Abstract
Purpose
We have shown that inadequate drug delivery to tumor cells is a major cause of failures in intravesical therapy of nonmuscle-invading bladder cancer. This is partly due to the dilution of drug concentration by urine production during treatment. To address this problem, we developed gelatin nanoparticles of paclitaxel (PNP) designed to yield constant drug concentrations. The hypothesis that constant, therapeutic concentrations in urine, bladder tissue and tumors can be attained was evaluated in dogs.
Materials and methods
We studied the drug release from PNP in culture medium in vitro. In vivo studies were performed in tumor-free dogs and in pet dogs with naturally occurring transitional cell carcinoma, where the pharmacokinetics (plasma, urine and tumors) of PNP was determined.
Results
The release of paclitaxel from PNP in vitro and in vivo was rate-limited by the drug solubility in aqueous medium. This property yielded constant drug concentrations independent of changes in the urine volume over the 2-hr treatment. Intravesical PNP showed low systemic absorption and favorable bladder tissue/tumor targeting and retention properties, with pharmacologically active concentrations retained in tumors for at least 1 week.
Conclusions
The constant drug release from PNP may overcome the problem of drug dilution by newly produced urine and the sustained drug levels in tumors may reduce the treatment frequency.
Keywords: Intravesical chemotherapy, bladder cancer, transitional cell carcinoma, gelatin nanoparticles, paclitaxel
INTRODUCTION
Patients presenting with nonmuscle-invading tumors (i.e., Ta tumors located in the urothelium, T1 tumors located in the lamina propria, and/or carcinoma in situ Tis) are typically managed by transurethral tumor resection, plus neoadjuvant or adjuvant intravesical immunotherapy or chemotherapy 1. The most commonly used agents are Bacillus Calmette-Guérin (BCG) and mitomycin C (MMC). We have identified inadequate drug delivery and chemoresistance as the two major causes for the highly variable and incomplete response of intravesical therapy in patients. Our data further indicate that a major source in the variability of drug exposure (and hence variable treatment efficacy) is due to the dilution of the instilled drug by residual urine and newly produced urine 2-4.
American Urological Association recommends adjuvant intravesical treatment as standard of care for high risk patients. However, a recent study based on the SEER database shows that only 42% of eligible patients receive intravesical therapy 5; the authors’ conclusion that overcoming the under-utilization is a priority is echoed by others 6. The potential reasons of the under-utilization include inconvenience due to the multiple weekly treatments, the required pharmacokinetic interventions such as dehydration and ultrasound-guided bladder emptying, and the unfamiliarity or difficulty with dose administration.
We propose that the problems of inadequate drug delivery, chemoresistance, and under-utilization of intravesical therapy may be overcome by using chemotherapeutics that (a) have equal or greater activity than MMC, (b) penetrate bladder tissues better than MMC, (c) require less frequent treatments, and (d) are easy to administer (e.g., without exhaustive bladder emptying or patient dehydration). We identified paclitaxel as a candidate since it shows higher activity against human bladder cancer cells 7, and produced a 42% response in advanced and/or metastatic bladder cancer in a phase II trial 8. In addition, paclitaxel, due to its lipophilicity, can penetrate the urothelium more readily than MMC 9. Paclitaxel is tightly bound to intracellular macromolecules such as tubulin and microtubule, resulting in significant drug accumulation (70-1,500 folds) and retention in tumor cells 10, and thereby offers the possibility of extending the drug action beyond the typical 2 hr treatment duration. Finally, paclitaxel induces apoptosis through p53-dependent and - independent pathways, which, in view of the high frequency of p53 mutations in bladder cancer 11, is an advantage over agents such as MMC that depend on functional p53 pathways for apoptosis 12. Accordingly, we studied the paclitaxel formulation approved for intravenous administration, i.e., paclitaxel dissolved in Cremophor and ethanol; our results show that during intravesical therapy, paclitaxel remains entrapped in Cremophor micelles, which reduces the free drug fraction and consequently lowers the drug penetration into the bladder tissue 13. We then explored using a surface-active agent dimethyl sulfoxide (DMSO) to disrupt the micelle structure; the results show that while DMSO increased the free drug fraction, it had other counteracting effects (increased urine production and increased drug removal by perfusing capillaries) that reduced the tissue drug concentrations 14. We eventually developed a new formulation, paclitaxel-loaded gelatin nanoparticles (PNP), that rapidly releases paclitaxel and has activity in vitro, and yields high paclitaxel concentration in bladder tissues in 3 tumor-free dogs in vivo 15.
The present study evaluated whether intravesical PNP provides constant drug concentrations in urine and yields favorable concentration-time-depth profiles in bladder tissues and tumors. The study was conducted in tumor-free dogs and pet dogs with naturally occurring bladder tumors.
MATERIALS AND METHODS
Chemicals
Cephalomannine was obtained from the National Cancer Institute (Bethesda, MD); paclitaxel from Handetech (Houston, TX); high performance liquid chromatography (HPLC) supplies from Fisher Scientific (Fair Lawn, NJ); procine skin Type A gelatin (175 bloom), Sephadex G50, pronase and glutaraldehyde (25% in water) and all other chemicals from Sigma Chemical (St. Louis, MO); and ELISA kits from Hawaii Biotech (Aiea, HI). All chemicals were used as received.
Preparation of PNP
PNP were prepared using the desolvation method, as described previously15,16. The drug loading was 0.52 ± 0.14% (mean ± SD, n=4). The particle size, determined using a particle size analyzer (Nano-ZS90, Malvern), was 638 ± 61 nm (mean ± SD, n=4)
Release of paclitaxel from PNP in vitro and in vivo
For in vitro release, PNP (1 to 50 μg/ml) was incubated with 20 ml phosphate-buffered saline (PBS) at 37°C. The in vivo drug release study was studied in tumor-free dogs (see below).
Animal Protocols
Two types of dogs were studied. Tumor-free beagle dogs were used to determine the in vivo drug release, systemic absorption, and pharmacokinetics in bladder tissues, under protocols approved by The Ohio State University Institutional Animal Care and Use Committee. Tumor-bearing pet dogs (various breeds) with pathologically confirmed transitional cell carcinoma (TCC) were used to determine the paclitaxel/PNP levels and the drug effects in tumors. These dogs were patients seen at The Ohio State University Veterinary Medicine Hospital and received intravesical therapy for disease management under protocols approved by the Veterinary Medicine Hospital Institute Review Board and with informed consents from owners. Animals received weekly intravesical PNP (1 mg/20 ml physiological saline) for three weeks. We previously showed that this dose would yield pharmacologically active drug concentrations in urothelium and lamina propria 13,14.
Studies of plasma, urine and bladder tissue pharmacokinetics in tumor-free dogs
Following intubation, dogs were anesthetized with isofluorane inhalation. A jugular vein was catheterized for blood sample collection and a urethral catheter for administering drug solution and for collecting urine samples. All experiments were conducted between 7 and 10 am. The bladder was emptied and PNP was instilled into the bladder through the urethral catheter. After 2 hr, the bladder was emptied through the catheter. For the experiments that required tissue samples at time points beyond 8 hr, animals were allowed to wake from anesthesia and return to their housing units until the times when they were re-anesthetized. For tissue harvesting, the bladder was emptied and excised at different times (i.e., 4, 8, 24, 72, 168 hr). Note, for example, the 4 hr time point corresponded to 2 hr after voiding the bladder. Euthanasia was accomplished by pentobarbital overdose. Tissue processing was as described previously 9,15.
Studies of drug concentrations of intravesical PNP in dog tumors
Whenever medically feasible and with informed consent of owners, tumor specimens were obtained from pet dog patients before and/or immediately after the weekly treatments.
Analysis of paclitaxel
Samples were analyzed for free and total (sum of free plus PNP-bound) drug concentrations, as follows. An aliquot was incubated with the enzyme pronase (1 mg/ml) at 37°C for 1 hr to digest the gelatin, and the resulting solution was analyzed for the total drug concentration. A second aliquot was centrifuged at 2500 × g for 30 min using a membrane with a MW cutoff of 10,000 Dalton, at room temperature. The filtrates were analyzed for the free drug concentrations. Paclitaxel was extracted and analyzed by two methods, i.e., HPLC for urine and drug release media samples that contained high drug levels and ELISA (competitive inhibition enzyme immunoassay) for plasma and tissue samples that contained lower drug levels, as described previously15,14. The lower limit of detection was 5 ng/ml for HPLC. ELISA detects all taxanes including paclitaxel and its metabolites; the lower detection limit was 0.1 ng/ml for 1 ml of plasma, 2 ng/g for 50 mg of bladder tissue, and 100 ng/g for 1 mg of bladder tumor.
Calculation of systemic drug absorption
Calculation of systemic bioavailability of intravesical PNP requires the area-under-plasma concentration-time curve (AUC) and the plasma clearance of paclitaxel; bioavailability is the multiplication product of AUC and clearance. AUC was calculated using the linear trapezoidal rule. Due to the severe hypersensitivity of dogs to Cremophor, paclitaxel clearance in dogs has not been studied. Hence, the calculation used instead the drug clearance values in other species, i.e., mice, rats, and humans (see Table 1) 17-22.
Table 1.
Systemic bioavailability of paclitaxel in tumor-free dogs given an intravesical dose of PNP (1 mg/20 ml).
| Literature data on paclitaxel clearance in other species, median (range), l/hr*kg | Calculated systemic bioavailability in dogs using clearance values in other species (%)
|
||
|---|---|---|---|
| 0-4 hr | 4-8 hr | 0-8 hr | |
|
| |||
| Mice: 0.301 (0.15-0.56) 17,18 | 0.60 | 0.35 | 0.95 |
| Rats: 0.366 (0.243-0.837) 19-21 | 0.73 | 0.43 | 1.16 |
| Humans: 0.430 (0.296-0.577) 22 | 0.84 | 0.50 | 1.35 |
Systemic bioavailability in dogs was calculated using the plasma data shown in Figure 2 and the median values of the clearance of paclitaxel (formulated in Cremophor micelles) in rodents and humans. This was necessary because the clearance in dogs has not been studied due to the severe Cremophor hypersensitivity in this species. The AUC in dogs was 119.2 ng*min/ml from 0-4 hr, 70.3 ng*min/ml from 4-8 hr, and 189.5 ng*min/ml from 0-8 hr.
Statistical Analysis
Comparison of values between groups was performed using two-tailed Student’s t tests. A value of P < 0.05 was considered statistically significant.
RESULTS
Solubility-limited drug release from PNP in vitro and in vivo
Figure 1A shows the release of paclitaxel from PNP in PBS at 37°C. The free paclitaxel concentration released into the medium increased only 2.5-fold and reached a plateau level when the starting PNP concentration was increased 50-fold from 1 to 50 μg/ml. This lack of dose-concentration proportionality indicates a nonlinear process that was rate-limited by a saturable property. The similarity between the plateau free concentration (between 1.3-1.4 μg/ml) and the aqueous solubility of paclitaxel (~1 μg/ml)23 suggests that its release from PNP was rate-limited by drug dissolution.
Figure 1. Release of paclitaxel from PNP.
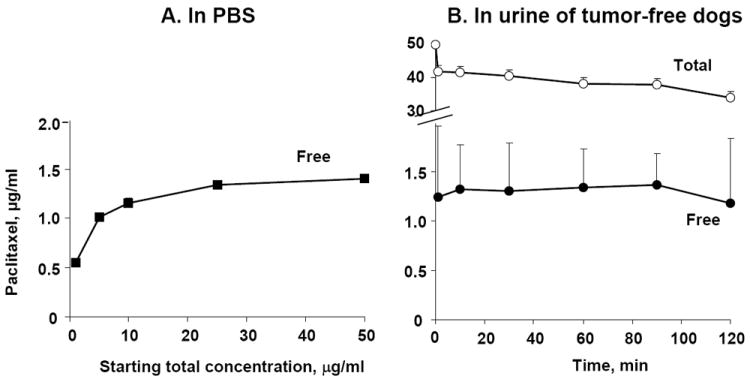
(A) In vitro release of paclitaxel from PNP (1 to 50 μg/ml paclitaxel-equivalent) in PBS. Data are mean + 1 SD (n=3). Note that SD are smaller than symbols. (B) Release of paclitaxel in urine of tumor-free dogs given a dose of intravesical PNP (1 mg/20 ml paclitaxel-equivalent). Note the break in the y axis. Over the 2-hr treatment, the volume of urine increased from 20 to 28 ml and the total concentration (PNP-bound and free drug) declined from 50 to 34 μg/ml, whereas the free paclitaxel concentrations remained relatively constant at 1.18-1.36 μg/ml. Data are mean + 1 SD (n=8).
Figure 1B shows the results of the in vivo drug release in tumor-free dogs (n=8). The urine results showed that over the 2-hr treatment duration, the urine volume increased 40% from 20 ml to 28±3 ml (mean±SD), and the total paclitaxel concentrations in the urine (sum of free and PNP-bound drug) decreased about proportionally from 50 μg/ml in the dosing solution to 34±4 μg/ml. In contrast, the free drug concentration remained nearly constant at 1.29 ±0.07 μg/ml (range, 1.18-1.36 μg/ml), or nearly identical to the free plateau concentration obtained under in vitro release.
Plasma pharmacokinetics of intravesical PNP in tumor-free dogs
In dogs treated with intravesical PNP, paclitaxel was detectable in the plasma at all time points (up to 8 hr after initiation of the 2-hr treatment); the average total concentrations were 0.54 ± 0.25 ng/ml (mean ± SD; range, 0.1 to 1.72 ng/ml; n= 8 dogs; Figure 1). Table 1 shows the AUC and systemic bioavailability at different time intervals; the bioavailability was about 0.60-0.84% of the administered dose during 0 to 4 hr and 0.35-0.50% during 4-8 hr, with a cumulative value of 0.95-1.35% over 0-8 hr.
Bladder tissue pharmacokinetics of intravescial PNP in tumor-free dogs
Figure 3 shows the drug concentration-depth profiles at 4 to 168 hr after initiation of the 2-hr treatment (3 animals per time point). Total drug concentrations (sum of free and PNP-bound concentrations) throughout the bladder were the highest at the first time point of 4 hr. The concentrations then declined with time and reached a relatively constant level that was sustained between 24 to 168 hr. The average total paclitaxel concentration of in tissue sections comprising the urothelium and lamina propria (300 μm depth from the inner surface of the bladder in dog) was 15.1 μg/g at 4 hr, and 0.18 μg/g after 1 week. The average drug concentrations in deeper tissues were 7.9 μg/g at 4 hr and 0.24 μg/g after 1 week in the first mm, and 4.4 μg/g at 4 hr and 0.19 μg/g after 1 week for the entire bladder. This data indicates significant drug retention throughout the bladder for at least 1 week.
Figure 3. Bladder tissue pharmacokinetics of paclitaxel in tumor-free dogs given intravesical PNP.
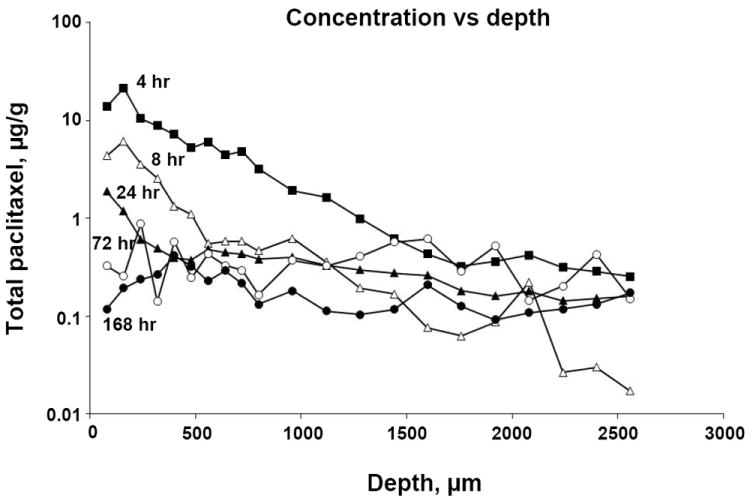
Animals were given a single dose of intravesical PNP (1 mg/20 ml paclitaxel-equivalent). Bladders were removed at predetermined times, frozen and cryo-sectioned. The first two sections (40 μm) were discarded to avoid contamination of tissue by urine that contained high drug concentrations. Drug concentrations in tissue sections were analyzed by ELISA. Total paclitaxel concentrations as a function of tissue depth, at five time points, i.e., 4, 8, 24, 72 and 168 hr after initiation of the 2-hr treatment. Data are mean values (n=3 per group). The average SD was 68% (range 6-180%, median: 61%) of the mean values (not shown).
Bladder tumor pharmacokinetics of intravesical PNP in pet dog patients
A total of 13 tumor-bearing dogs were studied. One dog was found not to have TCC and was excluded. The remaining 12 dogs (8 females and 4 males) received three weekly treatments. Tumor biopsy samples were obtained from a total of 7 dogs (all females), immediately after the first treatment, and again on day 7 and day 14 (samples taken before administration of second and third weekly doses). One dog was re-treated 3 months later again with three weekly treatments.
Figure 4 shows the drug concentrations in tumors. As expected, there were substantial inter- and intra-subject variations. The total paclitaxel concentrations showed a 13-fold range immediately after the first treatment, a 7-fold range on day 7 and a 20-fold range on day 14. The time-dependent variations were observed for all animals, i.e., there were no animals with consistently high or consistently low drug concentrations, indicating the variations were random. Interestingly, the mean drug concentrations at the three time points remained relatively constant (~40 μg/g) and the median concentrations were within 20-40% of the mean concentrations, indicating relatively constant drug exposure in individual animals upon repeated treatments.
Figure 4. Bladder tumor concentrations of paclitaxel in dogs with naturally occurring tumors after intravesical PNP.
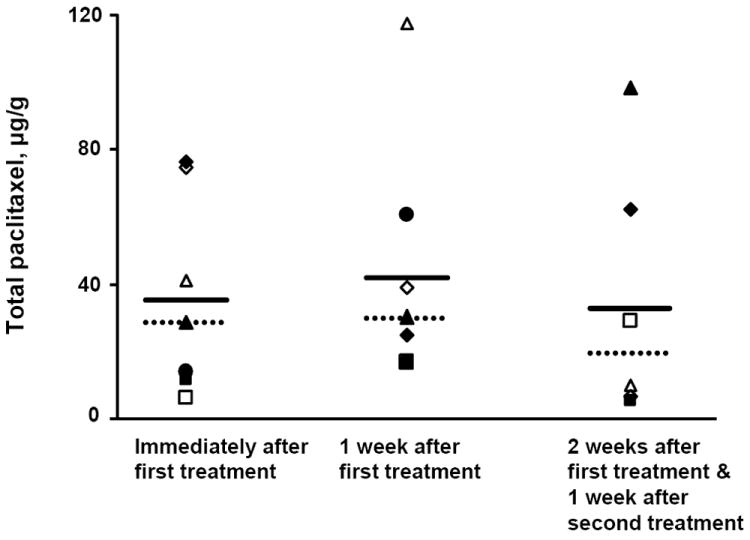
Tumor-bearing pet dogs were treated with three weekly doses of intravesical PNP (1 mg/20 ml paclitaxel-equivalent). Tumor samples were obtained immediately after the first treatment, and again on day 7 and day 14. The day 7 and 14 samples were taken before animals received their second and third weekly treatments. Hence, the day 7 samples were obtained 1 week after the first treatment, and the day 14 samples were obtained 2 weeks after the first treatment and 1 week after the second treatment. Drug concentrations, measured by ELISA, were 36.3±29.4 μg/g at 2 hr after initiation of treatment (mean±SD; median was 28.8 μg/g; range was 6.4-76.6 μg/g; n=7), 43.9±35.9 μg/g at 1 week after initiation of the first treatment (mean±SD; median was 30.3 μg/g; range was 16.8-117.6 μg/g; n=6), and 35.5±37.8 μg/g at 2 weeks after initiation of the first treatment and 1 week after the second treatment (mean±SD; median was 19.7 μg/g; range was 5.5-98.8 μg/g; n=6). Solid lines: mean values. Dotted lines: median values.
A comparison of the results in Figures 3 and 4 shows a 360-fold higher average total paclitaxel concentration in bladder tumors compared to the concentrations in the urothelium of tumor-free dogs (i.e., the first tissue sections of 80 μm thickness) one week after treatment. This indicates greater drug delivery/retention in tumor tissues.
DISCUSSION
Our major findings are as follows. First, the drug release data indicate a drug solubility-limited release from PNP in vivo, yielding constant drug concentrations irrespective of changes in urine volume. This feature will minimize the substantial inter- and intra-subject variability in drug exposure due to residual urine at the time of dose administration and newly formed urine (e.g., 20-fold variations for MMC) 4, thereby eliminate the need of dehydrating the patient and exhaustively emptying the bladder before treatment, and hence may improve the efficacy and enhance the ease of using intravesical therapy.
Second, only ~1% of the intravesical PNP dose (1 mg paclitaxel-equivalent) was absorbed, yielding average plasma paclitaxel concentrations of <2 ng/ml. These levels are 1000-times below the threshold levels associated with clinical toxicity 22, indicating intravesical PNP is not likely to result in systemic toxicity.
Third, the tissue pharmacokinetic data in tumor-free dogs indicate the average drug concentration in the urothelium and lamina propria at 4 hr was at least 13-times the free drug concentration in the urine at 2 hr and that a single dose of intravesical PNP yielded substantial drug levels sustained for at least 1 week. These results indicate preferential drug accumulation in tissues.
Fourth, compared to the concentrations previously observed with the Cremophor micelle formulation at 2 hr, the average drug concentrations derived from PNP at 4 hr were 5.4-times higher in tissue sections comprising the urothelium plus lamina propria (300 μm depth from the inner surface of the bladder in dog) and 4.4 times higher throughout the entire bladder, (15.1 vs 2.8 and 4.4 vs 1.0 μg/g, dose-adjusted to 1 mg/20 ml). These results indicate superior drug delivery from PNP.
Fifth, a comparison of the urine, tissue and plasma pharmacokinetics indicates the fate of the drug in tissues over time. For example, the sum of the drug amounts in all bladder tissue sections (data in Figure 3) equaled about 0.49% of the dose at 4 hr and 0.16% at 8 hr, indicating a loss of 0.33% over the 4 hr interval. This fraction is comparable to the sum of the amount of drug recovered in the urine over the same duration (0.09% at 4 hr and 0.12% at 8 hr, or a gain of 0.03%) plus the amount of drug absorbed into the systemic circulation (a gain of 0.4%). Hence, the drug in tissue was removed primarily by systemic absorption and, to a lesser extent, by diffusion back to urine.
Finally, we observed significant drug retention in bladder tissues from 24 to 168 hr. This is likely due to the drug binding, as we previously found a 100-to-1 ratio between the bound and free extracellular concentrations 24. We further observed 360-fold higher drug concentrations in tumors relative to normal tissues at the same tissue depth, indicating greater delivery/retention of PNP in tumors. One possibility is the loss of an intact urothelium in tumors resulting in enhanced drug penetration, as we previously observed for MMC 25. Another possibility is preferential adsorption and/or trapping of PNP on/in tumors (e.g., papillary tumors in humans show a cauliflower-like structure, with grooves and creases), which would have resulted in higher and more sustained drug concentrations. The drug concentrations in bladder tissues (above 100 ng/g or 120 nM at all tissue depths) are pharmacologically active. For example, the calculated AUC of paclitaxel from 2 to 168 hr in the tumors located in urothelium and lamina propria of tumor-bearing dog bladders, derived from a single dose of intravesical PNP, is about 6660 μg*hr/g. This value is about 60-times higher than the pharmacologically active AUC of paclitaxel in tumor-bearing mice given an intravenous dose of paclitaxel dissolved in Cremophor/ethanol or formulated as albumin-coated nanoparticles (respective AUC of 110 and 145 μg*hr/g for a 20 mg/kg dose 26). Based on these tissue/tumor pharmacokinetic data, we propose a single intravesical PNP dose would yield pharmacologically active drug levels in urothelium and lamina propria sustained for 1 week or longer.
In summary, intravesical PNP shows the desired properties for intravesical therapy, i.e., favorable bladder tissue/tumor targeting, penetration and retention properties, and low systemic absorption. We propose that clinical evaluation of intravesical PNP is warranted, and are pursuing computational studies to integrate the pharmacokinetic data described herein and our previously reported pharmacodynamic data of paclitaxel in human bladder tumor histocultures 27, for the purpose of defining the clinical trial design (i.e., dose, dosing frequency, number of patients, statistical power). We used a similar computational approach was successfully applied to design a phase III trial of intravesical mitomycin C, where the clinical outcome closely aligns with the computation-predicted outcome 28,29.
Figure 2. Plasma pharmacokinetics of paclitaxel in tumor-free dogs given intravesical PNP.
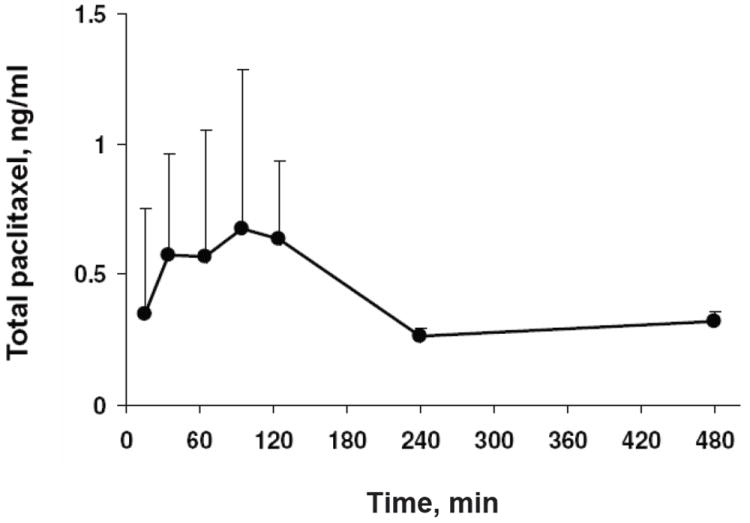
Animals were given a single dose of intravesical PNP (1 mg/20 ml paclitaxel-equivalent). Plasma samples were analyzed by ELISA for the total paclitaxel concentrations. Data are mean + 1 SD (n=3 at 240 and 480 min, n=8 for other times). Some SD are smaller than symbols.
Acknowledgments
This work was supported in part by research grants R43 CA107743 and R44 CA107743 from the National Cancer Institute, NIH, DHHS.
Abbreviations used are
- AUC
area-under-concentration-time curve
- BCG
Bacillus Calmette-Guérin
- DMSO
dimethyl sulfoxide
- ELISA
competitive inhibition enzyme immunoassay
- HPLC
high performance liquid chromatography
- MMC
mitomycin C
- PBS
phosphate-buffered saline
- PNP
paclitaxel gelatin nanoparticles
- TCC
transitional cell carcinoma
Footnotes
Some of the results were presented at the 2008 Surgical Urology Oncology Annual Meeting and at the 2009 American Urological Association Annual Meeting.
References
- 1.Smith JA, Jr, Labasky RF, Cockett AT, et al. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162:1697. [PubMed] [Google Scholar]
- 2.Wientjes MG, Dalton JT, Badalament RA, et al. A method to study drug concentration-depth profiles in tissues: mitomycin C in dog bladder wall. Pharm Res. 1991;8:168. doi: 10.1023/a:1015827700904. [DOI] [PubMed] [Google Scholar]
- 3.Wientjes MG, Dalton JT, Badalament RA, et al. Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res. 1991;51:4347. [PubMed] [Google Scholar]
- 4.Dalton JT, Wientjes MG, Badalament RA, et al. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. 1991;51:5144. [PubMed] [Google Scholar]
- 5.Huang GJ, Hamilton AS, Lo M, et al. Predictors of intravesical therapy for nonmuscle invasive bladder cancer: results from the surveillance, epidemiology and end results program 2003 patterns of care project. J Urol. 2008;180:520. doi: 10.1016/j.juro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan DP. Words of wisdom. Re: predictors of intravesical therapy for nonmuscle invasive bladder cancer: results from the Surveillance, Epidemiology and End Results Programme (SEER) 2003 Patterns of Care Project. Eur Urol. 2008;54:1443. doi: 10.1016/j.eururo.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Kugler A, Haschemi R, Zoller G, et al. In vitro investigations of new therapeutic agents on bladder tumor cell lines. Urol Res. 1997;25:247. doi: 10.1007/BF00942093. [DOI] [PubMed] [Google Scholar]
- 8.Roth BJ. Preliminary experience with paclitaxel in advanced bladder cancer. Semin Oncol. 1995;22:1. [PubMed] [Google Scholar]
- 9.Song D, Wientjes MG, Au JLS. Bladder tissue pharmacokinetics of intravesical taxol. Cancer Chemother Pharmacol. 1997;40:285. doi: 10.1007/s002800050660. [DOI] [PubMed] [Google Scholar]
- 10.Kuh HJ, Jang SH, Wientjes MG, et al. Computational model of intracellular pharmacokinetics of paclitaxel. J Pharmacol Exp Ther. 2000;293:761. [PubMed] [Google Scholar]
- 11.Cote RJ, Esrig D, Groshen S, et al. p53 and treatment of bladder cancer. Nature. 1997;385:123. doi: 10.1038/385123b0. [DOI] [PubMed] [Google Scholar]
- 12.Woods CM, Zhu J, McQueney PA, et al. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med. 1995;1:506. [PMC free article] [PubMed] [Google Scholar]
- 13.Knemeyer I, Wientjes MG, Au JL. Cremophor reduces paclitaxel penetration into bladder wall during intravesical treatment. Cancer Chemother Pharmacol. 1999;44:241. doi: 10.1007/s002800050973. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Song D, Wientjes MG, et al. Effect of dimethyl sulfoxide on bladder tissue penetration of intravesical Paclitaxel. Clin Cancer Res. 2003;9:363. [PubMed] [Google Scholar]
- 15.Lu Z, Yeh TK, Tsai M, et al. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin Cancer Res. 2004;10:7677. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 16.Tsai M, Lu Z, Wang J, et al. Effects of Carrier on Disposition and Antitumor Activity of Intraperitoneal Paclitaxel. Pharm Res. 2007 doi: 10.1007/s11095-007-9298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiseman JL, Eddington ND, Leslie J, et al. Plasma pharmacokinetics and tidssue distribution of paclitaxel in CD2F1 mice. Cancer Chemother Pharmacol. 1994;6:465. doi: 10.1007/BF00685656. [DOI] [PubMed] [Google Scholar]
- 18.Sparreboom A, van Tellingen O, Nooijen W, et al. Nonlinear pharmacokinetics of paclitaxel in mice results from thepharmaceutical vehicle cremophor EL. Cancer Res. 1996;56:2112. [PubMed] [Google Scholar]
- 19.He L, Wang GL, Zhang Q. An alternative paclitaxel microemulsion formulation: hypersensitivity evaluation and pharmacokinetic profile. Int J Pharm. 2003;250:45. doi: 10.1016/s0378-5173(02)00478-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Qu G, Sun Y, et al. Pharmacokinetics, biodistribution, efficacy and safety of N-octyl-O-sulfate chitosan micelles loaded with paclitaxel. BIOMATERIALS. 2008;29:1233. doi: 10.1016/j.biomaterials.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Sparreboom A, Scripture CD, Trieu V, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 22.Huizing MT, Keung AC, Rosing H, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol. 1993;11:2127. doi: 10.1200/JCO.1993.11.11.2127. [DOI] [PubMed] [Google Scholar]
- 23.Liggins RT, Hunter WL, Burt HM. Solid-state characterization of paclitaxel. J Pharm Sci. 1997;86:1458. doi: 10.1021/js9605226. [DOI] [PubMed] [Google Scholar]
- 24.Kuh HJ, Jang SH, Wientjes MG, et al. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290:871. [PubMed] [Google Scholar]
- 25.Gao X, Au JL, Badalament RA, et al. Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin Cancer Res. 1998;4:139. [PubMed] [Google Scholar]
- 26.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12:1317. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 27.Au JL, Kalns J, Gan Y, et al. Pharmacologic effects of paclitaxel in human bladder tumors. Cancer Chemother Pharmacol. 1997;41:69. doi: 10.1007/s002800050709. [DOI] [PubMed] [Google Scholar]
- 28.Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 29.Au JL, Badalament RA, Wientjes MG, et al. Optimized intravesical mitomycin C treatment for superficial bladder cancer: long-term follow-up. J Urol. 2006;175:268. [Google Scholar]


