Abstract
Background
Revision anterior cruciate ligament (ACL) reconstruction has worse outcomes than primary reconstructions. Predictors for these worse outcomes are not known. The Multicenter ACL Revision Study (MARS) Group was developed to perform a multisurgeon, multicenter prospective longitudinal study to obtain sufficient subjects to allow multivariable analysis to determine predictors of clinical outcome.
Purpose
To describe the formation of MARS and provide descriptive analysis of patient demographics and clinical features for the initial 460 enrolled patients to date in this prospective cohort.
Study Design
Cross-sectional study; Level of evidence, 2.
Methods
After training and institutional review board approval, surgeons began enrolling patients undergoing revision ACL reconstruction, recording patient demographics, previous ACL reconstruction methods, intra-articular injuries, and current revision techniques. Enrolled subjects completed a questionnaire consisting of validated patient-based outcome measures.
Results
As of April 1, 2009, 87 surgeons have enrolled a total of 460 patients (57% men; median age, 26 years). For 89%, the reconstruction was the first revision. Mode of failure as deemed by the revising surgeon was traumatic (32%), technical (24%), biologic (7%), combination (37%), infection (<1%), and no response (<1%). Previous graft present at the time of injury was 70% autograft, 27% allograft, 2% combination, and 1% unknown. Sixty-two percent were more than 2 years removed from their last reconstruction. Graft choice for revision ACL reconstruction was 45% autograft, 54% allograft, and more than 1% both allograft and autograft. Meniscus and/or chondral damage was found in 90% of patients.
Conclusion
The MARS Group has been able to quickly accumulate the largest revision ACL reconstruction cohort reported to date. Traumatic reinjury is deemed by surgeons to be the most common single mode of failure, but a combination of factors represents the most common mode of failure. Allograft graft choice is more common in the revision setting than autograft. Concomitant knee injury is extremely common in this population.
Keywords: revision, anterior cruciate ligament (ACL), reconstruction, epidemiology
Revision ACL reconstruction represents an infrequent but clinically important challenge in orthopaedic practice.8,18,20,24,25,29,31 Technical issues require specific revision techniques to address complications such as retained hardware, bone tunnel defects, and incorrect tunnel placement. Moreover, it is commonly reported that the results of revision surgery remain inferior to primary reconstructions.1,30 These poorer outcomes include inferior patient-based outcomes, increased laxity, higher graft failure rate, meniscal degeneration, and chondral lesions.
A number of reasons for the poorer outcome rate have been proposed, including compromised tunnel location, pathologic abnormalities untreated during the primary reconstruction, and greater reliance on allografts for revisions.6,14,27 To evaluate the contributions of these and other factors to outcome, large numbers of patients who are undergoing revisions must be identified and followed prospectively. Because of the relative infrequency of revision ACL reconstructions in any one center, a large multicenter, multisurgeon study is necessary to accumulate enough subjects over a reasonable time to allow multivariable analyses with a large number of predictor variables. The eventual goal of this project is to identify clinically useful (ie, modifiable) predictors of outcome that may inform practice decisions and improve revision ACL reconstruction outcomes.
The purposes of this article are to (1) describe the rationale for and development of a specialty society–organized, multicenter study to evaluate predictors of outcome following revision ACL reconstruction, (2) document the feasibility of collecting a large sample of relatively low-volume surgical cases in a short period of time using this study strategy, and (3) present preliminary baseline data from this study to characterize the cohort.
METHODS
Study Design
A prospective longitudinal cohort design with multiple sites and multiple surgeons was chosen to determine modifiable predictors of outcome. This study design permits the establishment of both high-level evidence on prognosis to better counsel patients and, more importantly, to discover predictors (risk factors) of these outcomes. This cohort study was designed to recruit and retain enough subjects with longitudinal follow-up to allow multivariable analyses of factors affecting outcome.
In keeping with the National Institutes of Health roadmap to revamp clinical research, practice-based research networks are recognized as a critical component of clinical research. These partnerships with private practice physicians are part of the Clinical and Translational Science Awards.28 Moreover, there have been calls for orthopaedic specialty societies to play a leading role in conducting multicenter clinical trials. The AOSSM has over 2000 members, including private practice and academic physicians from predominantly the United States and Canada; that group was the most practical venue to construct a large-scale research network involving revision ACL reconstruction surgery. All AOSSM members were notified regarding this society-level multicenter study and were given the opportunity to participate. Interested parties were required to attend 1 of 4 surgeon training sessions held in 2006–2008. At each session the manual of operating procedures was reviewed and items for data collection were discussed and revised based on group consensus. To establish standardization and uniformity among the group, all study-related forms were reviewed in detail and all questions were answered regarding how to appropriately complete them. Meniscal and articular cartilage arthroscopy videos were also reviewed, independently graded by each physician, and then discussed by the group to clarify any ambiguity in the classification of meniscal and articular cartilage injury. These videos were identical to the ones used in previous interobserver reliability studies evaluating meniscal and articular cartilage lesions.7,15
After the training sessions the surgeons were required to review the final manual of operating procedures, obtain institutional review board approval, complete a trial surgeon form, and sign a surgeon’s agreement to follow the manual of operating procedures before beginning patient enrollment. Since these training sessions, the MARS Study coordinators (L.J.H. and A.K.H.) have been available to address individual questions and concerns from surgeons and research assistants by e-mail and phone. Patient enrollment began May 1, 2006. The current study includes enrollment through March 31, 2009.
Participants
Inclusion criteria for patients enrolled in the study include all patients with ACL deficiency evaluated at the clinic between the ages of 12 and 65 years who are scheduled to have a revision ACL reconstruction by a participating (MARS study group) surgeon. All participants must have undergone an ACL reconstruction in the past, and are currently identified as having experienced failure of their ACL reconstruction, as defined by the surgeon by either MRI, knee laxity (>5 mm side-to-side difference on arthrometer testing), a positive pivot shift or Lachman test, functional instability, and/or by arthroscopic confirmation. Patients with concomitant injuries to the medial and lateral collateral ligaments, posterior cruciate ligament, or posterolateral complex are also included. Exclusion criteria for patients for the study were patients with graft failure secondary to prior intra-articular infection, arthrofibrosis, or complex regional pain syndrome. Patients unwilling or unable to complete their repeat questionnaire 2 years after their initial visit are also excluded. Surgeon enrollment logs demonstrate that 75% of eligible patients agreed to participate.
Treatment
Surgical reconstruction technique is left to the discretion of the operating surgeon. Because patients have already sustained failure of one or more ACL grafts, the surgeon is often forced to use a graft that is not his or her usual first choice. The following graft types are the only ones accepted for inclusion: (1) any autograft, including contralateral autografts; and (2) nonirradiated, fresh-frozen allografts from a single donor source (Musculoskeletal Transplant Foundation, Edison, New Jersey), including bone-patellar tendon-bone, tibialis anterior/posterior, Achilles tendon, semitendinosus, or gracilis. Required radiographs for the study include bilateral standing AP and a full-extension lateral. Recommended radiographs include bilateral 45° bent knee weightbearing Rosenberg view, patellofemoral view, and standing alignment (hip, knee, ankle).
Data Collection
After giving informed consent, subjects are provided a self-administered, 13-page patient questionnaire containing the validated outcome instruments of the Short Form-36 (version 2), Western Ontario and McMaster Osteoarthritis Index, Knee injury Osteoarthritis Outcome Score, International Knee Documentation Committee subjective form, and Marx activity scale. The surgeon questionnaire is completed at the time of surgery and includes sections on history of knee injury and/or surgery on both knees, the results of the general knee examination done under anesthesia, recording of all previous and new intra-articular injuries and treatments to the meniscus and articular cartilage, and the surgical technique used for the revision ACL reconstruction. Classification of the general knee examination findings follows the recommendations of the updated 1999 International Knee Documentation Committee guidelines.12,13 Surgeon documentation of articular cartilage injury is recorded on the modified Outerbridge classification4 and is based on an interobserver agreement study.15 Meniscal injuries are classified by size, location, and partial versus complete tears. Treatment of meniscal tears are recorded as none, repair, or extent of resection. Both of these classifications are based on a previous interrater agreement study.7 Rehabilitation issues are recorded including the use of postoperative and functional bracing, timing of initiating weightbearing, passive motion, and active motion.
Completed data forms are mailed from the participating sites to the central data collection site (Vanderbilt University). Data from both the patient and surgeon questionnaires are scanned with TeleForm software (Cardiff Software, Inc, Vista, California) that uses optical character recognition to avoid manual data entry, and the returned data are verified and then exported to a database.10,19 Both the patient and the surgeon questionnaire have a matched, barcoded identification number on each page to deidentify the data and to aid in database merging.
Statistical Design
Statistical power necessary to complete the MARS study could be used to calculate the sample size and duration of the proposed study, but power calculations have limitations in that they not only assume the magnitude of a single effect but also assign this single variable overriding importance. Because this study will rely on multivariable analysis to determine predictors of worse outcome, we elected to use sample size estimates based on estimating the number of variables and allowing a ratio of 10:1 for subjects to variables.11 Hence, sample size estimates are based on model complexity where m is the effective sample and m/10 variables (predictors plus nonlinearities and interactions) are possible in the model. A total of 900 to 1000 patients will be enrolled to ensure adequate power with expected 80% follow-up at 2 years.
RESULTS
As of April 1, 2009, 87 surgeons had enrolled 460 patients across 52 sites in 28 states and 2 Canadian provinces. Enrollment began July 1, 2006. Academic sites represented 54% of the total versus 46% private practice. Twenty-eight percent were performed by the surgeon who had performed the primary ACL reconstruction. Median age for the cohort is 26 years (range, 12-63 years) and there are 57% male patients. Age at the time of revision differed by sex. Most commonly, female patients underwent revision in the second decade of life and men most commonly underwent revision in the third decade (Figure 1). The most common races reported were white (83%) and black or African American (5%) (Table 1). The educational level completed ranged from sixth grade through 20 years of education (Table 2). Most adults had completed high school. The majority of the cohort reported that their injury was in a noncontact setting (71%; 31% of the total cohort reported that they were jumping at the time of injury, and 40% were cutting or changing direction). Eighty-three percent reported hearing a “pop” at the time of injury; 76% of the cohort reported that they were injured while playing a sport. Of this subgroup, subjects reinjured their ACL most commonly playing either soccer or basketball (Table 3).
Figure 1.
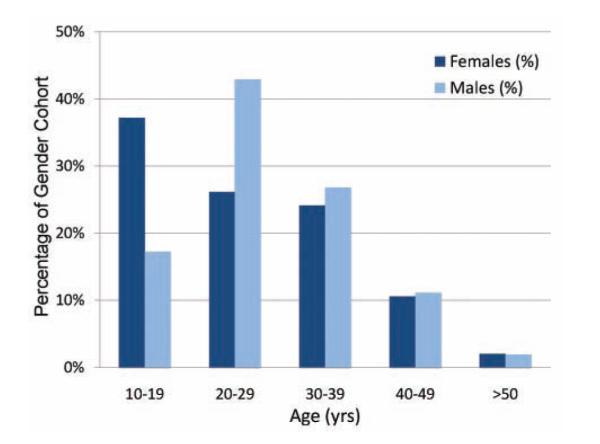
Age at time of revision (age range, 12-65 years).
TABLE 1.
Number of Subjects Enrolled, Stratified by Gender and Race
| Racial Categories | Females | Males | Total |
|---|---|---|---|
| American Indian/Alaska Native | 1 | 0 | 1 |
| Asian | 1 | 8 | 9 |
| Native Hawaiian or other Pacific Islander |
2 | 2 | 4 |
| Black or African American | 11 | 13 | 24 |
| White | 164 | 217 | 381 |
| Other | 6 | 7 | 13 |
| More than one race | 5 | 6 | 11 |
| Unknown or not reported | 9 | 8 | 17 |
| Racial categories: Total of all subjects | 199 | 261 | 460 |
TABLE 2.
Completed Education Level, Stratified by Gender
| Gender |
|||
|---|---|---|---|
| Years of School Completeda | Females | Males | Total |
| 6th grade | 1 | 1 | 2 |
| 7th grade | 1 | 0 | 1 |
| 8th grade | 3 | 3 | 6 |
| 9th grade | 9 | 2 | 11 |
| 10th grade | 9 | 8 | 17 |
| 11th grade | 16 | 12 | 28 |
| 12th grade | 25 | 48 | 73 |
| 13 | 24 | 26 | 50 |
| 14 | 21 | 29 | 50 |
| 15 | 8 | 17 | 25 |
| 16 | 42 | 64 | 106 |
| 17 | 11 | 17 | 28 |
| 18 | 15 | 20 | 35 |
| 19 | 2 | 3 | 5 |
| 20 | 11 | 8 | 19 |
| Unknown or not reported | 1 | 3 | 4 |
| Total | 199 | 261 | 460 |
For example, 12 = high school senior; 16 = college graduate.
TABLE 3.
Activity at the Time of Reinjurya
| Activity | No. (%) |
|---|---|
| Nonsport (ie, ADL) | 112 (24) |
| Sport | 348 (79) |
| Soccer | 83 (18%) |
| Basketball | 74 (16%) |
| Football | 56 (12%) |
| Other | 58 (13%) |
| Skiing | 39 (8%) |
| Baseball/softball | 14 (3%) |
| Volleyball | 13 (3%) |
| Gymnastics | 7 (2%) |
| Blank/not reported | 4 (<1%) |
| Total | 460 (100) |
“Other” includes sports such as wrestling, tennis, hockey, track and field, biking, cheerleading, rugby, lacrosse, racquetball, frisbee, dancing, martial arts, roller skating, and trampolining. ADL, activities of daily living.
This was the first revision for 89% of the patients, second for 9%, third for 2%, fourth for less than 1%, and not recorded for less than 1%. The time from last reconstruction was less than 1 year in 15% of patients, between 1 and 2 years for 22%, greater than 2 years for 62%, and unknown for less than 1%. Mode of failure as deemed by the revising surgeon was traumatic for 32% (148); technical, 24%; biologic, 7%; combination, 37% (surgeons marked all that applied); infection, less than 1%, and no response, less than 1% (Figure 2). Biologic failure is not well defined in the literature despite its use in previous studies. By consensus at the surgeon training sessions, biologic failure was defined as lack of incorporation of the graft as evidenced by early failure without a significant traumatic episode or obvious significant technical problems with the previous reconstruction.
Figure 2.
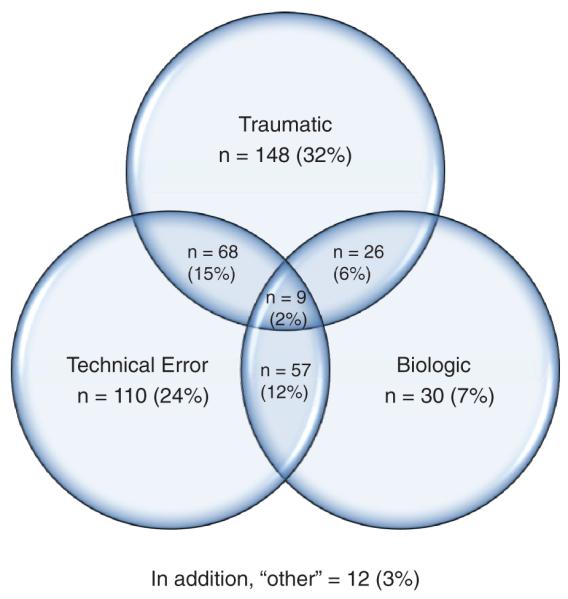
Mode of failure.
The type of technical failure was determined at the time of surgery by the surgeon using all available evidence (history, physical examination, radiographs, and arthroscopic evaluation). Surgeons were allowed to indicate more than one type of technical error. Femoral tunnel malposition was rated as the most common technical failure by far (80%), followed by tibial tunnel malposition (37%) (Table 4).
TABLE 4.
Technical Cause of Failurea
| Cause | No. (%) |
|---|---|
| Femoral tunnel malposition | 223 (80) |
| Tibial tunnel malposition | 104 (37) |
| Malalignment | 12 (4) |
| Femoral fixation | 17 (6) |
| Tibial fixation | 3 (1) |
| Autograft source | 4 (1) |
| Allograft source | 20 (7) |
| Posteromedial laxity | 6 (2) |
| Posterolateral laxity | 4 (1) |
| Other | 11 (4) |
Note the denominator is >100% because of the multiple choice option of this question (surgeons were instructed to “check all that apply”).
Graft source for the prior reconstruction was 70% autograft, 27% allograft, 2% both allograft and autograft, and 1% unknown (Table 5). Graft choice for the current revision ACL reconstruction was 45% autograft, 54% allograft, less than 1% combination, and less than 1% no response (Table 6). The single most common graft was an allograft bone-patellar tendon-bone (27%); autograft and allograft bone-patellar tendon-bone together represented 49% of all grafts chosen for revision reconstruction in this series. Prior approach was arthroscopic single incision in 81%, arthroscopic rear entry 2-incision in 16%, traditional arthrotomy in 2%, miniarthrotomy in less than 1%, and less than 1% were not recorded. Current surgical exposure and technique is arthroscopic single-incision transtibial drilling, 48%; arthroscopic single-incision anteromedial portal drilling, 35%; arthroscopic rear entry 2-incision, 15%; traditional arthrotomy, less than 1%; miniarthrotomy, less than 1%; and not recorded, less than 1%. Bone grafting of dilated tunnels was performed at the time of the revision in 3% of patients for the tibia and 3% of patients for the femur. It was performed as a staged procedure before revision reconstruction in 9% of patients for the tibia and in 8% of patients for the femur.
TABLE 5.
Prior Graft Source
| Source | Autograft, No. (%) |
Allograft, No. (%) |
|---|---|---|
| Bone-patellar tendon-bone | 205 (64) | 55 (44) |
| Quadriceps tendon bone | 3 (<1) | |
| Hamstring (semitendinosus [ST]) | 15 (5) | 1 (<1) |
| Hamstring (ST + gracilis) | 93 (29) | 6 (5) |
| Iliotibial band | 1 (<1) | |
| Achilles tendon | 14 (11) | |
| Tibialis anterior | 24 (19) | |
| Tibialis posterior | 4 (3) | |
| Other/unknown | 3 (1) | 21 (17) |
| Blank | 1 (<1) | 1 (<1) |
| Total | 321 (70) | 126 (27) |
TABLE 6.
Current Graft Source (Autografts and Allografts Only)
| Source | Autograft, No. (%) |
Allograft, No. (%) |
|---|---|---|
| Bone-patellar tendon-bone | 102 (49) | 123 (50) |
| Quadriceps tendon bone | 11 (5) | 2 (<1) |
| Hamstring (semitendinosus [ST]) | 11 (5) | 5 (2) |
| Hamstring (ST + gracilis) | 84 (40) | 1 (<1) |
| Iliotibial band | ||
| Achilles tendon | 30 (12) | |
| Tibialis anterior | 57 (23) | |
| Tibialis posterior | 28 (11) | |
| Other / unknown | 1 (<1) | |
| Blank | 1 (<1) | |
| Total | 209 (45) | 247 (54) |
Concomitant knee injury (meniscal and chondral) was common in this cohort (Tables 7 through 9). Current or previously treated meniscal injury was noted in 74% of patients. Articular cartilage damage grade 2 or worse using the modified Outerbridge classification system was noted in 73%. Both meniscal and articular cartilage damage was seen in 57%. Only 10% of the cohort had neither meniscal nor articular cartilage damage. Baseline time zero patient-based outcome measures were recorded (Table 10).
TABLE 7.
Meniscal and Articular Cartilage (AC) Condition
| AC Injury |
|||
|---|---|---|---|
| Meniscal Injury | Normal | Abnormal | Total |
| Normal | 44 (10%) | 74 (16%) | 118 (26%) |
| Abnormal | 79 (17%) | 263 (57%) | 342 (74%) |
| Total | 123 (27%) | 337 (73%) | 460 (100%) |
TABLE 10.
Baseline Outcome Instrument Scores
| Validated Outcome Instrument | Scale | Baseline (T0) |
|---|---|---|
| Marx activity level | 0–16 | 11 (4,16) |
| IKDC | 0–100 | 46 (34,57) |
| KOOS | 0–100 | |
| Symptoms | 68 (54,82) | |
| Pain | 75 (61,86) | |
| ADL | 87 (71,96) | |
| Sports/recreation | 45 (25,65) | |
| Knee-related quality of life | 31 (19,44) | |
| WOMAC | 0–100 | |
| Stiffness | 75 (50,88) | |
| Pain | 85 (70,95) | |
| ADL | 87 (71,96) |
Median (25%, 75% quartile). IKDC, International Knee Documentation Committee “subjective” form. KOOS, Knee injury Osteoarthritis Outcome Score; WOMAC, Western Ontario and McMaster Osteoarthritis Index; ADL, activities of daily living.
DISCUSSION
The primary purposes of this report were to describe the development of a large specialty society, multicenter study and to document the feasibility of collecting data rapidly on large numbers of relatively low-volume surgical cases using this strategy. Using this approach, MARS has been able to enroll over 400 ACL revision patients in the space of 2 years. This represents the largest multisurgeon, multicenter group assembled to investigate revision ACL reconstruction outcomes, and the cohort has reported here the largest series of revision patients reported in the literature. The strength of MARS lies in its society-wide, multicenter cohort design, the collective expertise of the group, and the establishment of the infrastructure. The study’s novelty is drawn from the unique combination of large numbers of both academic and private practice physician groups. Thus, the results become generalizable. The multisurgeon, multicenter sampling scheme has allowed rapid accumulation of a high number of patients that could not otherwise be achieved given the relative infrequency of revision ACL reconstructions in most surgeons’ practice. The prospective nature of the study ensures consistency in data collection, and the clinical study design uses multivariable analysis to determine the most important predictors of prognosis. Finally, the support of AOSSM ensures the ability of the findings to be widely and rapidly adopted by the sports medicine community.
A secondary purpose of this report was to provide representative demographic and clinical data from the cohort of patients enrolled in the initial 2 years of the study, as has been done for other large multicenter orthopaedic studies.31 These data will form the basis of our predictive analysis of patient outcomes. A prospective longitudinal cohort is the study design most useful for determining modifiable predictors of outcome. The Framingham study is the classic example of this type of study design to establish both high-level evidence on prognosis for outcome to counsel patients, and, more importantly, to discover independent predictors (risk factors) of these outcomes.5 Identifying these predictors will allow us to further investigate targeted subpopulations with poor outcome in future interventional trials. Similar to the Framingham study, our cohort is designed to collect enough subjects followed over time to allow multivariable analyses of the factors affecting outcome. Identification of these modifiable predictors will allow improved counseling of surgeons and patients and allow the focus of research resources toward improvement of revision ACL reconstruction outcomes.
The most common mode of failure was determined to be traumatic reinjury in this early report of the cohort. A combination of factors (technical, traumatic, and/or biologic) was overall believed to be the most common reason for failure. This is in contradistinction to some previous studies that have determined technical considerations to be the most common cause of failure of ACL reconstruction. In fact, previous studies have proposed that more than 50% of the failures were due to technical considerations.2,6,14,27 Carson et al2 in a review of 90 revision ACL reconstructions noted that 47 of 90 were due to technical considerations and 22 of 90 were traumatic reinjuries. Our numbers, while similar to those of Salmon et al,21 may differ from previous studies because of the inclusion of a “combination” category that likely decreases the numbers of both traumatic reinjuries and technical failures. Noyes and Barber-Westin16 demonstrated that multiple factors contributed to ACL graft rupture in 15 of 32 of their patient’s previous ACL reconstructions. This combination category in our cohort constitutes 170 of 460 (37%), which was larger than the technical failure group.
Most studies that have examined potential causes of ACL reconstruction failure did not delineate the specific type of technical failure and only list technical failure as a broad category. The preliminary findings from the present study are in general agreement with studies that did assess specific technical errors in showing the high prevalence of femoral tunnel malposition in failed ACL reconstructions. Garofalo et al9 demonstrated a 79% femoral tunnel and 21% tibial tunnel malposition in their revision ACL reconstruction series. Only 6 of 28 (21%) demonstrated appropriate tunnel position for both the femur and tibia. Taggart et al,26 in a series of revision ACL reconstructions, noted 12 of 20 (60%) with any femoral tunnel malposition. The findings in these 2 studies demonstrate the frequency of femoral tunnel malposition as a cause of failure, but unfortunately, as in most revision studies, the nature of the malposition (anterior vs vertical) was not delineated. Our study asks surgeons to determine if the femoral tunnel is too vertical or too anterior, and if the tibial tunnel is too anterior, posterior, medial, or lateral.
The best graft choice for revision ACL reconstruction continues to be an area of debate. Previous studies have demonstrated use of a variety of grafts in the revision ACL reconstruction setting.16,17,21-23 These included contralateral and ipsilateral hamstring, patellar tendon and quadriceps tendon autografts, and a variety of allografts. Graft choice is predominantly influenced by 2 factors: previous graft(s) used and surgeon preference. But it is also affected by a variety of other factors, including patient preference, tunnel dilatation, and contralateral knee status. Thus, comparison of our graft choice results with those of previous studies may be irrelevant because of the preponderance of small 1- or 2-surgeon case series in the literature. However, a few multisurgeon revision ACL reconstruction series do exist. Battaglia et al1 recently reported a 7-surgeon series. Graft choice included 43 of 63 (68%) autografts (19 ipsilateral bone patellar tendon bone, 10 ipsilateral hamstring, 3 ipsilateral quadriceps, 1 ipsilateral bone-patellar tendon-bone reharvest, and 10 contralateral bone-patellar tendon-bone grafts) and 20 of 63 (32%) allografts (type indeterminate).
We believe our results, given the much larger surgeon sample size, are more readily generalizable to the United States sports orthopaedic surgeon’s practice. The results in this study demonstrated a predilection for allograft (54%), especially bone-patellar tendon-bone grafts. Currently, graft source in general has not been determined to be a source for the worse outcomes in revision reconstructions compared with primary reconstruction. Given the large number of subjects and the near 50/50 split of autograft and allograft, we will be able to use multivariable analysis to determine if graft source is a predictor for outcome.
Limitations include the descriptive nature of this study that require further follow-up at 2 years after surgery to assess these factors as predictors for outcome of ACL revision reconstruction. An additional weakness is the potential lack of agreement between surgeons regarding contributions to failure. This was minimized as much as possible by the required training of all participating surgeons. To further address this potential weakness, intraobserver and interobserver studies are being designed and implemented for the MARS Group. Previous case series of revision reconstructions also suffer from this by the lack of defined standards of cause of failure. We are surprised by the small number of minorities represented in our cohort (Table 1). Based on the widespread geography, practice settings, and insurance plans (including Medicaid) represented by our 52 sites, we believe this may represent the actual racial distribution of revision ACL reconstruction currently performed in North America.
Potential limitations for MARS include the feasibility of coordinating 87 investigators and obtaining greater than 80% patient 2-year follow-up. However, based on our first year, we have been able to promote a collaborative atmosphere among the participating surgeons, have assisted the sites in obtaining individual institutional review board approvals, and have been successful in subsequent subject enrollment.
We believe this first study by the MARS group demonstrates the ability of the research consortium to rapidly collect a large series of revision ACL reconstruction cases. In addition, descriptive analysis provides the background for future studies and comparison with other cohorts. The overarching goal of this group is to identify modifiable predictors of revision ACL reconstruction outcomes to improve future outcomes, counsel patients on prognosis, educate surgeons within an entire society on evidence-based medicine and clinical research, and potentially perform randomized clinical trials on the most influential predictors. Variables to evaluate include, but are not limited to, those described in the Appendices (see online Appendix 1-3 for this article at http://ajs.sagepub.com/supplemental/). We await 2-year follow-up of this cohort to allow analysis of predictors of outcome.
Supplementary Material
TABLE 8.
Medial Versus Lateral Meniscal Tears
| Current Tears | Partial Tear | Complete Tear | Total |
|---|---|---|---|
| Medial | 53 (12%) | 129 (28%) | 182 (40%) |
| Lateral | 66 (14%) | 94 (20%) | 160 (35%) |
| Total | 119 (26%) | 223 (48%) | 342 (74%) |
TABLE 9.
Location and Frequency of Articular Cartilage Injurya
| Location | No. (%) |
|---|---|
| Medial femoral condyle | 204 (44) |
| Lateral femoral condyle | 131 (28) |
| Medial tibial plateau | 53 (12) |
| Lateral tibial plateau | 86 (19) |
| Patella | 158 (34) |
| Trochlea | 96 (21) |
Grade 2 or higher, seen at the time of revision surgery.
Acknowledgments
This study was supported by research grants from the Musculoskeletal Transplant Foundation, AOSSM, and grant No. 5 K23 AR052392-04 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
One or more authors has declared a potential conflict of interest
CONTRIBUTING AUTHORS Rick W. Wright, MD; Laura J. Huston, MS; Kurt P. Spindler, MD; Warren R. Dunn, MD, MPH; Amanda K. Haas, MA; Christina R. Allen, MD; Daniel E. Cooper, MD; Thomas M. DeBerardino, MD; Brett (Brick) A. Lantz, MD; Barton J. Mann, PhD; Michael J. Stuart, MD; John P. Albright, MD; Annunziato (Ned) Amendola, MD; Allen F. Anderson, MD; Jack T. Andrish, MD; Christopher C. Annunziata, MD; Robert A. Arciero, MD; Bernard R. Bach Jr, MD; Champ L. Baker III, MD; Arthur R. Bartolozzi, MD; Keith M. Baumgarten, MD; Jeffery R. Bechler, MD; Jeffrey H. Berg, MD; Geoffrey A. Bernas, MD; Stephen F. Brockmeier, MD; Robert H. Brophy, MD; Charles A. Bush-Joseph, MD; J. Brad Butler V, MD; John D. Campbell, MD; James L. Carey, MD, MPH; James E. Carpenter, MD; Brian J. Cole, MD; Jonathan M. Cooper, MD; Charles L. Cox, MD; R. Alexander Creighton, MD; Diane L. Dahm, MD; Tal S. David, MD; David C. Flanigan, MD; Robert W. Frederick, MD; Charles J. Gatt Jr, MD; Steven R. Gecha, MD; James Robert Giffin, MD; Donald B. Goodfellow, MD; Sharon L. Hame, MD; Jo A. Hannafin, MD, PhD; Christopher D. Harner, MD; Norman Lindsay Harris Jr, MD; Keith S. Hechtman, MD; Elliott B. Hershman, MD; Rudolf G. Hoellrich, MD; Timothy M. Hosea, MD; David C. Johnson, MD; Timothy S. Johnson, MD; Morgan H. Jones, MD; Christopher C. Kaeding, MD; Thomas E. Klootwyk, MD; Bruce A. Levy, MD; C. Benjamin Ma, MD; G. Peter Maiers II, MD; Robert G. Marx, MD; Matthew J. Matava, MD; Gregory M. Mathien, MD; David R. McAllister, MD; Eric C. McCarty, MD; Robert G. McCormack, MD; Bruce S. Miller, MD, MS; Ali R. Motamedi, MD; Carl W. Nissen, MD; Daniel F. O’Neill, MD, EdD; Richard D. Parker, MD; Mark L. Purnell, MD; Arun J. Ramappa, MD; Michael A. Rauh, MD; Arthur Rettig, MD; Jon K. Sekiya, MD; Kevin G. Shea, MD; Orrin H. Sherman, MD; James R. Slauterbeck, MD; Matthew V. Smith, MD; LTC Steven J. Svoboda, MD; Timothy N. Taft, MD; COL Joachim J. Tenuta, MD; Edwin M. Tingstad, MD; Armando F. Vidal, MD; Darius G. Viskontas, MD; Richard A. White, MD; James S. Williams Jr, MD; Michelle L. Wolcott, MD; Brian R. Wolf, MD; James J. York, MD.
REFERENCES
- 1.Battaglia MJ, 2nd, Cordasco FA, Hannafin JA, et al. Results of revision anterior cruciate ligament surgery. Am J Sports Med. 2007;35(12):2057–2066. doi: 10.1177/0363546507307391. [DOI] [PubMed] [Google Scholar]
- 2.Carson EW, Anisko EM, Restrepo C, Panariello RA, O’Brien SJ, Warren RF. Revision anterior cruciate ligament reconstruction: etiology of failures and clinical results. J Knee Surg. 2004;17(3):127–132. doi: 10.1055/s-0030-1248210. [DOI] [PubMed] [Google Scholar]
- 3.Cummins J, Lurie JD, Tosteson T, et al. Descriptive epidemiology and prior healthcare utilization of patients in the Spine Patient Outcomes Research Trial’s (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine. 2006;31(7):806–814. doi: 10.1097/01.brs.0000207473.09030.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 5.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36(5):851–860. doi: 10.1177/0363546507312381. [DOI] [PubMed] [Google Scholar]
- 7.Dunn WR, Wolf BR, Amendola A, et al. Multirater agreement of arthroscopic meniscal lesions. Am J Sports Med. 2004;32(8):1937–1940. doi: 10.1177/0363546504264586. [DOI] [PubMed] [Google Scholar]
- 8.Freedman KB, D’Amato MJ, Nedeff DD, Kaz A, Bach BR., Jr Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31(1):2–11. doi: 10.1177/03635465030310011501. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo R, Djahangiri A, Siegrist O. Revision anterior cruciate ligament reconstruction with quadriceps tendon-patellar bone autograft. Arthroscopy. 2006;22(2):205–214. doi: 10.1016/j.arthro.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Hardin JM, Woodby LL, Crawford MA, Windsor RA, Miller TM. Data collection in a multisite project: Teleform. Public Health Nurs. 2005;22(4):366–370. doi: 10.1111/j.0737-1209.2005.220410.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE., Jr . Regression Modeling Strategies. Springer; New York: 2001. [Google Scholar]
- 12.Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med. 2001;29(5):600–613. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 13.Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):107–114. doi: 10.1007/s001670050082. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DL, Swenson TM, Irrgang JJ, Fu FH, Harner CD. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop Relat Res. 1996;325:100–109. doi: 10.1097/00003086-199604000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Marx RG, Connor J, Lyman S, et al. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med. 2005;33(11):1654–1657. doi: 10.1177/0363546505275129. [DOI] [PubMed] [Google Scholar]
- 16.Noyes FR, Barber-Westin SD. Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon-patellar bone autograft. Am J Sports Med. 2006;34(4):553–564. doi: 10.1177/0363546505281812. [DOI] [PubMed] [Google Scholar]
- 17.Noyes FR, Barber-Westin SD. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. J Bone Joint Surg Am. 2001;83(8):1131–1143. doi: 10.2106/00004623-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Pinczewski LA, Deehan DJ, Salmon LJ, Russell VJ, Clingeleffer A. A five-year comparison of patellar tendon versus four-strand hamstring tendon autograft for arthroscopic reconstruction of the anterior cruciate ligament. Am J Sports Med. 2002;30(4):523–536. doi: 10.1177/03635465020300041201. [DOI] [PubMed] [Google Scholar]
- 19.Quan KH, Vigano A, Fainsinger RL. Evaluation of a data collection tool (TELEform) for palliative care research. J Palliat Med. 2003;6(3):401–408. doi: 10.1089/109662103322144718. [DOI] [PubMed] [Google Scholar]
- 20.Rappe M, Horodyski M, Meister K, Indelicato PA. Nonirradiated versus irradiated Achilles allograft: in vivo failure comparison. Am J Sports Med. 2007;35(10):1653–1658. doi: 10.1177/0363546507302926. [DOI] [PubMed] [Google Scholar]
- 21.Salmon LJ, Pinczewski LA, Russell VJ, Refshauge K. Revision anterior cruciate ligament reconstruction with hamstring tendon autograft: 5- to 9-year follow-up. Am J Sports Med. 2006;34(10):1604–1614. doi: 10.1177/0363546506288015. [DOI] [PubMed] [Google Scholar]
- 22.Shelbourne KD, Thomas J. Contralateral patellar tendon and the Shelbourne experience: part 1: revision anterior cruciate ligament reconstruction and rehabilitation. Sports Med Arthrosc Rev. 2005;13(1):25–31. [Google Scholar]
- 23.Shelbourne KD, Thomas J. Contralateral patellar tendon and the Shelbourne experience: part 2: results of revision anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev. 2005;13(2):69–72. [Google Scholar]
- 24.Singhal MC, Gardiner JR, Johnson DL. Failure of primary anterior cruciate ligament surgery using anterior tibialis allograft. Arthroscopy. 2007;23(5):469–475. doi: 10.1016/j.arthro.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE., Jr Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986–1995. doi: 10.1177/0363546504271211. [DOI] [PubMed] [Google Scholar]
- 26.Taggart TF, Kumar A, Bickerstaff DR. Revision anterior cruciate ligament reconstruction: a midterm patient assessment. Knee. 2004;11(1):29–36. doi: 10.1016/S0968-0160(02)00087-X. [DOI] [PubMed] [Google Scholar]
- 27.Uribe JW, Hechtman KS, Zvijac JE, Tjin-A-Tsoi EW. Revision anterior cruciate ligament surgery: experience from Miami. Clin Orthop Relat Res. 1996;325:91–99. doi: 10.1097/00003086-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Westfall JM, Mold J, Fagnan L. Practice-based research: “Blue Highways” on the NIH roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 29.Wright RW, Dunn WR, Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007;35(7):1131–1134. doi: 10.1177/0363546507301318. [DOI] [PubMed] [Google Scholar]
- 30.Wright RW, Dunn WR, Amendola A, et al. Anterior cruciate ligament revision reconstruction: two-year results from the MOON cohort. J Knee Surg. 2007;20(4):308–311. doi: 10.1055/s-0030-1248066. [DOI] [PubMed] [Google Scholar]
- 31.Yunes M, Richmond JC, Engels EA, Pinczewski LA. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17(3):248–257. doi: 10.1053/jars.2001.21242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


