Abstract
EMBO J (2013) 32 10, 1365–1380. doi:; DOI: 10.1038/emboj.2013.60
Long-term potentiation (LTP) of synaptic transmission has received widespread attention because it is thought to form the physiological basis of learning and memory. A new paper in The EMBO Journal identifies the atypical PKC family member PKCλ as an important contributor to the strengthening of the postsynaptic response in LTP.
Synaptic transmission in the brain is largely mediated by presynaptic glutamate release and postsynaptic activation of AMPA-type glutamate receptors (AMPARs). LTP is a permanent increase in synaptic transmission at individual synapses following a brief period of strongly enhanced synchronous activity of the very synapses and of the neurons the synapses connect (Lisman and Hell, 2008). LTP is typically mediated by an increase in postsynaptic AMPAR activity and requires Ca2+ flux through NMDA-type glutamate receptors (NMDARs) and the ensuing stimulation of CaMKII and, at least in certain cases, of PKC (Lisman and Hell, 2008).
The PKC family consists of ‘conventional’ PKCα, β, and γ, which are activated by Ca2+-induced binding of anionic phospholipid to their C2 domains and by binding of diacylglyerol (DAG) to their C1 domains, ‘novel’ PKCδ and ε, which are activated by DAG, and ‘atypical’ PKCζ and rodent PKCλ/ human PKCι, which are activated by lipids such as PIP3 or ceramide via binding to their unorthodox C1 domains (Steinberg, 2008). Proteolytic processing as well as differential splicing can give rise to constitutively active PKC isoforms (PKMs) that lack the regulatory domain including their inhibitory pseudosubstrate segments. Expression of PKMζ, which is formed by translation of an alternative transcript of the PKCζ gene, is induced by LTP whereas the full-length PKCζ gene product is usually undetectable in the hippocampus (Hernandez et al, 2003).
PKMζ has been implicated in the maintenance of LTP and memory (e.g., Ling et al, 2002; Pastalkova et al, 2006). Part of this evidence stems from the inhibition of LTP by the membrane-permeant myristoylated peptide ZIP that is derived from the autoinhibitory pseudosubstrate segment of PKCζ. However, the pseudosubstrate segment of PKCζ is identical to that of PKCλ raising the possibility that ZIP might also inhibit PKCλ and exert some of its effects by antagonizing PKCλ rather than PKCζ. In fact, pursuing this notion, the new work by Ren et al (2013) shows that 2 μM ZIP, which they call Myr-aPKC-PS, blocks not only PKMζ but also PKCλ. Application of ZIP/Myr-aPKC-PS resulted in short-lived LTP that decayed to baseline within 20 min after its induction in CA1 pyramidal cells. Knockdown (KD) of PKCλ also rendered LTP short-lived. Direct stimulation of PI3K, which generates PIP3, increased PKCλ activity, postsynaptic AMPAR content, and mEPSC and EPSC magnitude (mimicking LTP), all of which were blocked by Myr-aPKC-PS and PKCλ KD. PI3K activation also increased phosphorylation of the AMPAR GluA1 subunit on its PKC site S818. Phosphorylation of S818 by PKC is important for LTP under certain (Boehm et al, 2006) but not all conditions (Granger et al, 2013). It is possible that S818 phosphorylation plays a more important role in postsynaptic targeting of homomeric AMPAR that are formed by four GluA1 subunits rather than that of the more prevalent GluA1/GluA2 heterotetrameric receptors, with GluA1 homomers being important for LTP at certain but not all ages in rodents (Lu et al, 2007).
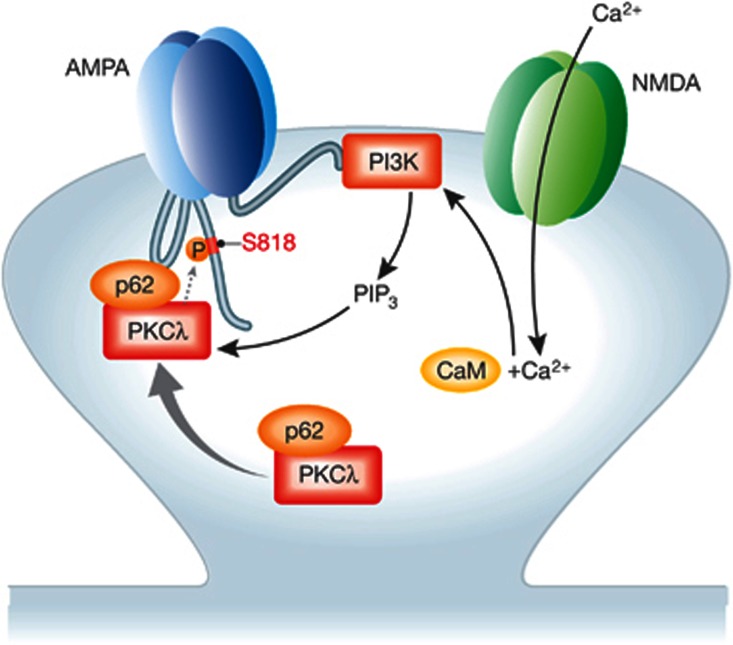
LTP is synapse specific. In fact, the following interactions are well suited to restrict PIP3–PKCλ signalling to activated postsynaptic sites. PI3K directly binds to GluA2 (Man et al, 2003) and the protein p62 links PKCλ to AMPARs (Jiang et al, 2009). Ren et al (2013) found that activation of PI3K increased the interaction of p62 and PKCλ with AMPARs, which was blocked by ZIP/Myr-aPKC-PS. Furthermore, KD of p62 blocked LTP. Acute application of membrane-permeant peptides derived from either the p62-binding site on GluA1 or the PKC-binding site on p62 displaced p62/PKCλ from GluA1 and PKCλ from p62, respectively, and abrogated upregulation of postsynaptic AMPAR content and EPSC magnitude by PIK3 activation and pairing-induced LTP. The emerging model for localized signalling via the Ca2+–PI3K–p62/PKCλ–AMPAR pathway is illustrated in Figure 1. The activity-driven increase in p62/PKCλ–AMPAR association might serve to recruit PKCλ to synapses that are undergoing LTP for prolonged signalling by PKCλ at these synapses to contribute to synapse specificity of LTP.
Figure 1.
The PI3K–PKCλ–AMPAR signalling pathway. The centre of the figure depicts a GluA1/A2 heterotetrameric AMPAR, which accounts for ∼80% of hippocampal AMPARs. PI3K binds to residues 833–853 in the cytosolic C terminus of the AMPAR GluA2 subunit for localized postsynaptic signalling (Man et al, 2003). p62 binds with its atypical PKC interaction domain (AID) to the N-terminal regulatory region of PKCλ and with its Zn finger domain to the second intracellular loop of GluA1 (Jiang et al, 2009). Ca2+ influx via the NMDAR during high-frequency synaptic transmission can activate PIP3K via calmodulin (CaM) (Joyal et al, 1997). The consequent production of PIP3,4,5 stimulates PKCλ, which might act in part by phosphorylating S818 on GluA1. How high-frequency activity augments the p62/PKCλ–AMPAR interaction is unclear.
PKMζ lacks a p62-binding region and full-length PKCζ is not expressed in the brain (Hernandez et al, 2003), leaving PKCλ as the only known candidate that matches the criteria for upregulating AMPAR by atypical PKCs in this p62-dependent manner. Recent work shows that knockout of the PKCζ/PKMζ-coding gene does not affect memory and that ZIP/Myr-aPKC-PS still reverses LTP in these mice (Lee et al, 2013; Volk et al, 2013). With the findings of Ren et al (2013), it is conceivable that PKCλ is an alternative target for this peptide in the maintenance of LTP and memory. However, given the strong run down of basal synaptic transmission in unpotentiated slices, it is also quite possible that ZIP/Myr-aPKC-PS affects yet other targets (Volk et al, 2013). The work by Ren et al (2013) will certainly inspire and guide further work on the potential complementary roles of PKCλ and PKMζ in LTP and memory, and stimulate the search for further PKC targets.
Footnotes
The authors declare that they have no conflict of interest.
References
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R (2006) Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 51: 213–225 [DOI] [PubMed] [Google Scholar]
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA (2013) LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 493: 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC (2003) Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem 278: 40305–40316 [DOI] [PubMed] [Google Scholar]
- Jiang J, Parameshwaran K, Seibenhener ML, Kang MG, Suppiramaniam V, Huganir RL, Diaz-Meco MT, Wooten MW (2009) AMPA receptor trafficking and synaptic plasticity require SQSTM1/p62. Hippocampus 19: 392–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JL, Burks DJ, Pons S, Matter WF, Vlahos CJ, White MF, Sacks DB (1997) Calmodulin activates phosphatidylinositol 3-kinase. J Biol Chem 272: 28183–28186 [DOI] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO (2013) Prkcz null mice show normal learning and memory. Nature 493: 416–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC (2002) Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci 5: 295–296 [DOI] [PubMed] [Google Scholar]
- Lisman JE, Hell JW (2008) Long-term potentiation. In Structural and Functional Organization of the Synapse Hell JW, Ehlers MD (eds) Heidelberg: Springer. [Google Scholar]
- Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usachev YM, McKnight GS, Hell JW (2007) Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J 26: 4879–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT (2003) Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron 38: 611–624 [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006) Storage of spatial information by the maintenance mechanism of LTP. Science 313: 1141–1144 [DOI] [PubMed] [Google Scholar]
- Ren S-Q, Yan J-Z, Zhang X-Y, Bu Y-F, Pan W-W, Yao W, Tian T, Lu W (2013) PKCλ is critical in AMPA recept phosphorylation and synaptic incorporation during LTP. EMBO J 32: 1365–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF (2008) Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL (2013) PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature 493: 420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]