Abstract
There is ample evidence that shows an inverse relationship between consumption of fruit/vegetable-rich diets and the risk of cancer at various anatomical sites. In this review, we will assess and summarize recent advances on cancer prevention by resveratrol, a natural stilbenoid present in red grapes, peanuts, some common drinks, and dietary supplements. We will focus on data published within the past few years on in vivo model tumor animal studies that reinforce the chemopreventive efficacy of resveratrol against a multitude of cancers, as well as on its sensitization/ enhancing activities against tumor cells when used in combination with established chemotherapeutic and pharmaceutical agents. In addition, we will review examples resveratrol-target proteins, denoted RTPs, including the 24-kDa cytosolic protein quinone reductase 2 (NQO2) discovered in our laboratory that may confer resveratrol responsiveness to cancer cells. We will discuss the possible role of NQO2 in mediating cancer prevention by resveratrol. Our analysis of published data strengthen support that resveratrol displays novel roles in various cellular processes, and help to establish an expanded molecular framework for cancer prevention by resveratrol in vivo.
Keywords: resveratrol, resveratrol targeting protein, chemoprevention
1. Background
The purpose of this review is to consider new, recent studies that suggest the need to accommodate and enlarge the scope of interpretation of resveratrol, in the context of using diet-derived phytochemicals for cancer prevention under the broad rubric of insights revealed by the study of Doll and Peto [1]. The Doll/Peto report provides compelling evidence for the integral role nutrition and dietary factors play in forestalling cancer incidence and mortality rates, thereby raising the tantalizing possibility that choice and frequency of food groups consumed in an average diet, most notably, fruits and vegetables, can be a deterrent in preventing neoplastic diseases. This landmark observation has further spawned a rapidly advancing scientific field in the ensuing years known as chemoprevention; research in subsequent years has been focused on the identification of food-derived small molecules with chemopreventive attributes. This has led to the discovery of resveratrol as a grape phytochemical showing cancer preventive activities.
As a polyphenolic stilbene, resveratrol is abundantly present in red wine, grape skin, peanuts, and several other foods/drinks consumed in a typical American diet. Interest in resveratrol as a chemopreventive agent stems largely from the report by Pezzuto and coworkers [2] regarding its activities to inhibit initiation and promotion of hydrocarbon-induced skin cancer and progression of breast cancer in mice. Since then, a voluminous body of literature has been published on resveratrol, and several excellent reviews have been written on its numerous biological activities [3–8]. Some of the notable bioactivities of resveratrol include: anti-oxidant and anti-inflammatory activity; inducer of phase II enzymes; ability to interact with membranes and the mitochondria; inhibition of lipoxygenase; and thus, the synthesis of proinflammatory lipid peroxides and by-products. Resveratrol also suppresses malignant cell growth, induces cell cycle arrest, restores apoptosis, and down regulates the expression of cancer cell type specific genes. In addition, resveratrol also prevents the activation of procarcinogens and inhibits a myriad of molecular and cellular events in cancer cell types, which provide gain of function for the cells to survive and thrive in their primary site and at distant, metastatic sites. The source, chemical structure, and biological/biochemical effects of resveratrol are summarized in Fig. 1.
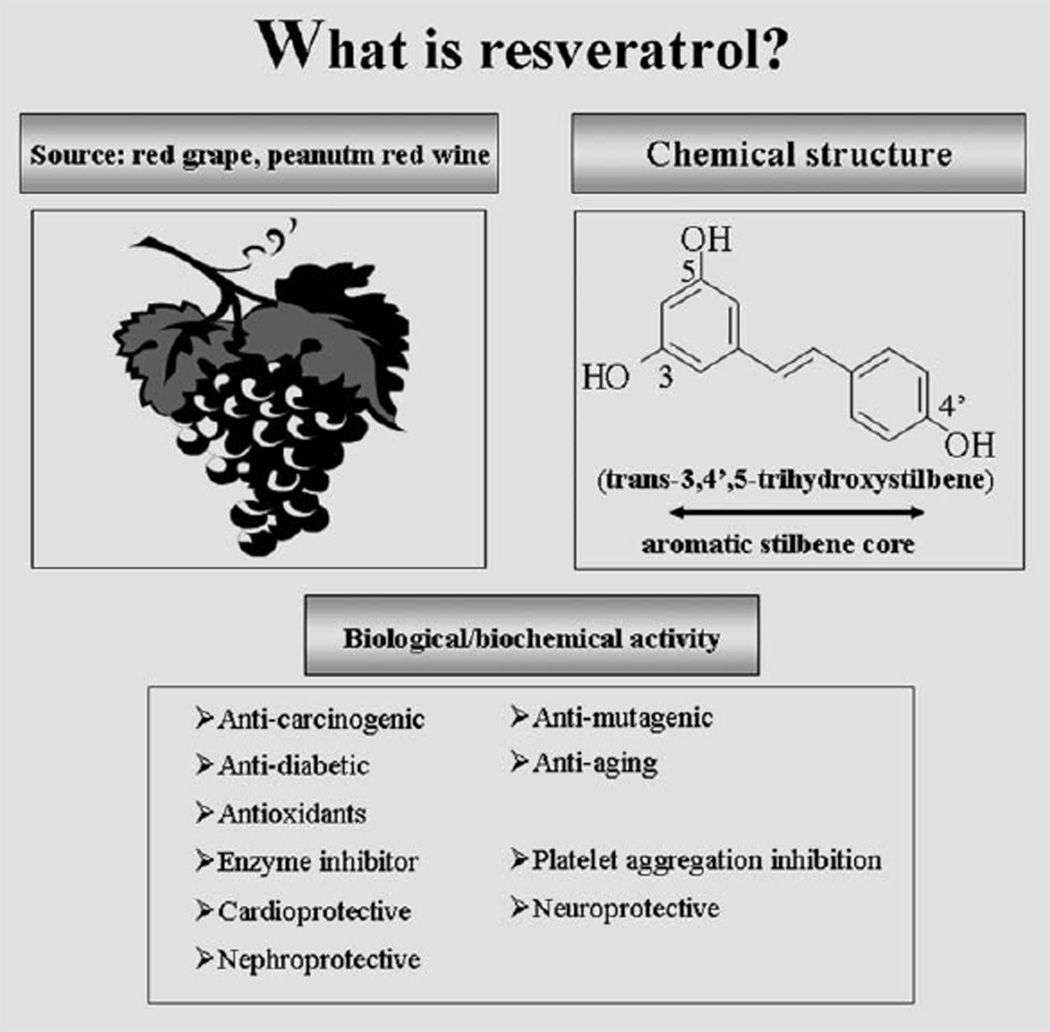
Fig. 1.
An overview showing the common source and chemical structure of resveratrol (trans-3,4′,5-trihydroxystilbene) and its multiple biological/ biochemical effects. The aromatic stilbene core structure and hydrophilic side groups of resveratrol support the hypothesis that its chemopreventive potential may in part lie in ability to interact with and modulate cellular target proteins, designated RTPs in resveratrol-responsive cells.
Interest on the cancer preventive and therapeutic effects of resveratrol has remained intense; published studies support the chemopreventive efficacy of resveratrol. Moreover, there is an abundance of publications documenting the activity of resveratrol in enhancing the sensitivity of tumor cells to commonly used chemotherapeutic and pharmaceutical agents. Developments in these two areas will be summarized in the first part of this review.
In addition to chemoprevention, resveratrol has shown multisystem beneficial biological activities including cardioprotection, neuroprotection, renal protection, and protection against inflammation, oxidative stress, aging, and diabetes. Unfortunately, use of resveratrol has been hampered by a number of unresolved issues, including low bioavailability [9].
To address the quandary of active bioefficacy of resveratrol coexisting with its poor bioavailability, we hypothesize that resveratrol exerts its effects and mechanisms in responsive biological systems by interacting and binding specific cellular targeting proteins, denoted resveratrol-target proteins (RTPs) that might function as its sensors and mediators [10–13]. We further surmise that qualitative and quantitative differences in RTPs might help explain in part the diverse dose-, organ-, and cell type-dependent effects of resveratrol. Using a resveratrol-immobilized affinity chromatography approach, we have discovered and identified quinone reductase 2 (NQO2) as an RTP that confers resveratrol responsiveness to many cancer cells at physiological concentrations [10,13]. Finally, we will present viewpoints regarding our current understanding of the signaling pathways that may mediate effects of NQO2 for cancer prevention. Our analysis of published data reinforces the notion that resveratrol displays novel roles in various cellular processes and helps to establish an expanded framework for understanding cancer prevention by resveratrol in vivo.
2. Advances supporting resveratrol as an anticancer molecule
2.1. In vivo studies reinforcing chemopreventive attributes of resveratrol
Animal studies offer a unique opportunity to assess the contribution of the physiological effects of consumption of resveratrol in different models of tumorigenesis. Most promising are the consistent findings showing that resveratrol administration inhibits carcinogenesis in prostate, breast, colon, skin, and various other solid tumor models [14–24]. Data for prostate and breast cancer as examples of malignant disorders most relevant to the interest of this laboratory are presented below.
2.1.1. Prostate cancer
Prompted by the seminal findings of Pezzuto and coworkers, we and others tested and reported that resveratrol suppressed proliferation in both androgen-dependent (AD) and androgen-independent prostate cancer (CaP) cells and also significantly inhibited the expression of prostate specific antigen, an established clinical biomarker for CaP [25–28]. These observations suggest that specific dietary constituents, for example, resveratrol, may have efficacy as a deterrent for CaP development.
The notion of CaP prevention by resveratrol is supported by animal studies. Harper and coworkers used the transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model developed in 1995 [29] to test whether administration of resveratrol incorporated into the feed might affect the development of orthotopic prostate tumors [18]. Because size of the prostate tumor in TRAMP mice may be a limiting factor in analysis, a the transgenic rat for adenocarcinoma of prostate (TRAP) rat model was recently developed [30]. Both TRAMP and TRAP rodent models use the minimal rat probasin promoter to drive the expression of SV40T antigen in the epithelium of the prostate, fostering the time-dependent development of orthotopic prostate tumors with AD features closely resembling pathogenesis of the human disease. Thus, in TRAMP mice, tumors are detected in the prostate by 10–12 weeks of age; animals subsequently develop premalignant changes resembling human prostatic intraepithelial neoplasia and poorly differentiated tumors, eventually resulting in the formation of prostatic adenocarcinomas that metastasize to distant sites, primarily the lymph nodes, bone, and lungs [29,31]. Feeding studies using both models showed that ingestion of resveratrol at a dose of 625 mg/kg diet for 28 weeks in the TRAMP model [18] or at a dose of 250 mg/kg diet for 30 weeks in the TRAP model [17] inhibited the progression of CaP. Similar results were obtained in TRAP when resveratrol was administered in the drinking water at doses ranging from 50 to 200 µg/mL for 7 weeks [32]. Studies using nude mice harboring LNCaP prostate cancer cells have also reported on the beneficial effects of feeding by resveratrol on CaP [28]. Taken together, these in vivo studies suggest that rodents fed resveratrol-fortified diets are suppressed and forestalled the progression of CaP.
2.1.2. Breast cancer
Evidence that resveratrol suppresses breast carcinogenesis has also been obtained using animal studies. Female nude mice orthotopically inoculated with 0.1 mg/kg once every 2 days 17β-estradiol-induced estrogen receptor-positive MCF-7 showed a 60% reduction in tumor formation when the animals were concurrently given resveratrol at 10 mg/kg every second day for a total of 45 days; in addition, the same treatment regimen with resveratrol resulted in a 15% reduction in orthotopic breast tumor growth assay in mice challenged with 0.1 mg/kg once every 2 days 17β-estradiol-induced estrogen receptor-negative MDA-MB-231 cells [33]. In female Sprague-Dawley rats challenged with 60 mg/kg DMBA (7,12-dimethylbenz(a)anthra-cene) at 50 days postpartum to induce mammary cancer, animals showed significantly lower tumor multiplicity rates and significantly longer tumor latency when they were fed 1 g/kg dietary resveratrol for the entirety of their life starting at birth compared with control rats not fed dietary resveratrol [34].
2.2. Resveratrol potentiates the efficacy of cancer therapeutic agents—In vitro and in vivo evidence
Radiation therapy and various forms of chemotherapy have been historically considered as the mainstay of treatment for individuals diagnosed with cancer. Both therapeutic modalities rely on reactive oxygen species (ROS) toxicity to eradicate tumor cells; as such, their efficacy is complicated by the need to maintain a proper balance between tolerance by the normal cells/tissues against development of resistance in many tumors. In recognition of the limitations of radiation therapy and chemotherapy, biotherapies with drastically different mechanism of efficacy have been introduced to the armamentarium of cancer therapies in recent years; nevertheless, development of variable intrinsic sensitivity and resistance of tumor cells are still lingering clinical concerns.
Discovery of adjuvants that potentiate sensitivity to biopharmaceuticals, ionizing radiation, or chemicals without invoking the untoward adverse effects represent a major challenge in the treatment of cancer patients. Increasingly, studies have shown that cancer chemopreventive phytochemicals may be used in combination with existing primary therapeutic modalities, and in the case of resveratrol, there is now a wealth of evidence showing that this grape-derived stilbenoid enhances the effects of chemotherapeutic drugs, for example, doxorubicin, death receptor ligand (TRAIL), and cisplatin in various tumor models. Importantly, sensitization by resveratrol to anticancer drugs is selective for tumor cells and does not affect normal cells.
2.2.1. Potentiation of effects of TRAIL by resveratrol
TRAIL is one of three extrinsic pathways of cell death; binding of different receptors by specific ligands elicit signaling cascades leading to the induction of apoptosis. Activation of TRAIL requires binding of ligands to cognate TRAIL receptors death receptors 4 and 5 resulting in rapid induction of apoptosis by a p53-independent mechanism selectively in human cancer cells without affecting normal cells [35,36]. Studies have shown that TRAIL treatment significantly suppresses growth of TRAIL-sensitive human cancer xenografts in mice, thereby offering the promise of specific and selective eradication of cancer cells. Unfortunately, cancer cell types do develop resistance to TRAIL, prompting searches for complementary agents, particularly those that lack the toxic side effects of chemotherapy or radiotherapy and yet contain attributes that may increase sensitivity of cells to TRAIL. Search for such candidates has revealed efficacy in dietary agents including resveratrol. Resveratrol reportedly enhances TRAIL-induced apoptosis in prostate [37,38], melanoma [39], and colon cancer cells [40]. Mechanisms proposed for the TRAIL-potentiating effects of resveratrol include inhibition of Akt phosphorylation [37], upregulation of TRAIL receptors [38,41], increased expression of proapoptotic proteins such as Bax, PUMA [38,41], and down regulation of antiapoptotic proteins [39].
2.2.2. Resveratrol sensitizes efficacy of chemotherapeutic agents
Chemotherapy is a primary treatment modality for individuals diagnosed with cancer; its efficacy is based on targeted killing of rapidly dividing cancer cells by interaction with their DNA, thereby impairing cell division and/or inducing apoptosis. At the time, when they were first introduced into clinical oncology after the Second World War, cytotoxic chemotherapeutic agents were used mainly in patients admitted into the clinic already diagnosed with advanced neoplastic disease, who rarely survived without highly cytotoxic therapy, and for whom concerns about later complications associated with the use of such aggressive treatments were a minor consideration. Today, with the advent of early diagnostic tools and advances in prevention, treatment landscape involving use of highly cytotoxic chemotherapeutic agents must weigh selectivity between cancer and normal cells in terms of risks and benefits. This consideration has provided impetus for the discovery of cancer therapies and adjuvants that selectively target neoplastic cells without affecting normal cells.
The observation by Doll and Peto [1] implicitly recognizes cancer as a chronic disease of the genome amenable to intervention at early stages in its natural history by nutritional factors. Therefore, by inference one might surmise that there are dietary factors that affect chemotherapeutic agents by enhancing their efficacy while at the same time lowering the threshold for toxicity. Recent studies have suggested that resveratrol can fulfill such a role. Thus, resveratrol has been shown to exert additive growth-inhibitory effect with cisplatin and doxorubicin in ovarian and uterine cancer cells [42]. At doses of 10–100 µM resveratrol induces reversal of doxorubicin resistance in acute myeloid leukemia cells by downregulating expression of multiple drug resistance protein, MRP1, a member of the ATP-binding cassette-related transporter functioning as drug efflux pumps to extricate structurally diverse lipophilic anions [43]. Resveratrol also reportedly attenuates the anticancer activity of paclitaxel in human breast cancer cells in vitro (40 µM) and in vivo (16.5 mg/kg, three times per week, intraperitoneal injection) [44]. In addition, resveratrol also enhances antitumor activity of gemcitabine in vitro and in an orthotopic mouse model of human pancreatic cancer [45].
2.2.3. Potentiation of ionizing radiation effects by resveratrol
Radiation has long been used as therapy for neoplastic diseases. Treatment using higher radiation doses can cause side effects during treatment, in the months or years after treatment, or after retreatment. Accordingly, a significant aim of modern radiotherapy is to reduce side effects to a minimum, and to help the patient to understand and better cope with the unavoidable side effects.
A limitation of radiotherapy in treating tumors is the prevalent establishment of a hypoxic state, which decreases the effectiveness of radiation by suppressing the formation of free radicals. Accordingly, much research has been devoted to overcoming this problem; approaches include use of naturally occurring compounds with established safety profiles and the identification of novel and potent radiation sensitizers. Of note, resveratrol has shown increased sensitivity to radiation in melanoma [46], glioblastoma [47], skin [48], and CaP cells [49].
3. Discovery and identification of RTPs as sensors and mediators of resveratrol
3.1. Shifting focus from resveratrol to discovery of resveratrol targeting proteins, RTPs
A lingering unresolved issue for considering use of resveratrol in chemoprevention is the seemingly irreconcilable disparity between high doses of resveratrol required for demonstrating bioactivity in laboratory settings, with low bioavailability of resveratrol shown in animal and human studies. Thus, 10–20 µM resveratrol is typically added to show bioactivity in tissue culture experiments; this amount is substantially more than peak levels of 1–2 µM “total” resveratrol appearing 30–60 min in plasma in oral feeding studies of resveratrol [50–53]. For instance, rodents fed resveratrol at increasing doses of up to 50 mg/kg show peak values of <10 µM resveratrol in plasma [54–56]. It is noteworthy that >70–90% administered resveratrol becomes bioavailable within minutes to hours, although typically as sulfated and glucuronidated forms, probably by enzymes present in the intestine and liver, and only trace unaltered resveratrol is detectable in the plasma [55,57]. From the chemopreventive viewpoint, these findings suggest that only low dose of resveratrol is likely to be delivered to the tissue of interest, raising doubt whether the cancer preventive activities of resveratrol can be attributed to this grape polyphenol per se.
The bioavailability-efficacy issue of resveratrol may be viewed from other perspectives. Studies have shown that the efficacious dose of resveratrol is dependent on cell type: in breast cancer cells submicromole resveratrol suffices to induce phosphatase and tensin homolog [58]; by comparison, in human B-cell lymphoma cells and lymphoid and myeloid cells ≤3–5 µM resveratrol suppresses cell growth by >50% [59,60], and in human Hep G2 cells, no effect of resveratrol is evident at all doses tested [61]. Conceivably, suboptimal dose of resveratrol given as part of a diet may interact synergistically with other dietary agents as to increase bioavailability. It is also possible that bioavailable resveratrol dosed as a single bolus to humans or animals, is different than when it is presented at a lower dose as part of a diet over an extended time period. The possibility of resveratrol binding to plasma proteins, membrane, and intracellular receptor targets, which could alter its biodelivery, availability, and storage, should also be considered. Whether the conjugated metabolites of resveratrol also contain antitumorigenic activity also warrant consideration and testing. Moreover, resveratrol may be classified as a “tag-and-flee” biophytochemical, acting by mechanisms similar to the bioactive alkaloid, monocrotaline which, presented as a single dose, has a half-life in aqueous medium of ~3 sec yet has been shown to predictably induce pulmonary hypertension 10–14 days postexposure [62]. Implicit in this “tag–flee” idea is that resveratrol interacts with cellular targets to “register”’ its presence with “implementation/execution” of its biological activity occurring later when it is no longer detectable in the system under study.
3.2. A resveratrol affinity approach identifies NQO2 as an RTP
A key component and test for the “tag–flee” hypothesis of resveratrol is the presence of RTPs. We advance this hypothesis by proposing that resveratrol acts as a cancer preventive agent, including prevention of CaP, through its ability to interact and bind RTPs [10,13,63]; binding may occur through its aromatic stilbenoid core and hydrophilic functional side groups. Studies have shown that aromatic centers are significant “sensor/detector” molecular recognition elements in biological systems: they confer stability on duplex DNA, contribute to unique properties of thermophilic proteins, and promote the formation/assembly of macromolecular complexes [64,65]. In other words, resveratrol can “sense” the presence of cellular RTPs by binding to their “functional pockets,” which are intrinsically present in RTPs or alternatively, are induced on binding of RTPs to resveratrol.
To isolate and identify RTPs, we designed an affinity approach by immobilizing resveratrol to epoxy-activated agarose, forming a biospecific ligand-directed proteomic platform that serves to retain, purify, and identify RTPs and to generate RTP profiles (Fig. 2). The resveratrol affinity approach separates proteins based on specificity and affinity for an immobilized ligand, independent of size, surface charge, and other physicochemical properties of proteins, and conceivably, could capture distinct targeting proteins having a more relevant or direct role in the chemopreventive attributes of resveratrol. In a typical fractionation scheme, nonspecifically bound proteins were first eluted by washing the column with lysis buffer. The column was next sequentially eluted with buffer containing 0.35 M and then 1.0 M NaCl to remove proteins with low affinity for resveratol. Buffer containing 1 mM adenosine-5′-triphosphate (ATP) was then applied to the columns as a more stringent condition to displace proteins with higher affinity for resveratrol. Finally, a 1–2-mM resveratrol solution was applied to quantitatively elute proteins avidly bound to the affinity matrix. Proteins displaced using different elution conditions were concentrated, separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by silver staining followed by visual inspection of the stained gels. A protein displaced from the affinity column only with the addition of resveratrol implying that it has tight and quantitative binding to resveratrol, was identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) and cloning as NQO2. Resveratrol binds tightly to NQO2 with an apparent Kd 50 ± 15 nM [66]. Resveratrol also potently inhibits NQO2 [66]. X-ray crystal analysis of NQO2 in complex with resveratrol shows that resveratrol binds NQO2 at a deep and narrow cleft located in close proximity and juxtaposition to cofactor flavin adenine dinucleotide (FAD) [66]. In CaP cells, the expression of NQO2 increases as CaP progresses from AD to a transition state and decreases when the hormone refractory stage of CaP is reached [10].
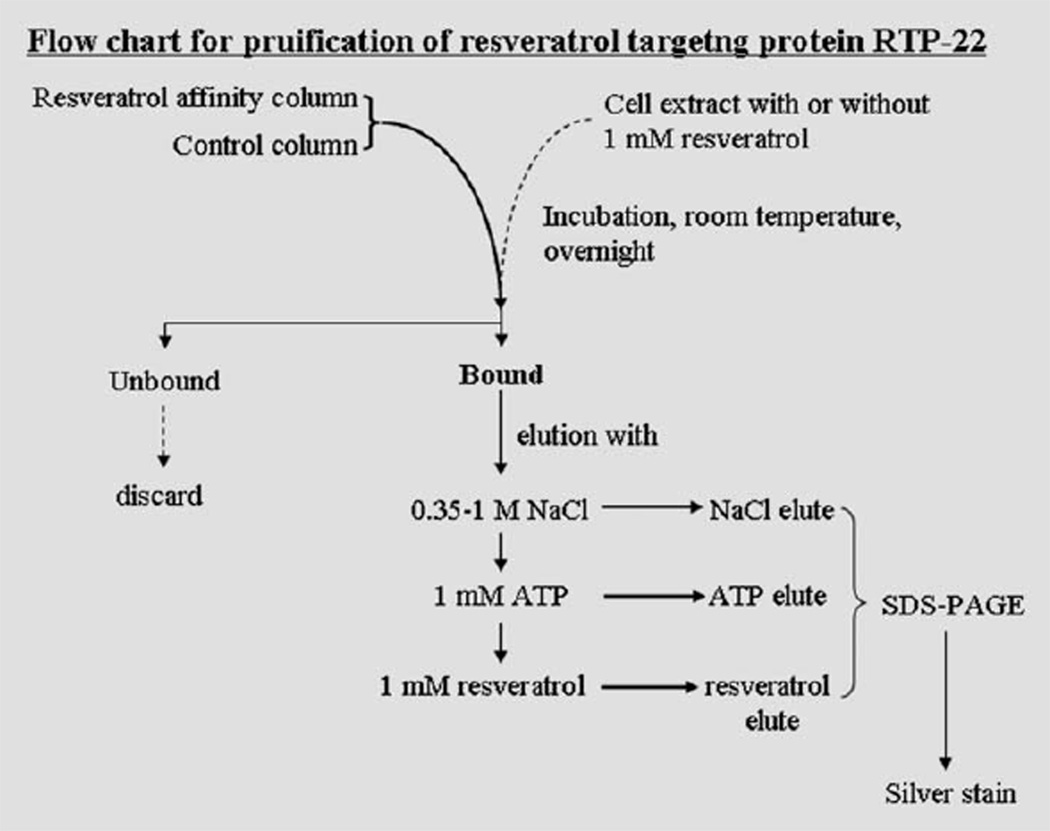
Fig. 2.
Flow chart for purification of resveratrol targeting protein from cultured mammalian cell extracts. It is hypothesized that ligand (resveratrol)-immobilized agarose columns could “capture” proteins with a high affinity for resveratrol. The column is sequentially eluted with varying ionic strength buffers, or with nucleotides such as ATP to displace proteins with modest affinity for resveratrol. Proteins binding most tightly to the affinity column, denoted RTPs, are eluted with resveratrol.
The identification of RTPs may be significant in helping us to understand cancer prevention including CaP by resveratrol: cell-type specific RTPs and quantitative and qualitative differences in their expression, coupled with differential uptake and metabolism of resveratrol may explain the large dose spread of resveratrol required for efficacy in vitro, and provide a rationale framework for the selective activity of resveratrol between normal and tumor epithelial CaP cells; the RTP profile in CaP cells may be significantly different from that generated from normal prostate stromal or epithelial cells. Conceivably, RTP identification may also obviate the need to resolve the issue of low bioavailability of resveratrol in the tissue/organ involved.
3.3. Other target proteins of resveratrol
Use of the resveratrol affinity column chromatography has resulted in identification of other RTPs, for example, glutathione sulfotransferase (GSTP1) and estrogen receptor ERβ [13]. Both NQO2 and GSTP1 are detoxification enzymes; therefore, it may be suggested that resveratrol exerts its antiCaP chemopreventive activities in part by interacting and binding detoxification enzymes, modulating their activity, stability, and signaling events integrally linked to CaP prevention by resveratrol. Thus, these findings have broadened the scope of our understanding of resveratrol as a chemopreventive agent for cancer in general, and CaP in particular. Other studies have independently demonstrated candidate proteins displaying strong affinity for resveratrol. Thus, 1 µM resveratrol effectively inhibited 12-o-tetradecanoylphorbol-13-acetate (TPA)-induced IκB kinase activity, thereby reducing phosphorylation and proteasome-mediated degradation of IκB and suppressing the activation and nuclear translocation of nuclear factor NFκB [67]. Resveratrol affinity chromatography revealed direct interaction between resveratrol and recombinant cyclo-oxygenase 2 (COX-2) with a Kd of 58 µM [68]. Figure 3 shows identity of RTPs discovered to date and their proposed functions.
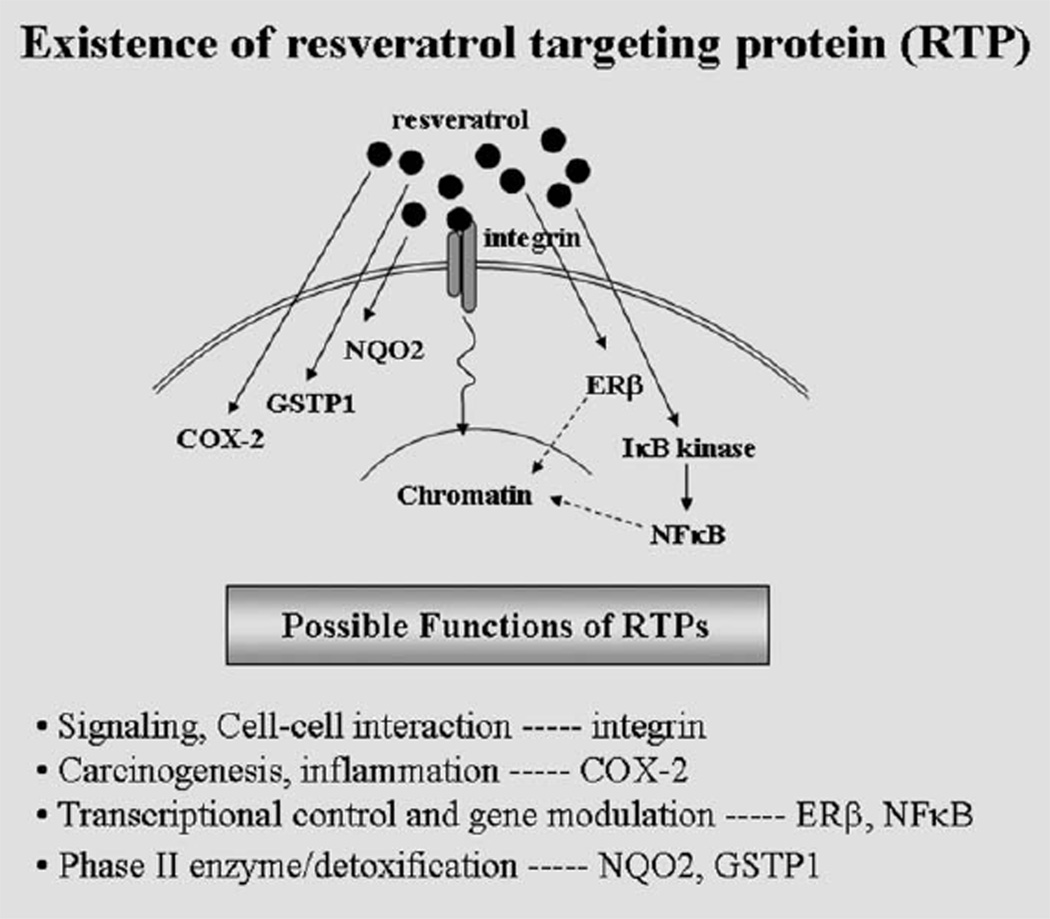
Fig. 3.
Identity and proposed functions of RTPs, spanning from membrane, cell–cell interaction signaling events, for example, integrins, to targets involved in carcinogenesis/inflammation, for example, COX-2, to transcription control and gene modulation, for example, ERβ and NFκB, to phase II detoxification enzymes, for example, NQO2 and GSTP1.
4. Multiplicity of function and mechanism of NQO2 in cancer prevention
Because NQO2 displays exquisite high affinity for resveratrol (Kd < 50 nM), it is of interest to ask whether it plays an obligatory role in cancer prevention by resveratrol or, alternatively, only functions to facilitate the biological activity of this grape-derived polyphenol.
Development of a proper perspective for the biological function of NQO2 may be helped by first reviewing “function” associated with a protein-encoding gene product, seemingly a definitive and yet can be an elusive concept in biochemistry. A protein’s function may be concurrently and variably described as a “kinase” from a biochemical viewpoint, or as a “cell-cycle phase regulator” based on a cellular perspective, or as “apoptosis” when viewed from the vantage point of genetics [69]. If such a multidimensional, mosaic analysis of biological functions of proteins is applied to NQO2, in the context of its discovery as a distinct RTP, one might envisage that NQO2 acts as sensor and/or mediator of resveratrol. A proposed direct and indirect model depicting the participation of NQO2 in mediating cancer preventive activities of resveratrol is illustrated in Fig. 4.
Fig. 4.
A direct and indirect model depicting participation of NQO2 as sensor and mediator of the cancer preventative activities of resveratrol. In the direct model, binding of NQO2 to resveratrol may induce a conformational change resulting in the change in its subcellular distribution. The indirect model posits that binding of NQO2 to resveratrol promotes further interaction with other client proteins forming complexes that participate integrally in cancer prevention.
4.1. NQO2 functions directly as an apical molecular mediator in a cell/tissue-dependent manner
NQO2 was discovered in 1960s as a FAD- and metallo-containing oxidoreductase. Because NQO2 shares 50% structural homology with NQO1, it was originally classified as a phase II detoxification enzyme functioning with a significant degree of redundancy with NQO1. Furthermore, studies show NQO2 to be distinct from NQO1, based on chromosomal locations, nonoverlapping physicochemical properties and substrate/inhibitor preferences. For example, NQO2 employs dihydronicotinamide riboside as an electron donor, while NQO1 uses nicotinamide adenine dinucleotide (NAD(P)H) [70,71]. NQO2 is 3,000 times more effective than NQO1 in the bioreduction of the prodrug CB-1954 [70–72]. In rodent studies, knockout of NQO1 results in mice showing an increase in sensitivity to menadione [73], whereas the reverse was found in NQO2-knockout animals [74].
Recent studies have revealed significant functional versatility in NQO2. Boutin and coworkers [75,76] provide evidence that NQO2 acts as a third melatonin receptor, MT3. Using puromycin affinity chromatography, Kodama et al. [77] purified a 25-kD protein from porcine renal extracts showing (i) 82% sequence identity to sequences deduced from nucleotides of liver cDNA encoding human NQO2 [78], (ii) high binding affinity for puromycin aminonucleoside, and (iii) existence as a nonglobular monomer [77]. Studies have shown that NQO2 plays a role in control of cell number/size, signaling, and integrating the cellular damage/repair-related events with replication, cell-cycle progression, maintenance of genomic stability against deleterious effects of quinones, modulation of the tumor suppressor p53, and prevention and protection against skin tumor formation induced by environmental toxicants [70,74,79]. NQO2-null mice show myeloid hyperplasia of bone marrow and decreased sensitivity to menedione [74,80], and increased susceptibility to DMBA and benzo(a)pyrene-induced skin carcinogenesis [81,82], and sensitivity to development of radiation-induced B-cell lymphomas [83]. These findings as a whole suggest that NQO2 acts as modifiers of risk for carcinogenesis [82], and additionally may take on different functions in a cell/ tissue-dependent manner.
Is acquisition of cell/tissue-specific functions by NQO2 dependent on binding with resveratrol? A review of scientific literature suggests that this is possible since examples abound where assumption of novel biological functions are predicated on binding of small molecules with specific target proteins; recent examples include: binding of novel 2′,5′-oligoadenylates to ankyrin 2/4-repeats in ribonuclease RNase L induces a conformational change lifting repression of the nuclease and concomitantly inducing RNase L dimerization and activation [84]; human hepatocytes incubated with taxol results in shuttling of taxol into the nucleolus [85]; binding of adenosine monophosphate (AMP)-activated protein kinase (AMPK) by AMP increases its phosphorylation and activation by upstream LKB1 gene-encoded kinase (LKB1), fosteing maintenance of metabolic homeostasis and response to change in nutrient status [86]; and incubation of human hepatoma cells with resveratrol resulted in translocated presence of resveratrol in the nucleolus [87]. Taken together, these findings suggest that interaction of chemicals with appropriate target proteins could result in newly acquired functions for the proteins as well as trafficking of the chemicals into distinct cellular compartments.
We propose that NQO2 can control cellular and signaling events involved in cancer prevention by acting directly as an apical molecular mediator, with or without requisite binding to resveratrol. For example, NQO2 may act as a classical oxidoreductase to regulate the activities of enzymes and transcription/signaling factors or proteins. Furthermore, NQO2 can also function as a stabilizing factor for other proteins. These roles of NQO2 may explain the reduced p53 expression observed in NQO2-knockdown keratinocyte studies which showed that p53 was destabilized in NQO2-null cells [88]. It is noteworthy that NQO2 reportedly protect p53 against 20S proteasomal degradation leading to stabilization and activation of p53 [88], apparently using a molecular feature in proximity with the flavin cofactor domain located in a highly conserved segment of the quinone reductases including NQO2 [89,90]. Interestingly, this segment of NQO2 also appears to overlap with the resveratrol-interacting domain [66].
4.2. NQO2 functions in cooperation with its client proteins
NQO2 may also act in cooperation with its client proteins. Haystead and coworkers [91] queried the identity of antimalarial quinoline-binding proteins by fractionating red blood cell extracts on γ-phosphate-linked ATP-Sepharose, and identified NQO2 as a distinct quinoline cellular protein target. In studies aiming to characterize nature of proteins binding to a kinase inhibitor, imatinib, NQO2 was shown to be a nonkinase protein target [92,93]. On the basis of these observations, we propose that NQO2 can interact with other proteins to form dynamic complexes, and that the composition and/or stability of these complexes can be further modulated by NQO2 binding to resveratrol. Studies of these and other possibilities should provide additional information on the biochemical characteristics of NQO2 and also other RTPs, in the context of their role in cancer prevention.
5. Conclusion
Early diagnosis and chemoprevention are the most economically feasible and effective means to reduce the incidence, mortality, and morbidity of most cancers. Diet and nutritional supplementations have proven to be useful adjunctive strategies during the active surveillance phase before and/or after cancer surgery. Hence, there is increasing interest and concerted effort in identifying such bioactive food ingredients.
In this review, we have summarized current advances on resveratrol, a grape-derived phytochemical with chemo-preventive potentials owing to its demonstrated activity to inhibit cancer cell proliferation, reactivate apoptosis, and additional properties that can allay growth of cancer. It is hoped that the perspectives we have presented will offer vital answers to the quest to reduce the pain and suffering engendered by neoplastic diseases and to promote optimal health. The hope offered by diet-derived ingredients and phytochemicals is worthy of further attention and research.
Acknowledgements
The authors thank Dr. Luciano Pirota for the invitation to write this review. Studies on resveratrol in the authors’ laboratories over the years were supported in part by: JMW, NCI CA104424, and Army grant W81XWH-04-1-0059; TCH, NCI CA 29502, CA109932, and NYMC Intramural Research Award.
References
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 3.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 4.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev. Res. (Phila Pa) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 8.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid. Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 9.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Hsieh TC, Zhang Z, Ma Y, Wu JM. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Biochem. Biophys. Res. Commun. 2004;323:743–749. doi: 10.1016/j.bbrc.2004.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Chen Y, Labinskyy N, Hsieh TC, Ungvari Z, Wu JM. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem. Biophys. Res. Commun. 2006;346:367–376. doi: 10.1016/j.bbrc.2006.05.156. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh TC. Antiproliferative effects of resveratrol and the mediating role of resveratrol targeting protein NQO2 in androgen receptor-positive, hormone-non-responsive CWR22Rv1 cells. Anticancer Res. 2009;29:3011–3017. [PubMed] [Google Scholar]
- 13.Hsieh TC, Wang Z, Deng H, Wu JM. Identification of glutathione sulfotransferase-pi (GSTP1) as a new RTP and studies of resveratrol-responsive protein changes by resveratrol affinity chromatography. Anticancer Res. 2008;28:29–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer. 2001;39:102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 15.Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans-resveratrol—a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. 2006;27:1038–1046. doi: 10.1093/carcin/bgi286. [DOI] [PubMed] [Google Scholar]
- 16.Sengottuvelan M, Deeptha K, Nalini N. Resveratrol attenuates 1,2-dimethylhydrazine (DMH) induced glycoconjugate abnormalities during various stages of colon carcinogenesis. Phytother Res. 2009;23:1154–1158. doi: 10.1002/ptr.2770. [DOI] [PubMed] [Google Scholar]
- 17.Harper CE, Cook LM, Patel BB, Wang J, Eltoum IA, Ara-bshahi A, Shirai T, Lamartiniere CA. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate. 2009;69:1668–1682. doi: 10.1002/pros.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007;28:1946–1953. doi: 10.1093/carcin/bgm144. [DOI] [PubMed] [Google Scholar]
- 19.Kowalczyk MC, Walaszek Z, Kowalczyk P, Kinjo T, Hanausek M, Slaga TJ. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: implications for skin cancer prevention. Carcinogenesis. 2009;30:1008–1015. doi: 10.1093/carcin/bgp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, Walaszek Z, Slaga TJ. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev. Res. (Phila Pa) 2010;3:170–178. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf N, Nasti TH, Meleth S, Elmets CA. Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis. Mol Carcinog. 2009;48:713–723. doi: 10.1002/mc.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Choi BY, Kundu JK, Shin YK, Na HK, Surh YJ. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009;69:7449–7458. doi: 10.1158/0008-5472.CAN-09-1266. [DOI] [PubMed] [Google Scholar]
- 23.Chen JC, Chen Y, Lin JH, Wu JM, Tseng SH. Resveratrol suppresses angiogenesis in gliomas: evaluation by color Doppler ultrasound. Anticancer Res. 2006;26:1237–1245. [PubMed] [Google Scholar]
- 24.Yang HL, Chen WQ, Cao X, Worschech A, Du LF, Fang WY, Xu YY, Stroncek DF, Li X, Wang E, Marincola FM. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J. Transl Med. 2009;7:22–34. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Exp Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 26.Narayanan BA, Narayanan NK, Re GG, Nixon DW. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer. 2003;104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 27.Wang TT, Schoene NW, Kim YS, Mizuno CS, Rimando AM. Differential effects of resveratrol and its naturally occurring methylether analogs on cell cycle and apoptosis in human androgen-responsive LNCaP cancer cells. Mol. Nutr. Food Res. 54:335–344. doi: 10.1002/mnfr.200900143. [DOI] [PubMed] [Google Scholar]
- 28.Wang TT, Hudson TS, Wang TC, Remsberg CM, Davies NM, Takahashi Y, Kim YS, Seifried H, Vinyard BT, Perkins SN, Hursting SD. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis. 2008;29:2001–2010. doi: 10.1093/carcin/bgn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc. Natl. Acad. Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asamoto M, Hokaiwado N, Cho YM, Takahashi S, Ikeda Y, Imaida K, Shirai T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001;61:4693–4700. [PubMed] [Google Scholar]
- 31.Greenberg NM, DeMayo FJ, Sheppard PC, Barrios R, Lebovitz R, Finegold M, Angelopoulou R, Dodd JG, Duckworth ML, Rosen JM. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol. 1994;8:230–239. doi: 10.1210/mend.8.2.8170479. [DOI] [PubMed] [Google Scholar]
- 32.Seeni A, Takahashi S, Takeshita K, Tang M, Sugiura S, Sato SY, Shirai T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac. J. Cancer Prev. 2008;9:7–14. [PubMed] [Google Scholar]
- 33.Su JL, Yang CY, Zhao M, Kuo ML, Yen ML. Fork-head proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J. Biol Chem. 2007;282:19385–19398. doi: 10.1074/jbc.M702452200. [DOI] [PubMed] [Google Scholar]
- 34.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. J. Carcinog. 2006;5:15–25. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shah-rokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J. Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 37.Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate. 2007;67:1641–1653. doi: 10.1002/pros.20653. [DOI] [PubMed] [Google Scholar]
- 38.Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava RK. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007;2:7–23. doi: 10.1186/1750-2187-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp. Cell Res. 2008;314:1163–1176. doi: 10.1016/j.yexcr.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishibashi M, Ohtsuki T. Studies on search for bioactive natural products targeting TRAIL signaling leading to tumor cell apoptosis. Med. Res. Rev. 2008;28:688–714. doi: 10.1002/med.20123. [DOI] [PubMed] [Google Scholar]
- 41.Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol. Cell Biochem. 2007;304:273–285. doi: 10.1007/s11010-007-9510-x. [DOI] [PubMed] [Google Scholar]
- 42.Rezk YA, Balulad SS, Keller RS, Bennett JA. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am. J. Obstet. Gynecol. 2006;194:e23–e26. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 43.Kweon SH, Song JH, Kim TS. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010;395:104–110. doi: 10.1016/j.bbrc.2010.03.147. [DOI] [PubMed] [Google Scholar]
- 44.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur. J. Cancer. doi: 10.1016/j.ejca.2010.02.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer. 2010;127:257–268. doi: 10.1002/ijc.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson GE, Ivanov VN, Hei TK. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis. 2008;13:790–802. doi: 10.1007/s10495-008-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leone S, Fiore M, Lauro MG, Pino S, Cornetta T, Cozzi R. Resveratrol and X rays affect gap junction intercellular communications in human glioblastoma cells. Mol. Carcinog. 2008;47:587–598. doi: 10.1002/mc.20416. [DOI] [PubMed] [Google Scholar]
- 48.Reagan-Shaw S, Mukhtar H, Ahmad N. Resveratrol imparts photoprotection of normal cells and enhances the efficacy of radiation therapy in cancer cells. Photochem. Photobiol. 2008;84:415–421. doi: 10.1111/j.1751-1097.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 49.Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, Botturi M, Ghidoni R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007;253:124–130. doi: 10.1016/j.canlet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 51.Vitaglione P, Sforza S, Galaverna G, Ghidini C, Caporaso N, Ves-covi PP, Fogliano V, Marchelli R. Bioavailability of trans-resveratrol from red wine in humans. Mol. Nutr. Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 52.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 53.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol. Biomarkers Prev. 2003;12:953–957. [PubMed] [Google Scholar]
- 54.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002;33:387–398. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 55.Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and metabolism of trans-resveratrol orally administered to Wistar rats. Mol. Nutr. Food Res. 2005;49:482–494. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 56.Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JP. Distribution of [3H]. trans-resveratrol in rat tissues following oral administration. Br. J. Nutr. 2006;96:62–70. doi: 10.1079/bjn20061810. [DOI] [PubMed] [Google Scholar]
- 57.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides—two novel resveratrol metabolites in human plasma. Mol. Nutr. Food Res. 2008;52:549–557. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 58.Waite KA, Sinden MR, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005;14:1457–1463. doi: 10.1093/hmg/ddi155. [DOI] [PubMed] [Google Scholar]
- 59.Roman V, Billard C, Kern C, Ferry-Dumazet H, Izard JC, Mohammad R, Mossalayi DM, Kolb JP. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia. Br. J. Haematol. 2002;117:842–851. doi: 10.1046/j.1365-2141.2002.03520.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb JP, Marit G, Reiffers J, Mossalayi MD. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327–1333. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- 61.Kuo PL, Chiang LC, Lin CC. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Life Sci. 2002;72:23–34. doi: 10.1016/s0024-3205(02)02177-x. [DOI] [PubMed] [Google Scholar]
- 62.Shah M, Patel K, Sehgal PB. Monocrotaline pyrrole-induced endothelial cell megalocytosis involves a golgi blockade mechanism. Am. J. Physiol. Cell Physiol. 2005;288:C850–C862. doi: 10.1152/ajpcell.00327.2004. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh TC. Uptake of resveratrol and role of resveratrol-targeting protein, quinone reductase 2, in normally cultured human prostate cells. Asian J. Androl. 2009;11:653–661. doi: 10.1038/aja.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waters ML. Aromatic interactions in model systems. Curr. Opin. Chem. Biol. 2002;6:736–741. doi: 10.1016/s1367-5931(02)00359-9. [DOI] [PubMed] [Google Scholar]
- 65.Waters ML. Aromatic interactions in peptides: impact on structure and function. Biopolymers. 2004;76:435–445. doi: 10.1002/bip.20144. [DOI] [PubMed] [Google Scholar]
- 66.Buryanovskyy L, Fu Y, Boyd M, Ma Y, Hsieh TC, Wu JM, Zhang Z. Crystal structure of quinone reductase 2 in complex with resveratrol. Biochemistry. 2004;43:11417–11426. doi: 10.1021/bi049162o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcino-genesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 68.Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sander C. Genomic medicine and the future of health care. Science. 2000;287:1977–1978. doi: 10.1126/science.287.5460.1977. [DOI] [PubMed] [Google Scholar]
- 70.Long DJ, II, Jaiswal AK. NRH:quinone oxidoreductase2 (NQO2) Chem. Biol. Interact. 2000;129:99–112. doi: 10.1016/s0009-2797(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 71.Wu K, Knox R, Sun XZ, Joseph P, Jaiswal AK, Zhang D, Deng PS, Chen S. Catalytic properties of NAD(P)H:quinone oxidoreductase-2 (NQO2), a dihydronicotinamide riboside dependent oxidoreductase. Arch. Biochem. Biophys. 1997;347:221–228. doi: 10.1006/abbi.1997.0344. [DOI] [PubMed] [Google Scholar]
- 72.Knox RJ, Burke PJ, Chen S, Kerr DJ. CB 1954: from the Walker tumor to NQO2 and VDEPT. Curr. Pharm. Des. 2003;9:2091–2104. doi: 10.2174/1381612033454108. [DOI] [PubMed] [Google Scholar]
- 73.Joseph P, Long DJ, II, Klein-Szanto AJ, Jaiswal AK. Role of NAD(P)H:quinone oxidoreductase 1 (DT diaphorase) in protection against quinone toxicity. Biochem. Pharmacol. 2000;60:207–214. doi: 10.1016/s0006-2952(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 74.Long DJ, II, Iskander K, Gaikwad A, Arin M, Roop DR, Knox R, Barrios R, Jaiswal AK. Disruption of dihydronicotinamide riboside:quinone oxidoreductase 2 (NQO2) leads to myeloid hyperplasia of bone marrow and decreased sensitivity to menadione toxicity. J. Biol. Chem. 2002;277:46131–46139. doi: 10.1074/jbc.M208675200. [DOI] [PubMed] [Google Scholar]
- 75.Boussard MF, Truche S, Rousseau-Rojas A, Briss S, Descamps S, Droual M, Wierzbicki M, Ferry G, Audinot V, Delagrange P, Boutin JA. New ligands at the melatonin binding site MT(3) Eur. J. Med. Chem. 2006;41:306–320. doi: 10.1016/j.ejmech.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Calamini B, Santarsiero BD, Boutin JA, Mesecar AD. Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem. J. 2008;413:81–91. doi: 10.1042/BJ20071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kodama T, Wakui H, Komatsuda A, Imai H, Miura AB, Tashima Y. Purification and localization of a 25-kD porcine renal puromycin aminonucleoside-binding protein. Nephrol. Dial. Transplant. 1997;12:1453–1460. doi: 10.1093/ndt/12.7.1453. [DOI] [PubMed] [Google Scholar]
- 78.Jaiswal AK, Burnett P, Adesnik M, McBride OW. Nucleotide and deduced amino acid sequence of a human cDNA (NQO2) corresponding to a second member of the NAD(P)H:quinone oxidore-ductase gene family. Extensive polymorphism at the NQO2 gene locus on chromosome 6. Biochemistry. 1990;29:1899–1906. doi: 10.1021/bi00459a034. [DOI] [PubMed] [Google Scholar]
- 79.Celli CM, Tran N, Knox R, Jaiswal AK. NRH:quinone oxidoreductase 2 (NQO2) catalyzes metabolic activation of quinones and anti-tumor drugs. Biochem. Pharmacol. 2006;72:366–376. doi: 10.1016/j.bcp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 80.Iskander K, Gaikwad A, Paquet M, Long DJ, II, Brayton C, Barrios R, Jaiswal AK. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005;65:2054–2058. doi: 10.1158/0008-5472.CAN-04-3157. [DOI] [PubMed] [Google Scholar]
- 81.Iskander K, Paquet M, Brayton C, Jaiswal AK. Deficiency of NRH:quinone oxidoreductase 2 increases susceptibility to 7,12-dimethylbenz(a)anthracene and benzo(a)pyrene-induced skin carcinogenesis. Cancer Res. 2004;64:5925–5928. doi: 10.1158/0008-5472.CAN-04-0763. [DOI] [PubMed] [Google Scholar]
- 82.Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 70:1006–1014. doi: 10.1158/0008-5472.CAN-09-2938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Iskander K, Barrios RJ, Jaiswal AK. NRH:quinone oxidoreductase 2-deficient mice are highly susceptible to radiation-induced B-cell lymphomas. Clin. Cancer Res. 2009;15:1534–1542. doi: 10.1158/1078-0432.CCR-08-1783. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Silverman RH. A scientific journey through the 2–5A/RNase L system. Cytokine Growth Factor Rev. 2007;18:381–388. doi: 10.1016/j.cytogfr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nicolaou KC, Guy RK, Potier P. Taxoids: new weapons against cancer. Sci. Am. 1996;274:94–98. doi: 10.1038/scientificamerican0696-94. [DOI] [PubMed] [Google Scholar]
- 86.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 87.Lancon A, Delmas D, Osman H, Thenot JP, Jannin B, Latruffe N. Human hepatic cell uptake of resveratrol: involvement of both passive diffusion and carrier-mediated process. Biochem. Biophys. Res. Commun. 2004;316:1132–1137. doi: 10.1016/j.bbrc.2004.02.164. [DOI] [PubMed] [Google Scholar]
- 88.Gong X, Kole L, Iskander K, Jaiswal AK. NRH:quinone oxidoreductase 2 and NAD(P)H:quinone oxidoreductase 1 protect tumor suppressor p53 against 20s proteasomal degradation leading to stabilization and activation of p53. Cancer Res. 2007;67:5380–5388. doi: 10.1158/0008-5472.CAN-07-0323. [DOI] [PubMed] [Google Scholar]
- 89.Sollner S, Schober M, Wagner A, Prem A, Lorkova L, Palfey BA, Groll M, Macheroux P. Quinone reductase acts as a redox switch of the 20S yeast proteasome. EMBO Rep. 2009;10:65–70. doi: 10.1038/embor.2008.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deller S, Macheroux P, Sollner S. Flavin-dependent quinone reductases. Cell Mol. Life Sci. 2008;65:141–160. doi: 10.1007/s00018-007-7300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graves PR, Kwiek JJ, Fadden P, Ray R, Hardeman K, Coley AM, Foley M, Haystead TA. Discovery of novel targets of quinoline drugs in the human purine binding proteome. Mol. Pharmacol. 2002;62:1364–1372. doi: 10.1124/mol.62.6.1364. [DOI] [PubMed] [Google Scholar]
- 92.Winger JA, Hantschel O, Superti-Furga G, Kuriyan J. The structure of the leukemia drug imatinib bound to human quinone reductase 2 (NQO2) BMC Struct. Biol. 2009;9:7. doi: 10.1186/1472-6807-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat. Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]