Abstract
We have previously demonstrated high pathologic response rates after neoadjuvant concurrent chemoradiation in patients with locally advanced breast cancer (LABC). We now report disease-free survival (DFS) and overall survival (OS) in the context of pathologic response. 105 LABC patients (White 46%, Non-White 54%) were treated with paclitaxel (30 mg/m2 intravenously twice a week) for 10–12 weeks. Daily radiotherapy was delivered to breast, axillary, and supraclavicular lymph nodes during weeks 2–7 of paclitaxel treatment, at 1.8 Gy per fraction to a total dose of 45 Gy with a tumor boost of 14 Gy at 2 Gy/fraction. Pathological complete response (pCR) was defined as the absence of invasive cancer in breast and lymph nodes and pathological partial response (pPR) as the persistence of <10 microscopic foci of invasive carcinoma in breast or lymph nodes. Pathologic response (pCR and pPR) after neoadjuvant chemoradiation was achieved in 36/105 patients (34%) and was associated with significantly better DFS and OS. Pathological responders had a lower risk of recurrence or death (HR = 0.35, P = 0.01) and a longer OS (HR = 4.27, P = 0.01) compared with non-responders. Median DFS and OS were 57 and 84 months for non-responders, respectively, and have not yet been reached for responders. Importantly, pathologic response was achieved in 54% of patients with HR negative tumors (26/48). In conclusion, pathologic response to concurrent paclitaxel-radiation translated into superior DFS and OS. Half of the patients with HR negative tumors achieved a pathologic response.
Keywords: Concurrent chemoradiation, Neoadjuvant, Locally advanced breast cancer, Survival, Pathologic response
Introduction
Neoadjuvant chemotherapy for breast cancer was introduced for women with locally advanced disease to render inoperable tumors resectable, and later extended toward enhancing the likelihood of breast conservation. In addition to providing information about tumor sensitivity to cytotoxic agents in vivo, pathological response (pCR) to systemic therapies has demonstrated to be an invaluable intermediate endpoint, since it is a surrogate of DFS and OS after neoadjuvant therapy [1]. Patients achieving a pCR following anthracycline-based neoadjuvant chemotherapy achieve significantly superior DFS and OS compared with non-responders in large randomized trials such as the NSABP B-18 and B-27 [2]. However, with the exception of the subset of patients with tumors over-expressing human epidermal growth factor receptor-2 (Her2), that have high pCR rates when treated with regimens including trastuzumab [3–6], pCR rates following standard multi-agent chemotherapy remain relatively low, ranging from 7 to 31% [2, 7–17]. Pre-operative radiotherapy after neoadjuvant, anthracycline-based chemotherapy failed to significantly enhance the rate of pCR [18].
While superior outcomes after concurrent versus sequential chemotherapy and radiation (RT) have been demonstrated in other tumor types [19–23] with better local control and improved survival, studies of concurrent regimens are uncommon in breast cancer.
A prospective clinical trial of twice a week paclitaxel and concurrent radiotherapy in the neoadjuvant setting of LABC was conducted at three academic institutions, New York University (NYU), University of Southern California (USC), and Vanderbilt University School of Medicine: a consistent and encouraging pathologic response rate of 34% has been previously reported [24–26]. The current report focuses on the 5-year results of the 105 patients accrued to the trial, and analyzes outcomes in the context of pathologic response and original tumor characteristics.
Methods
Patients and procedures
Women≥18 years of age with biopsy-proven LABC (stages IIB–IIIC), Eastern Cooperative Oncology Group performance status of 0 to 1, adequate bone marrow and organ function were eligible. Prior publications provide additional details on patient entry criteria, acute toxicity, and pathological response [24, 25]. Specifically, informed consent was obtained from all the patients in accordance with guidelines of the institutional review board of each institution. Tumor measurements were obtained by physical exam, mammography, and/or ultrasound at study entry. To guide the surgeon during the operation and facilitate evaluation for residual disease by the pathologist, the location of the tumor bed before neoadjuvant treatment was marked by tattoos (two largest perpendicular tumor diameters on the frontal plane). All the patients underwent further staging by computed tomographic (CT) scans of chest, abdomen, and pelvis, as well as bone scans to exclude detectable distant metastases.
Pathology
Standard immunohistochemistry was performed on the diagnostic core biopsy to determine estrogen receptor (ER) and progesterone receptor (PR) status on paraffin-embedded tissues. Staining in ≥10% of tumor cells was considered a positive result. ER+/PR+, ER+/PR−, and ER−/PR+ breast cancer were grouped as hormone receptor (HR) positive disease; ER−/PR− constituted the HR negative group. In more recent patients (85/105 patients), Her2 status was evaluated on paraffin-embedded tissue by immunohistochemistry; positivity was defined as 3+ over-expression or by fluorescence in situ hybridization (FISH), where positivity was defined as gene amplification (Her2 gene copy/chromosome 17 ratio ≥2.2).
Preoperative treatment
Primary therapy consisted of 30 mg/m2 paclitaxel administered as a 1-h intravenous infusion twice weekly for a total of 10–12 weeks (Fig. 1). Pre-medication with dexamethasone, diphenhydramine, and H2 blockade was administered at the discretion of the treating physician. External-beam RT was initiated within 1 week of the first paclitaxel dose; daily radiotherapy was delivered to the breast, axillary, and supraclavicular lymph nodes during weeks 2–7, at 1.8 Gy per fraction to a total dose of 45 Gy followed by a boost of 14 Gy at 2 Gy per fraction to the originally palpable tumor. No deliberate attempt was made to encompass internal mammary nodes within the treated volume. After the FDA approval of this agent, patients enrolled after 2006 who had HER-2 positive tumors, received weekly trastuzumab (2 mg/kg) during the paclitaxel treatment (eight patients). Thirty-six patients at Vanderbilt University received three cycles of paclitaxel monotherapy (175 mg/m2 every 3 weeks) prior to concurrent paclitaxel/RT. Their results were analyzed separately and in combination with the remaining patients.
Fig. 1.
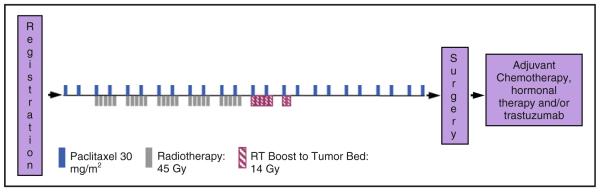
Treatment schema
Surgery
Patients underwent definitive breast cancer surgery approximately 4 weeks after completion of preoperative therapy or upon recovery of chemoradiation-induced dermatitis. Level I/II axillary lymph node dissection was required for all the patients. The type of surgery (breast conserving vs. mastectomy) was decided by the surgeon.
Adjuvant treatment
Postoperatively, combination chemotherapy was recommended for all the patients regardless of pathologic response, as well as a minimum of 5 years of endocrine therapy for patients with HR positive cancer.
Pathologic response
Response was assessed in the surgical specimen obtained at definitive cancer surgery. Pathologic complete response (pCR) was defined as the absence of invasive cancer in breast and axillary lymph nodes (persistent ductal carcinoma in situ (DCIS) was allowed). Pathologic partial response (pPR) was defined as persistence of less than 10 microscopic foci of invasive tumor cells in breast and/or axillary contents. All the other patients were classified as having achieved no pathologic response (pNR).
Statistical analysis
The distribution of patient characteristics at entry was compared across the three institutions. Frequency distributions (for qualitative variables, e.g., stage, hormone receptor status) were compared using contingency table methods (chi-square and Fisher’s exact tests). Age distributions were compared using analysis of variance methods. Response rates (pCR + pPR) were compared for the three institutions as well as by baseline characteristics using chi-square tests.
Overall survival was defined as time from definitive breast cancer surgery until death from any cause, with living patients censored at date of last contact. Disease-free survival was defined as time from definitive breast cancer surgery to first recurrence of breast cancer, or death from any cause. OS and DFS were summarized using Kaplan–Meier methods. Point estimates and 95% confidence intervals for 5-year OS and DFS are reported. Log-rank methods and Cox proportional hazards models were used to examine the effects of pathologic response and baseline disease characteristics individually and jointly on DFS and OS. Univariate and multivariable Cox proportional hazards models were developed to predict OS and DFS based on patient and disease characteristics using stepwise regression procedures. Final models were selected using SAS stepwise procedures and likelihood ratio tests. Hazard ratios and 95% confidence intervals are reported. All the statistical tests are two-sided.
Results
105 patients were treated with concurrent preoperative paclitaxel-radiation between March 1997 and August 2009; 33 at USC, 36 at NYU, and 36 at Vanderbilt University.
Patient baseline characteristics are reported by institution in Table 1. With the exception of differences in the distributions of race and stage, all characteristics are similar across the three institutions.
Table 1.
Baseline characteristics and pathologic response rates for the entire cohort (105 patients) and by institution
| Patient characteristics | NYU (N = 36) | USC (N = 33) | Vanderbilt (N = 36) | Total (N = 105) | All group P-value |
|---|---|---|---|---|---|
| Race | |||||
| Caucasian | 13 (36%) | 5 (15%) | 30 (83%) | 48 (46%) | <0.001a,^ |
| Non-Caucasian | 23 (64%) | 28 (85%) | 6 (17%) | 57 (54%) | |
| Hispanic | 7 (19%) | 21 (64%) | 0 | 28 (27%) | |
| African American | 7 (19%) | 1 (3%) | 5 (14%) | 13 (12%) | |
| Asian | 9 (25%) | 6 (18%) | 1 (3%) | 16 (15%) | |
| Age | Mean = 50.0 | Mean = 48.5 | Mean = 47.4 | Mean = 48.6 | 0.64c |
| SD = 11.06 | SD = 12.3 S | D = 11.4 | SD = 11.5 | ||
| Age ≤ 50 | 20 (56%) | 21 (64%) | 20 (56%) | 61 (58%) | 0.74a |
| Age > 50 | 16 (44%) | 12 (36%) | 16 (44%) | 44 (42%) | |
| AJCC Stage | |||||
| II | 19 (53%) | 10 (31%) | 22 (61%) | 51 (49%) | 0.04a |
| III | 17 (47%) | 22 (69%) | 14 (39%) | 53 (51%) | |
| Missing | 1 | 1 | |||
| Tumor Grade | |||||
| I | 1 (3%) | 0 | 4 (11%) | 5 (7%) | 0.44b |
| II | 14 (41%) | 0 | 14 (39%) | 28 (38%) | |
| III | 19 (56%) | 3 (100%) | 18 (50%) | 40 (55%) | |
| Missingd | 2 | 30 | 32 | ||
| ER | |||||
| Negative | 12 (33%) | 19 (58%) | 19 (53%) | 50 (48%) | 0.10a |
| Positive | 24 (67%) | 14 (42%) | 17 (47%) | 55 (52%) | |
| PR | |||||
| Negative | 22 (61%) | 20 (61%) | 22 (61%) | 64 (61%) | 1.00a |
| Positive | 14 (39%) | 13 (39%) | 14 (39%) | 41 (39%) | |
| HR status | |||||
| Negative | 12 (33%) | 17 (52%) | 19 (53%) | 48 (46%) | 0.18a,^^ |
| Positive | 24 (67%) | 16 (48%) | 17 (47%) | 57 (54%) | |
| ER+PR− | 10 (28%) | 3 (9%) | 3 (8%) | 16 (15%) | |
| ER+PR+ | 14 (39%) | 11 (33%) | 14 (39%) | 39 (37%) | |
| ER−PR+ | 0 | 2 (6%) | 0 | 2 (2%) | |
| Her2 status | |||||
| Negative | 26 (72%) | 11 (79%) | 21 (60%) | 58 (68%) | 0.36a |
| Positive | 10 (28%) | 3 (21%) | 14 (40%) | 27 (32%) | |
| Missing | 19 | 1 | 20 | ||
| Pathologic response | |||||
| pCR/pPR | 11 (31%) | 10 (30%) | 15 (42%) | 36 (34%) | 0.52a |
| pCR | 8 (22%) | 5 (15%) | 11 (31%) | 24 (23%) | |
| pPR | 3 (8%) | 5 (15%) | 4 (11%) | 12 (11%) | |
| pNR | 25 (69%) | 23 (70%) | 21 (58%) | 69 (66%) |
NYU New York University, USC University of Southern California, N number, SD standard deviation, AJCC American Joint Commission on Cancer, ER estrogen receptor, PR progesterone receptor, Her2 human epidermal growth factor receptor, pCR pathologic complete response, pPR pathologic partial response, pNR no pathologic response
P-values based on Caucasian vs. non Caucasian
P-value base on positive versus negative
Chi-square test
Fisher exact test
F-Statistic
Only nuclear grade was reported
Pathologic response
Pathologic response (pCR and pPR) after preoperative chemoradiation was achieved in 36/105 patients (34%, 95% confidence interval: 25–44%). Response rates were similar across the three institutions, including the cohort at one institution that received three cycles of paclitaxel monotherapy prior to concurrent chemoradiation (Table 1).
The pathologic response rate was significantly higher in patients with HR negative tumors (26/48 patients, 54%, 95% CI 39–69%) than in patients with HR positive tumors (10/57 patients, 18%, 95% CI 9–30%, P<0.0001).
Among the 85 patients for whom Her2 status is available, a pathologic response was achieved in 6/34 patients with HR+/Her2− tumors (17.6%), 3/13 patients with HR+/Her2+ tumors (23.1%), 13/24 patients with HR−/Her2− (triple negative) tumors (54.2%). Pathological response occurred in 7/14 patients with HR−/Her2+ cancers (50%), with 3/7 having received trastuzumab. Of the patients for whom Her2 status was not available, 60% (6/10) of HR negative patients had a pathologic response compared to 10% (1/10) of HR positive patients (2-sided Fisher’s exact test, P = 0.06). Table 2 describes the pathological response rate for breast cancer subtypes, based on HR and Her2 status and trastuzumab therapy.
Table 2.
Pathologic response rate for breast cancer subtypes based on HR and Her2 status
| Subtype | Total | Proportion of patients who received trastuzumab |
Proportion of patients with pathologic response (pCR + pPR) |
|---|---|---|---|
| Entire cohort (n = 105) | |||
| HR positive | 57 | 5/57 (2 with pathologic response) | 10/57 (18%) |
| HR negative | 48 | 3/48 (3 with pathologic response) | 26/48 (54%) |
| Cohort with Her2 status available (n = 85) | |||
| HR positive/Her2 negative | 34 | N/A | 6/34 (17.7%) |
| HR positive/Her2 positive | 13 | 5/13 (2 with pathologic response) | 3/13 (23.1%) |
| HR negative/Her2 positive | 14 | 3/14 (3 with pathologic response) | 7/14 (50.0%) |
| HR negative/Her2 negative (triple negative) | 24 | N/A | 13/24 (54.2%) |
HR hormone receptor, Her2 human epidermal growth factor receptor, pCR pathologic complete response, pPR pathologic partial response
Recurrences and second primary breast cancers
Recurrences manifested themselves mainly as distant metastases. Distant metastases were observed in 24/105 patients (22.9%): 6/36 patients with a pathologic response recurred (13%) compared with 23/69 non-responders (33%). Five-year loco-regional control was achieved in 95.2% of patients. Five patients experienced a local recurrence, one being an isolated event and the remaining four occurring synchronously with or following systemic recurrence. Invasive new contralateral breast cancers occurred in five patients (4.8%).
Disease-free and overall survival
At a median follow-up of 60 months, the median DFS and OS have not been reached. The estimated 5-year DFS is 61.4% (95% CI: 50.1–70.8%), the 5-year OS is 71.6% (95% CI: 60.5–80.1%) (Fig. 2).
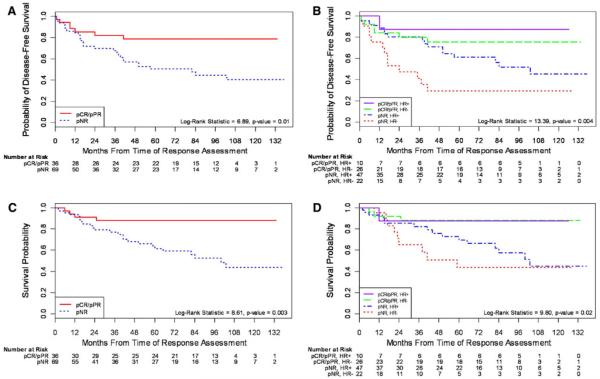
Fig. 2.
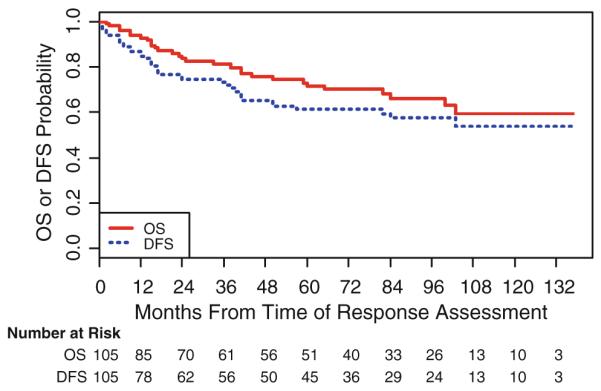
Overall survival and disease-free survival for the entire cohort
Association of pathologic response with disease-free and overall survival
Pathologic response was associated with an improved long-term outcome, regardless of HR status (Fig. 3). Patients with a pathologic response had a significantly longer DFS than patients without a pathologic response (hazard ratio = 2.85, 95% CI: 1.25–6.51, log-rank P-value = 0.01) (Fig. 3a, b). Similarly, responders had a longer OS (4.27 times greater) over the time period than non-responders (95% CI: 1.48, 12.29) (Fig. 3c, d).
Fig. 3.
Kaplan Meier estimates of overall survival and disease-free survival a DFS by pathologic response for entire cohort, b DFS by pathologic response and HR status, c OS by pathologic response for entire cohort, and d OS by pathologic response and HR status. HR hormone receptor, pCR pathologic complete response, pPR pathologic partial response, pNR no pathologic response
The median DFS was 57 months for non-responders and has not yet been reached for responders. The median OS was 84 months for non-responders and has not yet been reached for patients achieving a pathological response.
Among all variables tested (listed in Table 3), pathologic response was the only predictor of OS. HR negative patients achieving a pathologic response had a comparable OS to that of the HR positive responders (Fig. 3d).
Table 3.
Hazard ratios for disease-free survival and overall survival by baseline characteristics (Cox proportional hazards models)
| Patient characteristics | Disease-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariable analysis |
Univariate analysis |
||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Race | ||||||
| Asian vs. Caucasian | 1.49 (0.51–4.39) | 0.47 | 1.62 (0.47–5.56) | 0.45 | ||
| Black vs. Caucasian | 1.24 (0.42–3.64) | 0.69 | 0.92 (0.31–2.75) | 0.88 | ||
| Hispanic vs. Caucasian | 1.27 (0.57–2.80) | 0.56 | 2.06 (0.75–5.68) | 0.16 | ||
| Age | 1.004 (0.98–1.03) | 0.78 | 1.00 (0.97–1.03) | 0.99 | ||
| AJCC Stage | ||||||
| II vs. III | 2.01 (1.02–3.94) | 0.04 | 1.98 (0.92–4.25) | 0.08 | ||
| Tumor grade | ||||||
| I/II vs. III | 1.12 (0.55–2.27) | 0.76 | 0.99 (0.45–2.19) | 0.57 | ||
| ER | ||||||
| Positive vs. Negative | 1.29 (0.68–2.47) | 0.44 | 0.83 (0.40–1.73) | 0.62 | ||
| PR | ||||||
| Positive vs. Negative | 1.41 (0.72–2.77) | 0.32 | 1.05 (0.50–2.20) | 0.91 | ||
| HR status | ||||||
| Positive vs. Negative | 1.30 (0.68–2.47) | 0.43 | 2.18 (1.10–4.33) | 0.03 | 0.80 (0.38–1.69) | 0.56 |
| Her2 status | ||||||
| Positive vs. Negative | 0.67 (0.32–1.43) | 0.3 | 0.63 (0.27–1.49) | 0.29 | ||
| Pathologic response | ||||||
| pCR/pPR vs. pNR | 2.85 (1.25–6.51) | 0.01 | 4.08 (1.70–9.80) | 0.002 | 4.27 (1.48–12.29)a | 0.01 |
Best fit model chosen from stepwise selection procedures based on likelihood ratio criterion; only pathologic response influences survival when additional variables are included in overall survival models
HR hazard ratio, CI confidence interval, AJCC American Joint Commission on Cancer, ER estrogen receptor, PR progesterone receptor, HR hormone receptor, Her2 human epidermal growth factor receptor, pCR pathologic complete response, pPR pathologic partial response, pNR no pathologic response
We note that when institution is included in these models, there is no effect on DFS or OS.
Discussion
To our knowledge, this is the first report of the effect of pathological response to neoadjuvant concurrent chemoradiation on DFS and OS for LABC patients treated in a prospective clinical trial. With a median follow-up of 60 months, the estimated 5-year OS for the entire cohort treated with neoadjuvant paclitaxel/RT is 71.6%, a result comparable to that achieved in LABC patients treated by neoadjuvant chemotherapy, followed by surgery and adjuvant radiotherapy [10, 12, 27–34].
Of 105 patients treated with neoadjuvant paclitaxel/RT, 36 (34%) achieved a pathologic response. This response rate was consistently achieved at each of the three academic centers. The criteria for assessment of pathological response used in this series were originally defined in the mid-1990s, at USC [26]. Currently, there are more than eight proposed classifications for pathological response [11, 35–40]. To standardize response definitions, the Breast Cancer Task Force included a post-neoadjuvant therapy staging category in the revised American Joint Committee on Cancer (yAJCC) staging system for breast cancer [41]. Complete response is defined as the absence of invasive tumor in breast and lymph nodes (yAJCC stage 0), consistent with our definition of pCR [35, 38]. However, in contrast to some other studies, we combined pCR and pPR as pathologic response, therefore including patients with less than 10 microscopic foci of invasive cancer in our cohort of responders. The rationale for this criterion is based on the short duration of the concurrent chemoradiation approach (less than 2 months for most patients), possibly a period of time insufficient to enable complete clearance of tumor cells, hence the inclusion of pPR. Others have reported residual minimal invasive disease in the breast (Residual Breast Cancer Burden (RCB)-1) as prognostically similar to cases with absent invasive disease (RCB-0) [38]. Noticeably, when clinical outcomes were analyzed separately for pCR and pPR in our series, there were no differences in OS or DFS between these two groups (Log-rank Test, data not shown).
The median follow-up for the study cohort is 60 months. For the entire cohort, achievement of a pathologic response was associated with significantly fewer breast cancer recurrences and deaths at 5-year analysis. To our knowledge, this is the first prospective study that reports an association of pathologic response with survival after primary concurrent chemoradiation. The results reported are comparable to those from a large retrospective cohort of 1,117 LABC patients treated with concurrent chemoradiation in South India: pathologic response occurred in 33% of patients and was associated with improved DFS, consistent with our findings [42].
In the current series, the pathologic response rate of 54% in patients with triple-negative breast cancer compares favorably to the results achieved by neoadjuvant chemotherapy [43–45]: the fact that HR negative cancer carriers who achieved a pathological response had a similar OS as HR positive responders warrants further exploration in this subset of breast cancer patients.
This finding that a single agent regimen, like the one tested, when given with concurrent radiotherapy could achieve a pathological response prognostically similar to that achieved by standard neoadjuvant poly-chemotherapy is noteworthy. Several hypotheses can explain these results. First of all, it is possible that in tumors more sensitive to taxanes and radiation, the concurrent administration of the two modalities successfully eliminated the subset of cancer cells later destined to become circulating cells with seeding potential [46] and/or eradicated CD44+/CD24−/low or aldehyde dehydrogenase 1 (ALDH1)+ tumor cells, shown to possess tumor-initiating potential if they persist after treatment [47, 48]. In a study conducted on tumor specimens of the patients in this cohort who had adequate pre- and posttreatment biopsies, we reported the association of pathological response with over-expression of MAP2 at micro-array analysis [49], a possible explanation of enhanced sensitivity to taxanes [50].
A novel hypothesis for the systemic benefit of concurrent chemoradiation is the effective induction of protective anti-tumor immunity. In the study mentioned above, we also found an association of pathological response with distinct immune signatures [49]. At proteomic analysis a-defensins (DEFA) [51] expression was associated with increased immune infiltrates, and significantly correlated with pCR, particularly among triple-negative patients (58%). These findings were confirmed at genomic analysis and are consistent with those reported by Kreike et al. [52] who demonstrated that clusters of interferon-regulated and immunoglobulin genes were >3-fold increased in triple-negative tumors who achieved a pathological response. In addition to their cytotoxic effects standard anti-cancer modalities such as certain chemotherapy agents and radiotherapy can generate an immunogenic cell death, converting the original tumor into an in situ immunogenic hub [53–57]. In preclinical models, the acquired anti-tumor immune response contributes to tumor pathological responses that can also control micro-metastatic foci [54].
Finally, half of the patients in this series were Non-Caucasian women (54%), and the majority of women were treated at a public hospital. When analyzed, ethnicity did not have an impact on the achievement of pathologic response or outcomes. The population with LABC in the US often consists of minority and underserved women, more likely to experience challenges to adherence because of cultural and language barriers, resident status, lack of medical insurance, etc. It is therefore not surprising that 12 patients (11% of study population) were lost to follow-up despite intense efforts by the multidisciplinary team and patient navigators.
In summary, in spite of adverse characteristics in our patient population, our data suggest that preoperatively administered concurrent paclitaxel/radiation achieves comparable results to those of modern neoadjuvant chemotherapy with respect to pathologic response rate and OS. Most importantly, patients with HR negative tumors had a 54% chance of achieving a pathologic response, which translated into improved DFS and OS. Since paclitaxel is available in its generic form and the radiotherapy regimen of this trial is comparable to that currently used in standard regimens of neoadjuvant poly-chemotherapy followed by surgery and postoperative radiation, the comparative effectiveness of this approach warrants further study. However, only a prospective randomized study comparing concurrent chemo-radiation to the sequential standard protocol can establish the role of this approach.
Acknowledgments
We thank all the participating patients, treating physicians, and research teams at NYU, USC, and Vanderbilt University. The study was supported by the: Department of Defense Breast Cancer Research Program Center of Excellence (W81XWH-04-1-0905, to S.C.F., J.D.G., T.H. and R.J.S.), American Cancer Society (TURSG CCE 103174, to S.C.F.), National Institutes of Health Grants 5P30 CA016037-30 (NYU Core Services), K23CA125205P50 (to S.A.), CA95131 (Specialized Program of Research Excellence in Breast Cancer, to J.A.P.); CA105436 and CA070856 (to J.A.P.); ES00267 and CA68485 (Vanderbilt Core Services); CA009385 and CA138106 (to J.A.B.); US Army grant DAMD17-99-1-9422 (to J.A.P.); California Breast Cancer Research Program BCRP TRC 4E-6000 (to S.C.F.), Breast Cancer Research Foundation (to S.C.F. and R.J.S.), Avon Foundation 15D1500-36300 (to S.C.F. and R.J.S.), New York University General Clinical Research Center (NIH/NCRR M01 RR000096), and a Vanderbilt-Ingram Cancer Center Discovery Grant (to A.B.C.).
Footnotes
Conflicts of interest A.B.C received research funding from Bristol-Meyers Squibb.
Contributor Information
Sylvia Adams, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
A. Bapsi Chakravarthy, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA.
Martin Donach, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
Darcy Spicer, Kenneth Norris Jr. Comprehensive Cancer Center, University of Southern California Keck School of Medicine, Los Angeles, CA, USA.
Stella Lymberis, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
Baljit Singh, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
Joshua A. Bauer, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA
Tsivia Hochman, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
Judith D. Goldberg, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA
Franco Muggia, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA.
Robert J. Schneider, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA
Jennifer A. Pietenpol, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN, USA
Silvia C. Formenti, Department of Radiation Oncology, New York University School of Medicine, 160 E 34th Street, New York, NY 10016, USA
References
- 1.Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR. Pathological assessment of response to induction chemotherapy in breast cancer. Cancer Res. 1986;46(5):2578–2581. [PubMed] [Google Scholar]
- 2.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols -18 and b-27. J Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. doi:10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 3.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. doi:10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233. doi: 10.1158/1078-0432.CCR-06-1345. doi:10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 5.Sikov WM, Dizon DS, Strenger R, Legare RD, Theall KP, Graves TA, Gass JS, Kennedy TA, Fenton MA. Frequent pathologic complete responses in aggressive stages ii to iii breast cancers with every-4-week carboplatin and weekly paclitaxel with or without trastuzumab: a brown university oncology group study. J Clin Oncol. 2009;27(28):4693–4700. doi: 10.1200/JCO.2008.21.4163. doi:10.1200/JCO.2008.21.4163. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with her2-positive locally advanced breast cancer (the noah trial): a randomised controlled superiority trial with a parallel her2-negative cohort. Lancet. 375(9712):377–384. doi: 10.1016/S0140-6736(09)61964-4. doi:10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 7.Chua S, Smith IE, A’Hern RP, Coombes GA, Hickish TF, Robinson AC, Laing RW, O’Brien ME, Ebbs SR, Hong A, Wardley A, Mughal T, Verrill M, Dubois D, Bliss JM. Neoadjuvant vinorelbine/epirubicin (ve) versus standard adriamycin/cyclophosphamide (ac) in operable breast cancer: analysis of response and tolerability in a randomised phase iii trial (topic 2) Ann Oncol. 2005;16(9):1435–1441. doi: 10.1093/annonc/mdi276. doi:10.1093/annonc/mdi276. [DOI] [PubMed] [Google Scholar]
- 8.Buzdar AU, Singletary SE, Theriault RL, Booser DJ, Valero V, Ibrahim N, Smith TL, Asmar L, Frye D, Manuel N, Kau SW, McNeese M, Strom E, Hunt K, Ames F, Hortobagyi GN. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer. J Clin Oncol. 1999;17(11):3412–3417. doi: 10.1200/JCO.1999.17.11.3412. [DOI] [PubMed] [Google Scholar]
- 9.Evans TR, Yellowlees A, Foster E, Earl H, Cameron DA, Hutcheon AW, Coleman RE, Perren T, Gallagher CJ, Quigley M, Crown J, Jones AL, Highley M, Leonard RC, Mansi JL. Phase iii randomized trial of doxorubicin and docetaxel versus doxorubicin and cyclophosphamide as primary medical therapy in women with breast cancer: An anglo-celtic cooperative oncology group study. J Clin Oncol. 2005;23(13):2988–2995. doi: 10.1200/JCO.2005.06.156. doi:10.1200/JCO.2005.06.156. [DOI] [PubMed] [Google Scholar]
- 10.Low JA, Berman AW, Steinberg SM, Danforth DN, Lippman ME, Swain SM. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22(20):4067–4074. doi: 10.1200/JCO.2004.04.068. doi:10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 11.Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK, Eggleton SP, Ogston KN. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20(6):1456–1466. doi: 10.1200/JCO.2002.20.6.1456. [DOI] [PubMed] [Google Scholar]
- 12.Gradishar WJ, Wedam SB, Jahanzeb M, Erban J, Limentani SA, Tsai KT, Olsen SR, Swain SM. Neoadjuvant docetaxel followed by adjuvant doxorubicin and cyclophosphamide in patients with stage iii breast cancer. Ann Oncol. 2005;16(8):1297–1304. doi: 10.1093/annonc/mdi254. doi:10.1093/annonc/mdi254. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Kaufmann M. Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase iii randomized gepartrio trial. J Natl Cancer Inst. 2008;100(8):542–551. doi: 10.1093/jnci/djn085. doi:10.1093/jnci/djn085. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Raab G, Caputo A, Schutte M, Hilfrich J, Blohmer JU, Gerber B, Costa SD, Merkle E, Eidtmann H, Lampe D, Jackisch C, du Bois A, Kaufmann M. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the Geparduo study of the German Breast Group. J Clin Oncol. 2005;23(12):2676–2685. doi: 10.1200/JCO.2005.05.078. doi:10.1200/JCO.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 15.Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for her2 overexpressing stage ii or iii breast cancer: a pilot study. J Clin Oncol. 2003;21(1):46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 16.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, Cristofanilli M, Booser DJ, Pusztai L, Rivera E, Theriault RL, Carter C, Frye D, Hunt KK, Symmans WF, Strom EA, Sahin AA, Sikov W, Hortobagyi GN. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23(25):5983–5992. doi: 10.1200/JCO.2005.06.232. doi:10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 17.Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN, Gonzalez-Angulo AM. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24(7):1037–1044. doi: 10.1200/JCO.2005.02.6914. doi:10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 18.Colleoni M, Nole F, Minchella I, Noberasco C, Luini A, Orecchia R, Veronesi P, Zurrida S, Viale G, Goldhirsch A. Preoperative chemotherapy and radiotherapy in breast cancer. Eur J Cancer. 1998;34(5):641–645. doi: 10.1016/s0959-8049(97)10091-0. [DOI] [PubMed] [Google Scholar]
- 19.Lorvidhaya V, Chitapanarux I, Sangruchi S, Lertsanguansinchai P, Kongthanarat Y, Tangkaratt S, Visetsiri E. Concurrent mitomycin c, 5-fluorouracil, and radiotherapy in the treatment of locally advanced carcinoma of the cervix: a randomized trial. Int j Radiat Oncol Biol Phys. 2003;55(5):1226–1232. doi: 10.1016/s0360-3016(02)04405-x. [DOI] [PubMed] [Google Scholar]
- 20.Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P, Pecen L. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer. 2004;46(1):87–98. doi: 10.1016/j.lungcan.2004.03.004. doi:10.1016/j.lungcan.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, Saijo N. Phase iii study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin, etoposide for limited-stage small-cell lung cancer: results of the japan clinical oncology group study 9104. J Clin Oncol. 2002;20(14):3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 22.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 23.Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC, Wu PM, Law MW, Kwok CC, Yau CC, Wan KY, Chan RT, Choy DD. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol. 2004;22(13):2643–2653. doi: 10.1200/JCO.2004.05.173. doi:10.1200/JCO.2004.05.173. [DOI] [PubMed] [Google Scholar]
- 24.Formenti SC, Volm M, Skinner KA, Spicer D, Cohen D, Perez E, Bettini AC, Groshen S, Gee C, Florentine B, Press M, Danenberg P, Muggia F. Preoperative twice-weekly paclitaxel with concurrent radiation therapy followed by surgery and postoperative doxorubicin-based chemotherapy in locally advanced breast cancer: a phase I/II trial. J Clin Oncol. 2003;21(5):864–870. doi: 10.1200/JCO.2003.06.132. [DOI] [PubMed] [Google Scholar]
- 25.Chakravarthy AB, Kelley MC, McLaren B, Truica CI, Billheimer D, Mayer IA, Grau AM, Johnson DH, Simpson JF, Beauchamp RD, Jones C, Pietenpol JA. Neoadjuvant concurrent paclitaxel and radiation in stage II/III breast cancer. Clin Cancer Res. 2006;12(5):1570–1576. doi: 10.1158/1078-0432.CCR-05-2304. doi:10.1158/1078-0432.CCR-05-2304. [DOI] [PubMed] [Google Scholar]
- 26.Formenti SC, Dunnington G, Uzieli B, Lenz H, Keren-Rosenberg S, Silberman H, Spicer D, Denk M, Leichman G, Groshen S, Watkins K, Muggia F, Florentine B, Press M, Danenberg K, Danenberg P. Original p53 status predicts for pathological response in locally advanced breast cancer patients treated preoperatively with continuous infusion 5-fluorouracil and radiation therapy. Int J Radiat Oncol Biol Phys. 1997;39(5):1059–1068. doi: 10.1016/s0360-3016(97)00506-3. [DOI] [PubMed] [Google Scholar]
- 27.Morrell LE, Lee YJ, Hurley J, Arias M, Mies C, Richman SP, Fernandez H, Donofrio KA, Raub WA, Jr, Cassileth PA. A phase II trial of neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin in the treatment of patients with locally advanced breast carcinoma. Cancer. 1998;82(3):503–511. doi: 10.1002/(sici)1097-0142(19980201)82:3<503::aid-cncr12>3.0.co;2-5. doi:10.1002/(SICI)1097-0142(19980201)82:3<503:AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Yerushalmi R, Hayes MM, Gelmon KA, Chia S, Bajdik C, Norris B, Speers C, Hassell P, O’Reilly SE, Allan S, Shenkier TN. A phase II trial of a neoadjuvant platinum regimen for locally advanced breast cancer: pathologic response, long-term follow-up, and correlation with biomarkers. Clin Breast Cancer. 2009;9(3):166–172. doi: 10.3816/CBC.2009.n.027. doi:10.3816/CBC.2009.n.027. [DOI] [PubMed] [Google Scholar]
- 29.Moon YW, Rha SY, Jeung HC, Yang WI, Suh CO, Chung HC. Neoadjuvant chemotherapy with infusional 5-fluorouracil, adriamycin and cyclophosphamide (ifac) in locally advanced breast cancer: an early response predicts good prognosis. Ann Oncol. 2005;16(11):1778–1785. doi: 10.1093/annonc/mdi360. doi:10.1093/annonc/mdi360. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GF, Birchansky CA, Komarnicky LT, Mansfield CM, Cantor RI, Biermann WA, Fellin FM, McFarlane J. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer. 1994;73(2):362–369. doi: 10.1002/1097-0142(19940115)73:2<362::aid-cncr2820730221>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 31.Zambetti M, Oriana S, Quattrone P, Verderio P, Terenziani M, Zucali R, Valagussa P, Bonadonna G. Combined sequential approach in locally advanced breast cancer. Ann Oncol. 1999;10(3):305–310. doi: 10.1023/a:1008345901178. [DOI] [PubMed] [Google Scholar]
- 32.Frasci G, D’Aiuto G, Comella P, D’Aiuto M, Di Bonito M, Ruffolo P, Iodice G, Petrillo A, Lastoria S, Oliviero P, Capasso I, Montella M, Siani C, Santangelo M, Vizioli L, Comella G. Preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) improves prognosis in locally advanced breast cancer patients: an update of the Southern Italy Cooperative Oncology Group (SICOG) randomised trial 9908. Ann Oncol. 2009 doi: 10.1093/annonc/mdp356. doi:10.1093/annonc/mdp356. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez RH, Booser DJ, Cristofanilli M, Sahin AA, Strom EA, Guerra L, Kau SW, Gonzalez-Angulo AM, Hortobagyi GN, Valero V. Phase 2 trial of primary systemic therapy with doxorubicin and docetaxel followed by surgery, radiotherapy, and adjuvant chemotherapy with cyclophosphamide, methotrexate, and 5-fluorouracil based on clinical and pathologic response in patients with stage IIB to III breast cancer: long-term results from the University of Texas M. D. Anderson Cancer Center Study id97-099. Cancer. 2010;116(5):1210–1217. doi: 10.1002/cncr.24901. doi:10.1002/cncr.24901. [DOI] [PubMed] [Google Scholar]
- 34.Ezzat AA, Ibrahim EM, Ajarim DS, Rahal MM, Raja MA, Tulbah AM, Al-Malik OA, Al-Shabanah M, Sorbris R. Phase II study of neoadjuvant paclitaxel and cisplatin for operable and locally advanced breast cancer: analysis of 126 patients. Br J Cancer. 2004;90(5):968–974. doi: 10.1038/sj.bjc.6601616. doi:10.1038/sj.bjc.6601616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, Ollila DW, Klauber-DeMore N, Halle J, Sawyer L, Moore DT, Graham ML. American joint committee on cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97(15):1137–1142. doi: 10.1093/jnci/dji206. doi:10.1093/jnci/dji206. [DOI] [PubMed] [Google Scholar]
- 36.Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, Zambetti M. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute. J Clin Oncol. 1998;16(1):93–100. doi: 10.1200/JCO.1998.16.1.93. [DOI] [PubMed] [Google Scholar]
- 37.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB, Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 38.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–4422. doi: 10.1200/JCO.2007.10.6823. doi:10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 39.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 40.Cance WG, Carey LA, Calvo BF, Sartor C, Sawyer L, Moore DT, Rosenman J, Ollila DW, Graham M., 2nd Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival. Ann Surg. 2002;236(3):295–302. doi: 10.1097/01.SLA.0000027526.67560.64. doi:10.1097/01.SLA.0000027526.67560.64. discussion 302–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AJCC cancer staging manual. 7th edn Springer; 2010. [Google Scholar]
- 42.Shanta V, Swaminathan R, Rama R, Radhika R. Retrospective analysis of locally advanced noninflammatory breast cancer from chennai, South India, 1990–1999. Int J Radiat Oncol Biol Phys. 2008;70(1):51–58. doi: 10.1016/j.ijrobp.2007.05.050. doi:10.1016/j.ijrobp.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 43.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. doi:10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 44.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. doi:10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 45.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. doi:10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 46.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. doi:10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. doi:10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. doi:10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 49.Bauer JA, Chakravarthy AB, Rosenbluth JM, Mi D, Seeley EH, De Matos Granja-Ingram N, Olivares MG, Kelley MC, Mayer IA, Meszoely IM, Means-Powell JA, Johnson KN, Tsai CJ, Ayers GD, Sanders ME, Schneider RJ, Formenti SC, Caprioli RM, Pietenpol JA. Identification of markers of taxane sensitivity using proteomic and genomic analyses of breast tumors from patients receiving neoadjuvant paclitaxel and radiation. Clin Cancer Res. 2010;16(2):681–690. doi: 10.1158/1078-0432.CCR-09-1091. doi:10.1158/1078-0432.CCR-09-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishio K, Arioka H, Ishida T, Fukumoto H, Kurokawa H, Sata M, Ohata M, Saijo N. Enhanced interaction between tubulin and microtubule-associated protein 2 via inhibition of map kinase and cdc2 kinase by paclitaxel. Int J Cancer. 1995;63(5):688–693. doi: 10.1002/ijc.2910630514. [DOI] [PubMed] [Google Scholar]
- 51.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. doi:10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 52.Kreike B, van Kouwenhove M, Horlings H, Weigelt B, Peterse H, Bartelink H, van de Vijver MJ. Gene expression profiling and histopathological characterization of triple-negative/basallike breast carcinomas. Breast Cancer Res. 2007;9(5):R65. doi: 10.1186/bcr1771. doi:10.1186/bcr1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. doi:10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 54.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. doi:10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Formenti SC, Demaria S. Effects of chemoradiation on tumor-host interactions: the immunologic side. J Clin Oncol. 2008;26(9):1562–1563. doi: 10.1200/JCO.2007.15.5499. doi:10.1200/JCO.2007.15.5499 author reply 1563. [DOI] [PubMed] [Google Scholar]
- 56.Formenti SC. Immunological aspects of local radiotherapy: clinical relevance. Discov Med. 2010;9(45):119–124. [PubMed] [Google Scholar]
- 57.Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through tlr4 signaling in mice. Cell Immunol. 2010 doi: 10.1016/j.cellimm.2010.03.001. doi:10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]