Abstract
Memantine provides clinically relevant efficacy in patients with Alzheimer's disease and Parkinson’s diseases. In addition to blockade of N-methyl-D-aspartate receptor on neurons, memantine has neurotrophic and neuroprotective effects in in vivo and in vitro studies, however, the mechanism underlying these effects remains unclear. To address this question, primary midbrain neuron-glia cultures and reconstituted cultures were used, and lipopolysaccharide (LPS), an endotoxin from bacteria, was used to produce inflammation-mediated dopaminegic neuronal death. Here, we show that memantine exerted both potent neurotrophic and neuroprotective effects on dopaminergic neurons in rat neuron-glia cultures. The neurotrophic effect of memantine was glia-dependent, since memantine failed to show any positive effect on dopaminergic neurons in neuron-enriched cultures. More specifically, it appears that astroglia, not microglia, are the source of the memantine-elicited neurotrophic effects through the increased production of GDNF. Mechanistic studies revealed that GDNF upregulaton was associated with histone hyperacetylation by inhibiting the cellular histone deacetylase activity. In addition, memantine also displays neuroprotective effects against LPS-induced dopaminergic neuronal damage through its inhibition of microglia over-activation revealed by both OX-42 immunostaining and by the reduction of pro-inflammatory factors production such as extracelluar superoxide anion, intracellular reactive oxygen species, nitric oxide, prostaglandin E2, and tumor necrosis factor-α. These results suggest that memantine therapy for neurodegenerative diseases acts in part through alternative novel mechanisms by reducing microglia-associated inflammation and stimulating the release of neurotrophic factors from astroglia.
Keywords: GDNF, neuroinflammation, neuroprotection, neurodegenerative disease, HDAC
INTRODUCTION
Memantine has been extensively investigated in recent years mainly because of its clinical efficacy in Alzheimer’s disease (AD) and neuroprotective effects in laboratory studies. In vitro studies using neuronal cell cultures demonstrated that neuronal damage induced by glutamate (Weller et al, 1993b), N-methyl-D-aspartate (NMDA) (Weller et al, 1993a), prion protein (Muller et al, 1993), and gp120 (Nath et al, 2000; Ushijima et al, 1993) were inhibited by memantine. In addition, in vivo studies show a protective effect of memantine against ischemiareperfusion injury in spontaneously hypertensive rats (Dogan et al, 1999). Recently, several clinical trials also revealed the beneficial effects of memantine on the treatment of AD (Reisberg et al, 2003), vascular dementia (Wilcock et al, 2002), and Parkinson’s disease (PD) (Merello et al, 1999). Since it is known that memantine is a NMDA receptor blocker, most of the reports attributed neuroprotective effects or clinical therapeutic benefits of memantine to its inhibitory effects on NMDA-receptor mediated excitotoxicity (Lipton, 2006).
Recent advances in research of the central nervous system (CNS) strongly suggest that glia (astroglia and microglia) are key players in neurodegenerative diseases and prime targets for therapy (Block et al, 2007; Ralay Ranaivo et al, 2006). The differential roles of astroglia and microglia in neuron survival/degeneration have been reported in our laboratory and others (Block and Hong, 2005; Liu and Hong, 2003b; Teismann et al, 2003). On one hand, astroglia have been shown to be a major source of a variety of neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), which are beneficial for the survival of neurons (Chen et al, 2006b; Lin et al, 1993) Chen et al, 2006). On the other hand, microglia mediate an inflammatory response, which is a critical component underlying the pathogenesis of a diverse range of neurodegenerative diseases, such as AD and PD (Rogers et al, 2007; Wyss-Coray, 2006). It has 6 been reported that many drugs exert neuroprotective effects by modulating functions of glial cells. Some of them are neuroprotectvie through their anti-inflammatory effects by reducing the release of pro-inflammatory factors from over-activated microglia (Liu et al, 2002; Ralay Ranaivo et al, 2006), while others enhance the production of neurotrophic factors from astroglia (Chen et al, 2006b; Darlington, 2005).
Current mechanistic studies of memantine focus mainly on its effect(s) on neurons (Lipton, 2007; Zhao et al, 2006), in contrast, its possible effects on other cell types in the CNS have not been investigated. Given the crucial role of glia in the pathogenesis of neurodegenerative diseases, it is important to examine these roles in the neuroprotective effect of memantine. A recent report from Wenk and his colleagues proposed a possible role of memantine on microglial activation (Rosi et al, 2006). Here, using a series of midbrain primary cultures, we report that memantine showed potent efficacy in protecting dopaminergic (DA) neurons against LPS-induced damage. Mechanistic studies revealed two novel mechanisms underling the neuroprotective effects of memantine: 1) increase the release of GDNF from astroglia through histone hyperacetylation on gdnf promoter region by inhibiting activity of the cellular histone deacetylase (HDAC), and 2) anti-inflammatory action by inhibiting the over-activation of microglia, which is independent of NMDA receptors.
MATERIALS and METHODS
Animals
Timed-pregnant adult female Fisher 344 rats were purchased from Charles River Laboratories (Raleigh, NC, USA). Experimental use of the animals was performed in strict accordance with the National Institutes of Health guidelines.
Mesencephalic neuron-glia cultures
Rat primary mesencephalic neuron-glia cultures were prepared using a previously described protocol (Liu and Hong, 2003a). Briefly, ventral mesencephalic tissues were dissected from day 14 embryos. Cells were dissociated by gentle mechanical trituration and immediately seeded at 5 × 105/well in 24-well plates pre-coated with poly D-lysine (20 µg/ml). Plates were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cultures were treated 7 days after plating. At the time of treatment, the composition of the cultures was approximately 48% astroglia, 12% microglia, 40% neurons, of which about 2% of cells represent tyrosine hydroxylase (TH)-immunoreactive neurons, based on immunocytochemical (ICC) analysis.
Neuron-Enriched Cultures and Microglia-Depleted Cultures
Forty-eight hours after seeding, cytosine β-D-arabinofuranoside (10 µM) and l-leucine methyl ester (LME) (1mM) were added to the mesencephalic neuron-glia cultures cultures. Cytosine β-D-arabinofuranoside and LME were removed 48 h later and replaced with fresh media. Neuronenriched cultures were 98% pure and microglia-depleted cultures were 95% pure, as indicated by ICC staining with OX-42 and GFAP antibodies.
Microglia-Enriched Cultures
Primary enriched-microglia cultures were prepared from the whole brains of 1-day-old Fisher 344 rat pups, using a previously described protocol (Liu et al, 2003a). Two weeks after seeding, 8 microglia were shaken off and either replated at 5 × 104 in a 96-well plate for superoxide and Intracellular reactive oxygen species (iROS) assays, or reseeded on top of a neuron-enriched culture in a 24-well plate at 7.5 × 104/well for a neuron-microglia co-culture.
Astroglia-Enriched Cultures
Primary enriched astroglia cultures, with a purity of 98%, were prepared, using the previously described protocol (Liu et al, 2003a). Astroglia-enriched cultures were treated with memantine 24 h after seeding. The conditioned medium was aspirated from the cells, centrifuged, filtered through 0.22-µm-pore-diameter Millipore filters, and then dialyzed overnight using the Slide-A-Lyzer Dialysis Cassette (Pierce Biotechnology Inc., Rockford, IL) to remove memantine. Conditioned media were stored at -80°C until use.
[3H] DA Uptake Assay
The [3H] DA uptake assay was performed as described previously (Liu et al, 2002). Radioactivity was determined by liquid scintillation counting with a Beckman Tri-carb 2900 TR liquid scintillation counter (Fullerton, CA). The nonspecific DA uptake observed in the presence of mazindol (10 µM) was subtracted.
Immunostaining
DA neurons were recognized with the polyclonal antibody against TH (kindly gift from Dr. John Reinhard of GlaxoSmithKline, Research Triangle Park, NC), and microglia were detected with the OX-42 antibody (PharMingen, San Diego, CA) against CR3 receptor, using previously described protocol (Liu et al, 2003a). For visual counting of TH-positive neurons, nine representative areas per well of the 24-well plate were counted under the microscope at 100 magnifications. Counting was performed in a double-blind manner by two individuals, and conclusions were drawn only when the difference was within 5%.
Superoxide Assay
Extracellular superoxide production from microglia was determined by measuring the superoxide dismutase inhibitable reduction of tetrazolium salt as previously described (Qin et al, 2002).
Intracellular ROS (iROS) Assay
The production of iROS was measured by DCFH oxidation as previously described (Qin et al, 2002).
TNF-α and PGE2 Assays
The production of TNF-α and PGE2 was measured with a commercial ELISA kit from R&D Systems (Minneapolis, MN), and from Cayman Chemical Company (Ann Arbor, MI), respectively.
Nitrite Assay
As an indicator of nitric oxide production, the amount of nitrite accumulated in culture supernatant was determined with a colorimetric assay using Griess reagent [1% sulfanilamide, 2.5% H3PO4, 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride] as previously described (Qin et al, 2002).
HDAC Activity Assay
HDAC activity was measured by using a fluorescence activity assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions. Assays were performed in 96-well plates in a final volume of 210 µl. Various concentrations of memantine (1 to 1000 µM) were incubated in the presence of 3 µg of HeLa nuclear extract HDACs in the 96-well plate. In this assay, valproic acid (VPA) (0.5 and 1 mM), being a well-known HDAC inhibitor, was included for comparison purpose. The reaction was initiated by adding acetylated substrate (100 µM) to all the wells. Plates were incubated on a shaker for 30 min at 37°C. After adding the developer incubating for 15 min at room temperature, The intensity of fluorescence was determined with the Spectra Max Plus microplate spectrophotometer (Molecular Devices), with an excitation wavelength of 340–360 nm and an emission wavelength of 440–465 nm.
HDAC Activity Assay of Astroglial Lysate
Primary astroglia cultures were treated with memanitne (1 to 1000 µM) and valproic acid (1 mM) for various time points, then total lysate was prepared following the previously described method (Wei et al, 2004). Ten µg of astroglial total lysates were used for the assay of HDAC activity.
RT-PCR Analysis
Total RNA was extracted from rat primary astroglia-enriched cultures according to the acidphenol method followed by isopropanol precipitation (Wei et al, 2004). The forward (F) and reverse (R) primers for PCR reaction were used below: rat GDNF gene (F), 5’-CACCATGAA GTTATGGGATGTCGTGGCT-3’ and (R), 5’-TCAGATACATCCACACCGTTTAG CGGA-3’; rat β-actin gene (F), TTGTAACCAACTGGGACGATATGG and (R), GAT CTTGATCTTCAT GGTGCTAGG. The protocol for touchdown PCR was as follows: denaturation (95°C, 15 sec), annealing (60°C, 30 sec) to (50°C, 30 sec), and extension (72°C, 2 min) for 20 cycles and denaturation (95°C, 15 sec), annealing (50°C, 30 sec), extension (72°C, 2 min) for 10 cycles. Reaction was carried out in a PerkinElmer 9700 thermal cycler (PerkinElmer Life And Analytical Sciences, Inc., Waltham, MA), and PCR products were analyzed using 2% agarose gels.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed on the basis of a protocol from Upstate Biotechnology ChIP kit (Upstate Biotechnology, Lake Placid, NY) with slight modifications. Briefly, astrocytes treated for indicated times were crosslinked with 1% (v/v) formaldehyde in culture medium at room temperature for 10 min. Cells were then washed, lysed, and sonicated to shear chromatin to about 200–1000 bp in length for further application to ChIP with anti-acetyl histone H4 antibodies or nonimmune rabbit IgG followed by RT-PCR as previously described (Wei et al, 2004). Primers were selected upstream of respective initiator site of gene trascription (Caumont et al, 2006b) to amplify from mononucleosomal DNA. Three primers were designed to amplify sequences proximal to the GDNF promoter region (GenBank accession no. AJ011432) as fellows: GDNF primer a (Pa), forward, 5’-CATGCTGACCTGGAAATGGGTACATTAAGC-3’; reverse, 5’-CA TCACTGTGAATGAGAGATTACACTG AGGGC-3’(−1148/−956 bp from respective initiator site ). GDNF primer b (Pb), forward, 5’-AAATCCACGCCTATGTGGATGGATCG-3’; reverse, 5’-TTTGGGGAA ACCTAA GCAAGGACAGGACT-3’ (−598/--390 bp from respective initiator site ). GDNF primer c (Pc), forward, 5’-CATGGAAATGGAGCCTAAGTCTGAGAAG-3’; reverse, 5’-CGCTGCAAGTGGGATGCATTTATAGAG-3’ (−251/−14 bp from respective initiator site ) (all the primers obtained from Sigma-Genosys (St. Louis, MO)). Levels of histone modifications at each pair set of GDNF gene promoter were determined by measuring the amount of that gene in ChIP by use of RT-PCR. Input or total DNA (nonimmunoprecipitated) and immunoprecipitated DNA were run in triplicate for each sample, and was repeated at least twice independently. The values of the ChIP DNA were normalized to the input DNA.
Western Blot Analysis
Cells were lysed in ice-cold modified radioimmunoprecipitation assay (RIPA) buffer consisting of 50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 150mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 µg/ml each aprotinin, leupeptin, and pepstatin, 1 mM Na3VO4, and 1 mM NaF. Immunobloting analysis was performed as previously described (Wei et al, 2004). Rabbit anti-GAPDH (1:1000, Abcam, Cambridge, MA) was used as a control to confirm equal loading. Protein quantitation was determined by ImageQuant software version 5.1 (Amersham Biosciences).
High Pressure Liquid Chromatography (HPLC)
Amino acids were detected and quantified by HPLC as previously described (Rawls and McGinty, 1997). Glutamate and aspartate were identified by overlaying absorption spectra at 420 and 440 nm and quantifying at 420 nm using HP Chemstation software (Hewlett Packard Company) based on peak area by comparison with an external standard calibration curve ranging from 0.1 to 5 M. The detection limit was 100 nM, based on signal to noise ratio.
Statistical Analysis
The data were expressed as the mean ± S.E.M. Statistical significance was assessed with an analysis of variance followed by Bonferroni's t test using the Statview program (Abacus concepts, Berkeley, Ca). A value of p<0.05 was considered statistically significant. Data are expressed as mean ± S.E.M. 13
RESULTS
Memantine Enhanced Survival of DA Neurons in Rat Primary Midbrain Cultures
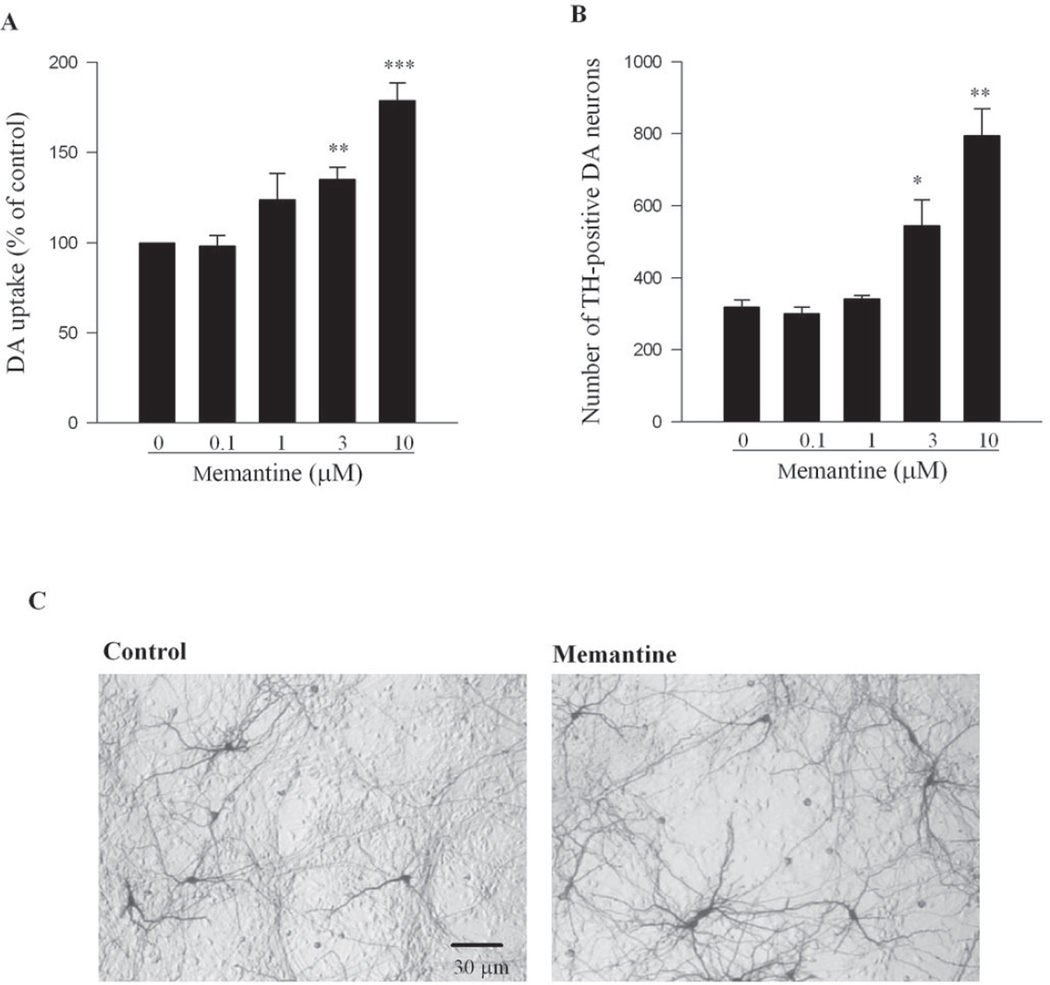
To assess the viability of DA neurons in rat primary midbrain neuron-glia cultures, [3H] DA uptake assay was used as a functional index, and immuno-cytochemical staining for TH-positive (a marker for DA neurons) neurons was used for both morphometric analysis and cell count. Various concentrations of memantine (0.1–30 µM) or vehicle were added to cultures seven days after seeding. One week later, [3H] DA uptake assay was performed. As shown in Figure 1A, memantine in the range of 3–10 µM enhanced the capacity of DA uptake in a dose-dependent manner. A higher concentration of memantine (30 µM) showed neurotoxicity (data not shown). Thus, a range of memantine concentration (0.1–10 µM) was used for the rest of the study. The increased DA uptake after memantine treatment was confirmed by the results from ICC studies (Figure 1B and 1C). Cell count analysis revealed that memantine increased the number of TH-positive neurons (DA neurons) in a dose-related manner. Furthermore, morphometric analysis indicated that memantine treatment promoted arborization of TH neurons, in both length and numbers of neurites per neuron, compared with vehicle controls.
Figure 1. Memantine enhanced the survival of DA neurons and its functional DA uptake capacity.
Rat primary midbrain neuron-glia cultures were treated with memantine (0.1– 10 µM) for 7 days. (A) The functional status of DA neurons was quantified by the [3H] DA uptake assay. Results were expressed as a percentage of the vehicle-treated control cultures. (B) The numeration of TH-positive neurons was shown and presented as TH-positive cellnumbers per well. (C) Examples of control and memantine treated neurons stained by TH antibody were shown. All results were the mean ± S.E.M. from three independent experiments in triplicate. *, p<0.05, **, p<0.01, and ***, p<0.001 compared with the vehicle-treated control cultures.
Neurotrophic Effects of Memantine Were Astroglia-Dependent
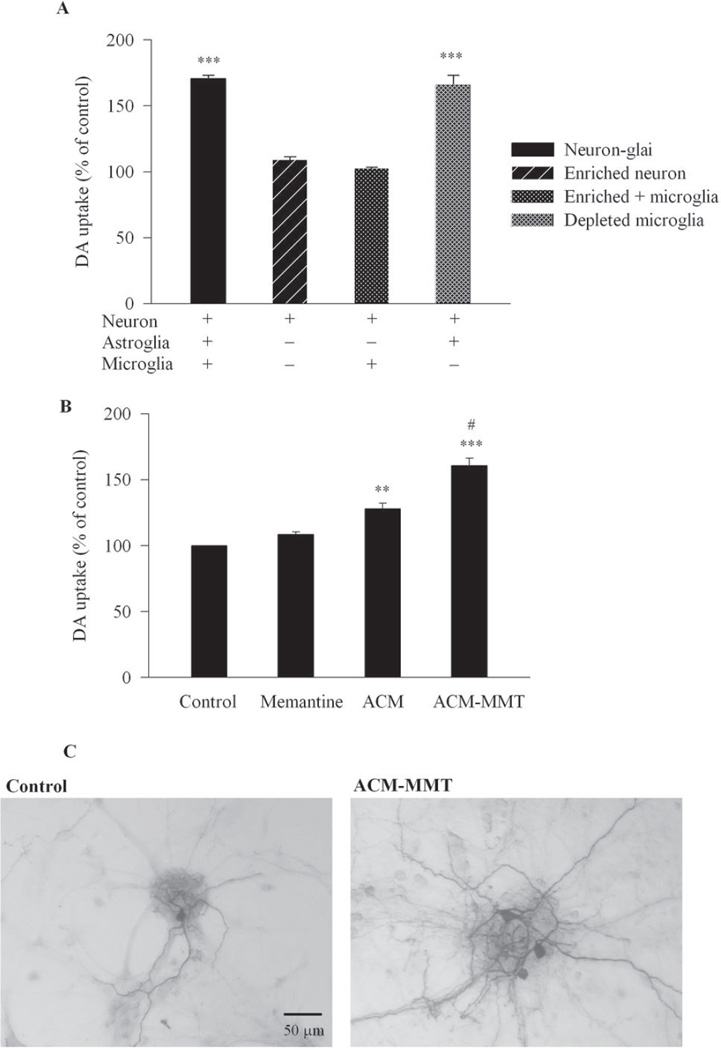
In an effort to understand the cellular mechanism underlying the enhanced number and activity of DA neurons after memantine treatment, a series of experiments using different types of cell cultures were conducted. First, neuron-enriched cultures (>98% purity) were used to investigate whether memantine has a direct effect on DA neurons. As shown in Figure 2A, while memantine (10 µM) enhanced [3H] DA uptake capacity in neuron-glia cultures, it failed to increase DA uptake capacity in neuron-enriched cultures, indicating that the observed neurotrophic effect of memantine was not due to a direct effect on DA neurons.
Figure 2. The neurotrophic effect of memanatine was astroglia-dependent.
(A) Memantine (10 µM) or vehicle was added to the following different cell cultures: neuron-glia cultures, neuron-enriched cultures, neuron-microglia co-cultures by adding 7.5 × 104/well of enriched microglia to the neuron-enriched cultures, and microglia-depleted cultures. DA neuronal function was quantified by the [3H] DA uptake assay. Results were expressed as a percentage of corresponding control cultures and were the mean ± S.E.M. from three independent experiments in triplicate. ***, p< 0.001 compared with corresponding control cultures. (B, C) The astrogliaconditioned media was prepared from astroglia-enriched cultures treated for 48 h with memantine (10 µM) (ACM-MMT) or vehicle (ACM). Seven days after adding the conditioned media to the neuron-enriched cultures, DA neuronal function was quantified by the [3H] DA uptake assay (B) and the cultures were stained with TH antibody (C). Results were expressed as a percentage of the vehicle-treated non-conditioned control cultures and were the mean ± S.E.M. from three independent experiments in triplicate. **, p<0.01, ***, p<0.001 compared with the control cultures; #, p<0.05 compared with the ACM-treated cultures.
To determine the possibility that glial cells (microglia or astroglia) mediated the memantineinduced neurotrophic effect, we used neuron-microglia co-cultures and microglia-depleted cultures (>95% purity). Neuron-enriched cultures supplemented with 7.5 × 104/well of microglia (neuron-microglia co-cultures) failed to increase DA uptake after memantine treatment (Figure 2A). On the other hand, microglia-depleted cultures that contain only neurons and astroglia, exhibited a [3H] DA uptake capacity with mematine that was comparable to that of neuron-glia cultures (Figure 2A). These three experiments suggest that astroglia, but not microglia, are potential targets for memantine-induced neurotrophic effects on DA neurons.
Conditioned Media from Memantine-Treated Astroglia Promoted Survival of DA Neurons in Neuron-Enriched Cultures
To confirm the role of astroglia in the memantine-induced survival-promoting effect and further investigate the underlying mechanism, conditioned media from astroglia-enriched cultures in the absence (ACM) or presence of 10 µM memantine (ACM-MMT) were prepared. Astroglia were incubated with or without memantine for 48 h, and conditioned media was collected and dialyzed to remove memantine. This conditioned media was then added to neuron-enriched cultures and incubated for 7 days before assays. Astroglia conditioned media significantly increased [3H] DA uptake compared with both non-conditioned media and memantine-treated cultures (Figure 2B). Menantine-treated astroglia conditioned media displayed a significant increase in DA uptake capacity, compared with astroglia conditioned media. Immunostaining analysis with anti-TH antibody showed higher levels of TH-positive neurons with more neurite configurations in enriched neuron cultures treated with menantine-treated astroglia conditioned media than those in control cultures (Figure 2C).
Memantine-Increased the Release of GDNF from Astroglia
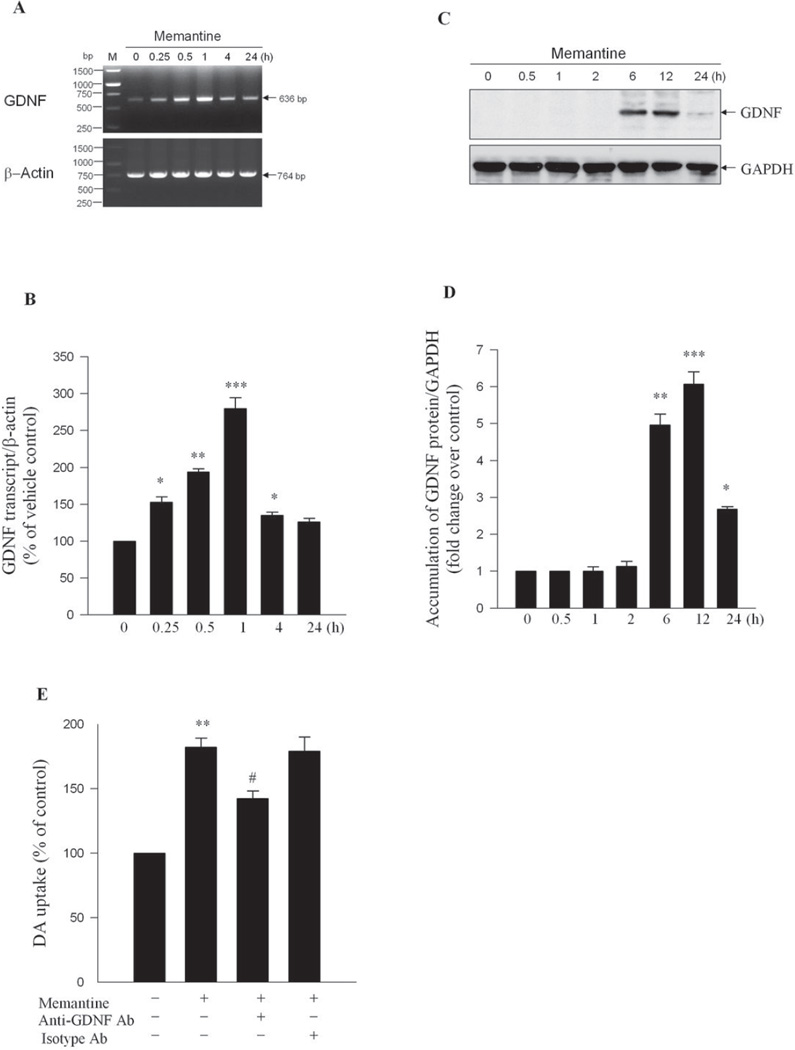
GDNF, and other growth factors have been shown to promote DA neurons survival, induce neurite outgrowth and sprouting, upregulate TH expression, and enhance synaptic efficacy (Baquet et al, 2005; Murer et al, 2001). GDNF is one of major neurotrophic factors in astroglia (Lin et al, 1993). Here, experiments were designed to test the possible involvement of GDNF in the neurotrophic effect of memantine. First, quantitative RT-PCR analysis showed that memantine treatment caused a time-dependent increase in GDNF mRNA levels in astroglial cultures. This increase reached two fold at 0.5 h, three fold at 1 h and returned to the control value at 24 h posttreatment (Figure 3A and 3B). Next, Western blot analysis revealed that memantine treatment increased the expression GDNF at 6 h and 12 h after treatment compared with 0 min (Figure 3C and 3D). A second experiment was conducted in order to provide evidence indicating GDNF was associated with the trophic effect of memantine. When the goat anti-GDNF antibody was mixed with memantine in neuron-glia cultures, the GDNF-neutralizing antibody significantly reduced memantine-enhanced DA uptake capacity; whereas treatment with goat isotype IgG control antibody has no effect (Figure 3E). Taken together, these two experiments strongly indicated a critical role of GDNF in mediating the neurotrophic effect of memantine.
Figure 3. GDNF mediated memantine-induced neurotrophic effects.
Rat primary astrocytes were exposed to 10 µM memantine for various time points ranging from 0 minute to 24 h. (A) Total RNA was extracted. Results of semiquantitative real-time PCR displayed the detection of a 635 bp band of GDNF. β-actin was used as loading control. (B) The ratio of densitometry values of GDNF and β-actin was analyzed and normalized to 0 min value. (C) Total protein of astroglial cells was extracted. Western blot analyses were performed with antibodies to GDNF. GAPDH was used as loading control. (D) The ratio of densitometry values of GDNF and GAPDH was analyzed and normalized to 0 min value. Results were expressed as mean ± S.E.M. from three experiments performed in triplicate. *, p<0.05 and **, p<0.01 ***, p<0.001 versus 0 min; (E) Neuron-glia cultures were treated with either control goat IgG (isotype Ab), or goat anti-GDNF, combined with memantine (10 µM) treatment. DA uptake capacity was measured 7 days later. **, p<0.01 compared with the vehicle-treated control cultures; #, p<0.05 compared with memantine or isotype Ab-treated cultures.
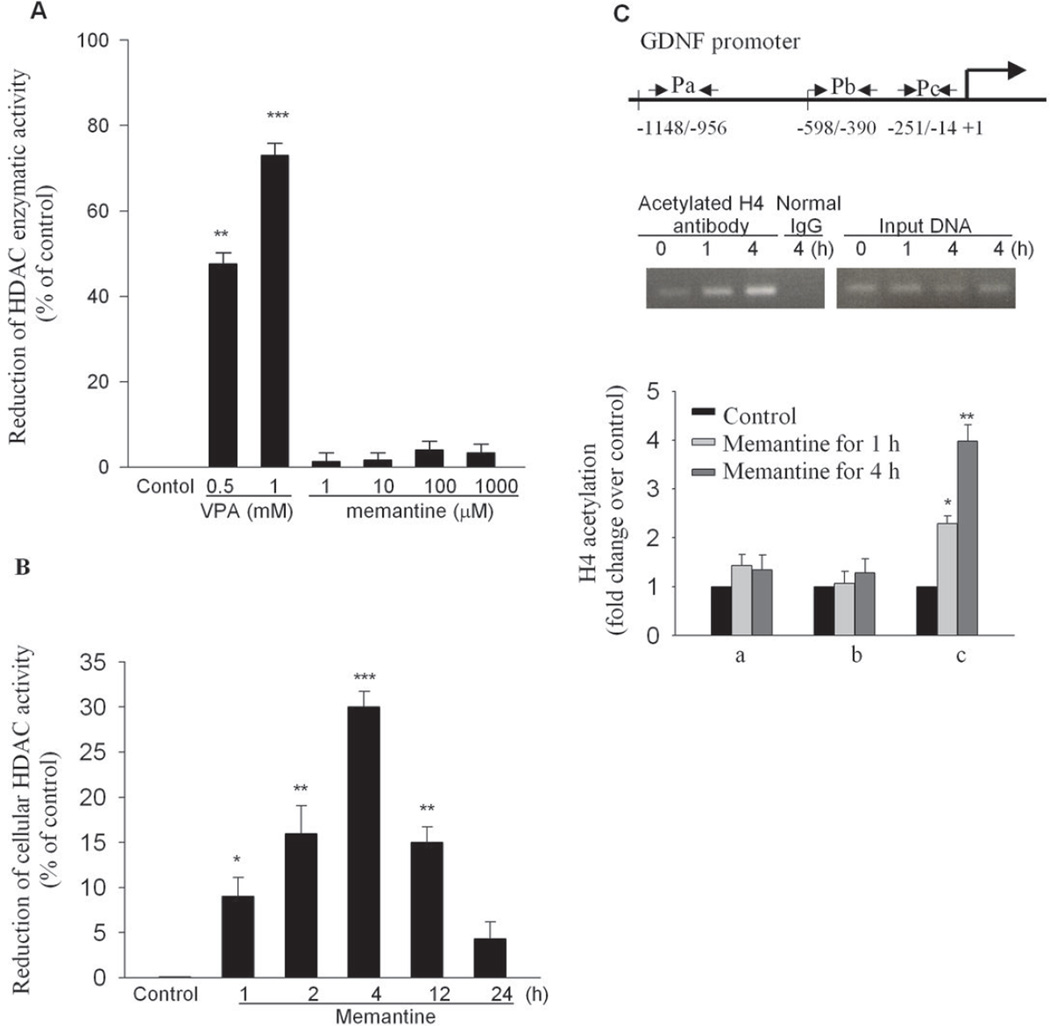
Memantine-Induced Suppression of Astroglia Cellular HDAC Activity Was Associated with Chromatin Remodeling at GDNF Promoter Region
We had previously reported that VPA increased the expression of neurotrophic factors through the inhibition of HDAC activity which was associated with the increase of histone hyperacetylation in astroglia (Wu et al, 2008). Thus, changes in histone acetylation were examined in order to further understand the molecular mechanism underlying memantine-elicited increase in the expression of GDNF. We first compared the mechanism by which these two compounds inhibit HDAC activity. HeLa nuclear extract was used as a source of HDAC enzymes for the inhibition of HDAC activity assay. As shown in Figure 4A, memantine failed to show any effect in inhibiting the HDAC activity. In contrast, VPA at clinically relevant concentrations, greatly inhibited the HDAC activity in a dose-dependent manner (Figure 4A). These findings indicate, despite their similar effects in the expression of GDNF, these two compounds are different in their effect on HDAC enzymes. Thus, we determined whether the HDAC activity is affected in the cellular levels of astroglia treated with memantine. The cellular HDAC activity in memantine-treated astroglia was significantly suppressed in a time-dependent manner and maximally at 4 hr, compared with the controls (Figure 4B). We also compared the effects of VAP (1 mM), a widely used HDAC inhibitor, and memantine (10 µM) on the HDAC activity of astrocglia at 4 hr after administration. The activity was almost equi-potently inhibited about 30% by either memantine or VPA. These results indicate that memantine and VPA inhibited HDAC activity in different mechanisms. Therefore, we examined if changes of histone acetylation levels occur at the GDNF promoter region by ChIP study. The result showed that markedly enhanced association of acetylated H4 for one (Pc) out of these three primer set regions was observed in the memantine-treated astroglia, indicating that memantine triggered recruitment of acetylated histone, specifically to the proximally close to the initiator site of GDNF gene (Figure 4C).
Figure 4. Inhibition of Cellular HDAC activity was associated with histone modification of GDNF promoter in memantine-treated astrglia.
(A) Various concentrations of memantine (1 to 1000 µM) and valproic acid (0.5 to 1 mM) were tested for inhibition of HeLa nuclear extract HDAC activity. (B) Rat primary astroglia were exposed to memantine (10 µM) for various time points. Total lysates were extracted for the cellular HDAC activity assay. (C) Chromatin was prepared from primary astroglia treated with memantine for 0, 1, and 4 h, using antibodies against acetylated H4 or normal rabbit IgG. Three pairs of primers referring to regions of GDNF promoter (Pa, Pb, and Pc) were used for ChIP study (upper panel). The levels of the GDNF promoter in the immunoprecipiates were measured by RT-PCR with these 3 primer sets (lower panel). The values of the ChIP DNA were normalized to the input DNA. Data are expressed as fold-change over the control. *, p<0.05 , **, p< 0.01, and ***, p< 0.001 compared with controls.
Neuroprotective Effects of Memantine against LPS-Induced Neurotoxicity Were Mediated through Microglia
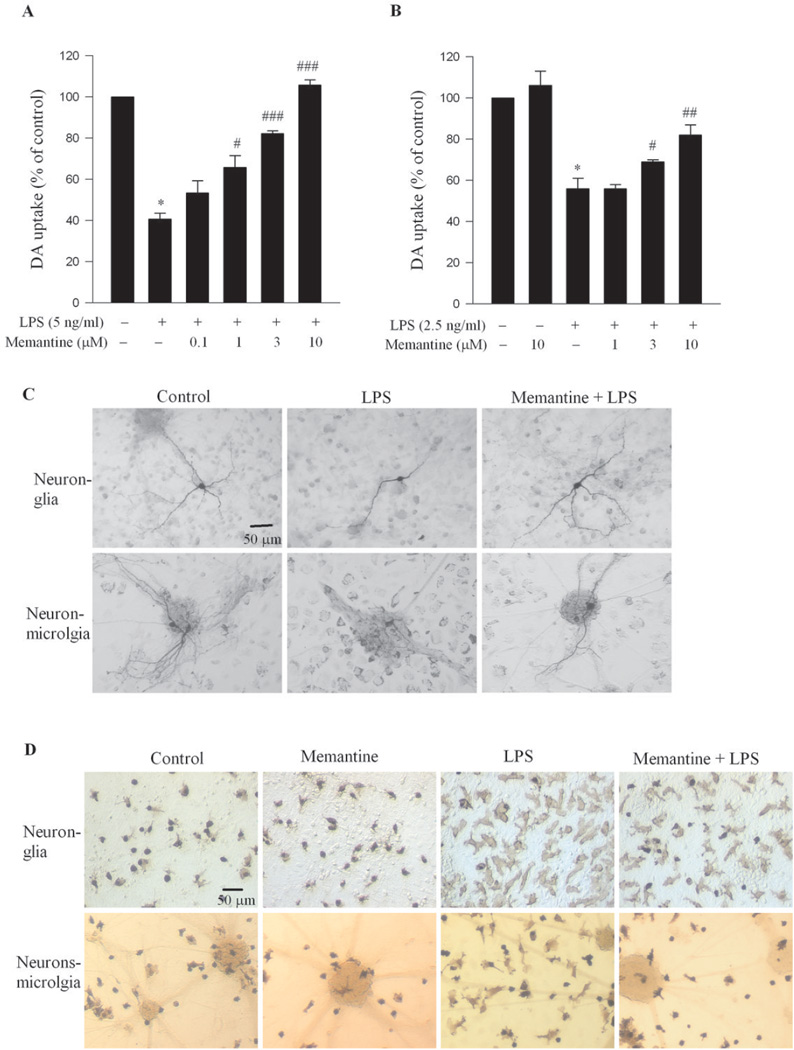
Neuroprotective effects of memantine on DA neurons in midbrain neuron-glia cultures were determined by using LPS, an endotoxin from bacteria, as pro-inflammatory stimulus to induce neurotoxicity. Neuron-glia cultures were treated with various concentrations of memantine for 30 min prior to LPS (5 ng/ml). After 7-day incubation, LPS decreased the [3H] DA uptake capacity by 60%, whereas memantine reduced LPS-induced loss of DA uptake in a dose-dependent fashion (Figure 5A). Morphological observation also revealed that DA neurons exhibited less damage in memantine-treated LPS cultures when compared with cells treated with LPS alone (Figure 5C; upper panel). Specifically, DA neurons treated with LPS in the presence of memantine displayed much longer and more elaborate TH-positive neurites compared with those from cultures treated with LPS alone (Figure 5C; upper panel).
Figure 5. Memantine was neuroprotective against LPS-induced neurotoxcity.
(A) Rat primary neuron-glia cultures were pretreated with memantine (0.1–10 µM) for 30 min before LPS (5 ng/ml) administration. [3H] DA uptake was determined seven days after LPS stimulation. (B) Neuron-enriched cultures were supplemented with 7.5 × 104/well of enriched microglia. Twenty-four hrs later, this neuron-microglia co-cultures were pre-treated with memantine (10 µM) for 30 min before LPS (2.5 ng/ml) administration. [3H] DA uptake was determined seven days after LPS stimulation. Results were expressed as a percentage of the vehicle-treated control cultures and were the mean ± S.E.M. from four independent experiments in triplicate. *, p<0.05, versus control; #, p<0.05 and ##, p<0.01, ###, p<0.001 versus LPS-treated. (C) Examples of control, LPS without or with memantine-treated neurons stained by TH antibody. Upper panel: neuron-glia cultures; lower panel: neuron-microglia co-cultures. (D) Examples of OX42 staining for activated microglia in neuron-glia cultures. (E) Examples of OX42 staining for activated microglia in neuron-microglia co-cultures.
Memantine alone produced astroglia-dependent neurotrophic effect in neuron-glia cultures (Figure 1). Therefore, astroglia will contribute to the neuroprotective effect of memantine against LPS-induced neurotoxicity. Since LPS is a potent inducer of microglial activation, the involvement of microglia would also be expected. Thus, neuron-microglia reconstituted cultures were used to determine whether microglia play a role in memantine-elicited neuroprotection. In the absence of astroglia in this culture, the role of microglia in memantine-elicited neuroprotection can be precisely evaluated. Cultures were pre-treated with various concentrations of memantine (1–10 µM) or vehicle for 30 min prior to LPS treatment (2.5 ng/ml). Lower concentration of LPS was used in neuron-microglia co-cultures because this type of culture is more sensitive to LPS treatment without the presumed protection of astroglia. Seven days later, the neurotoxic effects of LPS on DA neurons were assessed by [3H] DA uptake. In the absence of astroglia, memantine alone (10 µM) failed to increase the capacity of DA uptake in neuron-microglia co-cultures, indicating no trophic effect was observed under this condition (Figure 5B). However, memantine showed a pronounced neuroprotective effect on DA neurons against the LPS-induced neurotoxicity, strongly suggesting that microglia play a role in mediating the neuroprotective effect of memantine. The morphological changes elicited in TH-positive neurons in neuron-microglia co-cultures after LPS and/or memantine treatment were shown in figure 5C (lower panel).
The morphology of microglia in the primary midbrain neuron-glia cultures and in neuronmicroglia co-cultures was assessed by ICC staining with an OX42 antibody against the CR3 receptor, a marker for microglia. After LPS treatment, in both types of cultures, microglia dramatically changed the morphology from resting round and small shape to rod-and/or amoeboid shape with a significant enlargement of cell size (Figure 5D). Pre-treatment with memantine attenuated the LPS-induced change in morphology seen in microglial cells, indicating that memantine inhibits the activation of microglia.
Memantine Reduced LPS-Induced Production of Reactive Oxygen Species and Pro- Inflammatory Factors from Microglia
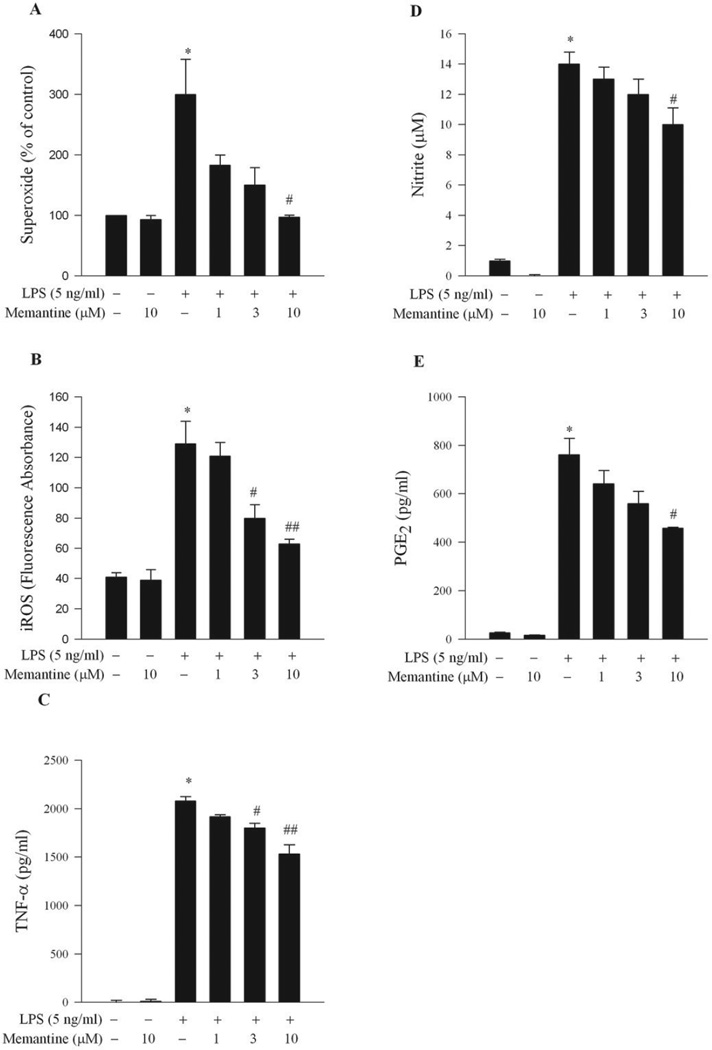
Activated microglia produces an array of pro-inflammatory factors that are key mediators underling LPS-induced DA neurotoxicity (Block et al, 2004). To provide more evidence of the anti-inflammatory effect of memantine, we determined several major inflammation-related factors released from microglia after LPS treatment. Because each factor released from microglia is different in terms of time and quantities, specific cultures and time points were tailored for each pro-inflammatory factor. Enriched microglia cultures were used for the determination of LPS-induced reactive oxygen species generation including extracellular superoxide and iROS. Memantine attenuated production of superoxide anion (Figure 6A) and iROS (Figure 6B) in a dose-dependent fashion compared with vehicle controls. In addition, in neuron-glia cultures, LPS-induced increase in TNF-α (4 h after LPS treatment) was significantly reduced by memantine (Figure 6C). The increased release of nitric oxide (NO, measured as nitrite) and PGE2 24 h after LPS stimulation was also significantly reduced in memantine-treated samples (Figure 6D and 6E).
Figure 6. Memantine inhibited LPS-induced production of reactive oxygen species (ROS), and proinflammatory factors.
(A, B) Microglia-enriched cultures seeded at 5 × 104/well in 96-well plates were pre-treated with memantine (10 µM) for 30 min before LPS (5 ng/ml) administration. (A) Production of extracellular superoxide was measured as SOD-inhibitable reduction of WST-1. Results were normalized to percentage of control level. (B) iROS production was determined by a fluorescence probe DCFH-DA. (C–E) Rat primary midbrain neuron-glia cultures were pre-treated with vehicle and memantine (1–10 µM) for 30 min before the LPS (5 ng/ml) stimulation. Supernatant was collected at 4 h for TNF-α assay (C), at 24 h for nitrite assay (D) and PGE2 assay (E). Results were expressed as mean ± S.E.M. from three 36 independent experiments in triplicate. #, p<0.05 and ##, p<0.01 versus LPS-treated cultures. *, p<0.05, versus control.
Supernatant Levels of Glutamate and Aspartate Remained Unchanged after LPS Treatment in Primary Midbrain Neuron-Glia Cultures
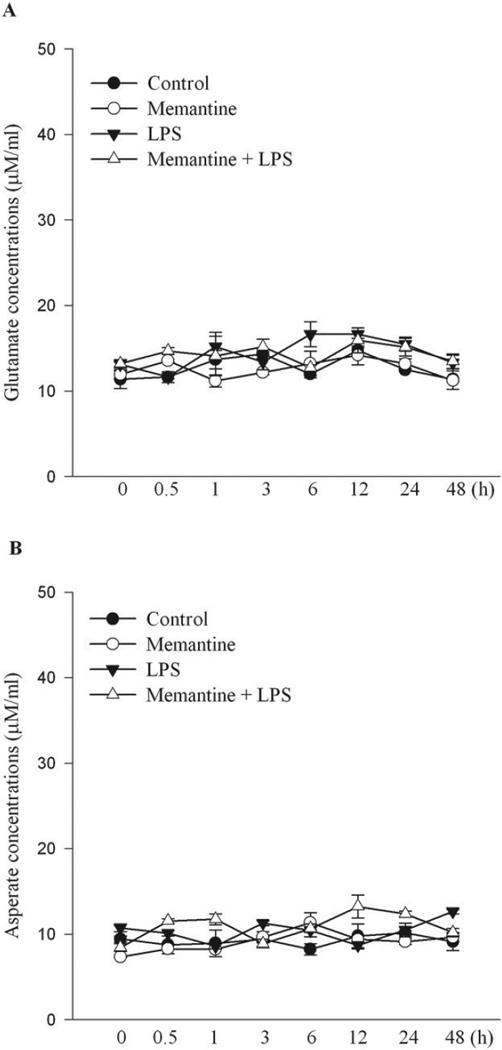
Since memantine is a NMDA receptor blocker, most of the reports attributed neuroprotective effects or clinical therapeutic benefits of this compound to its inhibitory effects on NMDA-receptor mediated excitotoxicity (Lipton, 2006). To determine whether NMDA receptors play a role in the neuroprotective effect of memantine in LPS-induced DA neurotoxicity, we measured the concentrations of two excitatory amino acids, glutamate and aspartate, in the supernatants of primary neuron-glia cultures treated with LPS and/or memantine (Figure 7). Basal levels of these two excitatory amino acids were low, around 12 µM for glutamate and 10 µM for aspartate. The levels remained unchanged for the entire time course (0.5 to 48 h) determined after LPS treatment. Supernatant concentrations of both glutamate and aspartate in cultures treated with memantine (10 µM) alone or added together with LPS did not differ from the levels of vehicle-or LPS-treated groups. These results suggested an NMDA receptor independent mechanism for glia-mediated neurotrophic and anti-inflammatory effects of memantine.
Figure 7. Concentrations of glutamate and asperate in supernatants of primary midbrain cultures.
Supernatants taken at indicated time points from primary neuron-glia cultures treated with LPS (5 ng/ml) with/without memantine (10 µM) were measured with HPLC for glutamate and asperate concentrations. The glutamate and asperate concentrations were remained in low and consistent concentrations during treatment, and not robustly fluctuated during LPS and/or memantine treatment.
DISCUSSION
This study is the first report describing a novel mechanism whereby glia mediated the neuroprotective effect of memantine. Our study showed that increase in the GDNF production from astroglia through histone remodeling enhanced the number and activity of DA neurons in the midbrain neuron-glia cultures. This action, plus a microglia-directed anti-inflammatory effect, likely underlies the neuroprotective effect of memantine against LPS-induced neurotoxicity. Furthermore, our study suggests that these glia-dependent actions of memantine are mechanistically remote from NMDA receptor.
Many reports have demonstrated potent neuroprotection by memantine in excitotoxin (such as glutamate, NMDA or gp 120)-induced neurodegeneration (Lipton, 2007; Weller et al, 1993a; Weller M, 1993). However, the majority of these in vitro studies use neuron cultures, which eliminate the opportunity to investigate the role of glial cells in the neuroprotective effect of this compound. In our study, we used a combination of various cultures including mixed neuron-glia cultures, neuron-microglia cocultures, and microglia-depleted cultures, which allow us to investigate the interaction between neurons and glial cells. With these in vitro models, we showed the main sites of action for memantine in protecting LPS-induced DA damage are the glial cells, but not the neurons.
Memantine Prolonged DA Neuron Survival in Neuron-Glia Cultures via Astroglia-Mediated GDNF Production
An important observation in this study was that memantine enhanced the release of GDNF from astroglia, which may account for its neuron survival-enhancing effect in primary neuron-glia cultures. Our data show that memantine increases DA uptake by about 70% in neuron-glia cultures (Figure 2A), compared to about only 10% increase in neuron-enriched cultures (Figure 2A). Further experiments using neuron-microglia coculture and microglia-depleted culture (Figure 2A) suggest astroglia as a critical component of memantine-mediated protection. Additionally, conditioned medium from memantine-treated astroglia significantly enhances the function of DA neurons (Figure 2B).
It has been demonstrated that neurotrophic factors play an important role in the development, maintenance, and survival of neurons, glia, and oligodendrocytes (Huang et al, 2004). Deficiency in the release of neurotrophic factors can lead to neuronal death and contribute to the pathogenesis of neurodegenerative diseases such as PD and AD (Phillips et al, 1991; Schindowski et al, 2008). Astroglia have been shown to be a major source of neurotrophic factors, especially GDNF (Darlington, 2005). Studies from both RT-PCR and Western blot analysis clearly showed elevated expression of GDNF in the memantine-treated astroglia (Figure 3). This finding is consistent with results from a previous report indicating an increase in GDNF production from C6 glioma cell line after memantine treatment (Caumont et al, 2006a). Moreover, we found that the neurotrophic effect of memantine was dramatically blocked by GDNF neutralizing antibodies (Figure 3E), suggesting that this neurotrophic factor is critical for memantine-enhanced neuronal survival.
Mechanistic studies showed a link between the inhibition of HDAC activity and the increase of GDNF transcripts in astroglia treated with memantine (Figure 4) and VPA (Wu et al, 2008), based on the enzyme activity and ChIP assays. Despite their similarity in producing neurotrophic effects, there is a critical difference in how HDAC activity was affected by these two compounds. Memantine failed to inhibit the HeLa nuclear HDAC activity, which is different from the typical HDAC inhibitors, such as valproate and Trichostain A (Di Gennaro et al, 2004; Finnin et al, 1999). Thus, we further examined the exact mechanism how memantine enhanced the epigenetic expression of neurotrophic factors. Our data showed that at the cellular level, memantine was capable of reducing the activity of astroglia HDAC in a potency similar to that of VPA. Therefore, it is likely that an indirect mechanism mediates the inhibitory effect of memantine on HDACs. We further postulated that the epigenetic effect on the expression of GDNF of memantine resulted from the inhibition of HDAC activity. This possibility was supported by a ChIP assay, which showed that memantine enhanced the association of acetylated H4 at gdnf promoter region close to the initiator site of gene transcription. This promoter-restricted localization of histone acetylation indicated that the local chromatin environment at the gdnf gene promoter region could be changed by memantine to facilitate gene transcription (Figure 4C)
Inhibition of Microglial Over-activation Underlies the Anti-inflammatory Effect of Memantine
It was recently reported that memantine treatment enhanced functional recovery and antiinflammatory effects in rat models of intracerebral hemorrhage and LPS-induced neuroinflammtion (Lee et al, 2006). Results from our study demonstrated the involvement of microglia in memantine-mediated DA neuroprotection against LPS-induced neuro-inflammation. We have previously reported that the release of microglail pro-inflammatory factors, such as superoxide production, TNF-α, nitric oxide, and prostaglandin E2 underlies LPS-induced DA neuronal toxicity in culture and animal studies (Qin et al, 2004; Qin et al, 2007). In this study, biochemical measurements of pro-inflammatory factors showed that the anti-inflammatory property of memantine is mediated through the inhibition of microglial over-activation. Release of pro-inflammatory factors, such as superoxide production, ROS (Figure 6A and 6B), TNF-α, NO, and PGE2 (Figure 6C–6E) was reduced by memantine.
Since memantine is a well-known low affinity uncompetitive antagonist of the NMDA receptor, the prevailing view as to how memantine is neuroprotective and beneficial to AD patients has focused on the blockade of NMDA receptors (Chen and Lipton, 2006a; Lipton, 2006). In excitotoxin-induced neurotoxicity models, memantine has been clearly shown to be a potent neuroprotective agent by inhibiting the opening of NMDA receptor-gated calcium channels on neurons. Several groups reported the release of excitatory amino acid from microglia by high concentration of LPS (100 ng/ml). The concentration of released glutamate, however, was limited to 10–15 µM, which may not be in sufficient concentrations to produce significant neuronal death (Obrenovitch et al, 2000). We measured the concentrations of excitatory amino acids, glutamate and aspartate, in the supernatant of neuron-glia cultures after LPS treatment by HPLC (Figure 7). Interestingly, with neurotoxic concentration of LPS (5 ng/ml), we could not detect any change in both glutamate and aspartate levels in the supernatant from 0.5 to 48 h after LPS (Figure 7). The discrapancy between our results and previous reports could come from the difference in LPS concentration. In our experiments, much lower concentration (5 ng/ml) of LPS was applied. In addition, previous reports have used microglial cultures, whereas, we used neuron-glia cultures. In our culture system, even if there was an increase in the release of glutamate or aspartate, their level would remain low due to the quick and efficient uptake by astroglia. Nevertheless, in agreement with a recent report by Wenk and colleagues who demonstrated a lack of NMDA receptors (NMDAR1) in microglia (Rosi et al, 2006), our results suggest that the anti-inflammatory effect of memantine is mediated through a novel mechanism, which is independent of NMDA receptors.
Finally it is interesting to compare the neuroprotective effects of memantine and valproate, a HDAC inhibitor. Although both compounds show anti-inflammatory effects by dampening microglial release of pro-inflamamtory factors, and increasing neurotrophic factor release from astroglia, there are major differences in their mode of actions: 1) memantine elicited its antiinflammatory effect by preventing the over-activation of microglia and reducing the release of proinflammatory factors (Figure 5 and 6). In contrast, we reported that the anti-inflammatory effect of valproate resulted from the decease in microglia number, since valproate induced apoptoic death of microglia (Chen et al, 2007) As mentioned above, despite their similar effects on the release of neurotrophic fectors from astroglia by both memantine and valproate, the mode of action mechanisms of these two compounds on HDAC inhibition was different. Unlikely the typical HDAC inhibitors, memantine does not directly inhibit HDAC (Figure 4A). Further detailed comparisons on molecular actions of these two drugs may provide insights to a possible choice of drug which can be used clinically for the neuroprotective purpose.
In conclusion, this study illustrates alternative mechanisms for neuroprotective effects of memantine by acting on glia: a neurotrophic effect mediated by astroglia through histone hyperacetylation, and an anti-inflammatory effect mediated by attenuation of microglia overactivation during inflammation. Our results also underline the emerging role of glia as active participants in neuronal survival, and further support the concept that astroglial dysfunction and aberrant activation of microglia contribute to the pathogenesis of various neurodegenerative disorders.
ACKNOWLEDGEMENTS
This project was supported by a grant from the National Cheng Kung University, Project of Promoting Academic Excellence & Developing World Class Research Centers, Taiwan, Republic of China. This research was also supported in part by the Intramural Research Program of the NIH/NIEHS.
ABBREVIATIONS
- ACM
Astrocyte conditioned media
- AD
Alzheimer’s disease
- GDNF
Glial cell line-derived neurotrophic factor
- HDAC
histone deacetylase
- LME
l-leucine methylester
- MMT
memantine
- LPS
lipopolysaccharide
- NMDA
N-methyl-D-aspartate
- PGE2
prostaglandin E2
- iROS
intracellular reactive oxygen species
- PD
Parkinson’s disease
- SOD
superoxide dismutase
- TH
tyrosine hydroxylase
- TNF-α
tumor necrosis factor-alpha
- VPA
valproic acid
REFERENCES
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Caumont AS, Octave JN, Hermans E. Amantadine and memantine induce the expression of the glial cell line-derived neurotrophic factor in C6 glioma cells. Neurosci Lett. 2006a;394:196–201. doi: 10.1016/j.neulet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Caumont AS, Octave JN, Hermans E. Specific regulation of rat glial cell line-derived neurotrophic factor gene expression by riluzole in C6 glioma cells. J Neurochem. 2006b;97:128–139. doi: 10.1111/j.1471-4159.2006.03711.x. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006a;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol Psychiatry. 2006b;11:1116–1125. doi: 10.1038/sj.mp.4001893. [DOI] [PubMed] [Google Scholar]
- Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149:203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CL. Astrocytes as targets for neuroprotective drugs. Curr Opin Investig Drugs. 2005;6:700–703. [PubMed] [Google Scholar]
- Di Gennaro E, Bruzzese F, Caraglia M, Abruzzese A, Budillon A. Acetylation of proteins as novel target for antitumor therapy: review article. Amino Acids. 2004;26:435–441. doi: 10.1007/s00726-004-0087-3. [DOI] [PubMed] [Google Scholar]
- Dogan A, Eras MA, Rao VL, Dempsey RJ. Protective effects of memantine against ischemia-reperfusion injury in spontaneously hypertensive rats. Acta Neurochir (Wien) 1999;141:1107–1113. doi: 10.1007/s007010050491. [DOI] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- Huang Y, Cheung L, Rowe D, Halliday G. Genetic contributions to Parkinson's disease. Brain Res Brain Res Rev. 2004;46:44–70. doi: 10.1016/j.brainresrev.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Kim J, Kim EH, Kim SJ, et al. Memantine reduces hematoma expansion in experimental intracerebral hemorrhage, resulting in functional improvement. J Cereb Blood Flow Metab. 2006;26:536–544. doi: 10.1038/sj.jcbfm.9600213. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Pathologically-activated therapeutics for neuroprotection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr Drug Targets. 2007;8:621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Primary rat mesencephalic neuron-glia, neuron-enriched, microgliaenriched, and astroglia-enriched cultures. Methods Mol Med. 2003a;79:387–395. doi: 10.1385/1-59259-358-5:387. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003b;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1-42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Merello M, Nouzeilles MI, Cammarota A, Leiguarda R. Effect of memantine (NMDA antagonist) on Parkinson's disease: a double-blind crossover randomized study. Clin Neuropharmacol. 1999;22:273–276. [PubMed] [Google Scholar]
- Muller WE, Ushijima H, Schroder HC, Forrest JM, Schatton WF, Rytik PG, et al. Cytoprotective effect of NMDA receptor antagonists on prion protein (PrionSc)-induced toxicity in rat cortical cell cultures. Eur J Pharmacol. 1993;246:261–267. doi: 10.1016/0922-4106(93)90040-g. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000;47:186–194. [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J, Zilkha E, Jay TM. Excitotoxicity in neurological disorders--the glutamate paradox. Int J Dev Neurosci. 2000;18:281–287. doi: 10.1016/s0736-5748(99)00096-9. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralay Ranaivo H, Craft JM, Hu W, Guo L, Wing LK, Van Eldik LJ, et al. Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26:662–670. doi: 10.1523/JNEUROSCI.4652-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF. L-trans-pyrrolidine-2,4-dicarboxylic acid-evoked striatal glutamate levels are attenuated by calcium reduction, tetrodotoxin, and glutamate receptor blockade. J Neurochem. 1997;68:1553–1563. doi: 10.1046/j.1471-4159.1997.68041553.x. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder? Int Rev Neurobiol. 2007;82:235–246. doi: 10.1016/S0074-7742(07)82012-5. [DOI] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142:1303–1315. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Schindowski K, Belarbi K, Buee L. Neurotrophic factors in Alzheimer's disease: role of axonal transport. Genes Brain Behav. 2008;7(Suppl 1):43–56. doi: 10.1111/j.1601-183X.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, et al. Pathogenic role of glial cells in Parkinson's disease. Mov Disord. 2003;18:121–129. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- Ushijima H, Ando S, Kunisada T, Schroder HC, Klocking HP, Kijjoa A, et al. HIV-1 gp120 and NMDA induce protein kinase C translocation differentially in rat primary neuronal cultures. J Acquir Immune Defic Syndr. 1993;6:339–343. [PubMed] [Google Scholar]
- Wei SJ, Trempus CS, Ali RC, Hansen LA, Tennant RW. 12-O-tetradecanoylphorbol-13-acetate and UV radiation-induced nucleoside diphosphate protein kinase B mediates neoplastic transformation of epidermal cells. J Biol Chem. 2004;279:5993–6004. doi: 10.1074/jbc.M310820200. [DOI] [PubMed] [Google Scholar]
- Weller M, Finiels-Marlier F, Paul SM. NMDA receptor-mediated glutamate toxicity of cultured cerebellar, cortical and mesencephalic neurons: neuroprotective properties of amantadine and memantine. Brain Res. 1993a;613:143–148. doi: 10.1016/0006-8993(93)90464-x. [DOI] [PubMed] [Google Scholar]
- Weller MMA, Finiels-Marlier F, Martin B, Paul SM. MK-801 and memantine protect cultured neurons from glutamate toxicity induced by glutamate carboxypeptidase-mediated cleavage of methotrexate. Eur J Pharmacol. 1993;248:303–312. doi: 10.1016/0926-6917(93)90004-a. [DOI] [PubMed] [Google Scholar]
- Weller M, Marini AM, Finiels-Marlier F, Martin B, Paul SM. MK-801 and memantine protect cultured neurons from glutamate toxicity induced by glutamate carboxypeptidase-mediated cleavage of methotrexate. Eur J Pharmacol. 1993b;248:303–312. doi: 10.1016/0926-6917(93)90004-a. [DOI] [PubMed] [Google Scholar]
- Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17:297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11:1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Zhao X, Marszalec W, Toth PT, Huang J, Yeh JZ, Narahashi T. In vitro galantaminememantine co-application: mechanism of beneficial action. Neuropharmacology. 2006;51:1181–1191. doi: 10.1016/j.neuropharm.2006.08.007. [DOI] [PubMed] [Google Scholar]