2 ml of citrated blood was drawn from the port-a-cath system and placed on ice. The samples were immediately transported to the clinical special coagulation laboratory, where they were analysed according to protocols used for human blood.
Results
Operative course
The weights of the donor swine were 10–15% lower than those of the recipient baboons. The reason for this was that we found in preliminary attempts [4] that the pig liver has a longer intrahepatic vena cava, which led to kinking in the recipient animals unless the donor animal was of slightly smaller size. No attempt was made to remove the anti-non-Gal antibodies that were present prior to transplant.
Post-operative course
B274 experienced a 20-min period of hypotension during the anhepatic phase while the vena cava and portal vein were clamped, but was observed to be sitting on the perch within 30 min of return to its cage, drinking water by six hours and eating by the next morning. Deteriorating LFT’s and renal function on POD 2 prompted re-exploration. All hepatic vessels were patent, suggesting the hepatic and renal dysfunction probably resulted from hypoxia secondary to fluid overload and pulmonary oedema. Following 36-h intubation and forced diuresis with lasix and mannitol, the animal’s condition improved. Liver function then deteriorated again on POD 5, concomitant with a fall in the platelet count to 20 000 per μl and below. Platelets (57 × 109) were administered, estimated to represent approximately one-third of the recipient’s total platelet count, but with no apparent salutary effect. The animal expired on POD 6 with diffuse bleeding. Final pathological examination showed the absence of acute cellular rejection in the liver, but pronounced centrilobular necrosis, attributed to the hypoxaemia in the early peri-operative phase. C4d staining was negative in all organs.
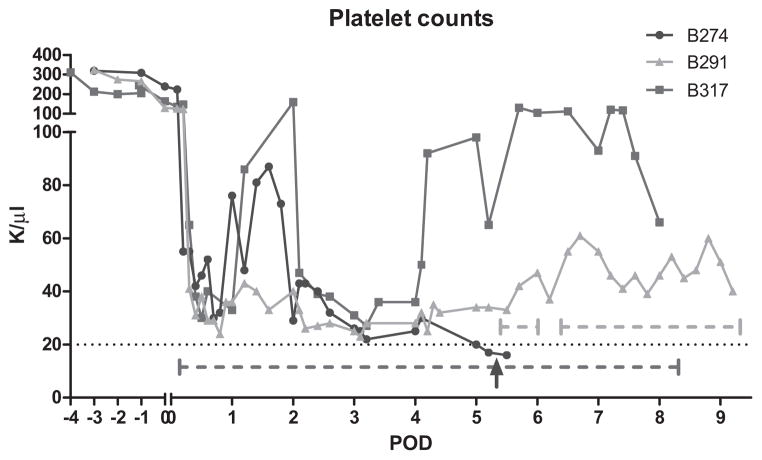
The second animal (B291) also recovered rapidly, sitting on the perch and eating by the next morning. On POD 2, the animal was explored for a falling hematocrit. Only a small omental bleeding site required coagulation. The volume of blood found in the peritoneal cavity appeared insufficient to explain the low hematocrit (Fig. 1). Over the next 3 days, the platelet count continued to fall, and D-dimer levels were highly elevated. In an attempt to inhibit fibrinolysis, we added Amicar to the therapeutic regimen on POD 5, first as a bolus of 500 mg iv, followed by a continuous infusion of 250 mg/h, similar to the dosing utilized clinically. This intervention was followed by an immediate rise in the platelet count (Fig. 2). When the infusion was inadvertently interrupted for several hours because of a technical problem, the platelet count fell again, but rose following resumption of the infusion. These observations suggested an efficacious effect of the Amicar. The platelet count subsequently remained at >40 000 per μl with continuous Amicar infusion.
Fig 1.
The hematocrit fell in all animals to critical levels, requiring the transfusion of substantial amounts of packed red blood cells (PRBC). The volume of transfused blood per day is represented by bar graphs.
Fig 2.
Thrombocytes were lost immediately after reperfusion. However, the levels remained slightly above the critical value of 20 000 per μl after Amicar treatment was started. The red and green dotted horizontal lines indicate Amicar infusion in B291 and B317, respectively. The interruption of the green dotted line indicates the accidental discontinuation of Amicar in B291 overnight. The blue arrow marks the time point of transfusion of allogenic platelets in B274 (details see text). B274 did not receive Amicar, while B317 received Amicar throughout its post-operative course.
An abdominal culture taken on the second postoperative day became positive for enterococcus within 24 h, probably from peritoneal contamination during construction of the hepaticojejunostomy. This necessitated ongoing antibiotics and further wash-out procedures on POD’s 5 and 7. These measures had limited success, as the peritoneal cultures remained positive. These explorations revealed smaller amounts of blood in the peritoneal cavity, now without an identifiable bleeding source. On the final exploration, a large amount of blood was found in the jejunum, ileum and colon. The animal did not recover from this last procedure and died on POD 9. Autopsy revealed clot in small and large intestine, but only superficial erosions of the gastric mucosa. Bilateral pulmonary atelectasis and a 1 cm in diameter recent left ventricle infarction without vascular thrombus were also noted. These findings may have been due to hypotension and depressed respiratory drive in the final hours before the animal’s demise. The presumptive cause of death was therapy-refractory sepsis because of intra-abdominal enterococcal infection.
The third animal, B317, received Amicar at the same dose as the 2nd animal, but this time we started the infusion one hour before reperfusion of the graft. The platelet count is again depicted in Fig. 2. This animal also required multiple blood transfusions, similar to the previous two animals (Fig. 1), and surgical explorations were needed on POD 2 and 5. Serosanguinous ascites was found without visible source of bleeding. The animal was active in the first few days, but on POD 2 we detected enterococcus in the ascites culture, and the blood culture grew enterococcus (vancomycin resistant strain). Despite appropriate antibiotics, the animal died on POD 8.
Coagulation assays
Analyses of pig clotting factors following allogenic transplantation or in naïve pigs, revealed inherent differences in baseline values between the pig and baboon (Fig. 3A). Coagulation factors II, VII and X are lower in porcine than in baboon plasma. In contrast, factors V, VIII, IX and XI are noticeably higher in pigs. In general, baboon baseline levels closely resembled the normal range in humans. Some of these measurements were likely influenced by the fact that the assays were designed for human rather than pig plasma, but the levels nevertheless provided a means for assessing the relative contribution of pig vs. baboon factors to the total levels measured. Figure 3A illustrates a gradual transition in the baboon recipients’ baseline levels to porcine levels. These observations confirm the conclusion that the porcine liver resumed normal function in the recipient. Nevertheless, while specific clotting factors were quantitatively sufficient to normalize usual coagulation assays, effective blood clotting was not observed in the abdominal cavity during post-operative explorations. However, PTT and INR remained near normal levels throughout the animals’ courses (Fig. 3b).
Fig 3.
(A, B) Coagulation factors in naïve swine and baboon and in the recipient of a porcine liver (B274). Coagulation factors approached swine baseline levels after transplantation. The Y-axis depicts per cent, with 100% being the normal value in humans. The half-lives of the clotting factors ranged from 6 to 12 h, making a transition from baboon to pig highly likely (A). (B) Shows the stable PTT/INR in all three animals with the exception of B 274, which experienced post-operative ischaemia and hypoxia.
Post-transplant liver function tests
The aspartate aminotransferase (AST) in B274 showed an early peak and a 2nd peak on POD 2 before beginning to improve (Fig. 4). After POD4, the AST progressively increased, until the animal’s death on POD6. In contrast, in animals B291 and B317, the AST fell after an early peak on POD1 and then never rose to levels seen in B274. Our conclusion was that intraoperative hypotension and reperfusion injury in B274 must have contributed to the early peaks and that the late peak was caused by peri-mortem hypoxia and hypotension. Haemolysis also leads to release of AST, and therefore the data are difficult to interpret. The liver-specific alanine aminotransferase (ALT), in contrast, was increased slightly after surgery in both animals, but returned to almost normal levels in B291 and B317 and did not rise again. Again, in B274, it remained elevated and increased further during the immediate pre-mortem period. The alkaline phosphatase never increased after the transplant procedure to levels higher than preoperative baseline (data not shown). The bilirubin progressively rose after transplantation in all animals (Fig. 4C). As the bilirubin was predominantly indirect, we concluded that haemolysis played a leading role in its generation. The albumin (Fig. 4D) in all animals remained near baseline levels throughout the post-operative course, but large quantities of human albumin were infused daily to treat hypovolaemia.
Fig 4.
Liver function tests (A) Alanine aminotransferase (ALT) levels confirmed stable hepatic function in animal B291 and B317, while B274 exhibited a progressive release of ALT in line with declining liver function, likely due to hypoxia (B) aspartate aminotransferase levels peaked twice after surgery in recipient Baboon 274, and release could be from hepatocytes or haemolyzed red blood cells. AST release appeared less in animals treated with Amicar. (C) Total bilirubin levels rose progressively in all animals. This was likely caused by the continued haemorrhage and haemolysis as the differential analysis showed that it was mostly indirect bilirubin. (D) Albumin levels remained in normal range throughout the animals’ life, but frequent exogenous administration makes interpretation difficult.
To maintain the hematocrit above 20%, all animals received an average of 70–80 ml of PRBC’s per day (Fig. 1). Amicar administration did not appear to affect this requirement. Bone marrow function was very active, with the reticulocyte count reaching 32% on POD5 in B291, confirming that the drop in hematocrit was not attributable to bone marrow suppression. Other observed abnormal values were the lactate dehydrogenase, which was constantly elevated after transplantation, closely related to the constantly falling hematocrit.
The platelet count in all animals showed a consistent trend, dropping immediately after reperfusion with recipient blood (Fig. 2). A new baseline was then established around 20 000 to 40 000 per μl in animal B274 until POD 5, when the count dropped further. At that point, 57 × 109 platelets were administered with no salutary effect. The first measurement after the platelet transfusion was actually lower than the pre-infusion value. In B291, a similar drop occurred after reperfusion. But when exploratory laparotomy on POD 5 did not reveal an explanation for the loss of RBC’s and platelets in B291, we decided to administer Amicar, which promptly resulted in elevation of platelet counts. B317 received Amicar before reperfusion, and post-operative platelet counts remained 30 000–100 000 per μl throughout the animal’s life. In general, animals receiving Amicar never experienced the low platelet count seen in B274.
Histology
H and E stains of serial liver biopsies did not show any evidence of acute cellular or humoral rejection (Fig. 5 shows the POD 7 histology in B291). In an attempt to define where the platelets and RBC’s were degraded, we obtained high-power magnifications of sinusoidal lining cells and hepatocytes. In some areas, iron pigment was found within the hepatocytes, evidence that red blood cells had been degraded. This finding is in concordance with recent observations by Burlak et al., [5] who perfused pig livers ex vivo with human platelets and demonstrated extensive phagocytosis. Electron microscopy revealed occasional platelets within, or closely associated with, liver sinusoidal endothelial cells.
Fig 5.

Normal liver tissue without any evidence of rejection, haematoxylin and eosin. The sample was taken on POD 7, B 291. It was representative for all liver samples of all animals in that none of the biopsies ever showed histologic evidence of rejection.
Discussion
Initial attempts to develop liver xenotransplantation date back to 1968, when Calne and coworkers reported immunosuppression consisting of steroids ± azathioprine, provided survivals of 6–84 h in primate recipients of pig livers [6]. Anti-lymphocyte globulin for immunosuppression led to similarly limited survival times [7].
The pig has been cited as the most suitable xenograft donor, largely because of its unlimited availability, but also because of its favourable breeding characteristics and the similarity of many of its organ systems to those of humans with regard to most metabolic functions. The partially inbred miniature swine have additional advantages, including size [8,9], genetic homogeneity and, now, availability of the GalT-KO line. An analysis of pig and human coagulation factors has revealed that various levels in pigs are several folds higher than corresponding human levels, but differences also extend to anticoagulation factors like antithrombin-III. As a result, prothrombin time (PT) and activated partial thromboplastin time (PTT) are not different from primates [10–12]. This pattern of porcine liver production of anticoagulation factors was confirmed in our baboon transplant recipients; some clotting factors, as measured post-transplantation in assays designed for determination of human factor levels, exceeded normal human levels. Initial studies using genetically altered pig donors were reported in 2000 by Ramirez and coworkers, who performed pig-to-baboon liver transplantation using donors expressing the “human complement regulator decay accelerating factor” (hDAF) to diminish complement activation. Their two recipient animals died at 4 days because of aspiration and at 8 days owing to bronchopneumonia [13]. During this period, coagulation factors were produced in sufficient quantities to prevent bleeding and serum albumin levels remained in the 2g/dl range, which is lower than the physiologic range for baboons [14]. In contrast to our findings, platelet counts, while below physiologic range, were better preserved. In our experiments, normal serum albumin levels were preserved, in part because we infused human serum albumin for treatment of hypovolaemia. Also, in contrast to features of hyperacute rejection seen on the terminal histology [13,15] of hDAF donor livers, we saw no evidence of rejection in our study using GalT-KO donors, with a follow-up of 6, 8 and 9 days, respectively.
The Pittsburgh group has recently reported their first series of 10 GalT-KO liver transplants into baboons [16,17] with survivals of 12 h to 7 days. The primary cause of death in the longer-term survivors was microangiopathy with thrombocytopenia and clotting disturbances. Platelet counts decreased to levels comparable to the ones seen in B274. They suggested that the platelet consumption was likely triggered by endothelial damage resulting from the effects of anti-non-Gal antibodies, precipitating a more vigorous coagulation cascade than is seen in allotransplants. Others also hypothesize that insufficient depletion of anti-non-Gal antibodies plays an important role in limiting survivals and that additional genetic manipulation of the xenograft donor will be required [18–20]. The pathophysiology observed in these studies was similar to that reported by Rees et al., [21,22] who perfused pig livers with human blood and found a progressive drop of hematocrit over 72 h of perfusion, which was not observed if the grafts were perfused with pig blood. Scanning electron microscopy revealed that red blood cells were bound and destroyed by Kupffer cells, apparently without complement activation [23]. Perfusion of pig livers expressing the Human Decay Accelerating Factor (hDAF) did not influence the rate of degradation of human RBC’s, further supporting the suggestion that this loss is related to Kupffer cells rather than to complement-dependent mechanisms. Similar results were reported by Satoh et al. [24] who also perfused porcine livers with human blood and detected upregulation of Von Willebrand factor and diffuse deposition of human IgM. Finally, it has been shown that human peripheral blood macrophages can spontaneously phagocytose porcine RBC’s [25].
Our results suggest for the first time that the loss of platelets may be overcome by administration of aminocaproic acid (Amicar), a plasmin inhibitor, commonly used in cardiac surgery and liver transplantation to treat fibrinolysis. Plasmin, a serine protease, is the key enzyme in the fibrinolytic cascade. It is effectively inhibited by forming a reversible complex with Amicar [26], thereby interrupting the fibrinolytic process. Amicar administration in the treated animals led to stabilization of platelet counts for the longest reported survivals to date of 8 and 9 days. Supporting this conclusion is the observation that temporary interruption of the Amicar infusion led to a drop in platelet count, while restarting the infusion led to stable counts for the following 3 days. Starting the infusion at time of reperfusion led to platelet counts in the 40 000–100 000 per mm3 throughout the animals’ life. A remaining difficulty to achieve short-term xenotransplantation survival is the constant need to transfuse RBC’s to maintain the recipient’s hematocrit. We presume that this requirement was attributable to hemolysis and blood loss despite near normal in vitro clotting parameters and maintenance of platelet counts above 25 000 per mm3. This ongoing blood loss undoubtedly impaired further recovery of the platelet count, and reasons for this persistent coagulopathy remain under investigations.
Although these data do not suggest that liver xenografts can yet be considered for destination therapy, they do suggest that temporary implantation of porcine livers as a life-saving measure may be feasible in the near future. The very fact that all xenograft recipients awakened and survived for 6–9 days with relatively normal activity suggests that most vital functions of the liver are compatible between these two species, because without such functions, animals would not regain consciousness. Further work will be needed to prevent the bleeding complications we have encountered and which appear to be unrelated to thrombocytopenia, avoid post-transplant infectious complications possibly by using less immunosuppression and to provide a longer window of opportunity for such temporary transplants.
Abbreviations
- ALT
alanine aminotransferase
- Amicar
aminocaproic acid
- AST
aspartate aminotransferase
- CBC
complete blood count
- FACS
fluorescent activated cell sorter
- FITC
fluorescein isothiocyanate
- GalT-KO
alpha1,3-galactosyl transferase knockout
- hDAF
human decay accelerating factor
- LFT's
liver function tests
- PBMC
peripheral blood mononuclear cells
- PDS
polidioxanone
- POD
post-operative day
- RPM
revolutions per minute
- UW
University of Wisconsin
- VRE
vancomycin resistant enterococcus
Footnotes
Authors' contributions
KK, ABC, MH: research design, performance of research, data analysis, writing; CS: writing of paper, performance of research, data analysis; NE, GRV: performance of research, research design; IW: sample analysis, data analysis; MV, RNS: performance of research, data analysis; SCR: research design, data analysis; DHS: research design, writing of the paper, data analysis. *KK and *CS contributed equally in regards to performing the experiments, data collection, analysis, and preparation of the manuscript.
References
- 1.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 3.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of 1,3-galactosyltransferase gene-knockout donors and the cotransplantation of viscularized thymic tissue. Nat Med. 2005;11:29–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 4.Powelson J, Cosimi AB, Austen W, Jr, et al. Porcine-toprimate orthotopic liver transplantation. Transplant Proc. 1994;26:1353–1354. [PubMed] [Google Scholar]
- 5.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2005;17:350–361. doi: 10.1111/j.1399-3089.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 6.Calne RY, White HJ, Herbertson BM, et al. Pig-tobaboon liver xenografts. Lancet. 1968;1:1176–1178. doi: 10.1016/s0140-6736(68)91869-2. [DOI] [PubMed] [Google Scholar]
- 7.Calne RY, Davis DR, Pena JR, et al. Hepatic allografts and xenografts in primates. Lancet. 1970;1:103–106. doi: 10.1016/s0140-6736(70)90462-9. [DOI] [PubMed] [Google Scholar]
- 8.Sachs DH. Homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, IA: Iowa State University Press; 1992. pp. 3–15. [Google Scholar]
- 9.Sachs DH. The pig as a xenograft donor. Pathol Biol (Paris) 1994;42:217–219. [PubMed] [Google Scholar]
- 10.Zhang L, Li Y, Jiang H, Liu J, Zeng Y, Cheng J. Comparison of hepatic coagulant, fibrinolytic, and anticoagulant functions between banna minipig inbred line and humans. Transplantation. 2005;79:1128–1131. doi: 10.1097/00007890-200505150-00031. [DOI] [PubMed] [Google Scholar]
- 11.Tucker A, Belcher C, Moloo B, et al. The production of transgenic pigs for potential use in clinical xenotransplantation: microbiological evaluation. Xenotransplantation. 2002;9:191–202. doi: 10.1034/j.1399-3089.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 12.Tucker A, Belcher C, Moloo B, et al. The production of transgenic pigs for potential use in clinical xenotransplantation: baseline clinical pathology and organ size studies. Xenotransplantation. 2002;9:203–208. doi: 10.1034/j.1399-3089.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez P, Chavez R, Majado M, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Munitiz V, Ramirez P, Hernandez Q, et al. Hematologic and hepatic function profile comparison between pig and baboon in an orthotopic liver xenotransplantation model. Transplant Proc. 1999;31:2641–2642. doi: 10.1016/s0041-1345(99)00482-0. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez P, Yelamos J, Parrilla P, Chavez R. Hepatic xenotransplantation will benefit from strategies aimed to reduce complement activation. Liver Transpl. 2001;7:562–563. doi: 10.1002/lt.500070618. [DOI] [PubMed] [Google Scholar]
- 16.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 17.Ekser B, Echeverri GJ, Hassett AC, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483–493. doi: 10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YL, Moran K, Dor FJ, et al. Elicited antibodies in baboons exposed to tissues from alfa 1,3-galactosyltransferase gene-knockout pigs. Transplantation. 2006;81:1058–1062. doi: 10.1097/01.tp.0000197555.16093.98. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu A, Hisashi Y, Kuwaki K, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from 1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplantation from alfa 1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008;8:2516–2526. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees MA, Butler AJ, Davies HFS, et al. Porcine livers perfused with human blood mount a graft-versus-“host” reaction. Transplantation. 2002;73:1460–1467. doi: 10.1097/00007890-200205150-00016. [DOI] [PubMed] [Google Scholar]
- 22.Rees MA, Butler AJ, Chavez-Cartaya G, et al. Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73:1194–1202. doi: 10.1097/00007890-200204270-00003. [DOI] [PubMed] [Google Scholar]
- 23.Rees MA, Butler AJ, Negus MC, Davies HFS, Friend PJ. Classical pathway complement destruction is not responsible for the loss of human erythrocytes during porcine liver perfusion. Transplantation. 2004;77:1416–1423. doi: 10.1097/01.tp.0000121135.24688.a3. [DOI] [PubMed] [Google Scholar]
- 24.Satoh T, Aramini JM, Li S, et al. Bioactive peptide design based on protein surface epitopes. A cyclic heptapeptide mimics CD4 domain 1 CC’ loop and inhibits CD4 biological function. J Biol Chem. 1997;272:12175–12180. doi: 10.1074/jbc.272.18.12175. [DOI] [PubMed] [Google Scholar]
- 25.Ide K, Ohdan H, Kobayashi T, Hara H, Ishiyama K, Asahara T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Koster A, Schirmer U. Re-evaluation of the role of antifibrinolytic therapy with lysine analogs during cardiac surgery in the post aprotinin era. Curr Opin Anaesthesiol. 2011;24:92–97. doi: 10.1097/ACO.0b013e32833ff3eb. [DOI] [PubMed] [Google Scholar]