Abstract
Objective
To investigate hypoestrogenic “inactive phases” (IP) in the follicular phase of the menstrual cycle, with respect to age, reproductive stage, and follicular depletion.
Design
Analysis of prospectively collected menstrual bleed and estrone-3-glucuronide data.
Setting
Center for Population and Health, Georgetown University.
Patient(s)
White women (n = 88, aged 25–59 years, mean = 44.7 years) from the population-based Biodemographic Models of Reproductive Aging (BIMORA) project.
Intervention(s)
None.
Main Outcome Measure(s)
The IP durations by age and reproductive stage. Estimated follicular depletion rate based on IP durations.
Result(s)
Mean IP duration and variability decreased and then increased with age/reproductive stage. The proportion of very short (% 1 day) IP durations increased and then decreased with age/stage. Long IPs occurred most, but not exclusively, in the oldest age/latest stage. Follicular depletion rate estimates were a plausible 2%–4% per year of age, but these models were a poor fit because IP durations did not consistently increase across ages/stages.
Conclusion(s)
Follicular depletion models alone do not explain the observed pattern of IPs. Our data suggest that IPs reflect both follicular depletion and hyperstimulation in premenopausal and perimenopausal women. Knowledge of underlying IP patterns in the menstrual cycle could inform decisions about hormone sampling and contraception during the perimenopause.
Keywords: Follicular depletion, hypoestrogenic, inactive phase, female reproductive aging, perimenopause
Population-level, longitudinal studies show characteristic changes inmenstrual cycle length as women age (1, 2). Mean cycle length and variability reach a minimum during late premenopause, and shorter cycles are interspersed with longer cycles during perimenopause, even after mean cycle length begins to increase (1–5). Increased mean cycle length and substantially elongated menstrual cycles (>60 days) are hallmarks of late perimenopause (6).
A primary determinant of cycle length variability is follicular phase length, which varies in both premenopausal and perimenopausal women (7–9). Miro et al. (9) have shown that it is the early portion of the follicular phase, before an increase in E2 level, that varies most as women age. The proximate causes of shorter and longer follicular phases are not fully established; however, growing evidence implicates advanced, out-of-phase, and delayed initiation of follicular development with reproductive aging (7, 10–12).
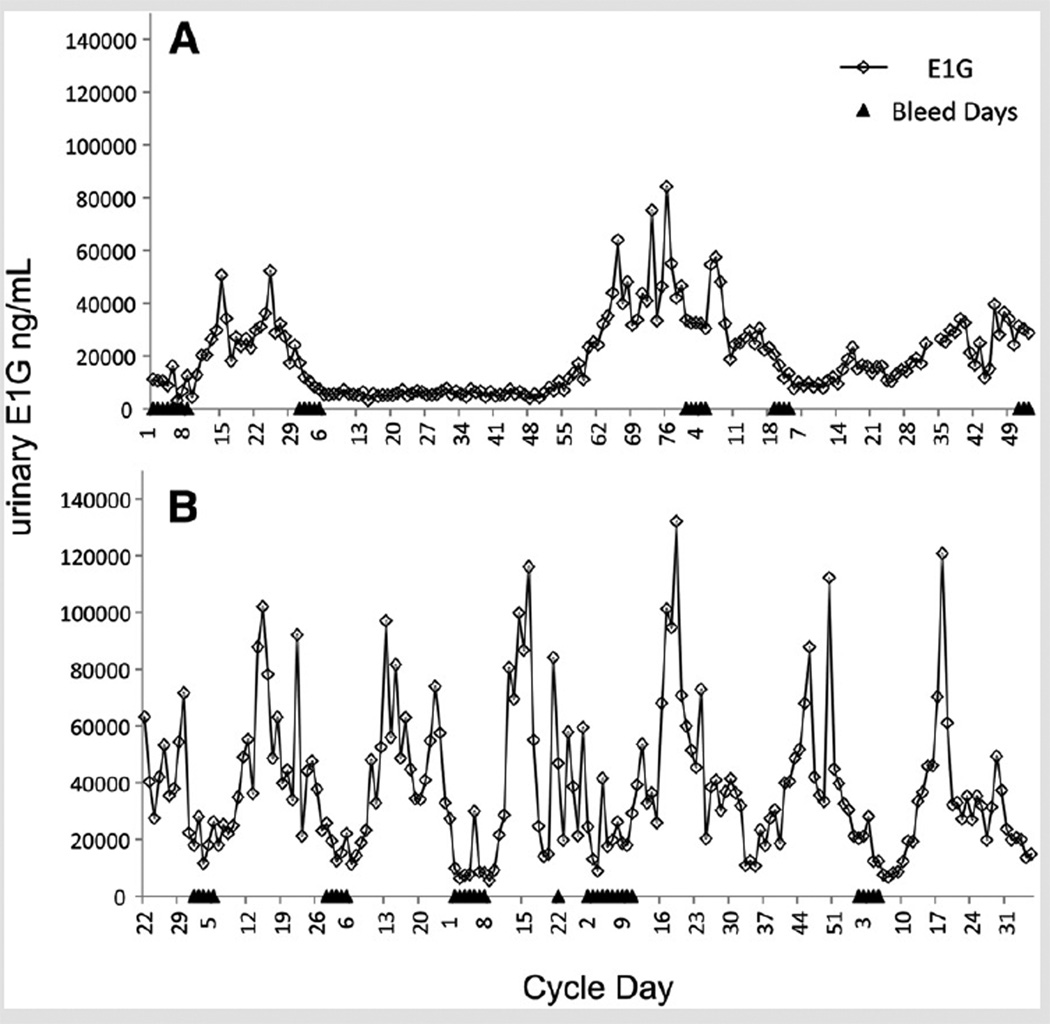
Short follicular phases appear to be related to follicular development that begins earlier in the menstrual cycle in older compared with younger reproductive-aged women (12). Hale et al. (10) identified out-of-phase onset of follicular growth from daily E2 in both long and short cycles in perimenopause, where follicular growth of a new cohort begins during the luteal phase of an already ovulatory cycle (3, 13, 14). Long follicular phases, in contrast, are thought to be related to delayed follicular development. Hypoestrogenic “inactive phases” (IP) (Fig. 1) have been observed at the start of menstrual cycles, most often in perimenopausal women (3, 7, 10, 15–20), and have been identified as a primary cause of elongated menstrual cycles associated with reproductive aging (7). During an IP, a woman’s hormone profile appears postmenopausal, with low steroid hormone levels and fluctuating gonadotropin levels resulting from release of steroid negative feedback (16, 18, 19, 21–23).
FIGURE 1.
Six-month profiles of estrone-3-glucuronide (E1G) and menstrual bleeds for two women. (A) age 47 years, perimenopausal. The long menstrual cycle in the center of the profile begins with an inactive phase of approximately 50 days in length. (B) age 41 years, late premenopausal, with several short inactive phases.
Here we define an IP as the interval (long or short) starting just after the decline of E2 level associated with the previous cycle’s luteal phase to the substantial increase in E2 level during the follicular phase. We use IP data from the Biodemographic Models of Reproductive Aging (BIMORA) project (24) to explore age-related changes in IP duration (length) using a flexible piecewise exponential hazards model. The outcomes of interest are the durations of IPs for four age categories (<40, 40–44, 45–49, 50+ years) and three reproductive stages (premenopausal, early perimenopausal, and late perimenopausal).
In addition, we test an existing model that explicitly connects IPs with the follicular depletion process, where age and IP duration are linked to depletion rate and follicle pool size (18, 19). This model is consistent with data from postmortem and ultrasound follicle counts (25–29) indicating constant or accelerating rates of depletion, with the accelerating rate (29) supported by recent analyses (30–32). The rationale for the model is that IPs are a stochastic consequence of a dwindling follicle pool, and there will be intervals by chance when no follicles initiate development and estrogen levels therefore remain low. The model predicts that the mean and variability in IP duration will increase with age as the follicular reserve approaches exhaustion (18, 19). We show that the observed IP durations are not consistent with this model of follicular depletion, and we consider the possibility that dual biological mechanisms explain the observed IP pattern.
MATERIALS AND METHODS
Sample
Women were participants in BIMORA, a 5-year prospective study of reproductive aging and the menopausal transition. The BIMORA participants were recruited from the second cohort of the TREMIN Research Program on Women’s Health (white, college-educated women at the University of Minnesota in the 1960s) for whom longitudinal menstrual and health data were available. The BIMORA eligibility requirements were 18–60 years old, at least one intact ovary, and not pregnant, breastfeeding, using exogenous hormones, or receiving cancer treatment. Participants provided written informed consent and received compensation of $150 per year; procedures were approved by the institutional review boards of Georgetown University, Pennsylvania State University, and University of Washington. Further details of recruitment, eligibility, and enrollment have been presented previously (24) (and see Supplemental Materials, Methods).
The BIMORA participants (n = 156) from which the present sample (n = 88) is drawn recorded menstrual bleed data and collected daily urine specimens for a 6-month interval (January 15–July 15) in each of 5 study years (1998–2002). Specimens were assayed in duplicate for estrone-3-glucuronide (E1G) (33), a urinary metabolite of E2, and corrected for hydration status using specific gravity (34).
For this analysis, we excluded women who were known at the start of BIMORA participation to be postmenopausal (i.e., 12+ months of amenorrhea). For eligible premenopausal and perimenopausal women, we excluded data collected within 3 months of a woman reporting exogenous hormone use, pregnancy, breastfeeding, or major medical procedures.
Menstrual Cycle Characteristics
We defined menstrual bleed episodes as at least 2 days of bleeding within a 6-day interval, preceded by two or more bleed-free days (35). “Cycle day 0” was the first day of a bleed episode; cycle length was the number of days from “day 0” of one menstrual cycle to the next.
We determined the reproductive stage of each cycle based on a rolling cycle length coefficient of variation (CV; the standard deviation of cycle length divided by the mean cycle length) calculated using the length of the current cycle and the five previous cycles (or all previous cycles if fewer than five) (36). The CV value cutoffs (<20% = stage −3, premenopausal; 20%–40% = stage −2, early perimenopausal; >40% or a cycle >60 days long = stage −1, late perimenopausal) were designed to correspond to the Stages of Reproductive Aging Workshop (STRAW) stages (4, 37).
We identified IPs from graphs showing both menstrual bleeds and smoothed E1G profiles. The “IP start” was the end of the decline in E1G in the late luteal phase of the previous menstrual cycle or early follicular phase of the current cycle. The “IP end” was the beginning of an increase in E1G (estrogen take-off) (7) one or more days after the decrease in E1G from the previous cycle (see Supplemental Materials, Methods).
We calculated IP duration (length) using the start and end dates of each IP relative to the first day of the menstrual cycle. We produced a best estimate (1-day interval) of the start date for each IP. The IP end date was an interval of one or more days to incorporate uncertainty in the exact date of the increase in E1G. We used a woman’s date of birth, the start-date of the cycle, and the cycle day when an IP began, to compute a woman’s age at the start of an IP.
We sampled all cycles that started in the observation period (January 15–July 15), reduced by 5 days at either end to ensure that the smoothed E1G pattern was clear for the entire reduced observation period. We excluded cycles where missing E1G data precluded observation of the IP and where an E1G decrease/increase in the early follicular phase was absent.
Statistical Methods
We studied the duration of the IP using survival analysis techniques because the data are often censored, either because the IP was still going on at the end of the observation window (right censoring) or because we were able to ascertain lower and upper bounds rather than an exact length (interval censoring). Failure to account for these features of the data leads to biased estimates.
We used a piecewise exponential model, a flexible semiparametric approach where the underlying hazard or rate at which women exit the IP is allowed to vary during small duration intervals. We added a frailty term to allow for correlation among the IPs observed in the same woman. Results are presented stratified by four age groups and three reproductive stage groups, using summary statistics and graphs of the survival, hazard, and density functions.
We also estimated simple follicular depletion models with constant and accelerating depletion rates, as proposed by Wood et al. (18) and O’Connor et al. (19). These models assume that the hazard, or rate, at which women move from the IP to the late follicular phase, is proportional to the number of follicles (and thus the age of the woman) at the start of the IP, and remains constant during the IP.
All models were estimated by maximum likelihood. Calculations were done using the statistical packages Stata (release 12; StataCorp) and R (38), with custom programming to allow for interval censoring. For full details please refer to the supplementary materials.
RESULTS
We identified 1,252 eligible menstrual cycles from 88 BIMORA women (average age 44.7 years, range 25–59 years; median 13 cycles per woman), with each cycle contributing an IP length, from a total of 1,974 cycles from 102 premenopausal and perimenopausal women. Of the 1,252 included cycles, 32 are right-censored and 1,220 are interval-censored (see Materials and Methods section). Most interval-censored cases have little uncertainty; 75% of the cases are intervals of 2 days or less and only 5% exceed 7.5 days.
We excluded seven women and 195 cycles because of hormone use, and another seven women and 527 cycles because of start dates outside of the observation period, missing E1G data that made it impossible to observe the IP, or ambiguous E1G data across the luteal-follicular transition. Women excluded for hormone use had a higher mean age and lower mean E1G than the included women. Cycles excluded because of hormone use had lower mean urinary pregnanediol-3-glucuronide level and longer cycle length; those excluded for missing data had lower mean pregnanediol-3-glucuronide and E1G levels; and cycles excluded for ambiguous data had lower mean pregnanediol-3-glucuronide level and higher mean age.
We present our results stratified by age (<40 years, n = 297 cycles; 40–44 years, n = 227; 45–49 years, n = 447; 50+ years, n = 281) and by reproductive stage (premenopausal, n = 813 cycles; early perimenopausal, n = 204 cycles; late perimenopausal, n = 235 cycles).
Piecewise Exponential Model
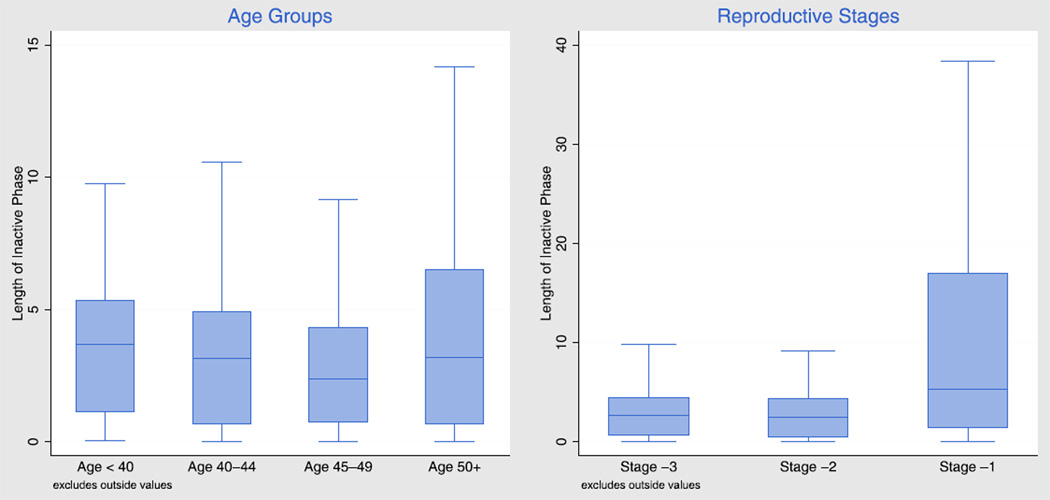
Figure 2 shows the results of our flexible semiparametric model using box plots to summarize key features of the distribution of IPs by age and reproductive stage for an average woman. All pairwise comparisons across age or reproductive stages are significant at the P<.001 level, except for the comparison of ages <40 years and 40–44 years.
FIGURE 2.
Box plots showing the distribution of inactive phase durations (lengths) in days, by age and by reproductive stage, for an average woman.
The central box in each plot represents the durations by which 75%, 50%, and 25% of the IPs were still in progress, with height proportional to the interquartile range. The median length of the IP is 3.0 days, and the interquartile range is 4.3 days, but both vary with age and with reproductive stage. In general, the IP becomes slightly shorter and somewhat less variable as women age from <40 to 40–44 and 45–49 years (or go through stages −3 and −2), but then become much longer and substantially more variable, for women aged 50+ years or in stage −1. The distribution for the older groups is very skewed and has a long right tail.
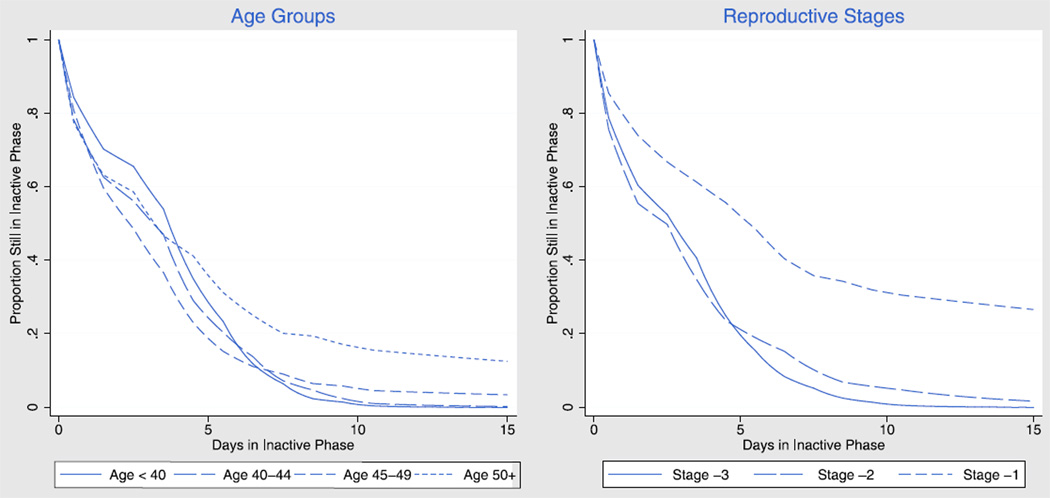
Figure 3 depicts the survival function, or proportion of women still experiencing the IP on each day, for up to 15 days, by age or reproductive stage. The most striking feature of these curves is the larger proportion of women aged 50+ years who remain in an IP after about 5 days, compared to the younger age groups. This result is even more striking when we stratify by reproductive stage, with more than a quarter of women in stage −1 still in the IP after 15 days. The other feature of interest is the fact that the curves for women less than 50 years crossover around 7 days, therefore younger women are somewhat slower to exit IPs at short durations but soon catch up and are less likely to remain in the IP at longer durations. The same pattern appears when we compare stages −3 and −2, with a crossover around 5 days. In short, IPs do not just become longer with reproductive age, but actually have different distributions.
FIGURE 3.
Probability of remaining in an inactive phase by inactive phase duration (length) in days, by age and reproductive stage, for an average woman.
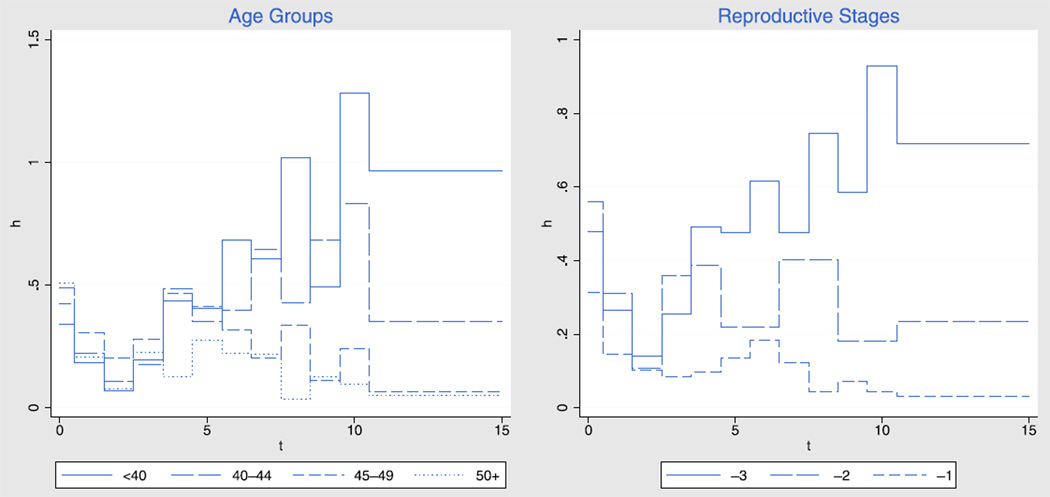
Another way to look at the results is in terms of the hazard function, or rate at which women progress from the inactive to the late follicular phase. Because the hazard reflects the proportion of women exiting the IP among women still in that phase, it provides a sharper focus on the underlying process. Figure 4 shows the hazard stratified by age and by reproductive stage, for IP durations up to 15 days.
FIGURE 4.
The rate (hazard, h) at which women transition from the inactive phase to the late follicular phase, by day of inactive phase (t), by age and reproductive stage, for an average woman.
In all groups, the rate at which women exit the IP declines during the first 3 days. For younger women this rate rebounds and then increases with IP duration, whereas for older women it tends to have a small rebound or none at all, followed by a decline with IP duration. The hazards by reproductive stage reveal the same pattern, perhaps even more clearly. Initially, older women have a slightly higher exit rate from the IP than younger women, but if they are still in an IP after about 3 days they move to the late follicular phase at a much lower rate, with differences by age or reproductive stage becoming increasingly marked at longer durations.
The supplementary materials (Supplemental Figure 1) also show results in terms of the frequency distribution or proportion of IPs ending each day for durations up to 15 days. These frequencies can be seen as the result of applying the hazard or exit rates in Figure 3 to the women who are still in the IP in Figure 2, therefore they reflect both types of results.
Follicular Depletion Models
A simple follicular depletion model where the hazard depends on a woman’s age at the start of the IP but is constant through the IP leads to an estimated follicular depletion rate of 2.15% per year of age. This result is biologically plausible, but the assumption of a constant hazard is contradicted by the results shown earlier, where the hazard declines with duration for the first 2 or 3 days and then follows different patterns depending on age or reproductive stage.
Fitting an accelerated depletion model, which allows for faster follicular depletion among older women, leads to a biologically implausible result where women would actually gain follicles up to age 38.6 years before depletion sets in, reflecting our observation that IPs get shorter before they become longer.
We also fitted the constant depletion model stratified by age and by reproductive stage. We obtained depletion rates of −6.0% for women less than 40 years, 12.6% for women 40–44 years, 0.2% for women 45–49 years, and 8.6% for women more than age 50 years. The results by stage show rates of −2.8% for premenopausal, 0.1 for early menopausal, and 3.1% for late perimenopausal women. In both cases the rates for younger women are negative, implying an implausible increase in the follicular pool with age.
In short, the depletion models do not account for the observed pattern of IPs by age and reproductive stage.
DISCUSSION
Hypoestrogenic intervals in the follicular phase have been called inactive phases (18, 19), delayed response cycles (20), lag phases (10), and “low-flat” estrogen profiles (3). As the terminology suggests, most of these studies refer specifically to elongated IPs. Previous studies—and our findings—show that IPs tend to occur at longer durations with increasing reproductive age (20) and appear to be responsible in large part for the elongation of menstrual cycles during perimenopause (7). However, we have expanded the investigation of IPs by considering IPs of all durations in both younger and older women, defining IPs by estrogen levels rather than tying them to the first day of menses, and using survival analysis techniques that account for variation within and across women and correct for bias that would otherwise underestimate IP lengths in our sample.
The analysis by reproductive stage shows the differences between groups most clearly, and our discussion therefore focuses primarily on the IP patterns by stage. Although it may appear that there is circularity between the staging of cycles and the lengths of inactive phases (longer cycles often have longer IPs), the staging of menstrual cycles, based on cycle length and variation in cycle length, was done independently and before this analysis of IPs.
The increased occurrence of both very short and longer IPs in the early perimenopausal stage is striking and underscores the unique nature of the perimenopause. Generally, across all reproductive stages, the increase and then decrease in very short IPs and the consistent increase in longer IPs suggest that there may be two biological mechanisms contributing to the observed pattern of IPs: one determining the immediate availability of a dominant follicle, with small differences by reproductive stage, and another related to the recruitment of a dominant follicle if one is not immediately available at the start of a cycle, with large differences by reproductive stage. The balance of these two mechanisms would explain why premenopausal women initially have a slower rate of exit from the IP but soon catch up to perimenopausal women, as well as why the chances of remaining in an IP at long durations are so much higher for women in the late perimenopause.
Follicular hyperstimulation is a likely cause of very short IPs. Elevated follicle-stimulating hormone (FSH) levels occurring in perimenopause have been shown to be associated with shorter early follicular phases (i.e., shorter IPs) (9), and other investigators have suggested that it is these elevated FSH levels that hyperstimulate the ovary and therefore advance (12, 39) follicular development for a given menstrual cycle. In addition, Baerwald et al. (14) recently suggested that there can be multiple follicle cohorts or waves under recruitment per ovarian cycle, and that this pattern increases in frequency with reproductive age (10). Multiple cohorts also could increase the likelihood of a follicle cohort being ready for stimulation by FSH at the start of a cycle.
Previous studies have pointed to follicular depletion as a potential cause of delays in follicular development that result in long IPs (7, 18, 19), although a proximate mechanism by which such delays occur is not entirely clear. Miro et al. (7) suggest that accelerating follicular depletion during perimenopause could result in increases in FSH levels that could disrupt the timing of early and dominant follicular development, implicating elevated FSH in the delay as well as the advance of follicular development (10). However, Wood et al. (18) and O’Connor et al. (19) suggest that as a woman’s follicle pool declines, there would increasingly be intervals when a cohort of developing follicles would simply be unavailable for stimulation by FSH, whether FSH levels were elevated or not. The poor fits of the constant and accelerating depletion models tested in the present study suggest that any relationship between IPs and follicular depletion is complex. Nevertheless, some of our findings (e.g., plausible follicular depletion rate similar to real follicle data [32], long right tail of IP durations in the latest reproductive stage) are consistent with general predictions of follicular depletion.
There are limitations to the current study. First, we capture several but not all types of follicular activity with our definition of the IP. Harlow et al. (3) characterize patterns of follicular phase estrogen and discuss the underlying follicular events likely to be associated with each pattern. Hale et al. (10) discuss luteal out-of-phase (LOOP) events in which follicular activity occurs in other parts of the menstrual cycle. Although our IP identification protocol is likely to have captured “low-flat” and “slow-rising” cycles (3), we explicitly excluded other patterns, such as extended peaks in estrogen at the luteal-follicular transition (3) and luteal out-of-phase events (10, 14). Second, the sample was drawn from the unique TREMIN study, thus it is homogeneous in terms of race and education. There may be genetic and other factors that affect menstrual cycles, follicular reserve, and follicular depletion that could vary by individual and by population (40–42). We are therefore limited in our ability to generalize the results to other populations (30) and may be missing women who reached menopause at early ages. The latter omission may result in underestimation of IP durations at a given age/reproductive stage and overestimation of the decline in IP duration for 40–44 year olds. Third, we excluded menstrual cycles affected by hormone use or ambiguous E1G patterns. The symptoms leading women to seek hormone therapy and the underlying cause of ambiguous E1G patterns also could be related to follicular depletion rates and follicle pool size. Finally, we do not address the relationship of IPs to reproductive hormone levels (e.g., FSH).
Our analysis emphasizes points of interest for clinicians and patients. First, long IPs do occasionally occur in premenopausal women, suggesting that the underlying processes have observable, albeit small effects, on the female reproductive axis well before the perimenopause. Second, during an elongated IP a woman will have a postmenopausal-like hormone profile, based on FSH and E1G, yet can still be premenopausal and/or perimenopausal for several more years (19, 23). The occurrence of IPs in all age groups and the median duration of 3 days for IPs indicate that single-day early follicular phase hormone levels (as tested with blood or urine samples) are not sufficient for determining a woman’s reproductive age or stage. Although cautions about single-day FSH measures are not new (e.g., cited by Metcalf et al. [23]), understanding that an IP is often the underlying reason that FSH is such a variable marker of reproductive aging should be emphasized, and underscores the need for alternative measures. The concept of the “skipped cycle” also can be clarified, as in many cases it will really be a long IP followed by ovulation. Indeed, O’Connor et al. (36) found that 25% of cycles longer than 60 days were ultimately ovulatory. Thus, understanding the underlying pattern of IPs relative to the menstrual cycle could be useful for making decisions about contraception late in reproductive life.
Supplementary Material
Acknowledgments
Profound gratitude is extended to the BIMORA participants. Thanks to Phyllis Mansfield, Susannah Barsom, Eleanor Brindle, Rebecca Miller, and the Georgetown University Graduate School of Arts and Sciences.
Supported by grants NIH R01AG015141, NIH R01HD034159, NIH F32HD007994, and NIH R24HD042828.
Footnotes
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/ferrellrj-hypoestrogenic-age-reproductive-stage-follicular-depletion/
R.J.F. has nothing to disclose. G.R. has nothing to disclose. D.H. has nothing to disclose. K.O. has nothing to disclose. J.W.W. has nothing to disclose. M.W. has nothing to disclose.
Dr. Ferrell contributed to this article in her personal capacity. The views expressed are her own and do not necessarily represent the views of the National Institutes of Health or the United States Government.
REFERENCES
- 1.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- 2.Ferrell RJ, Simon JA, Pincus SM, Rodriguez G, O'Connor KA, Holman DJ, et al. The length of perimenopausal menstrual cycles increases later and to a greater degree than previously reported. Fertil Steril. 2006;86:619–624. doi: 10.1016/j.fertnstert.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Harlow SD, Baird DD, Weinberg CR, Wilcox AJ. Urinary oestrogen patterns in long follicular phases. Human Reprod. 2000;15:11–16. doi: 10.1093/humrep/15.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW), Park City, Utah, July 2001. Menopause. 2001;8:402–407. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein M, Gorrindo T, Riley A, Mormino J, Niedfeldt J, Singer B, et al. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–791. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 6.Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF, et al. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10:112–119. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- 7.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE, et al. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: the FREEDOM Study. J Clin Endocrinol Metab. 2004;89:4910–4915. doi: 10.1210/jc.2003-031731. [DOI] [PubMed] [Google Scholar]
- 8.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35:376–384. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 9.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. Relationship between follicle-stimulating hormone levels at the beginning of the human menstrual cycle, length of the follicular phase and excreted estrogens: the FREEDOM study. J Clin Endocrinol Metab. 2004;89:3270–3275. doi: 10.1210/jc.2003-031732. [DOI] [PubMed] [Google Scholar]
- 10.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson SR, Miro F, Barrett S, Ellis JE. Levels of urinary human chorionic gonadotrophin (hCG) following conception and variability of menstrual cycle length in a cohort of women attempting to conceive. Curr Med Res Opin. 2009;25:741–748. doi: 10.1185/03007990902743935. [DOI] [PubMed] [Google Scholar]
- 12.Klein NA, Harper AJ, Houmard BS, Sluss PM, Soules MR. Is the short follicular phase in older women secondary to advanced or accelerated dominant follicle development? J Clin Endocrinol Metab. 2002;87:5746–5750. doi: 10.1210/jc.2002-020622. [DOI] [PubMed] [Google Scholar]
- 13.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocrinol Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 14.Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18:73–91. doi: 10.1093/humupd/dmr039. [DOI] [PubMed] [Google Scholar]
- 15.Shideler SE, DeVane GW, Kalra PS, Benirschke K, Lasley BL. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas. 1989;11:331–339. doi: 10.1016/0378-5122(89)90029-7. [DOI] [PubMed] [Google Scholar]
- 16.Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotrophins, immunoreactive inhibin, oestradiol and progesterone. Maturitas. 1993;18:9–20. doi: 10.1016/0378-5122(93)90026-e. [DOI] [PubMed] [Google Scholar]
- 17.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 18.Wood JW, Holman DJ, O'Connor KA. Did menopause evolve by antagonistic pleiotropy? In: Schultz M, editor. Homo - unsere Herkungt und Zukunft. Göttingen: Cuvillier; 2001. pp. 483–490. [Google Scholar]
- 19.O'Connor KA, Holman DJ, Wood JW. Menstrual cycle variability and the perimenopause. Am J Hum Biol. 2001;13:465–478. doi: 10.1002/ajhb.1078. [DOI] [PubMed] [Google Scholar]
- 20.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. Sequential classification of endocrine stages during reproductive aging in women: the FREE-DOM study. Menopause. 2005;12:281–290. doi: 10.1097/01.gme.0000147018.30796.25. [DOI] [PubMed] [Google Scholar]
- 21.Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, et al. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995;80:3537–3545. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- 22.Cramer DW, Barbieri RL, Fraer AR, Harlow BL. Determinants of early follicular phase gonadotrophin and estradiol concentrations in women of late reproductive age. Hum Reprod. 2002;17:221–227. doi: 10.1093/humrep/17.1.221. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol. 1981;14:245–55. doi: 10.1111/j.1365-2265.1981.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrell RJ, O'Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, et al. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause. 2005;12:567–577. doi: 10.1097/01.gme.0000172265.40196.86. [DOI] [PubMed] [Google Scholar]
- 25.Block E. Quantitative morphological investigations of the follicular system in women**variations at different ages. Acta anatomica. 1952;14:108–123. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]
- 26.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 27.Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- 28.Faddy MJ, Gosden RG. A mathematical model of follicle dynamics in the human ovary. Hum Reprod. 1995;10:770–775. doi: 10.1093/oxfordjournals.humrep.a136036. [DOI] [PubMed] [Google Scholar]
- 29.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 30.Coxworth JE, Hawkes K. Ovarian follicle loss in humans and mice: lessons from statistical model comparison. Hum Reprod. 2010;25:1796–1805. doi: 10.1093/humrep/deq136. [DOI] [PubMed] [Google Scholar]
- 31.Rosen MP, Sternfeld B, Schuh-Huerta SM, Reijo Pera RA, McCulloch CE, Cedars MI. Antral follicle count: absence of significant midlife decline. Fertil Steril. 2010;94:2182–2185. doi: 10.1016/j.fertnstert.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5:e8772. doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor KA, Brindle E, Shofer JB, Miller RC, Klein NA, Soules MR, et al. Statistical correction for non-parallelism in a urinary enzyme immunoassay. J Immunoassay Immunochem. 2004;25:259–278. doi: 10.1081/ias-200028078. [DOI] [PubMed] [Google Scholar]
- 34.Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- 35.O'Connor KA, Brindle E,Miller RC, Shofer JB, Ferrell RJ, Klein NA, et al. Ovulation detection methods for urinary hormones: precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod. 2006;21:1442–1452. doi: 10.1093/humrep/dei497. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16:1178–1187. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor KA, Ferrell RJ, Brindle E, Shofer J, Holman DJ, Miller RC, et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18:828–836. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Development Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 39.Santoro N, Isaac B, Neal-Perry G, Adel T, Weingart L, Nussbaum A, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502–5509. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 40.Westhoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril. 2000;74:624–628. doi: 10.1016/s0015-0282(00)01527-2. [DOI] [PubMed] [Google Scholar]
- 41.Voorhuis M, Broekmans FJ, Fauser BC, Onland-Moret NC, van der Schouw YT. Genes involved in initial follicle recruitment may be associated with age at menopause. J Clin Endocrinol Metab. 2011;96:E473–E479. doi: 10.1210/jc.2010-1799. [DOI] [PubMed] [Google Scholar]
- 42.Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early age at natural menopause in a British birth cohort study. Hum Reprod. 2010;25:791–798. doi: 10.1093/humrep/dep451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piessens R, de Doncker-Kapenga E, Überhuber C, Kahaner D. QUADPACK: a subroutine package for automatic integration. Berlin: Springer-Verlag; 1983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.