SUMMARY
Integrative organ crosstalk regulates key aspects of energy homeostasis, and its dysregulation may underlie metabolic disorders such as obesity and diabetes. To test the hypothesis that crosstalk between the liver and pancreatic islets modulates β cell growth in response to insulin resistance, we used the liver-specific insulin receptor knockout (LIRKO) mouse, a unique model that exhibits dramatic islet hyperplasia. Using complementary in vivo parabiosis and transplantation assays, as well as in vitro islet culture approaches, we demonstrate that humoral, nonneural, non-cell-autonomous factor(s) induces β cell proliferation in LIRKO mice. Furthermore, we report that a hepatocyte-derived factor(s) stimulates mouse and human β cell proliferation in ex vivo assays, independent of ambient glucose and insulin levels. These data implicate the liver as a critical source of β cell growth factor(s) in insulin-resistant states.
INTRODUCTION
Diabetes has reached epidemic proportions in both developed and developing countries, and the cost of treating individuals with complications resulting from uncontrolled hyperglycemia is a major economic burden in the world. A promising but still unrealized goal of efforts to improve diabetes therapy is the identification of novel factors that promote β cell regeneration, with the long-term goal of increasing functional β cell mass in patients with either type 1 or type 2 diabetes. Reduced functional β cell mass is a central feature in both forms of the disease and in diabetes associated with obesity (Muoio and Newgard, 2008). While autoimmune destruction of β cells is the major cause of β cell loss in type 1 diabetes, a failure of β cells to compensate for ambient insulin resistance leads to uncontrolled hyperglycemia in type 2 diabetes.
Lending encouragement to therapeutic strategies aimed at enhancing β cell mass, decades of research indicate that β cells possess the capacity to compensate for both physiological (pregnancy) and pathological (obesity) insulin resistance (Ogilvie, 1933; Van Assche et al., 1978). Although β cell growth in both humans and rodents has been documented to occur through self-duplication of preexisting β cells (Dor et al., 2004; Meier et al., 2008; Teta et al., 2007), albeit at low levels, the source of putative growth factor(s) mediating this process, especially in the context of insulin resistance, remains unknown. Among possible systemic regulators of β cell mass, gut-derived incretins such as glucagon-like peptide-1 (GLP-1), glucose-dependent insulin-tropic polypeptide (GIP) (Renner et al., 2010; Saxena et al., 2010), adipocyte-derived adipokines including leptin (Morioka et al., 2007) and adiponectin (Holland et al., 2011), muscle-derived myokines such as IL-6 (Ellingsgaard et al., 2008; Suzuki et al., 2011), macrophage-derived cytokines including IL-1β, IFNγ, and TNF-α (Wang et al., 2010), bone-derived osteocalcin (Ferron et al., 2008), thyroid-derived T3/T4 hormones (Jörns et al., 2010; Verga Falzacappa et al., 2010), platelet-derived growth factor (PDGF) (Chen et al., 2011), serotonin (Kim et al., 2010), and FGF21 (Wente et al., 2006) have each been implicated. However, the lack of significant and consistent alterations in these known factors in the peripheral blood that can fully account for the β cell proliferation in the insulin-resistant LIRKO mouse model (Table S1) prompted us to explore the presence of an as yet unidentified factor that is derived from an insulin-resistant liver. To test the hypothesis that crosstalk between the liver and pancreatic islets, communicated via a systemic humoral factor, mediates compensatory β cell regeneration in the LIRKO mouse, we used in vivo (parabiosis, transplantation) and in vitro (primary islet β cell proliferation assay) models to identify blood-borne and hepatocyte-produced soluble factors on β cell proliferation.
RESULTS AND DISCUSSION
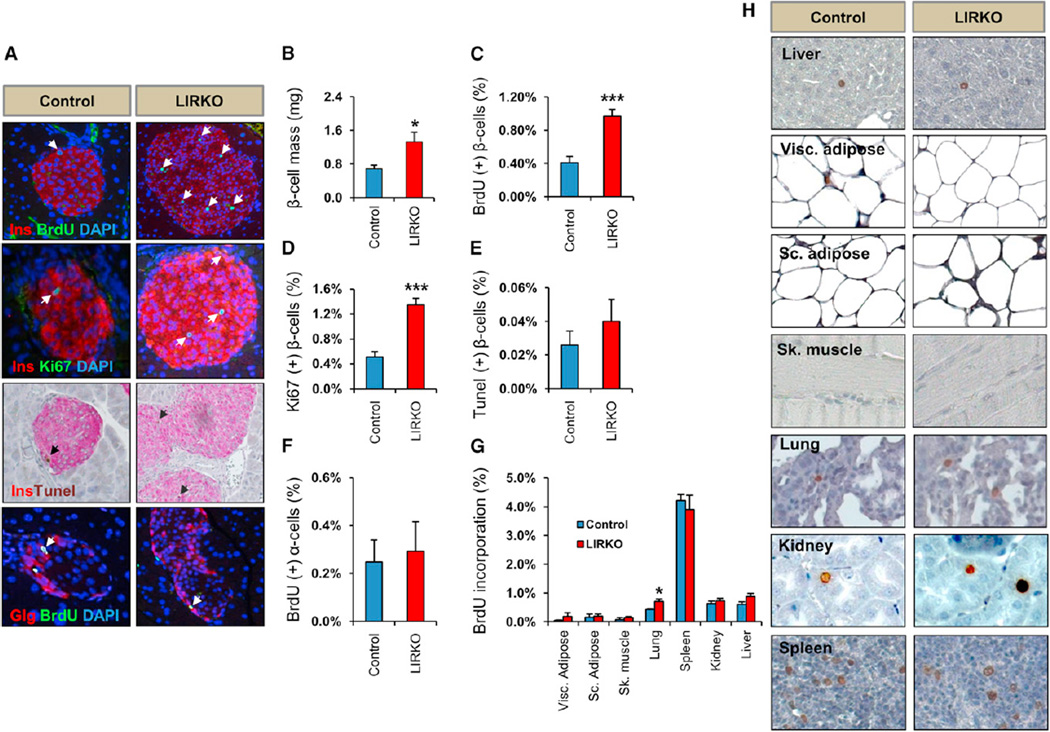
Concerted efforts in diabetes research are aimed at identifying molecules that specifically promote β cell regeneration without adverse proliferation of cells in other tissues. To determine whether LIRKO mice, which manifest a dramatic hyperplasia of the endocrine pancreas, exhibit increased proliferation in extrapancreatic tissues, we injected bromodeoxyuridine (BrdU; 100 mg/kg body weight) intraperitoneally in 3-month-old LIRKO mice and assessed proliferation of β cells, α cells, and cells in metabolic organs such as the liver, adipose and skeletal muscle, and in nonmetabolic tissues such as the lung, kidney, and spleen. We observed a 2-fold increase in β cell mass (LIRKO 1.32 ± 0.2 versus control 0.68 ± 0.08 mg; p < 0.05; n = 6) in LIRKO mice compared to littermate controls that was due to enhanced β cell proliferation evidenced by a 2.5-fold increase in BrdU incorporation (LIRKO 1% ± 0.08% versus control 0.4% ± 0.07% BrdU+ β cells; p < 0.001; n = 6) and Ki67 staining (LIRKO 1.34% ± 0.1% versus control 0.51% ± 0.08% Ki67+ β cells; p < 0.001; n = 6) in the LIRKOs. TUNEL staining did not reveal significant differences in the number of apoptotic β cells between groups. We also observed no difference in α cell proliferation (LIRKO 0.24% ± 0.09% versus control 0.29% ± 0.1% BrdU+ α cells; n = 6) (Figures 1A–1F), or in the proliferation of cells in multiple non-β cell tissues, including visceral adipose, subcutaneous adipose, muscle, kidney, liver, or spleen. Although we did observe some increase in proliferating lung cells (LIRKO 0.7% ± 0.02% versus control 0.43% ± 0.08% BrdU+ cells; n = 6; p < 0.05) (Figures 1G and 1H), histological analyses of tissues dissected from 12-month-old LIRKOs revealed no tumor-like phenotypes (Figure S1; Table S2), and the life span of the LIRKOs was similar to littermate controls. These data indicate that LIRKO mice exhibit a robust β cell-specific proliferation in response to insulin resistance.
Figure 1. Selective b Cell Proliferation in LIRKO Mice.
Three to 4-month-old LIRKO and control mice were intraperitoneally injected with BrdU (100 mg/kg body weight) 5 hr before animals were sacrificed, and tissues were dissected, fixed and stained as indicated.
(A) Pancreatic sections immunostained for insulin/BrdU/DAPI, insulin/Ki67/DAPI, insulin/TUNEL, or glucagon/BrdU/DAPI as indicated.
(B) β Cell mass quantification.
(C and D) Quantification of BrdU+ insulin+ and Ki67+ insulin+ cells: between 2,000 and 5,000 insulin+ cells per animal were counted in control versus LIRKO pancreases, respectively.
(E) Quantification of TUNEL+ insulin+ cells: between 2,000 and 5,000 insulin+ cells/mouse were counted in control versus LIRKO pancreases, respectively.
(F) Quantification of BrdU+ glucagon+ cells: between 2,000 and 5,000 insulin+ cells/mouse were counted in control versus LIRKO pancreases, respectively.
(G) Quantification of nuclei BrdU+ in indicated tissues: 4,000–5,000 cells/mouse were counted in each of liver, kidney, spleen, and lung, and 1,500 cells/mouse were counted in each for visceral (Visc.) and subcutaneous (Sc.) adipose tissue and skeletal muscle (Sk).
(H) Representative images of proliferating cells in tissue sections stained with BrdU.
Data represent mean ± SEM. *p ≤ 0.05 and ***p ≤ 0.001 (n = 6 in each group). See also Figure S1 and Table S2.
Circulating Nonneuronal Nonautonomous Factors Drive β Cell Replication in LIRKO Mice
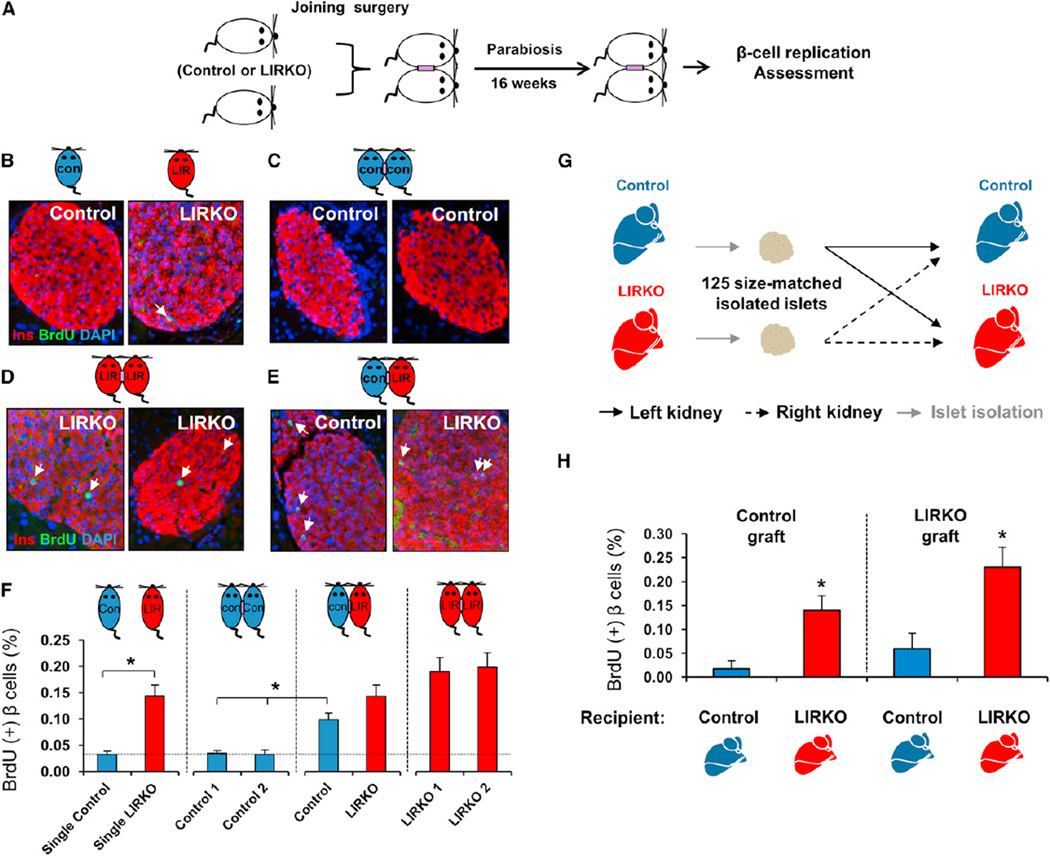
To directly address whether β cell proliferation in the LIRKO mouse is mediated by systemic factors, we first used a parabiosis model (Bunster and Meyer, 1933). Five to 6-week-old male mice were surgically joined at the shoulder and hip girdles, and successful anastomosis was confirmed within 2 weeks of joining by Evans Blue Dye injection (data not shown). Animals remained parabiosed for 16 weeks, and β cell replication was subsequently assessed by BrdU incorporation (Figure 2A). Three surgical models were generated: control/control, control/LIRKO, and LIRKO/LIRKO. All parabiont groups grew normally, with a weekly increase in their body weights, and the blood glucose of the parabiont partners was within the normal range and did not significantly differ between groups (Figures S2A–S2C). After 16 weeks of parabiosis, LIRKO and control parabionts displayed similar fasting blood glucose levels and circulating insulin levels and were higher in control partners joined with LIRKOs compared to nonparabiosed controls and controls parabiosed with controls (Figures S3A–S3D). As expected, BrdU incorporation revealed low β cell mitosis in control mice and a significant elevation in LIRKO animals (control 0.03% ± 0.005% versus LIRKO 0.14% ± 0.02% BrdU+ β cells; p < 0.005; n = 5–6). We also noted a low level of β cell proliferation in the control parabionts (Figures 2F and S4) compared to the single controls (Figures 1C and 1D). We believe this may be secondary to the parabiosis procedure itself and requires further investigation. BrdU incorporation was similar in pancreatic β cells of same-genotype parabionts: low in control/control (~0.03% BrdU+ β cells; n = 5–6); and high in LIRKO/LIRKO (~0.19% BrdU+ β cells; n = 5–6). Interestingly, BrdU incorporation was significantly increased in pancreatic β cells of control mice joined with LIRKO mice (control in control/LIRKO parabionts 0.09% ± 0.01% versus control in control/control parabionts [0.03% ± 0.004% and 0.03% ± 0.008%] BrdU+ β cells; p < 0.01; n = 5–6) (Figures 2B–2F). The latter observations were confirmed by immunostaining for phospho-Histone H3 (pHH3) (Figure S4). These data indicate the presence of cell nonautonomous, circulating factors produced in LIRKO mice that promote β cell replication. Previous studies have implicated neural pathways in modulating β cell proliferation in a cell-nonautonomous fashion (Imai et al., 2008). To evaluate a possible influence of such neural effects on β cell proliferation in the LIRKO model, we undertook transplantation studies to assess β cell replication. A total of 125 size-matched islets freshly isolated from either control or LIRKO mice were transplanted under the kidney capsule of either control or LIRKO recipients. To minimize systematic error, each recipient mouse (control or LIRKO) was transplanted with two islet grafts, one derived from control and the other derived from LIRKO donors, under the left and right kidney, respectively (Figure 2G). Sixteen weeks after transplantation, islet grafts were harvested, sectioned, and analyzed for β cell BrdU incorporation. As expected, control islets grafted into control animals exhibited minimal β cell proliferation (0.017% ± 0.017% BrdU+ β cells). Intriguingly, the same donor-derived control islets showed an ~8-fold increase in β cell replication when transplanted instead into LIRKO recipients (0.139% ± 0.03% BrdU+ β cells; p < 0.05; n = 3–5). Notably, LIRKO islets transplanted into LIRKO recipients exhibited robust β cell replication, reminiscent of the increased β cell proliferation seen in the pancreas of unmanipulated LIRKO mice, whereas this response was blunted when LIRKO islets were grafted instead into control animals (Figure 2H). Taken together, these two complementary experimental strategies provide evidence that circulating nonneural and non-cell-autonomous factors contribute to expanding β cell mass in response to insulin resistance.
Figure 2. Circulating Nonneuronal Nonautonomous Factors Drive b Cell Replication in LIRKO Mouse.
(A) Schematic of the parabiosis experiment. See also Figures S2, S3, and S4.
(B–E) Single and parabiont models were intraperitoneally injected with BrdU (100 mg/kg body weight) 5 hr before animals were sacrificed, and pancreases were dissected and immunostained for insulin, BrdU, and DAPI.
(F) Quantification of BrdU+ insulin+ cells: three sections separated by 80 µm were analyzed, and between 2,000 and 10,000 cells were counted in each group (n = 5–6 in each parabionts group).
(G) Schematic of the transplantation experiment.
(H) Quantification of BrdU+ insulin+ cells in islet grafts as indicated: three to six islet graft sections were analyzed and counted between 2,000 and 10,000 cells in each group (n = 3–5 in each group).
Data represent mean ± SEM. *p ≤ 0.05.
LIRKO Serum Induces Selective b Cell Replication In Vivo
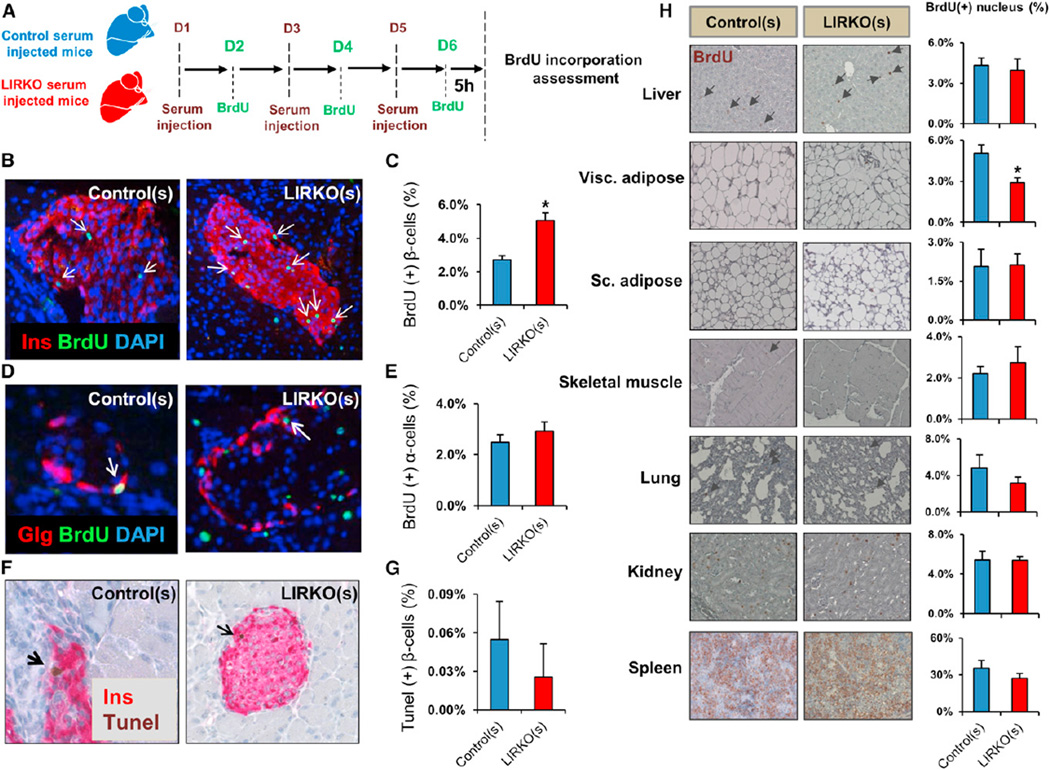
We next sought to evaluate the relative importance of blood-borne molecules versus cells in the induction of β cell proliferation in the LIRKO model. Five to 6-week-old male mice were injected intraperitoneally with freshly isolated serum from 6-month-old control or LIRKO mice, respectively, twice a day (150 µl per injection) on days 1, 3, and 5. The recipients were injected with BrdU (100 mg/kg body weight) once a day on days 2, 4, and 6. The pancreases were harvested on day 6 to assess β and α cell replication (Figure 3A). Control mice injected with LIRKO serum (LIRKO(s)) displayed an ~2-fold increase in their endogenous β cell, but not α cell, replication compared to littermates injected with control serum (control(s)) (Figures 3B–3E). We saw no significant difference in the number of TUNEL+ β cells (Figures 3F and 3G) between LIRKO(s) and control(s)-injected groups. Assessment of BrdU incorporation in extrapancreatic tissues, including liver, subcutaneous adipose, muscle, kidney, spleen, and lung, revealed no significant differences in proliferation between groups, whereas a mild decrease was observed in visceral adipose (Figure 3H). This in vivo study confirms that a circulating molecule(s), stable in serum, selectively promotes β cell proliferation in the LIRKO model.
Figure 3. LIRKO Serum Induces Selective β Cell Replication In Vivo.
Five to 6-week-old mice were injected intraperitoneally twice daily with 150 µl serum derived from 6-month-old control or LIRKO mice on days 1, 3, and 5. BrdU was injected intraperitoneally (100 mg/kg body weight) on days 2, 4, and 6. Animals were sacrificed 5 hr before the last BrdU injection, and tissues were dissected for immunostaining studies.
(A) Schematic of the experimental design.
(B and C) Representative images and quantification of BrdU+ insulin+ cells: two sections separated by 80 µm were analyzed, and at least 4,000 insulin+ cells were counted for each animal.
(D and E) Representative images and quantification of BrdU+ glucagon+ cells: 400–600 glucagon+ cells were counted in each animal.
(F and G) Representative images and quantification of TUNEL+ insulin+ cells: at least 2,000 insulin+ cells were counted in each animal.
(H) Representative images and quantification of nuclei BrdU+ in indicated tissues: for each animal, 4,000–5,000 cells were counted in sections from liver, kidney, spleen, and lung, and 1,500 cells were counted in sections from visceral (Visc.) and subcutaneous (Sc.) adipose tissue and skeletal muscle.
Data represent mean ± SEM. *p ≤ 0.05 (n = 3 in each group).
LIRKO Serum Increases Mouse and Human Islet β Cell Replication In Vitro
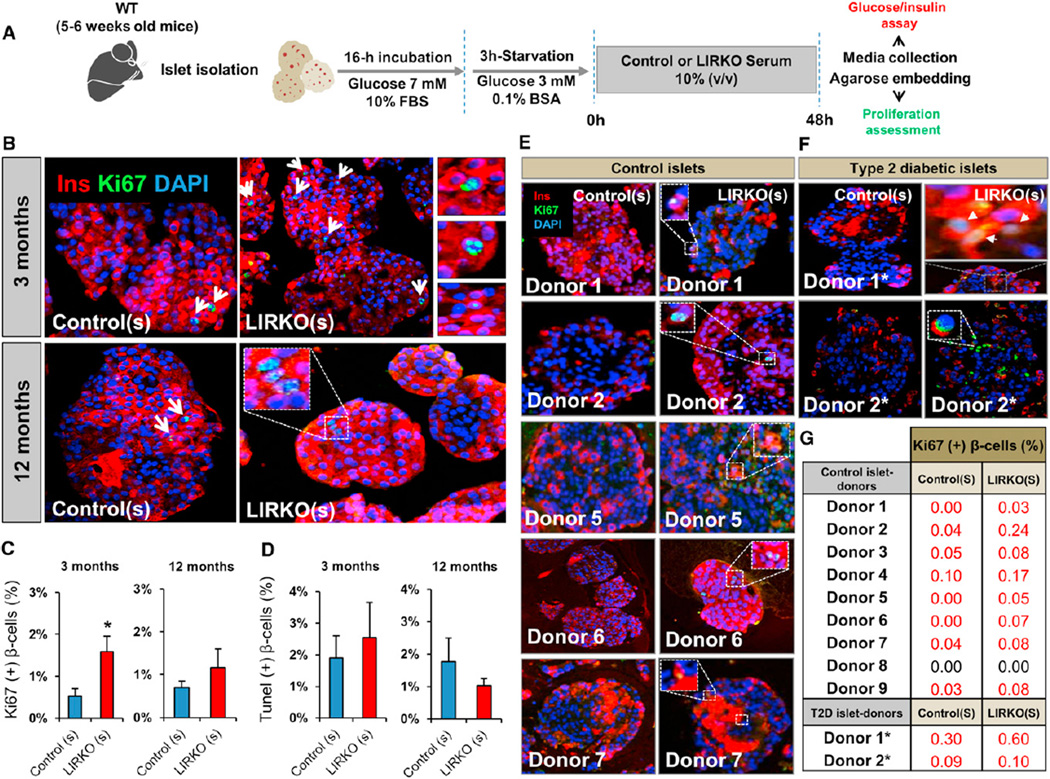
To gain further insight into the mode of action of this circulating β cell growth factor, we next established an in vitro functional assay to directly assess the impact of LIRKO or control serum on β cell replication in isolated mouse islets. We cultured islets in media containing serum from LIRKO or control mice and then assessed β cell proliferation using Ki67 immunostaining and fluorescence microscopy. Randomly selected Ki67+ β cells in each of the groups in all experiments were confirmed by confocal microscopy (Figure 4A). We first tested the ability of 6-month-old LIRKO serum to stimulate β cell proliferation and found that a 1:10 dilution of serum derived from LIRKO mice increased β cell proliferation in primary islets at 24 and 48 hr (Figure S5). LIRKO serum from 3-month-old mice also enhanced β cell proliferation in mouse islets (LIRKO(s) 1.58% ± 0.3% versus control(s) 0.52% ± 0.1% Ki67+ β cells; p < 0.05; n = 6). Moreover, mouse islets cultured in 12-month-old LIRKO serum showed a greater number of replicating β cells compared to islets incubated with age-matched control serum (LIRKO(s) 1.3% ± 0.5% versus control(s) 0.7% ± 0.2% Ki67+ β cells; p = 0.3; n = 4–6) (Figures 4B and 4C); this increase lost its statistical significance probably due to an elevated insulin resistance in aging controls that itself contributed to β cell proliferation (Kulkarni et al., 2003; Mori et al., 2010). TUNEL staining showed no significant difference in β cell apoptosis in islets cultured in LIRKO(s) versus control(s) (Figure 4D). Preliminary data indicate that the ability of the LIRKO serum to stimulate β cell proliferation is reduced when subjected to heat inactivation, suggesting that the putative circulating factor may be a protein (data not shown). To examine whether the proliferating effect of LIRKO serum is conserved across species, we next cultured human islets from nine healthy and two diabetic donors (for donor characteristics, see Table S3) in serum isolated from 12- to 18-month-old male LIRKO or control mice. Similar to the effects on mouse β cells, serum from LIRKO mice enhanced human islet β cell proliferation, albeit at a level lower than that reported in a recent study byRieck et al. (2012). Importantly, LIRKO serum was also effective in promoting proliferation of islet β cells from patients with type 2 diabetes (Figures 4E–4G). Thus, the β cell mitogen(s) present in the circulation of LIRKO mice shows conserved activity toward mouse and human islets, including islets from patients with type 2 diabetes. Glucose and insulin have been reported to promote β cell growth (Assmann et al., 2009a, 2009b; Bonner-Weir et al., 1989) and are potential candidates in the LIRKO model, which manifests glucose intolerance and hyperinsulinemia (Michael et al., 2000). However, our observations suggest that glucose is not a dominant factor in the LIRKO mouse for several reasons. First, control mice parabiosed to LIRKOs for 16 weeks demonstrate up to a 7-fold increase in proliferation despite normal blood glucose levels (~120 mg/dl) during the parabiosis period (Figures S2A and S2C). Second, serum used to examine the effects on β cell proliferation (see Figure 4A) was derived from either normoglycemic 3-month-old or hypoglycemic 12-month-old animals (data not shown). Finally, to further exclude a role for glucose, we cultured islets in a constant concentration of 5.5 mM glucose in experiments with serum from LIRKO or control mice (serum:culture media at 1:10 dilution) and observed an increase in proliferation in β cells only in the former group. Furthermore, the glucose levels in culture media at the beginning and at the end of islet incubation were similar in both groups (Figure S6A). We believe that insulin may be permissive but unlikely to account for the high level of β cell proliferation in our model because the levels of insulin in the diluted serum (Figure S6B) used in in vitro studies (see Figure 4) are significantly lower compared to the levels found in the circulation in LIRKO mice (11.63 ± 2.4 ng/ml [3-month-old LIRKOs] versus 2.59 ± 1 ng/ml [diluted serum in culture media] and 17.8 ± 4.4 ng/ml [12-month-old LIRKOs] versus 1.4 ± 1.3 ng/ml [diluted serum in culture media]) (Table S1; Michael et al., 2000). Together, these data support the presence of a glucose- and insulin-independent liver-derived factor that promotes the expansion of β cell mass.
Figure 4. LIRKO Serum Increases Mouse and Human Islet β Cell Replication In Vitro.
Five to 6-week-old mouse islets were stimulated with control or LIRKO serum for 48 hr. Islets were embedded in agarose and used for immunostaining studies. Culture media were assayed for glucose and insulin. WT, wild-type.
(A) Schematic of the experimental protocol. See also Figures S5 and S6.
(B) Representative images of mouse islets stimulated with sera derived from 3-month-old (upper panel) and 12-month-old animals (lower panel).
(C) Quantification of Ki67+ insulin+ cells in (B): two sets of three serial sections separated by 80 µm were analyzed. At least 4,000–5,000 cells were counted in each experimental group (n = 5 in each group).
(D) Quantification of TUNEL+ insulin+ cells in (B): at least 3,000–4,000 cells were counted in each group (n = 5 in each group).
(E and F) Representative images of healthy and type 2 diabetic donor islets stimulated with control versus LIRKO serum for 24 hr. See also Table S3.
(G) Quantification of Ki67+ insulin+ cells in (E) and (F): three sets of three serial sections separated by 80 µm were analyzed. At least 3,000–4,000 cells were counted in each group. See also Table S3.
Data represent mean ± SEM. *p ≤ 0.05. (See Serum Stimulation and Human Islet Studies sections in Experimental Procedures.)
Hepatocyte-Derived Factors Stimulate Mouse and Human Islet β Cell Replication In Vitro
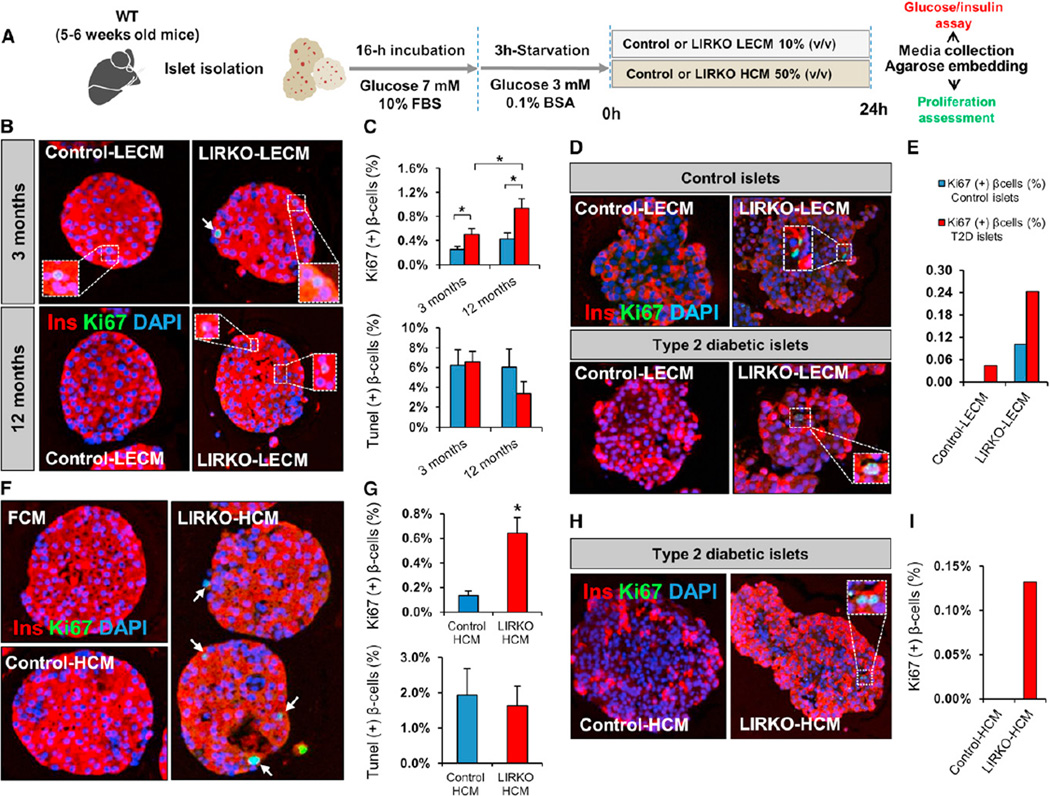
The common embryonic origin of the liver and the pancreas (Zaret, 2008) coupled with the robust β cell proliferation response to tissue-specific insulin resistance in the liver compared to the virtual lack of a compensatory response when insulin resistance was restricted to muscle (Brüning et al., 1998), adipose (Blüher et al., 2002), or brain (Brüning et al., 2000) prompted us to hypothesize that the liver serves as a source of β cell growth factor(s) in response to metabolic insults such as insulin resistance. To test this hypothesis, we collected conditioned media from liver explant cultures (LECM) from either 3- or 12-monthold LIRKO or control animals and evaluated their effects on β cell proliferation in mouse islets (Figure 5A). Ki67-positive β cells were significantly elevated in islets cultured in LECM from either 3- or 12- month-old LIRKO mice, compared to cells cultured inLECMderived from age-matched controls (Figure 5B). Interestingly, whereas mouse islets cultured in control LECM derived from 3- and 12-month-old animals displayed similar levels of proliferation, the levels were 2-fold higher in cultures containing 12-month-old LIRKO-LECM compared to 3-monthold control LECM (Figure 5C). This age-dependent effect of LIRKO-LECM is consistent with the age-dependent increase in β cell proliferation in LIRKO mice (Okada et al., 2007). Similarly, β cells in islets obtained from healthy human controls and patients with type 2 diabetes (for donor characteristics, see Table S3) cultured in LECM derived from LIRKO animals exhibited increased proliferation compared to islets from the same donors cultured in control LECM (Figures 5D and 5E).
Figure 5. Hepatocyte-Derived Factors Stimulate Mouse and Human Islet β Cell Replication In Vitro.
Five to 6-week-old mouse islets were stimulated for 24 hr with LECM or HCM obtained from control or LIRKO mice. Islets were embedded in agarose and subsequently analyzed by immunostaining. Culture media were assayed for glucose and insulin.
(A) Schematic of the experimental protocol. See also Figure S6.
(B) Representative images of mouse islets stimulated with LECM derived from 3-month-old (upper panel) and 12-month-old animals (lower panel).
(C) Quantification of Ki67+ insulin+ cells (upper panel) and TUNEL+ insulin+ cells (lower panel): at least 3,000–5,000 cells were counted in each experimental group (n = 5 in each group).
(D) Representative images of healthy human donor islets (upper panel) and type 2 diabetic donor islets (lower panel) treated for 24 hr with LECM derived from control versus LIRKO mice. See also Table S3.
(E) Quantification of Ki67+ insulin+ cells in (D): between 3,000 and 4,000 cells were counted in each condition (Control LECM versus LIRKO LECM) in control islets, and at least 2,000 cells were counted in each experimental group for type 2 diabetic islets. See also Table S3.
(F) Representative images of mouse islets stimulated with HCM derived from 6-month-old control versus LIRKO mice or fibroblast-conditioned media (FCM).
(G) Quantification of Ki67+ insulin+ cells (upper panel) and TUNEL+ insulin+ cells (lower panel): between 4,000 and 5,000 cells were counted in each experimental group (Control HCM versus LIRKO HCM) (n = 5 in each group).
(H) Representative images of type 2 diabetic donor islets stimulated with control or LIRKO HCM. See also Table S3.
(I) Quantification of Ki67+ insulin+ cells in (H): at least 2,000 cells were counted in each experimental condition. See also Table S3.
Data represent mean ± SEM. *p ≤ 0.05. (See LECM Stimulation, HCM Stimulation, and Human Islet Studies sections in Experimental Procedures.)
The liver contains multiple cell types, including hepatocytes, Kupffer cells, and endothelial cells (Si-Tayeb et al., 2010). To determine whether the growth factor activity in LIRKO serum is a product of hepatocytes or nonhepatic cells, we used conditioned media from cultures of primary hepatocytes (HCM), isolated from control or LIRKO mice in an in vitro β cell proliferation assay. Primary mouse islets cultured in LIRKO HCM exhibited markedly increased β cell proliferation compared to islets stimulated with control HCM (control HCM, 0.13% ± 0.03% versus LIRKO HCM, 0.64% ± 0.12%; p < 0.05; n = 5). The number of TUNEL+ β cells was similar in both conditions (Figures 5F and 5G). Furthermore, the proliferative effect of LIRKO HCM was also evident when human islets obtained from a patient with type 2 diabetes (for donor characteristics, see Table S3) were exposed to LIRKO HCM compared to control HCM (Figures 5H and 5I). Thus, insulin-resistant hepatocytes produce a β cell growth-promoting factor(s) that enhances proliferation of mouse and human β cells.
Although numerous signaling pathways impacting β cell growth have been documented (Kulkarni et al., 2012), specific blood-borne molecules that trigger β cell replication directly in response to insulin resistance have, to our knowledge, not been reported. The absence of a consistent increase in one or more growth factors in the serum of the LIRKOs (Table S1) supports the notion that additional unidentified factors are necessary to promote the full magnitude of proliferation observed in the LIRKO model. In summary, we provide evidence that a conserved systemic hepatocyte-derived growth factor(s) promotes β cell proliferation in mouse and human islets, supporting a liver-to-pancreas axis in the adaptive β cell growth response to insulin resistance.
EXPERIMENTAL PROCEDURES
Animals
Mice were housed in pathogen-free facilities and maintained on a 12 hr light/dark cycle in the Animal Care Facility at Joslin Diabetes Center, Boston, and the Foster Biomedical Research Laboratory, Brandeis University, Waltham, MA. All studies conducted and protocols used were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center and Brandeis University and were in accordance with NIH guidelines. LIRKO mice were generated by crossing Albumin-Cre to IRflox/flox on a mixed genetic background and were backcrossed for more than 15 generations on the C57/Bl6 background. LIRKO mice (Albumin-Cre+/−,IRflox/flox) and their littermate Lox controls (Albumin-Cre−/−,IRflox/flox) were genotyped as described previously by Okada et al. (2007). Blood glucose was monitored using an automated glucose monitor (Glucometer Elite; Bayer), and plasma insulin was detected by ELISA (Crystal Chem).
Parabiosis
Parabiosis surgery was performed as described earlier byEggan et al. (2006). Cross-circulation was determined 2 weeks after surgery by Evans Blue transmission (Pietramaggiori et al., 2009). Body weight and blood glucose of parabiont animals were monitored weekly. After a 16 week parabiosis period, animals were sacrificed, and pancreases were collected for morphometric analysis.
Islet Isolation and Transplantation
Islets were isolated from 9-month-old mice using the intraductal collagenase technique (Kulkarni et al., 1999). Islets were handpicked, concentrated in a pellet, and kept on ice until transplantation (Flier et al., 2001). Surgery was performed in mice after intraperitoneal injection (15 µl/g body weight) of a 1:1 (w/v) mixture of 2,2,2-tribromoethanol and tert-amyl alcohol and diluted 1:50 with PBS (pH 7.4). The capsules of the kidneys were incised, and the islets were implanted near the upper pole of each kidney in 5-month-old male mice. The capsules were cauterized, and the mice were allowed to recover on a heating pad.
Growth Factors and Hormones Assays
ELISA-based assays were used to measure growth factors and hormones, including IGF-1 (catalog #MG100; R&D Systems), HGF (catalog #ab100686; Abcam), EGF (catalog #IB39411; IBL-America), PDGFAA (catalog #DAA00B; R&D Systems), PDGFBB (catalog #MBB00; R&D Systems), VEGF (Millipore), FGF21 (catalog #EZRMFGF21-26K; Millipore), Gastrin (catalog #E91224mu; USCN Life Science), Adiponectin (catalog #EZMADP-60K; Millipore), Ostepontin (catalog #MOST00; R&D Systems), and Osteocalcin (catalog #EIA4010; International). Multiplex-based assays were used to measure endocrine hormones (catalog #MENDO-75; Millipore), gut hormones (catalog #MGT-78K; Millipore), adipokines (catalog #MADPK-71K; Millipore), and Cytokines/Chemokines (catalog #MPXMCYTO-70K.lxt; Millipore).
Serum Stimulation
Sera were obtained after coagulated blood was centrifuged twice for 15 min at 8,000 rpm at 4°C and stored at −80°C until use. Pancreatic islets were isolated from 5-week-old male mice by liberase and thermolysin digestion (Roche), handpicked, and cultured for 16 hr in RPMI 1640 with 7 mM glucose and 10% FBS (v/v). A total of 150 size-matched mouse islets were starved in RPMI 1640 with 0.1% BSA (v/v) containing 3 mM glucose for 3 hr and thereafter treated with RPMI 1640 with 5.5 mM glucose supplemented every 12 hr with 10% (v/v) serum obtained from 3- or 12-month-old LIRKO and control mice. Twenty-four to 48 hr later, islets were handpicked, fixed with 4% paraformaldehyde, embedded in agarose/paraffin, and sectioned for immunohistochemistry studies. To evaluate β cell replication, sections were analyzed by fluorescent microscopy subsequent to Ki67, TUNEL, and insulin immunostaining.
LECM Stimulation
Liver explant-conditioned medium preparation was adapted from Nicoleau et al. (2009). Mice were anesthetized with Avertin (240 mg/kg intraperitoneally), and 100 mg liver explants were dissected from LIRKO or control mice. Explants were washed twice in cold PBS, incubated in PBS at 37°C for 30 min, and then cultured in serum-free Dulbecco’s modified Eagle’s medium (DMEM) containing 5.5 mM glucose. After a 3 day incubation, LECM were collected, centrifuged, and kept at −80°C till use. Islets were initially starved for 3 hr in DMEM containing 3mM glucose and 0.1% BSA and thereafter stimulated for 24 hr with DMEM/5.5 mM glucose media containing 10% LECM. Islet β cell proliferation and apoptosis were analyzed by fluorescent microscopy after Ki67, TUNEL, and insulin immunostaining.
HCM Stimulation
Hepatocytes were isolated from 6-month-old LIRKO and controlmice by collagenase digestion via portal vein perfusion (Sun et al., 2005). Mice were anesthetized with Avertin (240 mg/kg intraperitoneally), and the portal vein was cannulated with JELCO 22G 3 1 inch catheter (Smiths Medical). The liver was perfused with EGTA solution (5.4 mmol/l KCl, 0.44 mmol/l KH2PO4, 140 mmol/l NaCl, 0.34 mmol/l Na2HPO4, and 0.5 mmol/l EGTA [pH 7.4]) and digested with DMEM containing 0.075% type I collagenase. Hepatocytes were washed twice in Hepatocyte Wash Medium (Invitrogen). The isolated mouse hepatocytes were seeded in collagen-coated 6-well plates (BD BioCoat) at a density of 106 cells/well in DMEM containing 25 mM glucose and 10% FBS (v/v). Sixteen hours later, hepatocytes were cultured for 24 hr in serum-free DMEM containing 5.5 mM glucose. HCM was collected, centrifuged, and kept at −80°C. Islets were initially starved for 3 hr in DMEM containing 3 mM glucose and 0.1% BSA and thereafter incubated for 24 hr in DMEM/5.5 mM glucose media containing 50% HCM. Islet β cell proliferation and apoptosis were analyzed by fluorescent microscopy after Ki67, TUNEL, and insulin immunostaining.
Human Islet Studies
Human islets were obtained from the Integrated Islet Distribution Program. All studies and protocols used were approved by the Joslin Diabetes Center’s Committee on Human Studies (CHS#5-05). Upon arrival, islets were cultured overnight in Miami Media #1A (Cellgro). The islets were then starved in Final Wash/Culture Media (Cellgro) for 3 hr prior to stimulation with serum (diluted to 10% v/v), LECM (diluted to 10% v/v), or HCM (diluted to 50% v/v) for 24 hr.
Immunostaining Studies
Pancreases and islets were analyzed by immunostaining using anti-Ki67 (BD), anti-insulin (Abcam), or anti-glucagon (Sigma-Aldrich) antibodies.
Counting Proliferating β Cells
In all experiments, cell counting was manually performed in a blinded fashion by a single observer. BrdU+ or Ki67+ β cells were assessed by immuno-fluorescence microscopy. Insulin+ cells showing nuclear DAPI staining were considered as β cells. Insulin+ cells showing nuclear colocalized staining for DAPI+ and Ki67+ (or BrdU+) were counted as proliferating β cells. The double-positive cells (Ins+/BrdU+ or Ins+/Ki67+) were confirmed in randomly selected cells in all experiments by confocal microscopy.
BrdU Injection Studies
Mice were injected with BrdU intraperitoneally (100 mg/kg body weight) 5 hr prior to animal sacrifice for immunostaining of the pancreas. BrdU injections in the in vivo serum administration experiments were performed on three occasions as denoted in Figure 2A.
Statistical Analysis
All data are presented as mean ± SEM and analyzed using unpaired, two-tailed Student’s t test. A p value of less than 0.05 is considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to C. Ronald Kahn for sharing the LIRKO model. We thank the laboratory of Gordon Weir for assistance in transplantation experiments, T. Roderick Bronson for assistance with histology, and Amarnath Kurpad, Marta Robledo, Samantha Haring, Rachael Martinez, and Ben Hambro for technical assistance. This work is supported by NIH RO1 DK 067536 (to R.N.K.); Société Francophone du Diabète, Association Française des Diabétiques, and American Diabetes Association (to A.E.O.); R01 DK 074795 (to W.-J.Q.); and the Burroughs Wellcome Fund, NIH 5 P30 DK36836-20, and NIH 1 DP2 OD004345 (to A.J.W.). A.J.W. is an Early Career Scientist of the Howard Hughes Medical Institute. Part of the study was supported by a Harvard Stem Cell Institute Seed Grant (to R.N.K.) and by a grant from the Juvenile Diabetes Research Foundation/Sanofi Aventis Strategic Alliance (17-2011-644) (to R.N.K.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.01.007.
LICENSING INFORMATION
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
REFERENCES
- Assmann A, Hinault C, Kulkarni RN. Growth factor control of pancreatic islet regeneration and function. Pediatr. Diabetes. 2009a;10:14–32. doi: 10.1111/j.1399-5448.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol. Cell. Biol. 2009b;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Bunster E, Meyer RK. An improved method of parabiosis. Anat. Rec. 1933;57:339–343. [Google Scholar]
- Chen H, Gu X, Liu Y, Wang J, Wirt SE, Bottino R, Schorle H, Sage J, Kim SK. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature. 2011;478:349–355. doi: 10.1038/nature10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441:1109–1114. doi: 10.1038/nature04929. [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, Kerr-Conte J, Pattou F, Berney T, Pipeleers D, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc. Natl. Acad. Sci. USA. 2008;105:13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc. Natl. Acad. Sci. USA. 2001;98:7475–7480. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai J, Katagiri H, Yamada T, Ishigaki Y, Suzuki T, Kudo H, Uno K, Hasegawa Y, Gao J, Kaneko K, et al. Regulation of pancreatic beta cell mass by neuronal signals from the liver. Science. 2008;322:1250–1254. doi: 10.1126/science.1163971. [DOI] [PubMed] [Google Scholar]
- Jörns A, Sennholz C, Naujok O, Lenzen S. Beta cell mass regulation in the rat pancreas through glucocorticoids and thyroid hormones. Pancreas. 2010;39:1167–1172. doi: 10.1097/MPA.0b013e3181dfce4f. [DOI] [PubMed] [Google Scholar]
- Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat. Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Winnay JN, Daniels M, Brüning JC, Flier SN, Hanahan D, Kahn CR. Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J. Clin. Invest. 1999;104:R69–R75. doi: 10.1172/JCI8339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RN, Almind K, Goren HJ, Winnay JN, Ueki K, Okada T, Kahn CR. Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes. 2003;52:1528–1534. doi: 10.2337/diabetes.52.6.1528. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- Mori MA, Liu M, Bezy O, Almind K, Shapiro H, Kasif S, Kahn CR. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes. 2010;59:2960–2971. doi: 10.2337/db10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, Li H, Elmquist JK, Kennedy RT, Kulkarni RN. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J. Clin. Invest. 2007;117:2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Nicoleau C, Benzakour O, Agasse F, Thiriet N, Petit J, Prestoz L, Roger M, Jaber M, Coronas V. Endogenous hepatocyte growth factor is a niche signal for subventricular zone neural stem cell amplification and self-renewal. Stem Cells. 2009;27:408–419. doi: 10.1634/stemcells.2008-0226. [DOI] [PubMed] [Google Scholar]
- Ogilvie RF. The islands of langerhans in 19 cases of obesity. J. Pathol. Bacteriol. 1933;37:473–481. [Google Scholar]
- Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl. Acad. Sci. USA. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietramaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J. Invest. Dermatol. 2009;129:2265–2274. doi: 10.1038/jid.2009.60. [DOI] [PubMed] [Google Scholar]
- Renner S, Fehlings C, Herbach N, Hofmann A, von Waldthausen DC, Kessler B, Ulrichs K, Chodnevskaja I, Moskalenko V, Amselgruber W, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59:1228–1238. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck S, Zhang J, Li Z, Liu C, Naji A, Takane KK, Fiaschi-Taesch NM, Stewart AF, Kushner JA, Kaestner KH. Overexpression of hepatocyte nuclear factor-4α initiates cell cycle entry, but is not sufficient to promote β-cell expansion in human islets. Mol. Endocrinol. 2012;26:1590–1602. doi: 10.1210/me.2012-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, Lyssenko V, Bouatia-Naji N, Dupuis J, Jackson AU, et al. GIANT consortium; MAGIC investigators. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat. Genet. 2010;42:142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sun R, Jaruga B, Kulkarni S, Sun H, Gao B. IL-6 modulates hepatocyte proliferation via induction of HGF/p21cip1: regulation by SOCS3. Biochem. Biophys. Res. Commun. 2005;338:1943–1949. doi: 10.1016/j.bbrc.2005.10.171. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Imai J, Yamada T, Ishigaki Y, Kaneko K, Uno K, Hasegawa Y, Ishihara H, Oka Y, Katagiri H. Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes. 2011;60:537–547. doi: 10.2337/db10-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Van Assche FA, Aerts L, De Prins F. A morphological study of the endocrine pancreas in human pregnancy. Br. J. Obstet. Gynaecol. 1978;85:818–820. doi: 10.1111/j.1471-0528.1978.tb15835.x. [DOI] [PubMed] [Google Scholar]
- Verga Falzacappa C, Mangialardo C, Raffa S, Mancuso A, Piergrossi P, Moriggi G, Piro S, Stigliano A, Torrisi MR, Brunetti E, et al. The thyroid hormone T3 improves function and survival of rat pancreatic islets during in vitro culture. Islets. 2010;2:96–103. doi: 10.4161/isl.2.2.11170. [DOI] [PubMed] [Google Scholar]
- Wang C, Guan Y, Yang J. Cytokines in the Progression of Pancreatic β-Cell Dysfunction. Int. J. Endocrinol. 2010;2010:515136. doi: 10.1155/2010/515136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- Zaret KS. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat. Rev. Genet. 2008;9:329–340. doi: 10.1038/nrg2318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.