Abstract
Direct phosphorylation of GluA1 by PKC controls α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) receptor (AMPAR) incorporation into active synapses during long-term potentiation (LTP). Numerous signalling molecules that involved in AMPAR incorporation have been identified, but the specific PKC isoform(s) participating in GluA1 phosphorylation and the molecule triggering PKC activation remain largely unknown. Here, we report that the atypical isoform of PKC, PKCλ, is a critical molecule that acts downstream of phosphatidylinositol 3-kinase (PI3K) and is essential for LTP expression. PKCλ activation is required for both GluA1 phosphorylation and increased surface expression of AMPARs during LTP. Moreover, p62 interacts with both PKCλ and GluA1 during LTP and may serve as a scaffolding protein to place PKCλ in close proximity to facilitate GluA1 phosphorylation by PKCλ. Thus, we conclude that PKCλ is the critical signalling molecule responsible for GluA1-containing AMPAR phosphorylation and synaptic incorporation at activated synapses during LTP expression.
Keywords: AMPA receptors, LTP, p62, PKCλ, PI3K
Introduction
Long-lasting changes in the synaptic efficacy, including long-term potentiation (LTP) and long-term depression (LTD), are considered as the cellular substrate of learning and memory (Bliss and Collingridge, 1993; Bear and Malenka, 1994; Zhuo and Hawkins, 1995; Malenka and Nicoll, 1999; Collingridge et al, 2010). The stable α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid receptor (AMPAR) incorporation into and removal from postsynaptic site is one of the main molecular mechanisms underlying LTP and LTD, respectively (Malenka and Nicoll, 1999; Malinow et al, 2000; Man et al, 2000; Sheng and Lee, 2001). AMPARs are ligand-sensitive tetrameric complex assembled from various subunits, GluA1, GluA2, GluA3 and GluA4 (Wisden and Seeburg, 1993; Hollmann and Heinemann, 1994). In the hippocampal CA1 synapse, AMPAR trafficking to cell surface during LTP has been suggested to be in the form of GluA1/GluA2 (Adesnik and Nicoll, 2007; Gray et al, 2007). Several lines of evidence point to the obligatory role of GluA1 in mediating N-methyl-D-aspartate (NMDA) receptor-dependent AMPAR insertion (Shi et al, 1999; Zamanillo et al, 1999; Hayashi et al, 2000; Lu et al, 2001; Passafaro et al, 2001; Shi et al, 2001; Esteban et al, 2003; Lee et al, 2003; Meng et al, 2003). In particular, PKC phosphorylation at the highly conserved serine 818 residue (Ser818) at the GluA1 C-terminal tail is critical for GluA1 synaptic incorporation during LTP (Boehm et al, 2006; Lin et al, 2009).
PKC is a multigene family consisting of at least 10 isoforms, 9 of which are expressed in the brain (Naik et al, 2000; Sossin, 2007; Steinberg, 2008). Based on the mechanisms of activation and domain structures, these isoforms are divided into three groups: conventional, novel and atypical PKC (aPKC; Nishizuka, 1988, 1995). PKC plays a variety of roles in numerous physiological and pathological processes, especially including synaptic plasticity in the brain (Malinow et al, 1989; Sacktor et al, 1993; Wang and Kelly, 1995; Boehm et al, 2006; but see Muller et al, 1992; Bortolotto and Collingridge, 2000). Only conventional and novel groups of PKC receive most attention, which are mainly activated by Ca2+ and/or DAG/phorbol ester. The aPKC subgroup contains PKCλ/ι, PKCζ and PKMζ. In the hippocampus, only PKCλ/ι and PKMζ but not PKCζ of the aPKC are expressed (Naik et al, 2000; Hernandez et al, 2003; Oster et al, 2004). Due to their atypical regulatory domain structure, PKCλ/ι is insensitive to DAG/phorbol ester and Ca2+, but can be activated by phosphatidylinositol 3,4,5-trisphosphate (PIP3; Hirai and Chida, 2003; Suzuki et al, 2003), while PKMζ has constitutively catalytic activity due to lacking regulatory domain. It is not until recently found that PKMζ plays a critical role in the maintenance of late LTP and long-term memory (Ling et al, 2002; Pastalkova et al, 2006; Yao et al, 2008; Migues et al, 2010). At the earlier stage of LTP expression, the maintenance of the potentiation is thought to be mediated by PKC phosphorylation of GluA1 Ser818 and subsequent incorporation of AMPARs at the activated synapse (Boehm et al, 2006; Lin et al, 2009). In vitro phosphorylation assay has revealed that almost all PKC isoforms can phosphorylate Ser818 site (Boehm et al, 2006). However, different isoforms of PKCs are activated by different activators and thus may function differentially at different LTP phases. Therefore, it is of importance to know which PKC isoform is specifically responsive to NMDA receptor (NMDAR) activation and responsible for subsequent GluA1 Ser818 phosphorylation during LTP expression.
It has been well accepted that synaptic activation of NMDAR is required for LTP and that the subsequent intracellular Ca2+ increase at activated synapses mediates further changes in AMPAR function, largely through sequentially triggering downstream critical molecules involving LTP expression and maintenance. However, how the activation of synaptic NMDARs is translated into PKC activation and subsequent GluA1 Ser818 phosphorylation remains elusive. It is known that Ca2+/calmodulin-dependent protein kinase (CaMKII) can be activated by Ca2+/Camoldulin (Lisman et al, 2002, 2012a) and in turn may facilitate AMPAR delivery into postsynaptic site by activating Ras (Zhu et al, 2002). Ras is shown to activate the class IA phosphatidylinositol 3-kinase (PI3K) via a direct interaction with the catalytic subunit of the lipid kinase (Rodriguez-Viciana et al, 1996; Downward, 1997). PI3K has been implicated in some forms of synaptic plasticity (Kelly and Lynch, 2000; Lin et al, 2000). PI3K can form complex with AMPAR and is required for AMPAR insertion during LTP expression (Man et al, 2003). The PI3K products, PIP3, are known to be involved in many synaptic functions including membrane trafficking processes (James and Piper, 1994; Carpenter and Cantley, 1996; De Camilli et al, 1996; Rapoport et al, 1997; Cheever et al, 2001; Arendt et al, 2010). Accumulation of PIP3 may recruits a PIP3 binding module-containing signalling complex, such as the serine/threonine kinases aPKC (Vanhaesebroeck et al, 1997; Hirai and Chida, 2003; Suzuki et al, 2003). This raises the possibility that PI3K-induced AMPAR insertion is mediated by aPKC phosphorylation of GluA1 Ser818.
In the present study, we provide evidence to suggest that PKCλ acts downstream of PI3K and is critical in AMPAR phosphorylation and subsequent incorporation into postsynaptic site during LTP expression. In addition, we show that p62 forms trimeric complex with both AMPAR and PKCλ and facilitates AMPAR phosphorylation by PKCλ. Thus, PKCλ may play an important role in mediating the selective AMPAR insertion at activated synapses during LTP expression.
Results
PKCλ is necessary and sufficient for LTP expression
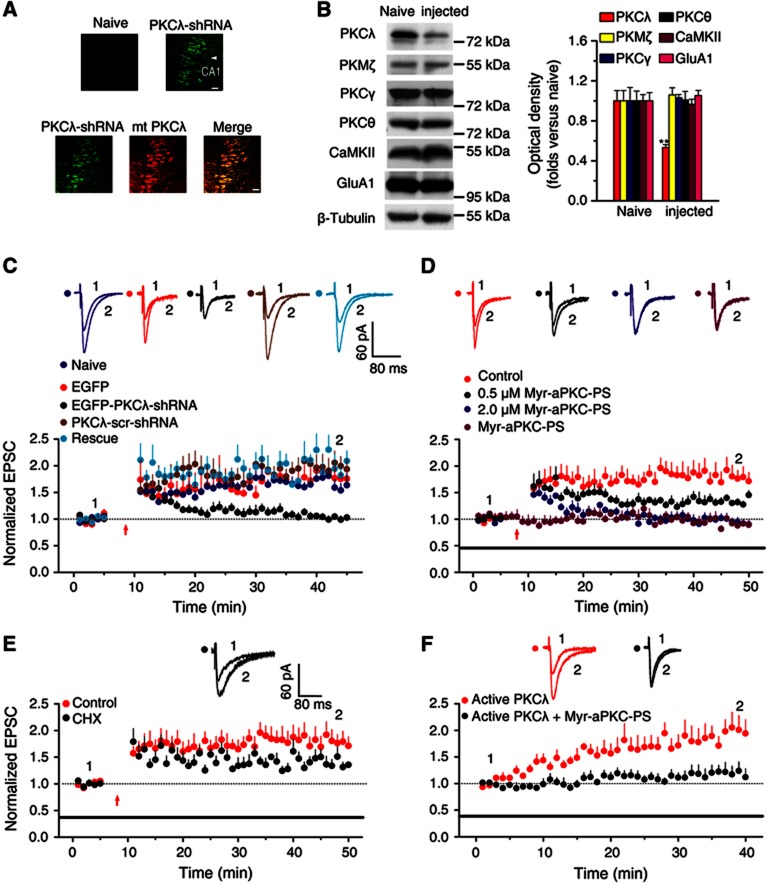
To investigate the potential role of PKCλ in LTP, we employed a method that could selectively target PKCλ in the hippocampus. A recombinant adeno-associated virus (rAAV1) particle constructed with rAAV2 coat proteins (rAAV2/1) is an especially effective tool for selectively transducing hippocampal neurons in vivo (Burger et al, 2004). We first utilized a strong enhanced green fluorescent protein (EGFP) expressing rAAV2/1 virus as a negative control to optimize injection location and volume. EGFP was expressed in most neurons in the dorsal CA1. We then designed and packaged rAAV particles that express short hairpin RNA (shRNA) in vivo and selectively target PKCλ. These virus particles were in the same virus package (rAAV2/1), though they contained two expression cassettes. The EGFP expression helped us to locate regions of CA1 and facilitated LTP studies.
We then injected either control or PKCλ-shRNA virus into one side of dorsal hippocampus and evaluated the possible physiological consequences of PKCλ knockdown (PKCλ KD). The rats were injected at postnatal 5–6 days and examined until 2 weeks after injection (Figure 1A). The uninjected side was used as an internal control for hairpin expression. PKCλ-shRNA driven by these particles selectively caused about 47% reduction in PKCλ expression from homogenates collected from dorsal hippocampus closest to the injection point compared to naive hippocampal samples (Figure 1B; Supplementary Figure S1, n=12). The knockdown effect of PKCλ-shRNA was also confirmed in HEK 293T cells (Supplementary Figure S2). We then utilized the LTP model induced by a classical pairing protocol (Xu et al, 2009) and determined how the reduction in PKCλ expression could affect LTP. Evoked AMPAR-mediated excitatory postsynaptic currents (AMPA EPSCs) were recorded under whole-cell configuration. We observed a marked and selective decrease in magnitude of potentiation during the stage of LTP expression in slices from PKCλ knockdown hippocampus (Figure 1C). In contrast, slices injected with PKCλ-shRNA scrambled (PKCλ-scr-shRNA) failed to display any obvious effect on LTP (Figure 1C). Importantly, the change in LTP caused by PKCλ KD was fully rescued by expression of a mutant PKCλ (mt-PKCλ) with silent mutations resistant to RNA knockdown by PKCλ-shRNA (Figure 1C). Moreover, PKCλ KD did not affect basal synaptic transmission, indicated by absence of changes in pair-pulse facilitation (PPF), AMPA EPSCs and NMDAR-mediated EPSCs (Supplementary Figure S3). These data support the notion that PKCλ is required for stabilization, but not induction, of early LTP.
Figure 1.
PKCλ is necessary and sufficient for LTP expression in acute hippocampal slices. (A) Images showing EGFP expression in dorsal hippocampal slices prepared from contralateral (naive; top left) and PKCλ-shRNA injected (top right) hemispheres following unilateral injections (4 μl) of a rAAV coexpressing PKCλ-shRNA and EGFP. A rescue experiment was also performed in parallel on slices prepared from hemisphere injected with both EGFP-PKCλ-shRNA (green; bottom) and mcherry-mt-PKCλ (red; bottom). The images were taken at 2 weeks after injection. Scale bar: 20 μm. (B) Examination of the selectivity of PKCλ knockdown. A 47% reduction in PKCλ, but not in other PKC isoforms or CaMKII, was observed in preparations from dorsal hippocampus. (C) PKCλ knockdown led to impaired LTP expression (1.02±0.04, n=10; compared with baseline, P>0.05, paired samples t test; compared with uninjected side cells, 1.59±0.16, n=5, P<0.01, one-way ANOVA LSD test), which was reversed by PKCλ rescue (2.01±0.14, n=5; compared with baseline, P<0.05; compared with PKCλ KD, P<0.01). As a control, LTP was normal in cells transfected with PKCλ-scr-shRNA or with EGFP only (PKCλ-scr-shRNA, 1.97±0.21, n=5, compared with baseline, P<0.01; compared with PKCλ KD, P<0.01; EGFP, 1.81±0.21, n=6, compared with baseline, P<0.05; compared with PKCλ KD, P<0.01). Overlaid traces above the graph show changes in averaged AMPAR-mediated EPSCs chosen at the times indicated on the graph. The red arrow refers to the time points that the pairing protocol was delivered. (D) PKCλ is required for LTP expression. 0.5 μM Myr-aPKC-PS, which was proved to significantly inhibit the activity of PKMζ (Ling et al, 2002), attenuated the maintenance of LTP (1.35±0.05, n=7; compared with control, 1.83±0.19, n=8, P<0.01, one-way ANOVA LSD test). When Myr-aPKC-PS was applied at 2.0 μM concentration, which inhibited both PKCλ and PKMζ, an additional inhibitory effect was observed at the stage immediately after LTP induction (0.94±0.06, n=6; compared with baseline, P>0.05, paired samples t test; compared with control, P<0.01). As a control, the peptide alone did not have any effect on the basal AMPA EPSCs (0.92±0.08, n=7; compared with baseline, P>0.05). The duration of the Myr-aPKC-PS treatment is indicated by the bar. (E) The protein synthesis inhibitor cycloheximide (CHX, 60 μM) attenuated late maintenance phase of LTP (1.40±0.08, n=7; compared with 0.5 μM Myr-aPKC-PS, P>0.05; compared with control, P<0.05; one-way ANOVA LSD test). The control is borrowed from (D) for comparison. (F) PKCλ is sufficient for LTP expression. Active PKCλ (1.5 nM), when applied in the intracellular solution, induced persistent enhancement of EPSCs (1.94±0.22, n=8; compared with baseline, P<0.05). This LTP was significantly attenuated by pretreatment with Myr-aPKC-PS (2.0 μM; 1.19±0.15, n=6; compared with baseline, P>0.05; compared with active PKCλ, P<0.05, independent samples t test).
Source data for this figure is available on the online supplementary information page.
To further confirm the role of PKCλ in LTP expression, we also employed a blocker of PKCλ to pharmacologically suppress PKCλ function to assess the physiological consequences. Myr-aPKC-Pseudosubstrate peptide (Myr-aPKC-PS) is a cell-membrane transducible zeta inhibitory peptide (ZIP) that has been shown to selectively inhibit PKMζ relative to conventional and novel PKCs and CaMKII (Ling et al, 2002). However, the amino-acid sequence of the peptide (myr-SIYRRGARRWRKL) is identical to the autoinhibitory pseudosubstrate domain of both PKCλ and PKMζ (Standaert et al, 2001; Bosch et al, 2004), thus it may competitively inhibit the activity of both PKMζ and PKCλ (Jiang et al, 2006). In order to determine whether Myr-aPKC-PS at a certain concentration can isolate one of the two aPKCs from the other, that is, only selectively inhibit one of the two aPKCs, we performed in vitro assay of protein kinase activity to investigate how this peptide affect the activity of these two aPKCs at different concentrations. We found that Myr-aPKC-PS at a concentration as low as 0.5 μM significantly inhibited activity of PKMζ (Ling et al, 2002) but hardly affect PKCλ or CaMKII activity (Supplementary Figure S4). When Myr-aPKC-PS increases to 2.0 μM, it began to display additional inhibitory effect on PKCλ. Thus, we utilized 0.5 μM Myr-aPKC-PS as a selective blocker of PKMζ. In good agreement with the previous reports that PKMζ display its action at the late maintenance of LTP that depends on protein synthesis (Osten et al, 1996; Migues et al, 2010), blocking PKMζ by constitutively perfusing slices with 0.5 μM Myr-aPKC-PS throughout the whole recording process only attenuated the late phase of LTP (Figure 1D), at which the protein synthesis inhibitor cycloheximide (CHX, 60 μM) began to display similar action (Figure 1E). By contrast, 2.0 μM Myr-aPKC-PS, which should inhibit activity of both PKCλ and PKMζ, significantly suppressed LTP expression at the stage immediately after LTP induction (Figure 1D). As a control, the peptide alone did not have any effect on the basal AMPA EPSCs (Figure 1D). Because the action of PKMζ on LTP only occurs at the late maintenance stage after LTP induction (Osten et al, 1996; Migues et al, 2010), we considered that the additional effect of 2.0 μM Myr-aPKC-PS on early state of LTP expression is attributable to suppression of PKCλ by the peptide. This assumption is consistent with above results on impaired LTP expression in PKCλ KD animals and obtains further support from the observation that PI3K-induced enhancement of PKCλ activity, indicated by increase in PKCλ autophosphorylation at Thr563 (P-Thr563 PKCλ), was reversed by 2.0 μM Myr-aPKC-PS (Figures 2A and B). Moreover, PKCλ KD occluded selected Myr-aPKC-PS effects on LTP (Supplementary Figure S5). Taken together, these results strongly suggest that the activation of PKCλ is required for LTP expression.
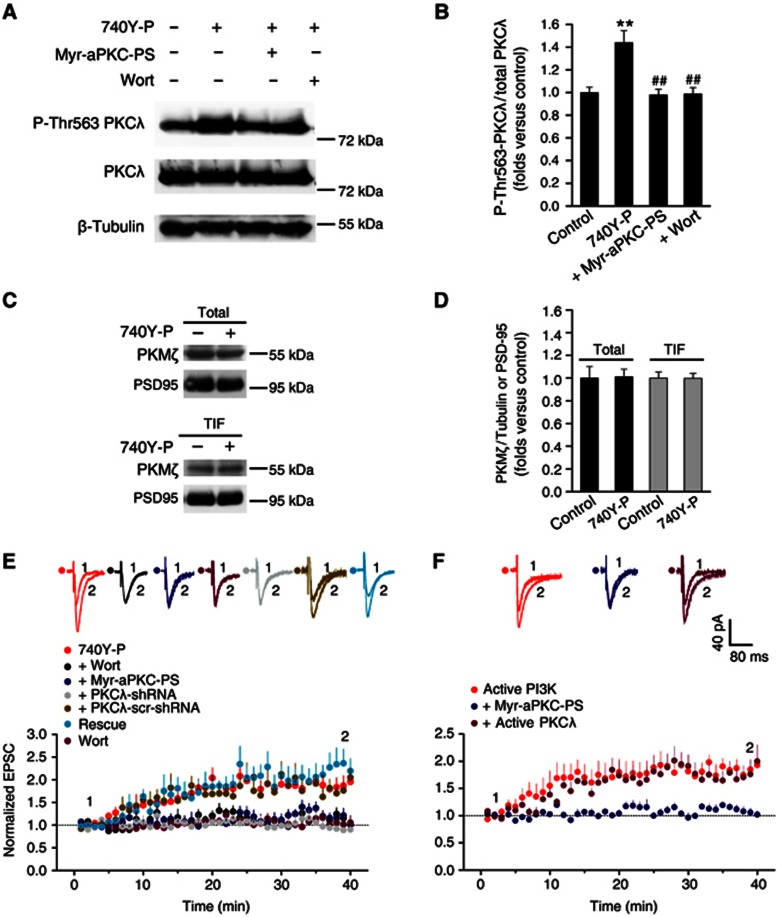
Figure 2.
PKCλ functions downstream of PI3K to induce LTP. (A) PI3K selectively activated PKCλ. PI3K activation by 740Y-P treatment specifically activated PKCλ, indicated by potentiated PKCλ phosphorylation at Thr563 site. This activation of PKCλ was abolished by PKCλ antagonist Myr-aPKC-PS or PI3K antagonist Wortmannin. By contrast, total PKCλ was not affected by PI3K activation. (B) Plots summarize data from the experiments in (A) showing the Thr563P-PKCλ/total PKCλ ratio under various treatments (740Y-P, 1.44±0.11, n=8, compared with control, P<0.01; Myr-aPKC-PS, 0.98±0.05, n=8, compared with 740Y-P, P<0.01; Wort, 0.99±0.06, n=8, compared with 740Y-P, P<0.01; one-way ANOVA LSD test). **Compared with control, P<0.01; ##compared with 740Y-P, P<0.01. (C) 740Y-P treatment did not change the total amount of PKMζ or PKMζ amount at PSD. (D) Plots summarize data from the experiments in (C) showing absence of changes in the total PKMζ/PSD95 (740Y-P, 1.01±0.07, n=7; compared with control, P>0.05, independent samples t test) or TIF PKMζ/PSD95 ratio under 740Y-P treatment (740Y-P, 1.00±0.04, n=4; compared with control, P>0.05). (E) PKCλ is required for LTP induced by PI3k activation. Activation of PI3K with PI3K agonist 740Y-P (15 μM) induced long-lasting potentiation of EPSCs (1.87±0.21, n=6; compared with baseline, P<0.05, paired sample t test). 740Y-P was applied in the recording pipette solution. This enhancement of AMPA EPSCs was significantly attenuated by blocking PKCλ with Myr-aPKC-PS (2.0 μM; 1.14±0.11, n=6; compared with 740Y-P, P<0.01, one-way ANOVA LSD test) or by selective PI3K antagonist Wort (0.5 μM; 1.15±0.13, n=6; compared with 740Y-P, P<0.01). As a control, Wort alone failed to exert any effect on basal EPSCs (0.97±0.13, n=7; compared with baseline, P>0.05). PKCλ knockdown attenuated enhancement of AMPA EPSCs (0.92±0.06, n=7; compared with baseline, P>0.05; compared with 740Y-P, P<0.01), which was reversed by PKCλ rescue (2.25±0.27, n=5; compared with 740Y-P, P>0.05; compared with PKCλ KD, P<0.01). In contrast, LTP was normal in cells transfected with PKCλ-scr-shRNA (1.79±0.16, n=5; compared with 740Y-P, P>0.05; compared with PKCλ KD, P<0.01). (F) PI3K-induced LTP occludes further enhancement induced by active PKCλ. Active PI3K (2 μg/ml), when applied in pipette solution, induced persistent enhancement of EPSCs (1.84±0.14, n=6; compared with baseline, P<0.01). This form of LTP was abolished by blocking PKCλ with Myr-aPKC-PS (2.0 μM; 1.08±0.08, n=6; compared with active PI3K, P<0.01). When co-applied with active form of PKCλ (1.5 nM), however, PI3K-induced LTP failed to display further enhancement in the magnitude of LTP (1.78±0.22, n=6; compared with active PI3K, P>0.05).
Source data for this figure is available on the online supplementary information page.
To test whether PKCλ is sufficient for LTP expression, we applied the active form of PKCλ (active PKCλ, 1.5 nM) in the solution of the recording pipette. Diffusion of active PKCλ into recorded cells enhanced EPSC amplitude within 6 min (Figure 1F), and by 40 min, EPSCs increased up to 1.94±0.22 (Figure 1F). Bath application of the PKCλ inhibitor Myr-aPKC-PS (2.0 μM) completely abolished the potentiation (Figure 1F). These data provide solid evidence that activation of PKCλ is sufficient for LTP expression.
In order to double check our deduction that the attenuation of LTP expression by 2.0 μM Myr-aPKC-PS was ascribe to the effect on PKCλ, we selectively applied Myr-aPKC-PS at different stages of LTP to investigate how these treatments would affect the LTP (Supplementary Figure S6). Our results confirm the conclusion that the attenuation of LTP expression by 2.0 μM Myr-aPKC-PS was ascribe to its inhibitory effect on PKCλ. Moreover, further results also exclude the possible role of conventional PKC in LTP expression (Supplementary Figure S7). Taken together, our above results provide strong evidence to the conclusion that PKCλ is both necessary and sufficient for LTP expression.
PKCλ functions downstream of PI3K to promote AMPAR incorporation at the synapse
One major mechanism underlying LTP is to increase AMPAR number at postsynaptic membrane via enhancing AMPAR insertion (Malenka and Nicoll, 1999; Malinow et al, 2000; Man et al, 2000; Sheng and Lee, 2001). It was reported that activation of PI3K was required for AMPAR insertion at activated CA1 synapses during LTP expression (Man et al, 2003). In addition, the PI3K product, PIP3, was known to be involved in many synaptic functions including membrane trafficking processes (James and Piper, 1994; Carpenter and Cantley, 1996; De Camilli et al, 1996; Rapoport et al, 1997; Cheever et al, 2001; Arendt et al, 2010). Moreover, PKCλ could bind to and be activated by PIP3 (Hirai and Chida, 2003; Suzuki et al, 2003). This raises the possibility that PKCλ may function as a downstream effector of PI3K during AMPAR insertion and LTP. To examine this hypothesis, we first investigated whether PI3K activation by its specific agonist 740Y-P (30 μM; Derossi et al, 1998; Williams and Doherty, 1999) could enhance the PKCλ activity. When PKCλ is activated, it undergoes autophosphorylation at Thr563 (Le Good et al, 1998; Standaert et al, 1999), therefore, the level of phosphorylated PKCλ at Thr563 is a good indicator of PKCλ activity. As shown in Figure 2, the level of phosphorylated Thr563 PKCλ (P-Thr563 PKCλ) detected in acute hippocampal slices was significantly elevated after 740Y-P incubation for 20 min (Figures 2A and B). This elevation in P-Thr563 PKCλ was blocked by PKCλ blocker Myr-aPKC-PS (Figures 2A and B) or by PI3k antagonist Wortmannin (Wort, Figures 2A and B). In contrast, the total PKC amount was not changed under these treatments. These results suggest that activation of PI3K promotes PKCλ activity. Because PKMζ has constitutively catalytic activity due to lacking an autoinhibitory regulatory domain, the role of PKMζ in LTP maintenance is dependent on the increase of its amount (Osten et al, 1996; Migues et al, 2010). However, the total amount of PKMζ was unaltered 20 min after 740Y-P (Figures 2C and D). To further examine the possible redistribution of PKMζ from cytoplasm to postsynaptic site upon 740Y-P treatment, we also determined PKMζ quantity at postsynaptic density (PSD) and failed to detect any obvious changes (Figures 2C and D; Supplementary Figure S8), indicating that PKMζ did not take a role during the early phase of 740Y-P administration.
We then investigated whether LTP induced by PI3K activation was also affected by inhibiting PKCλ. Intracellular application of the PI3K-specific agonist 740Y-P (15 μM) in the recording pipette solution induced a gradual enhancement of AMPA EPSCs, which became stable in 20 min (Figure 2E). This persistent enhancement of AMPA EPSCs was abolished by selective PI3K antagonist Wort (Figure 2E). Wort alone did not exert any effect on basal AMPA EPSCs (Figure 2E). Similarly, perfusing slices with 740Y-P (30 μM) for 10 min induced LTP (Supplementary Figure S9). Importantly, PKCλ inhibitor Myr-aPKC-PS (2.0 μM) significantly attenuated the PI3K-induced LTP expression (Figure 2E). Moreover, PKCλ KD inhibited PI3K-induced LTP expression (Figure 2E). In contrast, slices injected with PKCλ-scr-shRNA failed to display any obvious effect on PI3K-induced LTP expression (Figure 2E). Importantly, a rescue experiment performed in parallel reversed the changes in LTP caused by PKCλ KD (Figure 2E). In addition, the PI3K-induced LTP was also produced by putting the active form of PI3K in the recording pipette solution (active PI3K, 2 μg/ml; Figure 2F). Similarly, PKCλ inhibition with Myr-aPKC-PS (2.0 μM) completely inhibited the potentiation in EPSCs (Figure 2F). Moreover, co-application of the active form of PI3K (2.0 μg/ml) and PKCλ (1.5 nM) failed to display any further potentiation in EPSCs (Figure 2F). Taken together, our results indicate that PI3K and PKCλ act in a common pathway and that PKCλ functions downstream of PI3K during LTP. PI3K therefore exerts its effect via selectively activating PKCλ during LTP expression.
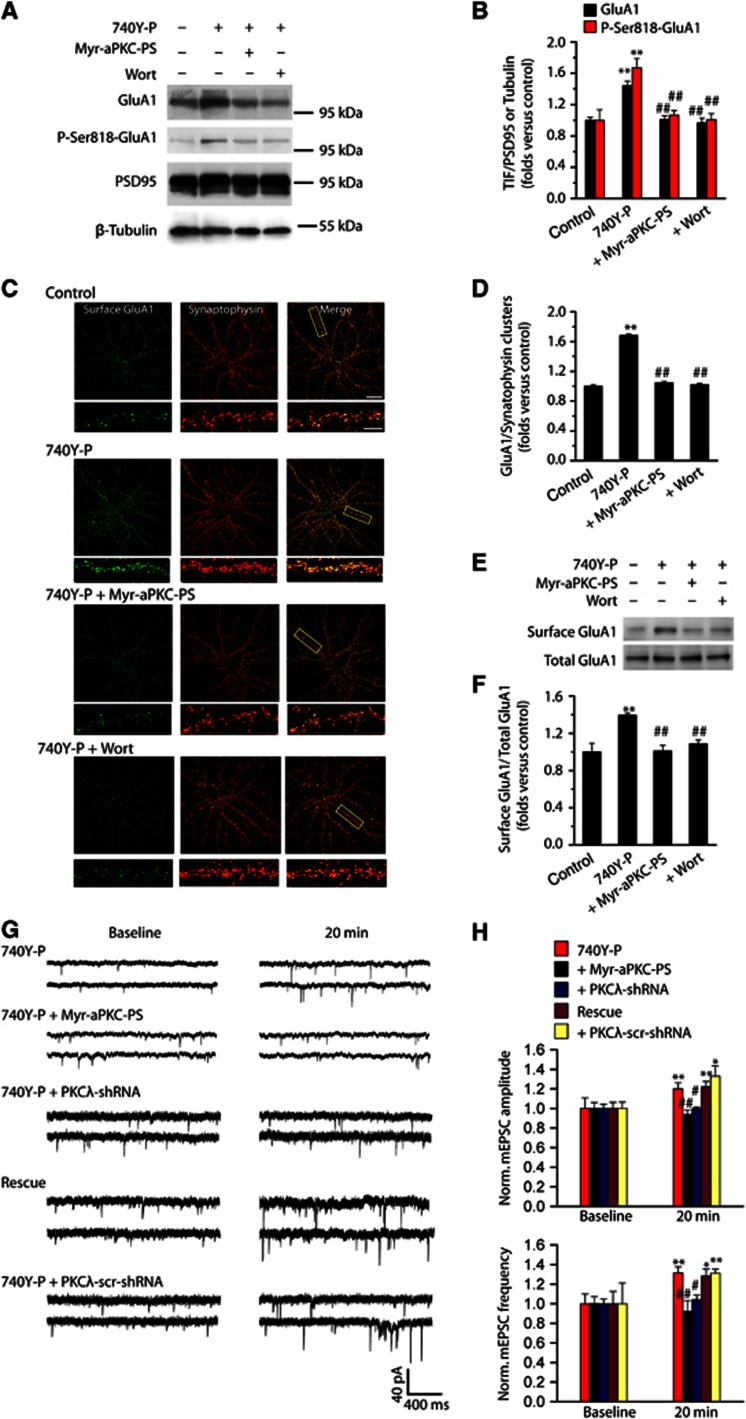
The AMPAR insertion at activated CA1 synapses contributes to PI3K-induced LTP (Man et al, 2003). We next examined whether LTP triggered by PKCλ activation was attributable to the postsynaptic incorporation of GluA1. Triton-insoluble fraction (TIF) was fractionated as postsynaptic compartment (Gardoni et al, 2001; Yan et al, 2011). PI3K activation increased the postsynaptic expression of GluA1 (Figures 3A and B) and GluA2 (Supplementary Figure S10), suggesting that AMPAR trafficking into PSD induced by PI3K activation are in the form of GluA1/GluA2. However, the enhancement in both postsynaptic GluA1 and GluA2 was inhibited by antagonist of either PI3K or PKCλ (Figures 3A and B; Supplementary Figure S10), suggesting that PKCλ activity was required for elevated AMPAR expression at postsynaptic site. Interestingly, a parallel enhancement in total and PSD phosphorylated GluA1 at the highly conserved serine 818 residue (P-Ser818-GluA1) was also observed upon PI3K activation (Figures 3A and B; Supplementary Figure S11), whereas no change in phosphorylated GluA1 at 831 (P-Ser818-GluA1) was detected (Supplementary Figure S11), suggesting that this increase in P-Ser818-GluA1 may contribute to the total increase in GluA1 in PSD. The specificity of the P-Ser818-GluA1 antibody was also examined (Supplementary Figure S12). These results were consistent with the immunofluorescence and western blot data obtained in cultured hippocampal neurons showing similar alterations in postsynaptic GluA1 and GluA2 (Figures 3C–F; Supplementary Figure S10). The synaptic GluA1 was determined by quantifying the degree of colocalization of GluA1 and synaptophysin using an N-terminal antibody against GluA1 under a non-permeable condition. We observed an increased GluA1 density at synapses following 740Y-P treatment (Figures 3C and D). This enhancement was completely blocked by Myr-aPKC-PS or Wort (Figures 3C and D). These results obtained further support from the standard surface biotinylation assay of GluA1 (Figures 3E and F).
Figure 3.
PKCλ activation is required for PI3K-induced increase in AMPA receptor expression and clustering at synapses. (A) PKCλ activation is required for PI3K-induced increase of GluA1 expression at synapses: western blot evidence. (B) Statistical plot of data as shown in (A). PI3K activation by 740Y-P treatment promoted postsynaptic expression of GluA1 (1.44±0.06, n=11; compared with control, P<0.01, one-way ANOVA LSD test). This potentiation was reversed by either PKCλ antagonist Myr-aPKC-PS (2.0 μM; 1.01±0.05, n=11; compared with 740Y-P, P<0.01) or PI3K antagonist Wort (0.5 μM; 0.97±0.06, n=11; compared with 740Y-P, P<0.01). A parallel enhancement in PSD phosphorylated GluA1 at the highly conserved serine 818 residue (P-Ser818-GluA1) was also observed upon PI3K activation (1.67±0.12, n=5; compared with control, P<0.01). This potentiation was reversed by either PKCλ antagonist Myr-aPKC-PS (1.07±0.06, n=5; compared with 740Y-P, P<0.01) or PI3K antagonist Wort (1.01±0.08, n=5; compared with 740Y-P, P<0.01). **Compared with control, P<0.01; ##compared with 740Y-P, P<0.01. (C) PKCλ activation is required for PI3K-induced increase of GluA1 clusters at synapses: immunofluorescence evidence. Example images of cell surface AMPARs (green; non-permeabilized) at synapses (red; synaptophysin) under control and various treatments are shown. Bar, 30 μm. Higher magnification of dendritic branches is in lower panels. Bar, 5 μm. The boxes in the Merge column indicated corresponding magnified regions. (D) Quantification of cell surface AMPA receptor clusters at synapses under various conditions, represented by normalized GluA1/synaptophysin clusters ratio. PI3K activation increased GluA1 clusters at synapses, represented by increased colocalization of surface GluA1 and synaptophysin staining (1.74±0.02, n=22; compared with control, P<0.01). This potentiation in synaptic AMPARs was reversed either by blocking PKCλ with Myr-aPKC-PS (2.0 μM; 1.11±0.02, n=24; compared with 740Y-P, P<0.01), or by blocking PI3K with Wort (0.5 μM; 1.10±0.02, n=25; compared with 740Y-P, P<0.01). (E) PKCλ activation is required for PI3K-induced increase of GluA1 expression at synapses: standard surface biotinylation assay. (F) Plot summarized the data from experiments shown in (E; 740Y-P, 1.39±0.02, n=4, compared with control, P<0.01; Myr-aPKC-PS, 1.01±0.06, n=4, compared with 740Y-P, P<0.01; Wort, 1.08±0.04, n=4, compared with 740Y-P, P<0.01). (G) Continuous sample mEPSCs traces taken at the times indicated from cultured hippocampal neurons under various treatments. (H) Plot summarized the data from experiments shown in (G). Adding 740Y-P (15 μM) in pipette solution significantly potentiated mEPSC amplitude (1.20±0.06, n=13; compared with baseline, P<0.01, paired samples t test) and frequency (1.31±0.06, n=13; compared with baseline, P<0.01) at 20 min after recording. The potentiation in both mEPSCs amplitude and frequency was abolished by blocking PKCλ with 2.0 μM Myr-aPKC-PS (amplitude, 0.94±0.04, n=11, compared with 740Y-P, P<0.01; frequency, 0.92±0.11, n=11, compared with 740Y-P, P<0.01; independent samples t test). This potentiation was absent in PKCλ KD group (amplitude, 1.00±0.02, n=7, compared with baseline, P>0.05; frequency, 1.04±0.05, n=7, compared with baseline, P>0.05), but reappeared in PKCλ rescue group (amplitude, 1.22±0.06, n=7, compared with baseline, P<0.01; frequency, 1.28±0.07, n=7, compared with baseline, P<0.01). As a control, potentiated mEPSC amplitude and frequency was normal in cells transfected with PKCλ-scr-shRNA (amplitude, 1.33±0.10, n=5, compared with baseline, P<0.05; frequency, 1.31±0.07, n=5, compared with baseline, P<0.01). *Compared with baseline, P<0.05; **compared with baseline, P<0.01; #compared with 740Y-P, P<0.05; ##compared with 740Y-P, P<0.01.
Source data for this figure is available on the online supplementary information page.
To further assess the functional consequence of potentiation of AMPAR insertion upon PI3K activation and the role of PKCλ in this process, we determined the possible changes in miniature EPSCs (mEPSCs). PI3K activation by 740Y-P (15 μM) treatment induced enhancement in both mEPSCs amplitude and frequency (Figures 3G and H). The increase in frequency was more likely caused by an unsilencing of synapses, because 740Y-P treatment did not display any significant effect on pair-pulse ratio (Supplementary Figure S13). The potentiation in both mEPSCs amplitude and frequency was abolished by blocking PKCλ with 2.0 μM Myr-aPKC-PS (Figures 3G and H). Moreover, no change in mEPSCs amplitude or frequency was detected in PKCλ KD group (Figures 3G and H). Notably, potentiation in mEPSCs reappeared in a PKCλ rescue experiment performed in parallel (Figures 3G and H). As a control, potentiated mEPSC amplitude and frequency was normal in cells transfected with PKCλ-scr-shRNA (Figures 3G and H). Taken together, these results provide compelling evidence that AMPAR incorporation upon PI3K activation was achieved through activation of PKCλ.
p62 may serve as a scaffolding protein to facilitate GluA1 phosphorylation by PKCλ
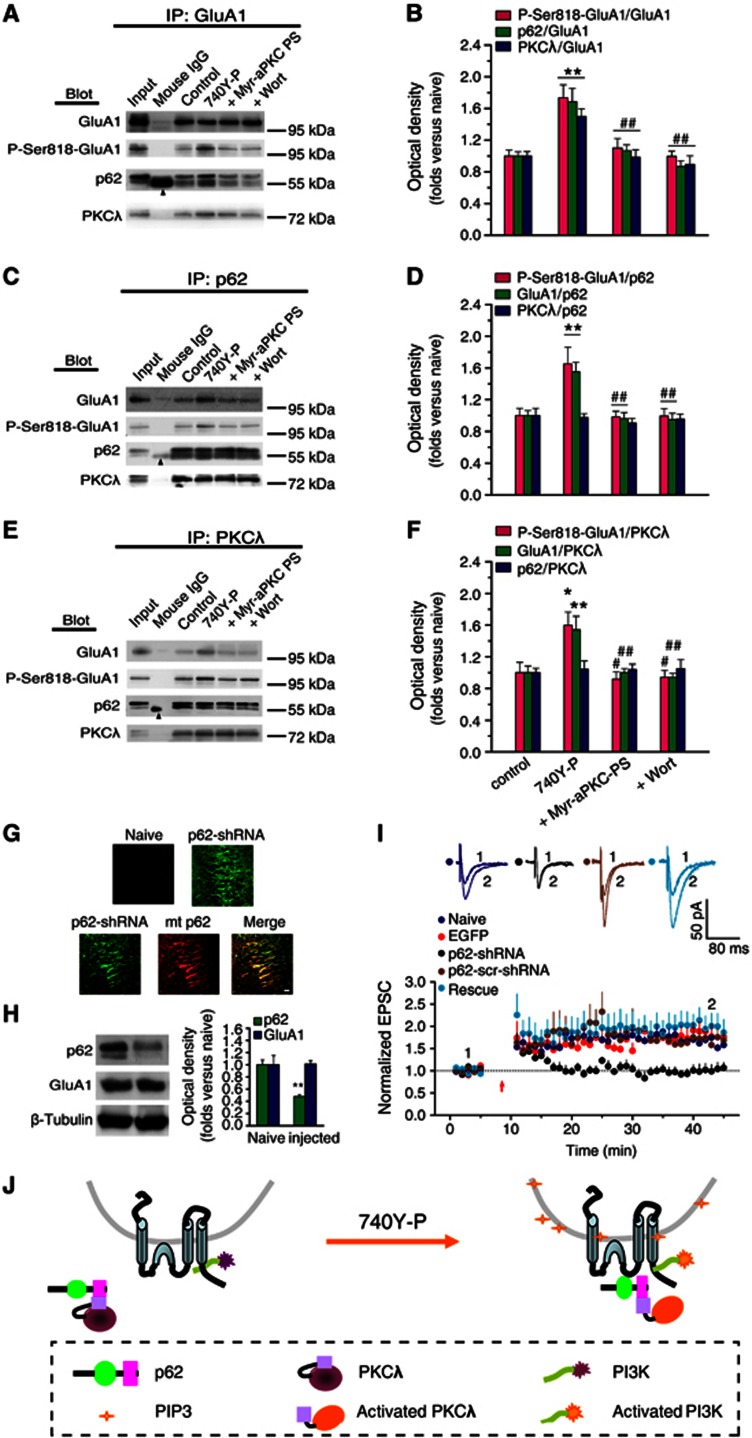
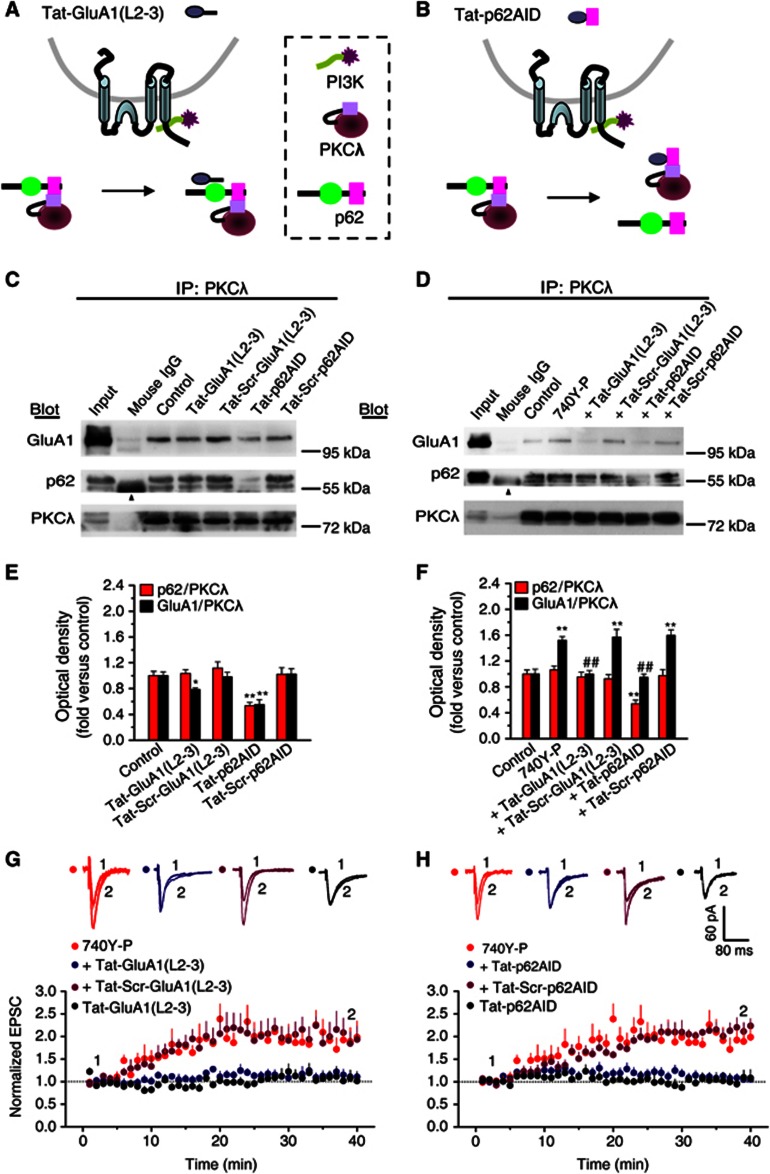
Since PKCλ activation is required for GluA1 Ser818 phosphorylation, PKCλ might be the kinase that directly phosphorylates GluA1 at Ser818. In order to phosphorylate GluA1, PKCλ needs to be recruited to a close vicinity of GluA1 at the activated synapses. This can be achieved through direct or indirect interactions between the kinase and GluA1. p62, an aPKC-associated protein that is highly expressed in the hippocampus (Gong et al, 1999), has been shown to be a multi-domain scaffolding protein that directly interacts with aPKCs and AMPARs through its aPKC interacting domain (AID) and ZZ-type finger domain, respectively (Geetha and Wooten, 2002; Jiang et al, 2009). Moreover, p62 knockout impairs hippocampal AMPAR trafficking and LTP expression (Jiang et al, 2009), suggesting that p62 plays a critical role in LTP. It is interesting to note that PKMζ lacks the regulatory region and thus cannot bind to p62 as PKCλ does (Puls et al, 1997; Sanchez et al, 1998; Jiang et al, 2006). Therefore, it is possible that p62 serves as a scaffold to link PKCλ and AMPARs to form a trimeric complex and thus to facilitate the AMPAR phosphorylation by PKCλ. To test this possibility, we used 10 000 × g ultracentrifugation for 20 min to remove non-solubilized material and employed co-immunoprecipitation (Co-IP) assay and confirmed that these molecules were indeed associated (Supplementary Figure S14). Then, we employed anti-GluA1 antibody to precipitate the protein complex and to examine how PI3K activation would affect the association between these molecules. We found that upon 740Y-P treatment the amounts of p62 and PKCλ that interacted with GluA1 (p62/GluA1 and PKCλ/GluA1) were significantly increased (Figures 4A and B), and the amount of P-Ser818-GluA1 was also increased simultaneously (Figures 4A and B). These results were further confirmed by Co-IP experiments with anti-p62 or anti-PKCλ antibody (Figures 4C–F). Interestingly, the association between PKCλ and p62 (PKCλ/p62) was not altered by the treatment (Figures 4C and D, also seen in Figures 4E and F). Antagonizing PKCλ activity with Myr-aPKC-PS (2.0 μM), however, abolished the enhancements in association induced by 740Y-P (Figures 4A and B). Similar results were obtained in Co-IP assays with anti-p62 or anti-PKCλ antibody (Figures 4C–F). In addition, blocking PI3K with Wort also suppressed the enhancements in association induced by 740Y-P (30 μM, Figures 4A–F). These results suggest that the activation of PKCλ is required for the increased association of GluA1 with p62 and PKCλ. The absence of change in association between PKCλ and p62 (PKCλ/p62) upon PI3K activation suggests that the level of their association is already high or even saturated under basal conditions in our experiments and was not further affected by PKCλ activity, as reported by a previous study (Sanchez et al, 1998). These data provide evidence to the notion that p62 serves as a scaffolding protein to link GluA1 and PKCλ during LTP.
Figure 4.
p62 serves as a scaffold protein to associate with both PKCλ and AMPAR during PI3K-induced LTP. (A, C, E) Co-IP assay with anti-GluA1, p62 or PKCλ antibody revealed the association of GluA1 with p62 or with PKCλ under various treatments. Arrowhead indicates the non-immune IgG heavy chain. (B, D, F) Statistical plot of data displaying the effects of various treatments on the association of GluA1 with p62 or with PKCλ as shown in (A), (C) and (E), respectively. PI3K activation potentiated the association between p62 and GluA1 or the association between PKCλ and GluA1 (p62/GluA1, 1.69±0.17, n=9, compared with control, P<0.01; PKCλ/GluA1, 1.50±0.09, n=9, compared with control, P<0.01; one-way ANOVA LSD test; A and B, also seen in C–F), but not affected the association between PKCλ and p62 (0.98±0.05, n=9; compared with control, P>0.05; C and D, also seen in E and F). The amount of P-Ser818-GluA1 was also increased simultaneously (P818-GluA1/GluA1, 1.74±0.16, n=5, compared with control, P<0.01; A and B, also seen in C–F). The potentiation was antagonized by blocking PKCλ with Myr-aPKC-PS (p62/GluA1, 1.07±0.07, n=9, compared with 740Y-P, P<0.01; PKCλ/GluA1, 0.99±0.09, n=9, compared with 740Y-P, P<0.01; P818-GluA1/GluA1, 1.10±0.11, n=5, compared with 740Y-P, P<0.01; A and B, also seen in C–F) or by PI3K antagonist Wort (p62/GluA1, 0.86±0.07, n=9, compared with 740Y-P, P<0.01; PKCλ/GluA1, 0.90±0.11, n=9, compared with 740Y-P, P<0.01; P818-GluA1/GluA1, 1.00±0.07, n=5, compared with 740Y-P, P<0.01; A and B, also seen in C–F. *Compared with control, P<0.05; **compared with control, P<0.01; #compared with 740Y-P, P<0.05; ##compared with 740Y-P, P<0.01. (G) Images showing EGFP expression in dorsal hippocampal slices prepared from p62-shRNA injected and contralateral (naive) hemispheres following unilateral injections (4 μl) of an rAAV coexpressing p62-shRNA and EGFP. A rescue experiment was also performed in parallel on slices prepared from hemisphere injected with both EGFP-p62-shRNA (green) and mcherry-p62 (red; bottom). Scale bar: 20 μm. (H) Confirmation of PKCλ knockdown. A 52% (52%±3%) reduction in p62 was observed in preparations from dorsal hippocampus. (I) p62 knockdown led to impaired LTP expression (1.03±0.13, n=9; compared with baseline, P>0.05, paired sample t test; compared with uninjected side cells, 1.63±0.05, n=6, P<0.01, one-way ANOVA LSD test), which was reversed by PKCλ rescue (1.99±0.29, n=5; compared with p62 KD, P<0.01; compared with uninjected or EGFP control, P>0.05). As a control, LTP was normal in cells transfected with p62-scr-shRNA (1.65±0.12, n=4; compared with baseline, P<0.05; compared with p62 KD, P<0.05). The EGFP control was borrowed from Figure 1C for comparison. (J) A schematic illustration describes the possible ‘briding’ role of p62 during PI3K-induced LTP. Under basal conditions, PKCλ/p62 association is already at high level, while relatively fewer GluA1 is associated with p62/PKCλ complex. PI3K activation by 740Y-P treatment causes accumulation of its products, PIP3, on the membrane of AMPAR-containing vesicles. PIP3 can thus activate downstream PKCλ, which in turn trigger the recruitment of the p62/PKCλ complex to vicinity of GluA1 via an unknown mechanism, thereby facilitating direct interaction between GluA1 and PKCλ. Thus, p62 may function as a ‘bridge’ between AMPAR and PKCλ to facilitate direct GluA1 phosphorylation by PKCλ.
Source data for this figure is available on the online supplementary information page.
If p62 really implements such an important ‘bridging’ function to facilitate direct GluA1 phosphorylation by PKCλ, then reduction in p62 should display significant physiological consequences, including possible alterations in synaptic plasticity. Thus, we utilized rAAV2/1 virus to deliver EGFP-tagged p62-shRNA into dorsal hippocampus to knockdown the expression of p62 in vivo (Figures 4G and H). p62-shRNA driven by these particles caused about 52% reduction in p62 expression from hippocampal homogenates closest to the injection point (Figure 4H). The knockdown effect of p62-shRNA also had been confirmed in HEK 293T cells (Supplementary Figure S2). Compared with naive hippocampal samples, p62 knockdown preparations displayed decreased potentiation magnitude during LTP expression (Figure 4I). Importantly, the changes in LTP caused by p62 KD were fully rescued by expression of a mutant p62 (mt-p62) with silent mutations resistant to RNA knockdown by p62-shRNA (Figure 4I).
These results support a working model illustrated in Figure 4J. According to this model, disrupting the interaction between p62 and GluA1 or between p62 and PKCλ should hinder the formation of the trimeric PKCλ/p62/GluA1 complex and in turn suppress GluA1 phosphorylation by PKCλ. In order to test this hypothesis, we utilized short cell-permeable peptides derived from p62 binding sequence in the loop L2-3 of GluA1 [Tat-GluA1(L2-3)] or from the AID sequence of p62 (Tat-p62AID), to interfere with p62/GluA1 and PKCλ/p62 interactions, respectively (Figures 5A and B). The efficacy and specificity of the peptides were evaluated by Co-IP assays. Tat-GluA1(L2-3) (2.0 μM) decreased the GluA1/PKCλ association (Figures 5C and E) but did not affect the p62/PKCλ association (Figures 5C and E), whereas Tat-p62AID (4.0 μM) significantly decreased both p62/PKCλ and GluA1/PKCλ association under basal condition (Figures 5C and E). In addition, as expected, Tat-GluA1(L2-3) blocked 740Y-P-induced potentiation in p62/GluA1 association (Supplementary Figure S15). On the other hand, although 740Y-P treatment failed to change p62/PKCλ association (Figures 5D and F; also see Figure 4), Tat-p62AID (4.0 μM) suppressed p62/PKCλ association to the degree below the basal level (Figures 5D and F). Moreover, Tat-GluA1(L2-3)-induced inhibition in p62/GluA1 association was accompanied by a decrease in GluA1/PKCλ association (Figures 5D and F and also see Supplementary Figure S15), whereas Tat-p62AID-induced inhibition in p62/PKCλ was accompanied by a decrease in GluA1/PKCλ (Figures 5D and F). These results are consistent with our prediction that both PKCλ and GluA1 directly interact with p62 and support the existence of a trimeric complex composing of PKCλ, p62 and GluA1 (PKCλ/p62/GluA1). This PKCλ/p62/GluA1 complex puts PKCλ in a strategic position to phosphorylate GluA1 during PI3K-induced LTP. In addition, these results support the notion that increased p62/GluA1 association upon 740Y-P treatment is a secondary event following PKCλ activation.
Figure 5.
Association of PKCλ or GluA1 with p62 is required for PI3K-induced LTP. (A, B) Schematic diagram illustrating the operation principles of two short cell membrane-permeable peptides which interfere GluA1/p62 association and p62/PKCλ, respectively. (C) Co-IP assay with antibody against PKCλ revealed how the two interfering peptides affect the association of PKCλ with p62 or with GluA1 under basal condition (in the absence of 740Y-P). Scrambled peptides were employed as controls. Tat-GluA1(L2-3), 2.0 μM; Tat-p62AID, 4.0 μM. Arrowhead indicates the non-immune IgG heavy chain. (D) Co-IP assay revealed how the two interfering peptides affected the increased association of PKCλ with GluA1 or with p62 upon PI3K activation. (E) Plot summarized the data from experiments shown in (C). Tat-GluA1(L2-3) decreased the GluA1/PKCλ association (0.78±0.02, n=4, compared with control, P<0.05; one-way ANOVA LSD test), but did not affect p62/PKCλ association (1.04±0.06, n=4, compared with control, P>0.05), whereas Tat-p62AID significantly inhibited both GluA1/PKCλ and p62/PKCλ association (in absence of 740Y-P, p62/PKCλ: 0.53±0.05, n=4; compared with control, P<0.01; GluA1/PKCλ: 0.55±0.08, n=4). As a control, scrambled peptides failed to show any significant effects on these associations. (F) Plot summarized the data from experiments shown in (D). Tat-GluA1(L2-3) reversed the PI3K-induced potentiation in GluA1/PKCλ association (1.00±0.05, n=7; compared with 740Y-P, 1.52±0.06, n=7, P<0.01). As a control, scrambled peptides failed to show any significant effects on these associations. On the other hand, although 740Y-P treatment failed to change p62/PKCλ association (1.06±0.06, n=7, compared with control, P>0.05), Tat-p62AID (4.0 μM) suppressed p62/PKCλ association to the degree below the basal level (0.54±0.06, n=7; compared with control, P<0.01). Moreover, Tat-GluA1(L2-3)-induced inhibition in p62/GluA1 association was accompanied by a decrease in GluA1/PKCλ association (1.00±0.05, n=7; compared with 740Y-P, P<0.01), whereas Tat-p62AID-induced inhibition in p62/PKCλ was accompanied by a decrease in GluA1/PKCλ (0.95±0.05, n=7; compared with 740Y-P, P<0.01). *Compared with control, P<0.05; **compared with control, P<0.01; ##compared with 740Y-P, P<0.01. (G, H) Interfering p62/GluA1 association (G) or p62/PKCλ association (H) attenuated PI3K-induced LTP (740Y-P, 1.88±0.27, n=5, compared with baseline, P<0.05, paired samples t test; 740Y-P+Tat-GluA1(L2-3), 1.14±0.11, n=6, compared with 740Y-P, P<0.05; 740Y-P+Tat-p62AID, 1.08±0.08, n=11, P<0.01; one-way ANOVA LSD test). Tat-GluA1(L2-3) or Tat-p62AID failed to show any significant effects on basal synaptic transmission (Tat-GluA1(L2-3), 1.06±0.13, compared with baseline, P>0.05; Tat-p62AID, 1.03±0.15, compared with baseline, P>0.05). As a control, the scrambled peptides had no effect on LTP (740Y-P+Tat-Scr-GluA1(L2-3), 2.00±0.32, n=5, compared with 740Y-P, P>0.05; 740Y-P+Tat-Scr-p62AID, 2.14±0.18, n=6, P>0.05). The data for 740Y-P group in (H) were borrowed from (G) for comparison. Overlaid sample traces on the top of the graph were recorded at indicated times were shown.
Source data for this figure is available on the online supplementary information page.
Protein interacting with C kinase 1 (PICK1) has been reported to be involved in both LTP and LTD (Terashima et al, 2008). GluA2 binds a dimeric PICK1, which may interact with PKC. This mechanism may also bring GluA1/GluA2 and PKC together. To examine the possible role of PICK1, we used a peptide Tat-pep2-SVKI that possessed YNVYGIESVKI sequence to disturb the association between GluA2 and PICK1 (Daw et al, 2000). We found that application of this peptide failed to produce any changes in the enhancement of GluA1 Ser818 phosphorylation induced by PI3K activation (Supplementary Figure S16), suggesting that PICK1 does not take a role in this PI3K-induced phosphorylation of GluA1.
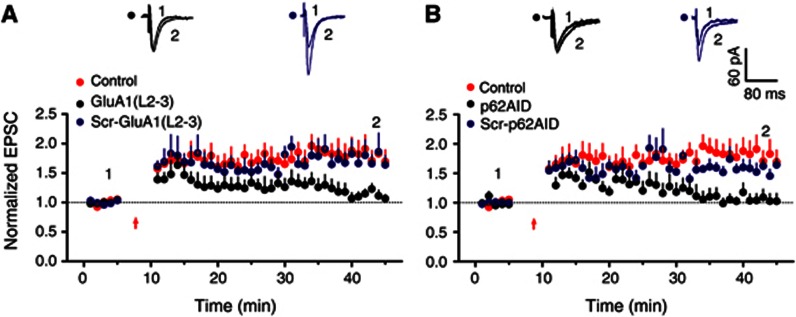
To further determine whether the direct interactions between p62 and PKCλ or between p62 and GluA1 were required for LTP induced by PI3K activation, we performed whole-cell recording in hippocampal CA1 neurons in the presence of Tat-GluA1(L2-3) or Tat-p62AID. As shown in Figures 5G and H, both Tat-GluA1(L2-3) and Tat-p62AID interfering peptides, which disturbed p62/GluA1 and PKCλ/p62 interactions, respectively, significantly attenuated LTP expression induced by PI3K activation. None of the peptides had an effect on the basal AMPA EPSCs. As a control, the scrambled peptides had no effect on LTP. These results suggest that interactions of p62 with both PKCλ and GluA1 are critical for the expression of LTP.
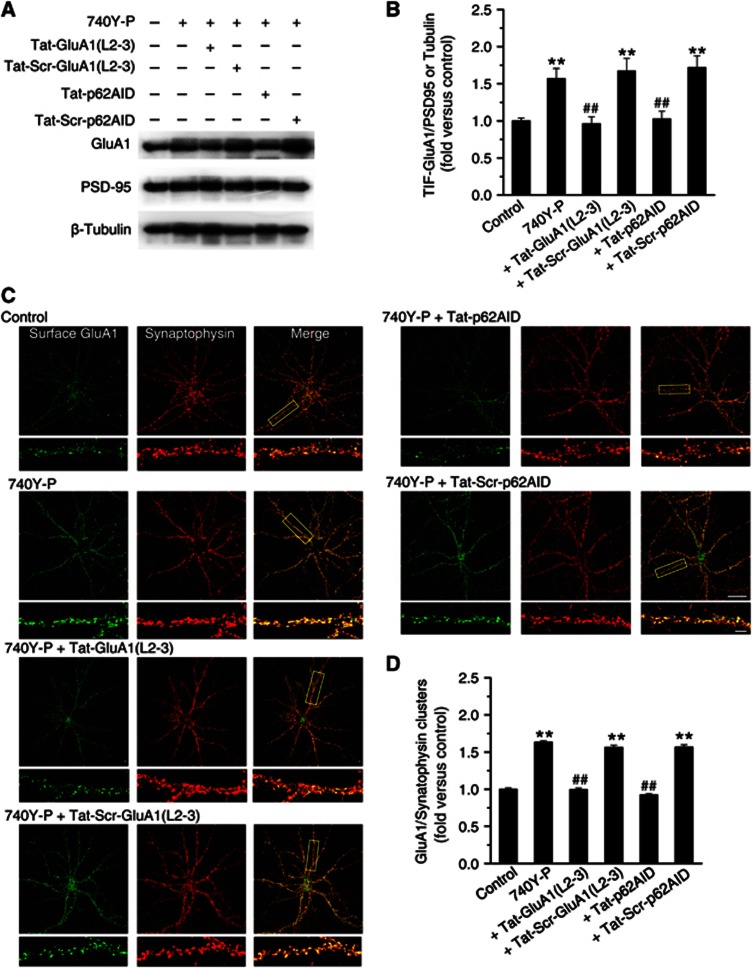
We next examined whether the attenuation of LTP by the interfering peptides was due to the disturbance of AMPAR insertion. As shown in Figures 6A and B, both peptides reversed 740Y-P-induced increase in postsynaptic GluA1 expression. The scrambled peptides did not have any effect. To further confirm these biochemical results, we performed immunofluorescence assays in cultured hippocampal neurons to determine the amount of synaptic GluA1 by quantifying colocalization of surface GluA1 and synaptophysin. Consistent with the western blotting data, both peptides significantly reduced GluA1 at synapses (Figures 6C and D). These effects were not observed in cultured cells incubated with the scrambled peptides. In addition, disturbing the p62/PKCλ or p62/GluA1 interaction with these interfering peptides also significantly attenuated expression of LTP that induced by a classical pairing protocol (Figures 7A and B). Together with the above biochemical results, these immunofluorescence data highly suggest that both p62/PKCλ and p62/GluA1 interactions are critical for both PI3K and physiologically induced LTP and further supports the notion that p62 serves as a scaffolding protein to facilitate the formation of trimeric PKCλ/p62/GluA1 complex upon PKCλ activation, which in turn place PKCλ in close proximity to facilitate GluA1 phosphorylation by PKCλ.
Figure 6.
Association of PKCλ or GluA1 with p62 is required for PI3K-induced AMPAR insertion at synapses. (A) Interfering peptides affected PI3K-induced increase in GluA1 expression at postsynaptic sites. (B) Plot summarized the data from experiments shown in (A). Increase in GluA1 postsynaptic clustering upon PI3K activation was completely reversed by interfering GluA1/p62 association with Tat-GluA1(L2-3) or by interfering p62/PKCλ association with Tat-p62AID (740-Y-P+Tat-GluA1(L2-3), 0.96±0.09, n=8, compared with 740-Y-P, 1.57±0.14, n=8, P<0.01; 740Y-P+Tat-p62AID, 1.03±0.10, n=8, compared with 740-Y-P, P<0.01; one-way ANOVA LSD test). The scrambled peptides (Tat-Scr-GluA1(L2-3) and Tat-Scr-p62AID) failed to display effect in GluA1 postsynaptic expression (compared with 740-Y-P, P>0.05). (C) Interfering peptides suppressed increase in AMAPR insertion upon PI3K activation. Example images of cell surface AMPARs (green; non-permeabilized) at synapses (red; synaptophysin) under control and various treatments are shown. Bar, 30 μm. Higher magnification of dendritic branches are in lower panels. Bar, 5 μm. The boxes in the Merge column indicated corresponding magnified regions. (D) Plot summarized the data from experiments shown in (C). Both of the two interfering peptides reversed increase in surface AMPAR insertion at synapses (740-Y-P+Tat-GluA1(L2-3), 1.00±0.02, n=19, compared with 740-Y-P, 1.63±0.03, n=22, P<0.01; 740Y-P+Tat-p62AID, 0.92±0.02, n=20, compared with 740-Y-P, P<0.01). As a control, the scrambled peptides failed to display any significant effect on AMPAR insertion (P>0.05). **Compared with control, P<0.01; ##compared with 740Y-P, P<0.01.
Source data for this figure is available on the online supplementary information page.
Figure 7.
Association of PKCλ or GluA1 with p62 is required for physiologically induced LTP expression. (A) Association of GluA1 with p62 is required for physiologically induced LTP. LTP was induced by a classical pairing protocol (200 synaptic stimuli at 2 Hz with a 2.5-min depolarization to 0 mV prior to stimulation). Interfering p62/GluA1 association with intracellular treatment of GluA1(L2-L3) peptide attenuated LTP magnitude at expression stage (1.14±0.08, n=9, compared with control, P<0.01, one-way ANOVA LSD test). As a control, the scrambled peptide (Scr-GluA1(L2-3)) failed to display any significant change in LTP (1.67±0.16, n=6, compared with control, P>0.05). (B) Association of PKCλ with p62 is required for physiologically induced LTP. Interfering p62/PKCλ association with p62AID peptide attenuated LTP magnitude at expression stage (1.04±0.12, n=8, compared with control, P<0.01), whereas the scrambled peptide (Scr-p62AID) failed to display any significant change in LTP (1.56±0.10, n=4, compared with control, P>0.05). The control in this figure is borrowed from Figure 1D for comparison.
Discussion
PKCλ exerts its effect in LTP expression
Several pieces of evidence support the role of PKCλ in LTP early expression. First, suppressing PKCλ function with either knockdown or pharmacological manipulation specifically attenuated the magnitude of potentiation during LTP expression, but not the induction of LTP (Figure 1). Second, the peptides disrupting the p62/PKCλ or p62/GluA1 association also specially affected LTP expression (Figures 6 and 7). Finally, PI3K, which can activate downstream PKCλ, is reported to be involved in the expression of LTP (Sanna et al, 2002). The action by PKCλ on LTP early expression is in clear contrast to the effect of PKMζ. PKMζ is considered as the constitutively active kinase that is responsible for the maintenance phase of LTP and is required for storage of spatial memory (Ling et al, 2002; Pastalkova et al, 2006; Yao et al, 2008; Migues et al, 2010). Moreover, the conventional PKC has been reported to be required for LTP induction (Abeliovich et al, 1993).
Importantly, the underlying mechanisms by which these isoforms of PKC take their roles in LTP are also different. PKCλ, as we demonstrate here, contributes to LTP by facilitating GluA1-dependent AMPAR incorporation at postsynaptic sites. PKMζ maintains LTP and memory either by inhibiting GluA2-dependent AMPAR removal (Migues et al, 2010) or by persistently modifying N-ethylmaleimide-sensitive factor/GluA2-dependent trafficking of postsynaptic AMPARs (Yao et al, 2008). Conventional PKC can be briefly activated by transient Ca2+ elevation during LTP induction, then phosphorylate Src and in turn modify NMDAR channel property to potentiate its opening (Lu et al, 1998, 1999). Thus, different isoforms of PKC may function differentially at different stages of LTP through distinct mechanisms.
The specificity of ZIP on aPKC
Recently, a debate is raised on the specificity of zeta inhibitory protein (ZIP) as a main pharmacological agent used to inhibit PKMζ (Sacktor and Fenton, 2012; Lisman et al, 2012a; Lisman, 2012b; Yao et al, 2013). The concern is that ZIP at relatively high dosage (>5.0 μM) may cause non-specific effects. It is worth noting that the sequence of the ZIP (myr-SIYRRGARRWRKL) is identical to the autoinhibitory pseudosubstrate domain of both PKCλ and PKMζ (Standaert et al, 1999; Bosch et al, 2004); therefore, it may inhibit the activity of both PKMζ and PKCλ (Jiang et al, 2006), even though the sensitivity of these two aPKCs to this ZIP might not be identical. In this study, we found that Myr-aPKC-PS at a relatively low dosage (0.5 μM) exerted its action on PKMζ and hardly affected PKCλ, while Myr-aPKC-PS at a higher level (2.0 μM) suppressed the activity of both PKMζ and PKCλ. These results call attention to the issue that the dosage of ZIP is critical to the effect it exerts. Our observation that Myr-aPKC-PS at a dosage that selectively inhibits PKMζ attenuates the protein synthesis-dependent late phase of LTP confirms the role of PKMζ in LTP maintenance. However, we cannot exclude the possibility that ZIP-induced inactivity of PKCλ may also make a contribution to inhibitory effect by ZIP at a dosage higher than 2.0 μM, even if it is only applied at the late stage of LTP. Thus, care should be taken in the future when interpretations on ZIP’s action are given.
The implication of the present study is further highlighted by two recent independent studies, both of which indicate that the suppressive effects of ZIP on LTP are independent of PKMζ (Lee et al, 2013; Volk et al, 2013; for comments of these studies, see Frankland and Josselyn, 2013). However, as Frankland and Josselyn mention in their comments, upon publication of the two studies, at least two questions still remained to be addressed. First, which molecule is the real target of ZIP independent of PKMζ? Second, is that targeted molecule required for synaptic plasticity? Our present study well addressed these two questions. We demonstrate that ZIP at a regular dosage used by previous studies (2 μM) can also inhibit the activity of PKCλ. More importantly, our present study explicitly demonstrates, for the first time, that PKCλ is the precise molecular target of ZIP as well as a molecule involved in synaptic plasticity. Till now the results obtained from knockout animals in these studies cannot completely exclude the possible compensation by other remained PKC isoforms, future study on acute PKMζ knockdown animals is needed to validate these findings. Nevertheless, if PKMζ is really not required for synaptic plasticity, learning and memory as the two recent studies point out, then most of the previous observations that have been ascribed to the effects of PKMζ, especially those findings based on ZIP treatment, should now be accredited to PKCλ.
One recent study reports that ZIP at 1 μM fails to inhibit PKMζ in cells overexpressing PKMζ (Wu-Zhang et al, 2012). This may be partially, if not at all, due to the overexpression system they used in all of their experiments. PKMζ overexpression can largely elevate the level of exogenous PKMζ. The resultant PKMζ level may exceed the level required for phosphorylation of all its endogenous substrate (Yao et al, 2013). As a result, almost all of the PKMζ endogenous substrate is phosphorylated. If ZIP only antagonizes the excess part of the PKMζ, then the remaining PKMζ can still keep its substrate phosphorylated. In contrast, as a general and potent kinase inhibitor, staurosporine inhibits both PKMζ and other PKC isoforms. Accordingly, the level of phospho-Ser PKC, which reflects the phosphorylation of all PKCs substrate (but not only for PKMζ substrate), is more easily be affected by staurosporine.
How does PKCλ affect AMPAR trafficking during LTP expression?
It has been widely accepted that LTP is mediated by synaptic insertion of GluA1-containing receptors via its C-tail interactions (Shi et al, 1999, 2001; Zamanillo et al, 1999; Hayashi et al, 2000; Lu et al, 2001; Passafaro et al, 2001; Esteban et al, 2003; Lee et al, 2003; Meng et al, 2003; Boehm et al, 2006; Lin et al, 2009). In this study, upon PKCλ activation, we detected a parallel increase in phosphorylation of Ser818 site in membrane proximal region of GluA1 C-tail. Since previous study has demonstrated that phosphorylation of this residue controls AMPAR incorporation into active synapses during LTP, we propose that PKCλ may take its role in LTP early expression through phosphorylation of this site. However, here we do not examine the direct causal link between PKCλ activation and Ser818 phosphorylation.
Recently, an important study used a single molecular replacement strategy to replace all endogenous AMPARs with transfected subunits and found no requirement of the GluA1 C-tail for LTP (Granger et al, 2012). Moreover, LTP induction was independent of GluA1 subunit type. These findings obviously contradict with some previous data and thus impel us to reconsider the core molecular events underlying synaptic plasticity. Taken into consideration of those compelling studies that demonstrates impaired LTP in mice with phospho-null knock-in mutations of two key phosphorylation sites (Lee et al, 2003), the study cannot rule out the possibility that C-tails have some modulatory effects on synaptic plasticity. The study also shows that GluA1 knockout can significantly reduce synaptic and extrasynaptic AMPARs and disrupt LTP expression, which is consistent with previous studies (Zamanillo et al, 1999). Lu et al (2010)have found that the first loop of GluA1 is required for synaptic targeting of AMPARs. Our present results also show that the domain between loop2 and loop3 of GluA1, which can bind p62-PKCλ complex, takes a role in this process. Whether other domains of GluA1 are necessary for AMPA trafficking or whether PKCλ has functions other than phosphorylating Ser818 need to be further investigated.
Materials and methods
Whole-cell recordings from acute hippocampal slices
Male Sprague Dawley rats, 2–3 weeks old, were anaesthetized with ethyl ether and decapitated. The whole-cell recording of CA1 neurons in hippocampal slices was performed using standard techniques. For details, see Supplementary Experimental Procedures.
shRNAs and shRNA rescue design, packaging and stereotactic virus injection
shRNA sequences targeting the rat isoforms of PKCλ and p62 were 5′-CTGGATTTGCTGACATCCAAG-3′ and 5′-GAACUCCAGUCUCUACAGA-3′ separately, and the scrambled shRNA sequences for PKCλ and p62 were 5′-GGCTATGTAAGTCCTTCCAAG-3′ and 5′-GCATAACCCTTCAATCAGG-3′, respectively. The PKCλ and p62 genes were synthesized according to Genebank NM_032059.1 and NM_175843.4, respectively, and the mt-PKCλ and mt-p62 genes were synthesized with silent mutations resistant to RNA knockdown by PKCλ-shRNA and p62-shRNA separately. These gene sequences were then cloned into plasmids for packaging into AAV2/1 virus. A comprehensive description of these procedures and virus injection is provided in Supplementary Experimental Procedures and Supplementary Figures S1 and S2.
Primary hippocampal cell culture, transfection and spontaneous EPSCs recordings
Low-density primary hippocampal cultures (0.1–0.2 × 105 cells/ml) from E18–19 rats were prepared as described previously (Brewer et al, 1993). A comprehensive description of cell culture, transfection and mEPSCs recording is provided in Supplementary Experimental Procedures.
Immunofluorescence labelling and analysis
DIV14–18 cultured neurons were treated with 740Y-P in the culture media for 20 min and then were fixed with paraformaldehyde for 30 min. A comprehensive description of labelling of surface GluA1 and confocal imaging is provided in Supplementary Experimental Procedures.
Subcellular fractionation, immunoblotting and immunoprecipitation
Hippocampal slices were prepared as described above. A comprehensive description of subcellular fractionation, immunoblotting and immunoprecipitation is provided in Supplementary Experimental Procedures.
In vitro kinase activity assay
A comprehensive description of in vitro kinase activity assay is provided in Supplementary Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by grants to WL from the National Natural Science Foundation of China (NFSC, No. 31025011), the Major State Basic Research Program of China (2010CB912002), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Doctoral Fund of Ministry of Education of China (20103234110004) and the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University.
Author contributions: The study was conceived and supervised by WL. SR and JY performed most of the experiments. XZ, YB, WP and WY contributed with biochemical and cell culture assays. TT contributed with confocal image analysis. WL wrote and edited the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S (1993) Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell 75: 1253–1262 [DOI] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Royo M, Fernandez-Monreal M, Knafo S, Petrok CN, Martens JR, Esteban JA (2010) PIP3 controls synaptic function by maintaining AMPA receptor clustering at the postsynaptic membrane. Nat Neurosci 13: 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC (1994) Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4: 389–399 [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39 [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R (2006) Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluA1. Neuron 51: 213–225 [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collingridge GL (2000) A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. Eur J Neurosci 12: 4055–4062 [DOI] [PubMed] [Google Scholar]
- Bosch RR, Bazuine M, Span PN, Willems PH, Olthaar AJ, van Rennes H, Maassen JA, Tack CJ, Hermus AR, Sweep CG (2004) Regulation of GLUT1-mediated glucose uptake by PKClambda-PKCbeta(II) interactions in 3T3-L1 adipocytes. Biochem J 384: 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ (1993) Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 35: 567–576 [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10: 302–317 [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Cantley LC (1996) Phosphoinositide kinases. Curr Opin Cell Biol 8: 153–158 [DOI] [PubMed] [Google Scholar]
- Cheever ML, Sato TK, de Beer T, Kutateladze TG, Emr SD, Overduin M (2001) Phox domain interaction with PtdIns(3)P targets the Vam7 t-SNARE to vacuole membranes. Nat Cell Biol 3: 613–618 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT (2010) Long-term depression in the CNS. Nat Rev Neurosci 11: 459–473 [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT (2000) PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28: 873–886 [DOI] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P (1996) Phosphoinositides as regulators in membrane traffic. Science 271: 1533–1539 [DOI] [PubMed] [Google Scholar]
- Derossi D, Williams EJ, Green PJ, Dunican DJ, Doherty P (1998) Stimulation of mitogenesis by a cell-permeable PI 3-kinase binding peptide. Biochem Biophys Res Commun 251: 148–152 [DOI] [PubMed] [Google Scholar]
- Downward J (1997) Role of phosphoinositide-3-OH kinase in Ras signaling. Adv Second Messenger Phosphoprotein Res 31: 1–10 [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Josselyn SA (2013) Memory and the single molecule. Nature 493: 312–313 [DOI] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M (2001) Hippocampal synaptic plasticity involves competition between Ca2+/calmodulin-dependent protein kinase II and postsynaptic density 95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci 21: 1501–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Wooten MW (2002) Structure and functional properties of the ubiquitin binding protein p62. FEBS Lett 512: 19–24 [DOI] [PubMed] [Google Scholar]
- Gong J, Xu J, Bezanilla M, van Huizen R, Derin R, Li M (1999) Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science 285: 1565–1569 [DOI] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA (2012) LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Fink AE, Sarinana J, Vissel B, O'Dell TJ (2007) Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol 98: 2488–2492 [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267 [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC (2003) Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem 278: 40305–40316 [DOI] [PubMed] [Google Scholar]
- Hirai T, Chida K (2003) Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem 133: 1–7 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108 [DOI] [PubMed] [Google Scholar]
- James DE, Piper RC (1994) Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol 126: 1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Parameshwaran K, Seibenhener ML, Kang MG, Suppiramaniam V, Huganir RL, Diaz-Meco MT, Wooten MW (2009) AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus 19: 392–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Suppiramaniam V, Wooten MW (2006) Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals 15: 266–282 [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch MA (2000) Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology 39: 643–651 [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281: 2042–2045 [DOI] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO (2013) Prkcz null mice show normal learning and memory. Nature 493: 416–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643 [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW (2000) A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 31: 841–851 [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12: 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC (2002) Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 5: 295–296 [DOI] [PubMed] [Google Scholar]
- Lisman J (2012b) Memory erasure by very high concentrations of ZIP may not be due to PKM-zeta. Hippocampus 22: 648–649 [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190 [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S (2012a) Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13: 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Isozaki K, Roche KW, Nicoll RA (2010) Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc Natl Acad Sci USA 107: 22266–22271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254 [DOI] [PubMed] [Google Scholar]
- Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, MacDonald JF (1999) G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci 2: 331–338 [DOI] [PubMed] [Google Scholar]
- Lu YM, Rode JC, Davidow J, Salter MW (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279: 1363–1367 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA (1999) Long-term potentiation--a decade of progress? Science 285: 1870–1874 [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y (2000) LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol 10: 352–357 [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW (1989) Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245: 862–866 [DOI] [PubMed] [Google Scholar]
- Man HY, Ju W, Ahmadian G, Wang YT (2000) Intracellular trafficking of AMPA receptors in synaptic plasticity. Cell Mol Life Sci 57: 1526–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT (2003) Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38: 611–624 [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z (2003) Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron 39: 163–176 [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K (2010) PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13: 630–634 [DOI] [PubMed] [Google Scholar]
- Muller D, Bittar P, Boddeke H (1992) Induction of stable long-term potentiation in the presence of the protein kinase C antagonist staurosporine. Neurosci Lett 135: 18–22 [DOI] [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Dow-Edwards D, Osman M, Sacktor TC (2000) Distribution of protein kinase Mzeta and the complete protein kinase C isoform family in rat brain. J Comp Neurol 426: 243–258 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1988) The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 334: 661–665 [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484–496 [PubMed] [Google Scholar]
- Osten P, Valsamis L, Harris A, Sacktor TC (1996) Protein synthesis-dependent formation of protein kinase Mzeta in long-term potentiation. J Neurosci 16: 2444–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Eichele G, Leitges M (2004) Differential expression of atypical PKCs in the adult mouse brain. Brain Res Mol Brain Res 127: 79–88 [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4: 917–926 [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313: 1141–1144 [DOI] [PubMed] [Google Scholar]
- Puls A, Schmidt S, Grawe F, Stabel S (1997) Interaction of protein kinase C zeta with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA 94: 6191–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T (1997) Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J 16: 2240–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J (1996) Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J 15: 2442–2451 [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC, Fenton AA (2012) Appropriate application of ZIP for PKMζ inhibition, LTP reversal, and memory erasure. Hippocampus 22: 645–647 [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E (1993) Persistent activation of the zeta isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA 90: 8342–8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, De Carcer G, Sandoval IV, Moscat J, Diaz-Meco MT (1998) Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol 18: 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W (2002) Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci 22: 3359–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH (2001) AMPA receptor trafficking and the control of synaptic transmission. Cell 105: 825–828 [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343 [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811–1816 [DOI] [PubMed] [Google Scholar]
- Sossin WS (2007) Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem 14: 236–246 [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV (2001) Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry 40: 249–255 [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Sajan MP, Cong L, Quon MJ, Farese RV (1999) Okadaic acid activates atypical protein kinase C (zeta/lambda) in rat and 3T3/L1 adipocytes. An apparent requirement for activation of Glut4 translocation and glucose transport. J Biol Chem 274: 14074–14078 [DOI] [PubMed] [Google Scholar]
- Steinberg SF (2008) Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Akimoto K, Ohno S (2003) Protein kinase C lambda/iota (PKClambda/iota): a PKC isotype essential for the development of multicellular organisms. J Biochem 133: 9–16 [DOI] [PubMed] [Google Scholar]
- Terashima A, Pelkey KA, Rah JC, Suh YH, Roche KW, Collingridge GL, McBain CJ, Isaac JT (2008) An esential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron 57: 872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci 22: 267–272 [DOI] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL (2013) PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature 493: 420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Kelly PT (1995) Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron 15: 443–452 [DOI] [PubMed] [Google Scholar]
- Williams EJ, Doherty P (1999) Evidence for and against a pivotal role of PI 3-kinase in a neuronal cell survival pathway. Mol Cell Neurosci 13: 272–280 [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH (1993) Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol 3: 291–298 [DOI] [PubMed] [Google Scholar]
- Wu-Zhang AX, Schramm CL, Nabavi S, Malinow R, Newton AC (2012) Cellular pharmacology of protein kinase Mzeta (PKMzeta) contrasts with its in vitro profile: implications for PKMzeta as a mediator of memory. J Biol Chem 287: 12879–12885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Chen RQ, Gu QH, Yan JZ, Wang SH, Liu SY, Lu W (2009) Metaplastic regulation of long-term potentiation/long-term depression threshold by activity-dependent changes of NR2A/NR2B ratio. J Neurosci 29: 8764–8773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JZ, Xu Z, Ren SQ, Hu B, Yao W, Wang SH, Liu SY, Lu W (2011) Protein kinase C promotes NMDA receptor trafficking by indirectly triggering CaMKII autophosphorylation. J Biol Chem 286: 25187–25200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC (2008) PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluA2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci 28: 7820–7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Shao C, Jothianandan D, Tcherepanov A, Shouval H, Sacktor TC (2013) Matching biochemical and functional efficacies confirm ZIP as a potent competitive inhibitor of PKMzeta in neurons. Neuropharmacology 66: 37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B (1999) Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811 [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R (2002) Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110: 443–455 [DOI] [PubMed] [Google Scholar]
- Zhuo M, Hawkins RD (1995) Long-term depression: a learning-related type of synaptic plasticity in the mammalian central nervous system. Rev Neurosci 6: 259–277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.