Abstract
Objective
Elevated pre-sleep arousal has been consistently associated with insomnia, yet the cognitive-emotional mechanisms involved in sleep-related arousal remain unclear. The purpose of this study was to identify predictors of pre-sleep arousal and trait hyperarousal from a set of variables that included self-reported affect, sleep-related cognitions, locus of control, and gender.
Methods
Cross-sectional data were analyzed for 128 participants (89 female) who met criteria for psychophysiological insomnia and completed a set of questionnaires that included the Beliefs and Attitudes about Sleep (BAS), Positive and Negative Affect Schedule (Negative Subscale (nPANAS) and Positive Subscale (pPANAS)), Sleep Locus of Control (SLOC), Pre-Sleep Arousal Scale (PSAS), Hyperarousal Scale (HAS) and demographic information. Step-wise regression was conducted with a set of independent variables, with PSAS and HAS serving as separate dependent variables.
Results
Trait hyperarousal was associated with higher levels of both negative and positive emotionality, as well as negative beliefs about sleep, in both genders. Pre-sleep arousal was associated with greater negative emotionality and internal sleep locus of control, varying by gender. Among women, high pre-sleep arousal was associated with negative emotionality, while in men greater pre-sleep arousal was associated with an internal sleep locus of control.
Conclusion
These findings have clinical implications, suggesting that men and women may require different cognitive targets when addressing pre-sleep arousal.
Keywords: insomnia, pre-sleep arousal, hyperarousal, gender differences
Introduction
Hyperarousal and Sleep Disturbance
Insomnia disorders are among the most common sleep disorders, with prevalence rates ranging from 6 to 10% of the general population (1–3). Psychophysiological insomnia is a specific type of insomnia characterized by heightened levels of physiological, cognitive, and emotional arousal that is present across the 24-hour day (4). Such hyperarousal may include trait-like tendencies toward excess arousal, or state hyperarousal at bedtime. Trait hyperarousal refers to a broad pattern of excessive, poorly modulated responsiveness to stimuli during wakefulness (5,6), which can serve as a predisposition or vulnerability for insomnia (7,8). In contrast, pre-sleep arousal is a narrower, state-like construct, referring specifically to cognitive (9–11) and somatic arousal while attempting to fall asleep (12–15). Pre-sleep hyperarousal is hypothesized to arise from conditioning factors and maladaptive responses that are a reaction to chronically disturbed sleep (8,16–18) or the inability to de-arouse (19). Although the precise mechanisms are not yet clear, it appears that insomnia disorders arise from the additive effects of trait factors such as the tendency for cognitive-emotional hyperarousal, combined with state factors, such as rumination during pre-sleep and times of stress, and coping strategies focused on negative emotions triggered by stressful events (8,19,20). To our knowledge no previous study has examined differences in the cognitive and emotional factors related to trait and state hyperarousal in the context of insomnia.
Psychological Constructs Related to Arousal
Cognition
Cognitive activity is characterized by its level, thought content, and emotional tone. Traditionally, cognitive hyperarousal has been associated with an increased level of cognitive activity (e.g., racing thoughts), and is a strong predictor of insomnia (10). Individuals with psychophysiological insomnia exhibited higher levels of pre-sleep cognitive arousal compared to good sleepers (21). Those with psychophysiological insomnia had higher cognitive arousal on the PSAS at bedtime and up to three hours before bedtime (22).
Besides the level of cognitive activity, cognitive hyperarousal may involve intrusive thoughts during the sleep onset period, dysfunctional beliefs about sleep, and maladaptive cognitions about control over sleep (16,23). Indeed, maladaptive beliefs about sleep may perpetuate pre-sleep arousal (16). Compared to normal sleepers, those with insomnia reported more dysfunctional beliefs about sleep, generally related to helplessness and hopelessness (9,24). These dysfunctional beliefs include maladaptive concerns about the consequences of insomnia, beliefs that sleep is unpredictable and uncontrollable, unrealistic expectations about sleep needs, misconceptions about causes of insomnia, and incorrect beliefs about sleep promoting practices (11,16,24,25). Dysfunctional beliefs about sleep are also associated with greater insomnia severity and lower sleep self-efficacy (24).
Among people with insomnia, a particularly important set of cognitions is the control over sleep, or sleep locus of control. An external, or “chance,” locus of control is the belief that one has little control over sleep, while an internal locus of control indicates belief that one can control his or her own sleep. In the face of pressure to control sleep, perceived inability to do so may perpetuate insomnia by increasing emotional arousal at bedtime, contributing to the “vicious cycle” of insomnia (16). Cognitions related to control may be particularly potent compared to other cognitive content. Poor self-efficacy predicted more severe insomnia, while dysfunctional beliefs did not demonstrate an independent effect on insomnia severity (26). As sense of control over sleep improved with cognitive behavioral therapy, sleep efficiency also improved (27), and among individuals who developed a more internal sleep locus of control during CBT for insomnia, insomnia severity decreased (28). While there appears to be a connection between locus of control and insomnia severity, the mechanism is not yet known. One possibility is that locus of control might be related to arousal. Among people with insomnia, an internal locus of control was associated with higher levels of sleep-related anticipatory anxiety (29), suggesting that pressure to control one’s sleep may be related to hyperarousal. Thus, further exploration into the relationship between sleep locus of control and arousal seems warranted.
Emotion
Previous research has examined mood and emotional functioning associated with insomnia (30,31) with more recent research examining the subjective experience of emotion, including both valence and arousal (32). Individuals with psychophysiological insomnia exhibit more negatively valenced mood (33). Following a night of poor sleep, those with insomnia had higher negative emotionality the following day (34); this was particularly robust in women (35). A recent study from our lab found that poor sleepers reported more negative affect and arousal at night, but only more negative affect during the day when compared to good sleepers (36). In a study comparing healthy controls to individuals with high levels of stress, those with high negative emotionality had the worst sleep quality (37). The cognitive model of insomnia posits that the valence of cognitive activity among insomnia patients is excessively negative (17). Dysfunctional cognitions about sleep may trigger negatively valenced emotions, thus perpetuating sleep disturbance (16,32). Recent work has attempted to classify subtypes of insomnia based on symptom constellations, including negative emotion. Psychophysiological insomnia diagnostic traits trended toward a pro le marked by negative affect (38).
Gender Differences in Insomnia
Insomnia disproportionately affects women, compared to men. A meta-analysis of 31 studies comprising over one million participants found that women suffered from insomnia significantly more than men (OR = 1.4) (39). A number of mechanisms have been proposed for this gender difference, including hormonal factors (40,41), differences in career and family obligations (42), tendency toward rumination (24), and differences in the prevalence of affective disorders between men and women (43).
Gender differences in stress perception and response may drive higher rates of insomnia in women. In a large study examining stressful life events and insomnia, men and women reported similar numbers of stressful life events (44). However, women rated the events as more stressful. Women were also more likely to report difficulty falling sleep and nighttime awakenings than men (44). Increased negative emotional reactivity in women may contribute to the gender difference in insomnia. Research indicates that women are more emotionally reactive to stress or negative stimuli than men (45–47). Specific to sleep, positive affective states were associated with greater sleep efficiency and total sleep time, while negative affective states were positively correlated with wake time after sleep onset among women (48,49). While women have a greater prevalence of insomnia compared to men, it is unclear if men and women have different cognitive-emotional profiles that could be related to arousal in the context of insomnia.
Objectives
The overall purpose of this study was to examine different factors related to state and trait hyperarousal using self-report measures. The primary aim was to examine the relationship between a set of cognitive and emotional variables with pre-sleep arousal and trait hyperarousal serving as separate dependent variables. It was hypothesized that cognitive-emotional variables including dysfunctional beliefs about sleep, sleep locus of control, and negative emotionality would predict both pre-sleep arousal and trait hyperarousal. The secondary aim was to examine gender differences across state and trait hyperarousal. It was hypothesized that women would report a higher level of negative affect compared to men and this would be significantly related to hyperarousal.
Methods
Participants
Selection Criteria
Participants were at least 18 years old and met International Classification of Sleep Disorders (ICSD-2) criteria (4) for psychophysiological insomnia, which included: 1) complaint of difficulty initiating sleep, maintaining sleep, waking too early, or non-restorative sleep despite adequate circumstances for sleep; 2) complaint of daytime impairment or distress such as fatigue, impaired concentration, or concerns or worries about sleep; 3) evidence of heightened arousal or conditioned inability to sleep such as an inability to shut off mind or physical tension (4). Minimum duration of these symptoms was at least six months. In addition, all participants reported at least 30 minutes of sleep onset latency and/or wake after sleep onset on sleep diaries during a one-week screening period. Participants who reported current use of sleep medications were not included in these analyses.
Procedures
Data from this study were collected as part of the screening and baseline assessment of a larger treatment study on non-pharmacological treatments for insomnia. Participants were recruited through fliers posted on the Rush University Medical Center campus, advertisements placed in the community, and referral though the Rush Sleep Clinic. Additional postings were made on internet bulletin boards and on the public transportation system in Chicago, Illinois.
Participants were initially interviewed over the telephone to determine general eligibility for the parent study. Those who were eligible and interested in the treatment study were asked to complete one week of sleep diary and scheduled for an in-person interview to further determine candidacy. During the in-person interview, participants provided informed consent and were administered the Duke Structured Clinical Interview for Sleep Disorders (DSISD), International Classification of Sleep Disorders (ICSD) and the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I) (50). Participants also completed a packet of questionnaires including demographic information (age, sex, race, ethnicity, education, and marital status) and the self-report measures below.
Measures
Pre-Sleep Arousal Scale
The Pre-Sleep Arousal Scale (PSAS) is a 16-item self-report scale that assesses physiological (“Heart racing, pounding or beating irregularly”) and cognitive (“Worry about falling asleep”) arousal prior to sleep. Participants were asked to rate the extent to which they experienced each item as they tried to fall asleep in the past week from 1 (“Not at all”) to 5 (“Extremely”) (13). The internal consistency of the scale in the current study was α = 0.80.
Hyperarousal Scale
The Hyperarousal Scale was developed by Pavlova et al. (5) and contains 26 items that are intended to measure trait arousal/alertness during wakefulness in participants with insomnia (“My mind is always going”). Participants were asked to consider the items in the questionnaire and indicate the degree to which it applied to them from 1 (“Not at all”) to 4 (“Extremely”). The internal consistency of the scale in the current study was α = 0.74.
Positive and Negative Affect Schedule
The Positive and Negative Affect Schedule (PANAS) is a 20-item questionnaire that assesses affect across two dimensions, positive (e.g., determined, enthusiastic, excited) and negative (e.g., afraid, guilty, distressed). Participants were asked to rank the extent to which they felt each item during the past week on a Likert scale from 1 (“Very little/Not at all”) to 5 (“Extremely”) (51). The internal consistency of the scale in the current study was α = 0. 77. Analyses were conducted on the positive and negative subscales of the PANAS. In this study, the timeframe used was the past week.
Beliefs and Attitudes About Sleep
The Beliefs and Attitudes about Sleep (BAS) comprises 30 statements that examine various cognitions (i.e., expectations, consequences, control) related to sleep. On a scale of 0 (“Strongly Disagree”) to 10 (“Strongly Agree”), participants were asked to indicate the degree to which they agreed or disagreed with each statement (16). The internal consistency of the scale in the current study was α = 0.87.
Sleep Locus of Control
The Sleep Locus of Control (SLOC) is an 8-item scale developed by Vincent et al. (29) that measures two dimensions of sleep locus of control: internal and chance. Participants indicated on a Likert scale from 1 (Strongly Disagree) to 6 (Strongly Agree) the amount to which they agreed or disagreed with each item. The internal consistency of the scale in the current study was α = 0.63.
Statistical Analyses
Prior to performing regression analyses, variables were examined to ensure no violation of the assumptions of normality, linearity, multicollinearity, or homoscedasticity. Correlations among independent variables were no higher than r = 0.7. Tolerance values were greater than 0.1, and variance inflation factor values were all less than 2. Normal P-P and scatter plots suggested no major deviations from normality. Pearson correlations were first performed to assess the relationship among the constructs. Independent-sample T-tests were performed to examine gender differences in all main variables. Main hypotheses were tested with step-wise multiple regression. Models assessed the relationship between the dependent variables (PSAS and HAS) and a set of independent variables including gender, nPANAS, pPANAS, BAS, SLOC, age, gender, or current Axis I diagnosis. Demographic factors of age, marital status, and current Axis I diagnosis were entered for the first step. Questionnaire measures of SLOC, nPANAS, pPANAS, and BAS were entered in the second step. Finally, to assess potential gender differences in these relationships, interactions between gender and the questionnaire measures were entered at the third step. Full model analyses and model comparisons were only performed for participants who had no missing values for any of the included independent variables (32 male and 79 female participants). All analyses were performed with SPSS Version 19 and the freeware statistical package R.
Results
Participants
Participants included 39 men and 89 women. The age of participants ranged from 19 to 76 years (M = 45.1, SD = 13.9). A majority of the sample was female (70%) and identified as White (58%) and non-Hispanic or Latino (89%). The overwhelming majority (89%) of the sample completed high school (17%) or college (72%). Among participants, 29.7% were married and 54.7% were single, while others were divorced, separated, engaged, or living with a partner. Duration of insomnia ranged from 6 months to 30 years. Based on Duke history, 10 participants indicated comorbid sleep disorders including restless leg syndrome (n = 4), suspected OSA (n = 3), symptoms of delayed sleep phase syndrome (n = 2), and insomnia related to a mental disorder (n = 1). Based on SCID interview, the sample included 42 subjects with a current Axis I diagnosis; the most common diagnoses were dysthymia (n = 12), generalized anxiety disorder (n = 11) and major depressive disorder (n = 6). Slightly more than half of the participants who met criteria for an Axis I disorder were women (64%). Mean scores on each measure for men, women, and the combined sample can be found in Table 1.
Table 1.
Mean scores on measures in women, men, and in the combined sample.
| Measure | Women | Men | Overall Sample | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Age | 45.1 | 14.32 | 45.33 | 13.01 | 45.1 | 13.9 |
| Weight (pounds) | 151.19 | 34.50 | 180.21 | 26.24 | 160.03 | 34.79 |
| Height (inches) | 64.60 | 2.74 | 70.05 | 2.65 | 66.26 | 3.69 |
| BMI | 25.44 | 5.45 | 25.82 | 3.70 | 25.56 | 4.67 |
| Years of education | 16.19 | 3.38 | 15.23 | 3.56 | 15.87 | 3.46 |
| BAS | 124.34 | 40.75 | 133.79 | 37.07 | 127.22 | 39.76 |
| nPANAS | 19.26 | 6.73 | 19.65 | 5.37 | 19.38 | 6.33 |
| pPANAS | 27.99 | 7.53 | 29.53 | 7.41 | 28.46 | 7.49 |
| SLOC | 26.83 | 6.71 | 28.29 | 37.07 | 27.28 | 6.62 |
| PSAS | 39.25* | 8.61 | 34.96* | 8.07 | 38.02 | 8.65 |
| pPSAS | 14.07 | 4.39 | 12.90 | 3.77 | 13.73 | 4.24 |
| cPSAS | 25.18 | 6.59 | 22.03 | 5.75 | 24.29 | 6.49 |
| HAS | 61.37 | 8.44 | 61.39 | 9.28 | 61.38 | 8.67 |
| Duke Sleep Disorder (n) | 7 | - | 3 | - | 10 | - |
| Axis I Disorder (n) | 27 | - | 15 | - | 42 | - |
Note: BAS (Behaviors and Attitudes about Sleep), n/pPANAS (Negative/Positive Subscale of Positive and Negative Affect Schedule), SLOC (Sleep Locus of Control), PSAS (Pre-Sleep Arousal Scale), p/cPSAS (Physiological and Cognitive Subscale of Pre-Sleep Arousal Scale), HAS (Hyperarousal Scale).
p < 0.01 level.
p < 0.05 level.
Correlations Among Variables
PSAS was significantly correlated with gender (r = −0.23, p = 0.02), age (r = −0.24, p = 0.01), and Axis I diagnosis (r = 0.39, p < 0.01) but not with race, education, or marital status. HAS was significantly associated with age (r = 0.33, p < 0.01), marital status (r = −0.22, p = 0.02), and Axis I diagnosis (r = 0.27, p < 0.01), but not with gender, race, or education (Table 2).
Table 2.
Pearson correlations among study variables.
| Gender | Age | Education | Race | Marital | Axis I | BAS | nPANAS | pPANAS | SLOC | PSAS | pPSAS | cPSAS | HAS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Age | 0.01 | — | — | — | — | — | — | — | — | — | — | — | — | — |
| Education | −0.13 | 0.17 | — | — | — | — | — | — | — | — | — | — | — | — |
| Race | −0.10 | 0.04 | 0.13 | — | — | — | — | — | — | — | — | — | — | — |
| Marital | −0.10 | 0.29** | 0.06 | 0.10 | — | — | — | — | — | — | — | — | — | — |
| Axis I | 0.08 | −0.07 | −0.08 | −0.03 | −0.17 | — | — | — | — | — | — | — | — | — |
| BAS | 0.11 | −0.08 | −0.10 | −0.08 | −0.12 | 0.25** | — | — | — | — | — | — | — | — |
| nPANAS | 0.03 | −0.19* | −0.11 | 0.036 | −0.23* | 0.47** | 0.36** | — | — | — | — | — | — | — |
| pPANAS | 0.09 | 0.35** | −0.01 | −0.07 | 0.10 | −0.08 | −0.16 | −0.18* | — | — | — | — | — | — |
| SLOC | 0.10 | −0.15 | −0.05 | −0.21* | −0.03 | 0.02 | −0.11 | 0.05 | −0.04 | — | — | — | — | — |
| PSAS | −0.23* | −0.24** | −0.08 | −0.01 | −0.15 | 0.39** | 0.23* | 0.40** | −0.19* | 0.08 | — | — | — | — |
| pPSAS | −0.12 | −0.17 | −0.07 | 0.01 | −0.11 | 0.26** | 0.22* | 0.39** | −0.26** | 0.02 | 0.33** | — | — | — |
| cPSAS | −0.22* | −0.21* | −0.07 | −0.02 | −0.12 | 0.34** | 0.17 | 0.27** | −0.08 | 0.09 | 0.38** | 0.27** | — | — |
| HAS | 0.001 | −0.33** | −0.06 | 0.05 | −0.22* | 0.27** | 0.39** | 0.53** | −0.03 | 0.09 | 0.45** | 0.33** | 0.38** | — |
Note: BAS (Behaviors and Attitudes about Sleep), n/pPANAS (Negative and Positive Subscale of Positive and Negative Affect Schedule), SLOC (Sleep Locus of Control Scale), PSAS (Pre-Sleep Arousal Scale), p/cPSAS (Physiological and Cognitive Subscale of Pre-Sleep Arousal Scale), HAS (Hyperarousal Scale).
p < 0.01 level.
p < 0.05 level.
Gender Differences
Chi-square and independent-sample T-tests were performed to examine gender differences in all main variables. There were no statistically significant differences between men and women in age, education level, or BMI. Women had higher PSAS scores (t (113) = −2.452, p = 0.016 (two-tailed)) than men (Table 1). Examining bivariate correlations within each gender, PSAS was significantly, positively associated with SLOC in men (r = 0.42). Among women, high PSAS was significantly, positively correlated with negative emotionality (nPANAS) (r = 0.46) and BAS (r = 0.29).
Main Analyses
Pre-Sleep Arousal
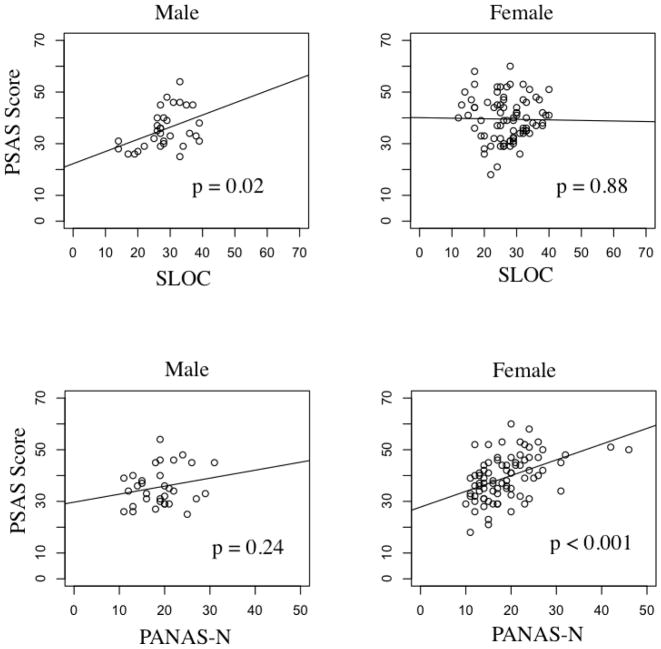
Step-wise multiple regression was conducted on the PSAS as the dependent variable. Gender, age, marital status, and Axis I psychiatric diagnoses were entered at the first step explaining 22.7% of the variance in the PSAS, (F(5,105) = 7.46, p < 0.001). After entry of pPANAS, nPANAS, BAS, SLOC, and their interactions with gender, the total variance explained by the model was 29.0% (F(13, 97) = 4.45, p < 0.001). After controlling for covariance, the addition of pPANAS, nPANAS, BAS, and SLOC and their interaction with gender explained an additional 6.3% of the variance in pre-sleep arousal (R squared change = 0.063, F change (8, 97) = 2.17, p = 0.037). In the final model, interactions between gender and nPANAS (p = 0.02) and gender and SLOC (p = 0.03) were statistically significant (Table 3). Post-hoc analyses revealed greater nPANAS, was associated with greater PSAS in women, but not in men; greater SLOC was associated with greater PSAS in men, but not in women (Figure 1). Post-hoc analyses conducted on each subscale of the PSAS revealed a similar pattern.
Table 3.
Summary of Regression Analysis for Pre-Sleep Arousal (PSAS).
| Variable | B | SE (B) | t | Sig. (p) | ΔR2 |
|---|---|---|---|---|---|
| Step 1 | 0.227 | ||||
| Gender | −1.867 | 0.758 | −2.472 | 0.015* | |
| Age | −0.114 | 0.051 | −2.153 | 0.033* | |
| Marital M | 1.093 | 1.520 | 0.726 | 0.476 | |
| Marital S | - | - | - | - | |
| Marital D | 1.458 | 7.272 | 0.208 | 0.842 | |
| Axis I Disorder | 7.377 | 1.473 | 5.009 | 0.000** | |
| Step 2 | 0.254 | ||||
| Gender | −2.050 | 0.754 | −2.725 | 0.008** | |
| Age | −0.103 | 0.056 | −1.836 | 0.069 | |
| Marital M | 1.544 | 1.529 | 1.002 | 0.316 | |
| Marital S | - | - | - | - | |
| Marital D | 1.721 | 7.265 | 0.241 | 0.812 | |
| Axis I Disorder | 5.211 | 1.672 | 3.128 | 0.002** | |
| nPANAS | 0.275 | 0.137 | 2.036 | 0.045* | |
| pPANAS | 0.037 | 0.103 | 0.323 | 0.753 | |
| BAS | 0.023 | 0.022 | 1.125 | 0.266 | |
| SLOC | 0.094 | 0.105 | 0.853 | 0.398 | |
| Step 3 | 0.289 | ||||
| Gender | −6.325 | 5.972 | −1.051 | 0.292 | |
| Age | −0.123 | 0.053 | −2.164 | 0.033* | |
| Marital M | 1.437 | 1.499 | 0.967 | 0.342 | |
| Marital S | - | - | - | - | |
| Marital D | 2.517 | 7.130 | 0.358 | 0.734 | |
| Axis I Disorder | 5.731 | 1.663 | 3.467 | 0.000** | |
| nPANAS | 0.053 | 0.169 | 0.294 | 0.775 | |
| pPANAS | 0.088 | 0.124 | 0.731 | 0.469 | |
| BAS | 0.032 | 0.026 | 1.542 | 0.128 | |
| SLOC | 0.240 | 0.121 | 2.011 | 0.047* | |
| Gender * nPANAS | −0.344 | 0.148 | −2.280 | 0.025* | |
| Gender * pPANAS | −0.010 | 0.108 | −0.093 | 0.926 | |
| Gender * BAS | 0.023 | 0.021 | 1.107 | 0.271 | |
| Gender * SLOC | 0.284 | 0.117 | 2.432 | 0.017* |
Note: n/pPANAS (Negative and Positive Subscales of Positive and Negative Affect Schedule), BAS (Behaviors and Attitudes about Sleep), SLOC (Sleep Locus of Control).
p < 0.01 level.
p < 0.05 level.
Figure 1. Relationship between Pre-Sleep Arousal and nPANAS, SLOC in Men and Women.
Note: PSAS (Pre-Sleep Arousal Scale), PANAS-N (Negative Subscale of Positive and Negative Affect Schedule), SLOC (Sleep Locus of Control).
Trait Hyperarousal
To examine trait hyperarousal, step-wise multiple regression was conducted with HAS as the dependent variable. Gender, age, marital status, and comorbid Axis I psychiatric diagnoses were entered at the first step, explaining 12.2% of the variance in the PSAS (F(5,105) = 4.06, p = 0.002). After entry of pPANAS, nPANAS, BAS, SLOC, and their interactions with gender, the total variance explained by the model was 33.8% (F(13, 97) = 6.88, p < 0.001). After controlling for covariance, pPANAS, nPANAS, BAS, SLOC, and their interactions with gender explained an additional 21.6% of the variance in hyperarousal (R squared change = 0.216, F change (8, 97) = 5.28, p < 0.001). In the final model, there were significant relationships between HAS and pPANAS (p = 0.002), nPANAS (p < 0.001), and BAS (p = 0.005), but none of the interactions with gender were statistically significant (Table 4).
Table 4.
Summary of Regression Analysis for Hyperarousal (HAS).
| Variable | B | SE (B) | t | Sig. (p) | ΔR2 |
|---|---|---|---|---|---|
| Step 1 | .192 | ||||
| Gender | −.098 | .834 | −.118 | .906 | |
| Age | −.173 | .057 | −3.033 | .003** | |
| Marital M | −1.073 | 1.682 | −.638 | .525 | |
| Marital S | - | - | - | ||
| Marital D | 6.556 | 8.024 | .817 | .416 | |
| Axis I Disorder | 4.335 | 1.626 | 2.666 | .009** | |
| Step 2 | .240 | ||||
| Gender | −.537 | .736 | −.730 | .466 | |
| Age | −.177 | .052 | −3.385 | .001** | |
| Marital M | −.079 | 1.488 | −.053 | .958 | |
| Marital S | - | - | - | - | |
| Marital D | 7.701 | 7.090 | 1.087 | .280 | |
| Axis I Disorder | −.111 | 1.633 | −.068 | .946 | |
| nPANAS | .565 | .130 | 4.339 | .000** | |
| pPANAS | .231 | .101 | 2.281 | .025* | |
| BAS | .051 | .018 | 2.772 | .006** | |
| SLOC | .087 | .103 | .849 | ||
| Step 3 | .023 | ||||
| Gender | −12.179 | 5.967 | −2.041 | .044* | |
| Age | −.167 | .053 | −3.121 | .002** | |
| Marital M | −.117 | 1.489 | −.078 | .938 | |
| Marital S | - | - | - | - | |
| Marital D | 8.551 | 7.121 | 1.201 | .233 | |
| Axis I Disorder | −.540 | 1.659 | −.326 | .745 | |
| nPANAS | .599 | .161 | 3.715 | .000** | |
| pPANAS | .300 | .110 | 2.720 | .008** | |
| BAS | .058 | .020 | 2.849 | .005** | |
| SLOC | .173 | .118 | 1.463 | .147 | |
| Gender * nPANAS | .082 | .148 | .561 | .576 | |
| Gender * pPANAS | .169 | .108 | 1.564 | .121 | |
| Gender * BAS | .007 | .021 | .356 | .722 | |
| Gender * SLOC | .153 | .117 | 1.308 | .194 |
Note: n/pPANAS (Negative and Positive Subscales of Positive and Negative Affect Schedule), BAS (Behaviors and Attitudes about Sleep), SLOC (Sleep Locus of Control).
p <0.01 level.
p< 0.05 level.
Conclusion
The primary purpose of this study was to examine different factors related to pre-sleep arousal and trait hyperarousal among individuals with psychophysiological insomnia. Results suggested different patterns for pre-sleep arousal compared to trait hyperarousal. Within this sample, pre-sleep arousal was associated with greater negative emotionality and internal sleep locus of control, varying by gender, while trait hyperarousal was associated with higher levels of both negative and positive emotionality, as well as negative beliefs about sleep, in both genders. Further analyses revealed that among women, high pre-sleep arousal was associated with negative emotionality, while in men greater pre-sleep arousal was associated with an internal sleep locus of control.
These findings have important conceptual implications. First, the association between negative affect and both state and trait hyperarousal is consistent with the findings on the importance of emotional valence in insomnia (30–34,36,38). Interestingly, positive affect was associated with the HAS, suggesting that elevations on both valences of affect are related to trait hyperarousal. Although hyperarousal is typically associated with anxiety or emotional activation, the relatively strong correlations and consistent associations between negative affect across both state and trait hyperarousal provide indications that the construct of emotion (as opposed to mood), merits closer investigation as it applies to insomnia. Since BAS was associated with HAS, which was used as a measure of trait hyperarousal, a more enduring construct than pre-sleep arousal, these findings would appear to support the hypothesis that individuals with insomnia who have elevations in trait hyperarousal are more likely to have maladaptive sleep-related cognitions as opposed to the hypothesis that maladaptive cognitions develop during a state of elevated pre-sleep arousal. In other words, these findings would argue against the notion that dysfunctional beliefs and attitudes about sleep are simply a reaction to sleep disturbance. Instead, it seems that dysfunctional beliefs about sleep are present in individuals with more general, trait-like tendencies towards hyperarousal that could be exacerbated during periods of elevated pre-sleep arousal. This conceptualization would appear to fit Spielman’s “3 P Model,” which posits that predisposing factors (such as tendency toward hyperarousal), precipitating factors (such as a stressor), and perpetuating factors (such as expectation of poor sleep) contribute to and maintain chronic insomnia (8).
These findings also indicate gender differences in the relationship between cognitions and pre-sleep arousal among individuals with insomnia. Among women, negative emotions were related to increased pre-sleep arousal while for men, the degree of perceived control over sleep was related to increased pre-sleep arousal. While previous work has found a greater tendency toward rumination among women than men, it may not be rumination itself that is associated with hyperarousal, but the emotional impact of rumination or maladaptive cognitions (9,18,32). Conversely, the present findings indicate that in men, excessive thinking about one’s control over sleep may induce hyperarousal at bedtime. Previous research suggests that an internal sleep locus of control may result in pressure to control one’s sleep, leading to hyperarousal (29). Our results suggest that this sense of pressure to control one’s own sleep is associated with hyperarousal among men, but not women. This research builds on previous work suggesting that the relationship between sleep and emotion might be stronger for women than men, and suggests the need to further examine the need for control of sleep among men (35).
The clinical implications of the gender profiles in this study are twofold. First, men and women may present differently in the clinic when seeking assessment or treatment of insomnia. Women might report more negative affect during the pre-sleep period while men might report more concerns about the ability to control sleep. This finding is consistent with research demonstrating gender differences in emotional expression and response to stress (44–46,49). Second, men and women might respond differently to insomnia treatment. Specifically, men and women might require different cognitive targets when addressing pre-sleep arousal. Research suggests that women and men perceive and cope with stress differently, and therefore may be differentially responsive to therapy targeting emotion regulation or cognitive restructuring (52). For women, emotion-regulation techniques such as mindfulness meditation (18,53,54) might be more helpful to reduce pre-sleep arousal while for men, more traditional cognitive strategies such as cognitive restructuring or behavioral experiments (25) aimed at shifting the locus of control might be more helpful.
Limitations and Future Directions
We acknowledge several limitations of this study. First, our study relied on self-report data and employed a cross-sectional design, so the direction and temporal precedence of the relationships between the variables cannot be confirmed. Future studies should employ a longitudinal design and incorporate physiological measures for sleep and arousal to compare the self-report findings with objective findings. Although Axis I diagnosis was not an a priori variable of interest, there was a strong relationship between comorbid psychiatric diagnoses and the PSAS suggesting that hyperarousal might play a role in other co-occurring psychiatric disorders, such as dysthymia, GAD, and MDD. While beyond the scope of the present study, future studies may explore the relationship between the psychiatric comorbidity and hyperarousal in the context of an insomnia disorder. In addition, the present study did not include a comparison group of good sleepers or healthy controls. Therefore, it is unclear whether these findings are specific to people with insomnia. Future studies might explore treatment preferences for men versus women with insomnia complaints. Additionally, treatment studies might compare efficacy of cognitive versus affective treatment targets for men versus women with insomnia complaints. We hope that the present findings provide a springboard for further investigation into the relationships between gender and sleep-related arousal.
Acknowledgments
This research was supported by grant K23AT003678 awarded to Jason Ong. We greatly appreciate the contributions of David Sholtes and Michael Lederman for their help with data collection and data management. We also acknowledge the mentorship of James Wyatt, Ph.D. and Rachel Manber, Ph.D. for the design and implementation of the parent study within which this study was conducted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jansson-Fröjmark M, Linton SJ. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008 Jun;31(6):881–6. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009 Aug;32(8):1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002 Apr;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2. American Academy of Sleep Medicine (AASM); Westchester, IL: American Sleep Disorders Association; 2005. (ICSD-2) [Google Scholar]
- 5.Pavlova M, Berg O, Gleason R, Walker F, Roberts S, Regestein Q. Self-reported hyperarousal traits among insomnia patients. Journal of Psychosomatic Research. 2001 Aug;51(2):435–41. doi: 10.1016/s0022-3999(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 6.Regestein QR, Dambrosia J, Hallett M, Murawski B, Paine M. Daytime alertness in patients with primary insomnia. Am J Psychiatry. 1993 Oct;150(10):1529–34. doi: 10.1176/ajp.150.10.1529. [DOI] [PubMed] [Google Scholar]
- 7.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004 Mar 15;27(2):285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 8.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987 Dec;10(4):541–53. [PubMed] [Google Scholar]
- 9.Carney CE, Edinger JD, Morin CM, Manber R, Rybarczyk B, Stepanski EJ, et al. Examining maladaptive beliefs about sleep across insomnia patient groups. J Psychosom Res. 2010 Jan;68(1):57–65. doi: 10.1016/j.jpsychores.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichstein KL, Rosenthal TL. Insomniacs’ perceptions of cognitive versus somatic determinants of sleep disturbance. J Abnorm Psychol. 1980 Feb;89(1):105–7. doi: 10.1037//0021-843x.89.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000 Jul;38(7):679–93. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- 12.Drummond SPA, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med Rev. 2004 Jun;8(3):227–42. doi: 10.1016/j.smrv.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behaviour Research and Therapy. 1985;23(3):263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 14.Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010 Feb;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001 Aug;86(8):3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993 Sep;8(3):463–7. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 17.Harvey A. A cognitive model of insomnia. Behaviour Research and Therapy. 2002 Aug;40(8):869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 18.Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: a metacognitive model of insomnia. Behav Res Ther. 2012 Nov;50(11):651–60. doi: 10.1016/j.brat.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Mendoza J, Vela-Bueno A, Vgontzas AN, Ramos-Platón MJ, Olavarrieta-Bernardino S, Bixler EO, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010 May;72(4):397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Rodrigue S, Ivers H. Role of Stress, Arousal, and Coping Skills in Primary Insomnia. Psychosom Med. 2003 Mar 1;65(2):259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 22.Robertson JA, Broomfield NM, Espie CA. Prospective comparison of subjective arousal during the pre-sleep period in primary sleep-onset insomnia and normal sleepers. Journal of Sleep Research. 2007;16(2):230–8. doi: 10.1111/j.1365-2869.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 23.Borkovec TD, Lane TW, VanOot PH. Phenomenology of sleep among insomniacs and good sleepers: Wakefulness experience when cortically asleep. Journal of Abnormal Psychology. 1981;90(6):607–9. doi: 10.1037//0021-843x.90.6.607. [DOI] [PubMed] [Google Scholar]
- 24.Carney CE, Edinger JD. Identifying critical beliefs about sleep in primary insomnia. Sleep. 2006 Apr;29(4):444–53. [PubMed] [Google Scholar]
- 25.Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007 Oct;45(10):2491–501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Bluestein D, Rutledge CM, Healey AC. Psychosocial Correlates of Insomnia Severity in Primary Care. J Am Board Fam Med. 2010 Mar 1;23(2):204–11. doi: 10.3122/jabfm.2010.02.090179. [DOI] [PubMed] [Google Scholar]
- 27.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behav Res Ther. 2002 Jul;40(7):741–52. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 28.Vincent N, Walsh K, Lewycky S. Sleep locus of control and computerized cognitive-behavioral therapy (cCBT) Behav Res Ther. 2010 Aug;48(8):779–83. doi: 10.1016/j.brat.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Vincent N, Sande G, Read C, Giannuzzi T. Sleep locus of control: report on a new scale. Behav Sleep Med. 2004;2(2):79–93. doi: 10.1207/s15402010bsm0202_1. [DOI] [PubMed] [Google Scholar]
- 30.Kales A, Caldwell AB, Preston TA, Healey S, Kales JD. Personality patterns in insomnia. Theoretical implications. Arch Gen Psychiatry. 1976 Sep;33(9):1128–1134. doi: 10.1001/archpsyc.1976.01770090118013. [DOI] [PubMed] [Google Scholar]
- 31.Kales A, Caldwell AB, Soldatos CR, Bixler EO, Kales JD. Biopsychobehavioral correlates of insomnia.II. Pattern specificity and consistency with the Minnesota Multiphasic Personality Inventory. Psychosom Med. 1983 Aug;45(4):341–56. doi: 10.1097/00006842-198308000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Baglioni C, Lombardo C, Bux E, Hansen S, Salveta C, Biello S, et al. Psychophysiological reactivity to sleep-related emotional stimuli in primary insomnia. Behav Res Ther. 2010 Jun;48(6):467–75. doi: 10.1016/j.brat.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, et al. Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Medicine. 2007 Apr;8(3):198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCrae CS, Wilson NM, Lichstein KL, Durrence HH, Taylor DJ, Riedel BW, et al. Self-Reported Sleep, Demographics, Health, and Daytime Functioning in Young Old and Old Old Community-Dwelling Seniors. Behavioral Sleep Medicine. 2008;6(2):106–26. doi: 10.1080/15402000801952906. [DOI] [PubMed] [Google Scholar]
- 35.Scott BA, Judge TA. Insomnia, Emotions, and Job Satisfaction: A Multilevel Study. Journal of Management. 2006 Oct 1;32(5):622–45. [Google Scholar]
- 36.Ong JC, Cardé NB, Gross JJ, Manber R. A two-dimensional approach to assessing affective states in good and poor sleepers. J Sleep Res. 2011 Dec;20(4):606–10. doi: 10.1111/j.1365-2869.2011.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norlander T, Johansson Å, Bood SÅ. The affective personality: its relation to quality of sleep, well-being and stress. Social Behavior and Personality: an international journal. 2005;33(7):709–22. [Google Scholar]
- 38.Sánchez-Ortuño MM, Edinger JD, Wyatt JK. Daytime symptom patterns in insomnia sufferers: is there evidence for subtyping insomnia? Journal of Sleep Research. 2011;20(3):425–33. doi: 10.1111/j.1365-2869.2010.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Wing Y-K. Sex differences in insomnia: a meta-analysis. Sleep. 2006 Jan;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 40.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001 Sep;98(3):391–7. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 41.Kravitz HM, Janssen I, Santoro N, Bromberger JT, Schocken M, Everson-Rose SA, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005 Nov 14;165(20):2370–6. doi: 10.1001/archinte.165.20.2370. [DOI] [PubMed] [Google Scholar]
- 42.Yoshioka E, Saijo Y, Kita T, Satoh H, Kawaharada M, Fukui T, et al. Gender differences in insomnia and the role of paid work and family responsibilities. Soc Psychiatry Psychiatr Epidemiol. 2012 Apr;47(4):651–62. doi: 10.1007/s00127-011-0370-z. [DOI] [PubMed] [Google Scholar]
- 43.Hale L, Do DP, Basurto-Davila R, Heron M, Finch BK, Dubowitz T, et al. Does mental health history explain gender disparities in insomnia symptoms among young adults? Sleep Med. 2009 Dec;10(10):1118–23. doi: 10.1016/j.sleep.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagen EW, Friedman EM, Hale M, Salzieder N, Peppard PE. Severity of stressfulness of major life events and insomnia symptoms in women and men. Journal of Sleep and Sleep Disorder Research. 2012;35(Abstract Supplement):A238. [Google Scholar]
- 45.Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry. 2008 Mar;39(1):87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen DK, Mehlsen MY, Viidik A, Sommerlund B, Zachariae R. Age and gender differences in negative affect—Is there a role for emotion regulation? Personality and Individual Differences. 2005 Jun;38(8):1935–46. [Google Scholar]
- 47.Nolen-Hoeksema S, Larson J, Grayson C. Explaining the gender difference in depressive symptoms. J Pers Soc Psychol. 1999 Nov;77(5):1061–72. doi: 10.1037//0022-3514.77.5.1061. [DOI] [PubMed] [Google Scholar]
- 48.Berry DT, Webb WB. State measures and sleep stages. Psychol Rep. 1983 Jun;52(3):807–12. doi: 10.2466/pr0.1983.52.3.807. [DOI] [PubMed] [Google Scholar]
- 49.Berry DT, Webb WB. Mood and sleep in aging women. J Pers Soc Psychol. 1985 Dec;49(6):1724–7. doi: 10.1037//0022-3514.49.6.1724. [DOI] [PubMed] [Google Scholar]
- 50.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 51.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 52.Sontag LM, Graber JA. Coping with perceived peer stress: gender-specific and common pathways to symptoms of psychopathology. Dev Psychol. 2010 Nov;46(6):1605–20. doi: 10.1037/a0020617. [DOI] [PubMed] [Google Scholar]
- 53.Ong J, Sholtes D. A mindfulness-based approach to the treatment of insomnia. J Clin Psychol. 2010 Nov;66(11):1175–84. doi: 10.1002/jclp.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008 Jun;39(2):171–82. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]