Abstract
Exposure to ambient nanoparticles (defined as particulate matter [PM] having one dimension < 100 nm) is associated with increased risk of childhood and adult asthma. Nanomaterials feature a smaller aerodynamic diameter and a higher surface area per unit mass ratio compared to fine or coarse-sized particles, resulting in greater lung deposition efficiency and an increased potential for biological interaction. The neurotrophins nerve growth factor and brain-derived neurotrophic factor are key regulatory elements of neuronal development and responsiveness of airway sensory neurons. Changes in their expression are associated with bronchoconstriction, airway hyperresponsiveness, and airway inflammation. The neurogenic-mediated control of airway responses is a key pathophysiological mechanism of childhood asthma. However, the effects of nanoparticle exposure on neurotrophin-driven airway responses and their potential role as a predisposing factor for developing asthma have not been clearly elucidated. In this study, in vivo inhalation exposure to titanium dioxide nanoparticles (12 mg/m13; 5.6 h/d for 3 d) produced upregulation of lung neurotrophins in weanling (2-wk-old) and newborn (2-d-old) rats but not in adult (12-wk-old) animals compared to controls. This effect was associated with increased airway responsiveness and upregulation of growth-related oncogene/keratine-derived chemokine (GRO/KC; CXCL1, rat equivalent of human interleukin [IL]-8) in bronchoalveolar lavage fluid. These data show for the first time that exposure to nanoparticulate upregulates the expression of lung neurotrophins in an age-dependent fashion and that this effect is associated with airway hyperresponsiveness and inflammation. These results suggest the presence of a critical window of vulnerability in earlier stages of lung development, which may lead to a higher risk of developing asthma.
Airborne particulate matter (PM) has been associated with increases in respiratory and cardiovascular morbidity and mortality (Peters et al., 2004; Atkinson et al., 2001; Dominici et al., 2005; Krewski et al., 2005; Mossman et al., 2007). In particular, environmental exposure to nanoparticles (defined as particulate matter [PM] having one dimension <100 nm), the level of which is increased in urban areas due to vehicle emissions, has been associated with an enhanced risk of childhood and adult asthma (Donaldson et al., 2000; von Klot et al., 2002; Wong & Lai, 2004; Krewski & Rainham, 2007; Karr et al., 2009). Results from animal studies consistently show a rise in pulmonary inflammation, oxidative stress, and distal organ involvement following respiratory exposure to inhaled or instilled nanoparticles (Zhang et al., 2003; Zhou et al., 2003). These particles pose a greater risk in terms of their effects on the respiratory and cardiovascular system due to their higher deposition efficiency in the peripheral lung compared to fine particles and their ability to penetrate into lung tissue crossing the pulmonary epithelium, reaching the interstitium and possibly translocating to other organs (Oberdorster, 2001; Kreyling et al., 2002; BeruBe et al., 2007; Warheit et al., 2007; Moller et al., 2008). Typically, the biological activity of particles increases as size decreases. Smaller particles occupy less volume, resulting in a larger number of particles with a greater surface area per unit mass and, therefore, an elevated potential for biological interaction (Huang et al., 2004; Yang & Watts, 2005). Due to the unique and novel nature of these particles, these interactions with the respiratory system may potentially result in airway responses of a nature that is poorly understood.
Neurotrophins are a family of proteins that have been shown to play a key role in neural survival, development, function, and plasticity of nerves (Huang & Reichard, 2001). The neurotrophin family includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and neurotrophin 4 (NT4). These neurotrophins share common structural features and act through their corresponding high-affinity tyrosine kinase (Trk) receptor subtypes and a common low-affinity receptor, p75 (Lewin & Barde, 1996; Chao, 2003). The prototypical neurotrophin nerve growth factor (NGF) is a key regulatory element of neuronal development and responsiveness (Levi-Montalcini, 1987; Kernie & Parada, 2000) and controls expression of the genes encoding the precursor of the pro-inflammatory neurotransmitter substance P (SP) and other neuropeptides in sensory neurons (Lindsay & Harmar, 1989). In addition, NGF modulates immune responses and has been associated with allergic inflammation and airway hyperresponsiveness in animal models as well as in humans, suggesting that neurotrophic factors may play an important pathophysiologic role in asthma (Bonini et al., 1996; Braun et al., 1998, 1999).
In previous studies it was shown that lower-respiratory-tract infection (LRTI) with respiratory syncytial virus (RSV) is associated with a marked increase in the expression of NGF, and both its high-affinity receptor, tyrosine kinase A (TrkA), and low-affinity receptor, p75, in lung tissues. Under the influence of NGF, the nociceptive fibers located in the airway epithelium increase both synthesis and release of SP. SP, in turn, produces bronchoconstriction, airway hyperresponsiveness and activation of inflammatory cells including T lymphocytes and monocytes, resulting in airway inflammation, also referred to as neurogenic inflammation (Piedimonte, 2003; Piedimonte et al., 2004). In addition to its bronchoconstrictive and pro-inflammatory effects, substance P is known to possess important immunomodulatory properties and specifically regulates the function of T and B lymphocytes, monocytes, and macrophages by affecting their migration, response to mitogens and allergens, and synthetic functions (Goetzl et al., 1995; Maggi, 1997). The RSV-induced increased NGF expression is also greater in weanling rats compared to adult rats, indicating a vulnerable window in infancy when the innervation of the lung is being determined (Hu et al., 2002). The critical importance of neurogenic-mediated control of airway functions is further confirmed by the poor response (or lack thereof) to conventional anti-inflammatory treatment in children with RSV-induced bronchiolitis (Richter & Sheddon, 1998; Fox et al., 1999; Cade et al., 2000). Collectively, these data suggest that changes of neurotrophin expression in the early stages of airway development may lead to irreversible neurosensory dysfunctions. This, in turn, may result in chronic inflammatory effects extending further in life and ultimately resulting in the development of asthma (Nassenstein et al., 2006).
Although the collective studies just cited provide convincing evidence that environmental exposure to either viruses (such as RSV) or nanoparticles results in the development of respiratory diseases, and that the incidence of asthma is increased in urban areas with elevated environmental pollution, the impact of nanoparticles on the neurogenic-mediated control of airway responses and their potential role as a predisposing factor for developing asthma have not been clearly elucidated. Therefore, in this study, the effects of nanoparticle exposure were investigated on the expression of lung neurotrophins and whether this effect was age-dependent.
METHODS
Animals
Adult rats (12 wk old) weighing 190 ±10 g (SE) of both genders, newborn rats (1–2 d old) weighing 6 ± 0.1 g (SE) of both genders, and weanling rats (2 wk old) weighing 23 ± 0.4 g (SE) of both genders born to specific-pathogen-free timed-pregnant dams of Fischer 344 (F-344) strain were obtained from Charles River Laboratories (Wilmington, MA). The animals were housed in the AAALAC-approved NIOSH Animal Facility and kept in polycarbonate micro-isolator cages in a strictly controlled (12-h light/dark cycle; 20–25°C; 36/605 relative humidity) pathogen-free environment to prevent any microbial contamination. These cages were placed on racks that provided positive individual ventilation with class 100 air to each cage at the rate of approximately 1 cage change of air per minute (Maxi-Miser; Thoren Caging System, Hazleton, PA) and were serviced by trained husbandry technicians. Bedding, water, and food were auto-claved prior to use and unpacked only under laminar flow. Cages and water bottles were run through a tunnel washer after every use and disinfected with both chemical and heat. The West Virginia University and the NIOSH Animal Care and Use Committees approved all experimental procedures followed in this study.
Exposure System
The unrestrained animals were exposed to titanium dioxide nanoparticles (TiO2) in a specialized inhalation system as previously described by Chen et al. (2006) and illustrated in Figure 1. TiO2 powder (P25) was obtained from DeGussa (Parsippany, NJ). Although the average primary size is reported as 21 nm by the manufacturer, the primary particles tend to form aggregates as a result of van der Waals forces. To reduce the size of the aggregates, the powders were carefully prepared for generation by sieving (to remove the big aggregates), drying (to avoid aggregate formation due to high humidity), and storage (to prevent aggregate attraction through contact charges). A TSI fluidized-bed aerosol generator was used (Marple et al., 1978). TiO2 mass concentration was monitored with a Data RAM (DR-4000, Thermo Electron Co, Franklin, MA) and gravimetrically measured with Teflon filters. In this study, a mean aerosol concentration of 10 mg/m3 was maintained in the exposure chamber by adjusting the powder feed rate in the generator. The particle size distribution of TiO2 aerosols was measured using a cascade impactor (MOUDI, MSP Co. Shoreview, MN), an electrical mobility classifier (SMPS, TSI Inc. Shoreview, MN), and an aerodynamic sizing instrument (APS, TSI, Inc., Shoreview, MN). The impactor was used for measuring mass-based aerodynamic size distributions, while the latter two sizing devices were combined for determining number-based mobility size distributions. Assuming a log-normal distribution, the size distribution of TiO2 aerosols has a mass median aerodynamic diameter (MMAD) of 1.6 μm and a geometric standard deviation of 2.5, while the count mean aerodynamic diameter was 138 nm. For a typical concentration of 10 mg/m3, the count mode (primary peak) of the distribution is approximately 100 nm (Figure 2).
FIGURE 1.
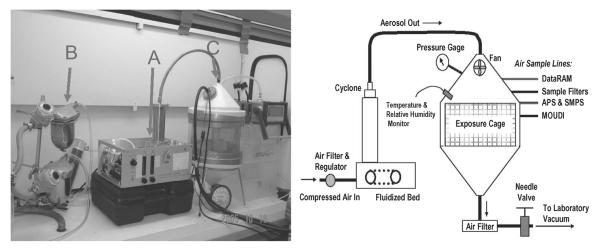
The left panel shows an actual picture of the exposure system used for this study. (A) Fluidized-bed aerosol generator containing TiO2 powder. (B) Air drier unit, which introduces dry clean air into the generator to fluidize the air and disperse the particles into the exposure chamber. (C) Exposure chamber containing the unrestrained animals. The right panel illustrates the schematic diagram of the inhalation exposure system.
FIGURE 2.
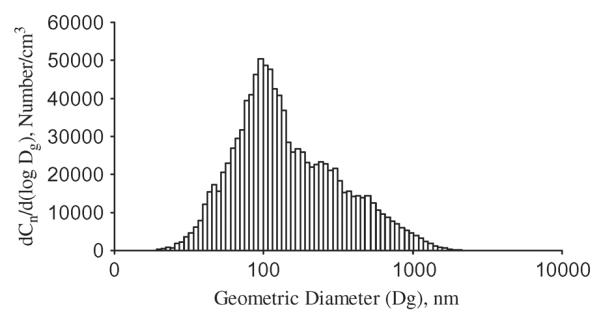
A typical number-based particle size distribution of TiO2 aerosol where Cn is the number concentration of the aerosol and Dg is the geometric (mobility) diameter.
Experimental Protocols
Adult pathogen-free rats (12 wk old; n = 6) were exposed to TiO2 nanoparticles (particle size 100 nm) at a concentration of 12 mg/m3 for 5.6 h/d for 3 consecutive days. This exposure regimen translates to a cumulative lung deposition of 240 μg of particulate. The deposited dose per animal is derived by the formula (aerosol concentration) × (minute ventilation) × (exposure duration) × (dpositon fraction), where minute ventilation was estimated to be 0.2 L/min (Brown et al., 2005) and deposition fraction is assumed to be 0.1 for particles with an MMAD of 1.6 μm. The deposition fraction was based upon Kreyling's alveolar deposition curve for inhaled ultrafine particles in rats (Kreyling, 2003). An equal number of age-matched animals were used as controls. Control animals were exposed to HEPA-filtered room air through a similar system except that dry air was fed directly into the exposure chamber and the chambers were located in a separate room. Upon completion of the last exposure, the animals were sacrificed by intraperitoneal (ip) injection of sodium pentobarbital (50 mg/kg) followed by exanguination as per the “AVMA Guidelines on Euthanasia,” June 2007, and their lungs were removed for subsequent analysis.
Newborn rats (1–2 d old; n = 12), born to specific-pathogen-free females, were exposed to the nanoparticle aerosol according to the same protocol as the adult animals except that they were kept with their mothers before, during, and after exposure in order to allow nursing and avoid any potential stress-related responses. The cumulative lung deposition per each newborn was equal to 60 μg of particulate. The deposited dose per animals is derived by the same formula described earlier except that minute ventilation was estimated to be 0.05 L/min (Brown et al., 2005). The lung deposition calculation may not reach a comparable level of accuracy for newborns due to the disruption of their breathing pattern during feeding and grooming from the mother. Age-matched control animals (1–2 d old; n = 10) born to different females were exposed to HEPA-filtered room air through a similar system except that dry air was fed directly into the exposure chamber and the chambers were located in a separate room. Upon completion of the last exposure, the animals were sacrificed by ip injection of sodium pentobarbital (50 mg/kg) followed by exanguination as per the “AVMA Guidelines on Euthanasia,” June 2007, and their lungs were removed for subsequent analysis.
Weanling rats (2 wk old; n = 11), born to specific-pathogen-free females, were exposed to the nanoparticle aerosol according to the same protocol as the adult animals except that they were kept with their mothers before, during, and after exposure in order to allow nursing and avoid any potential stress-related responses. The cumulative lung deposition per each weanling was equal to 120 μg of particulate. The deposited dose per animals is derived by the same formula described earlier except that minute ventilation was estimated to be 0.1 L/min (Brown et al., 2005). The lung deposition calculation may not reach a comparable level of accuracy for weanlings due to the disruption of their breathing pattern during feeding and grooming from the mother. Age-matched control animals (2 wk old; n = 8) born to different females were exposed to HEPA-filtered room air through a similar system except that dry air was fed directly into the exposure chamber and the chambers were located in a separate room. Upon completion of the last exposure, the animals were sacrificed by ip injection of sodium pentobarbital (50 mg/kg) followed by exanguination as per the “AVMA Guidelines on Euthanasia,” June 2007, and their lungs were removed for subsequent analysis.
Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
The presence of RNA in lung tissue was detected by reverse-transcription polymerase chain reaction (RT-PCR). The left lung from each animal was removed upon sacrifice and immediately flash frozen in liquid nitrogen and stored at −80°C. The frozen specimens were then placed in RLT disruption buffer and homogenized using a conventional rotor-stator homogenizer (Brinkmann Instruments, Westbury, NY) for 45–60 s until the sample was uniformly homogenous. Total RNA was extracted from lung homogenates using RNEasy Midi-Kits (Qiagen GmbH, Hilden, Germany) according to the manufacturer's specifications. RNA samples (1 pg–2 mg) were added to a 50-μl master mix consisting of 400 μM each of deoxynucleotide triphosphates (dNTPs), 10 U RNAse inhibitor, 2 μl enzyme mix containing Taq DNA polymerase (one-step RT-PCR Promega, Madison, WI), and 50 pmol each of primers flanking the nucleotide sequence for nerve growth factor (NGF) and its high- and low-affinity receptor (TrKa and p75), respectively, brain-derived neurotrophic factor (BDNF) and its receptor (TrKb), and the housekeeping gene (β-actin). The same master mix without the RNA sample was used as a negative control. The primers pairs were designed on the basis of previously published protocols (Hu et al., 2002) and were used to differentiate cDNA-generated PCR products from genomic DNA contamination. The specific primer sequences (sense and antisense) and their expected size are illustrated in Table 1. Amplification was performed using a Gene-Amp PCR System 9600 thermal cycler (Perkin-Elmer, Waltham, MA). The process was started with an initial step of 50°C/30 min, then 95°C/15 min, followed by 25–35 cycles with a denaturing step, followed by an annealing step, an extension step, and then one final extension step at 68–72°C for 10 min. All programs included a 4°C hold step at the end. Amplified PCR products were size-fractionated by electrophoresis through a 2% agarose gel and stained with ethidium bromide. The gels were then photographed using an imaging system (FOTO/Analyst Luminary Workstation, Fotodyne, Hartland, WI). The initial input/product-response curve was obtained to confirm that the selected cycle was within the “linear” range for each primer. The intensity of DNA bands was analyzed by computerized densitometry (TotalLab TL-101 Image Analysis Software) and expressed as the ratio of the densitometric score measured for each target normalized by the β-actin control.
TABLE 1.
Sequence of Primers Used for Analysis of Rat Neurotrophins and Neurotrophin Receptor Transcripts
| Gene | Product size, bp | Accession number | Gene ID | Sense | Anti-sense |
|---|---|---|---|---|---|
| BDNF | 293 | M61178 | 24225 | 5′ - AGC TGA GCG TGT GTG ACA GTA T - 3′ | 5′ - GTC TAT CCT TAT GAA CCG CCA G - 3′ |
| TrKb | 664 | M55291 | 25054 | 5′ - OH CCG CTA GGA TTT GGT GTA CTG AGC CTT CT- 3′ | 5′ - OH CCA CTG TCA TCA GAT GAA ATG TTC GTT ATC CT- 3′ |
| NCF | 395 | M36589 | 310738 | 5′ - CTG GAC TAA ACT TCA GCA TTC - 3′ | 5′ - TGT TGT TAA TGT TCA CCT CGC - 3′ |
| TrKa | 690 | M021589 | 59109 | 5′ - GCC TTC GCC TCA ACC ACC CCA - 3′ | 5′ - CTC TTC ATG TGC TGT TAG TGT - 3′ |
| p75 | 663 | M012601 | 24596 | 5′ - OH AGC CAA CCA GAC CGT GTG TG - 3′ | 5′ - OH TTG CAG CTG TTC CAC CTC TT - 3′ |
| Beta-actin | 285 | C005111.2 | 81822 | 5′ - OH TCA TGA AGT GTG ACG TTG ACA TCC GT - 3′ | 5′ - OH CTT AGA AGC ATT TGC GGT GCA CGA TG - 3′ |
Histopathology
The right lung from each animal was fixed in 10% buffered formalin, embedded in paraffin, and cut in 3-μm-thick sections. Hematoxylin and eosin (H&E) staining was performed for histopathologic analysis. All slides were coded and interpreted by two independent board-certified pathologists who were blinded as to whether the specimens were from a treated animal or a control. Histopathologic changes were graded as mild (5–10 inflammatory cells in 3 or more adjacent alveoli), moderate (10–20 inflammatory cells per alveolus), or severe (>20 inflammatory cells per alveolus). The scores of three different animals per each experimental group were averaged and then compared.
Airway Mechanics
A newly developed acoustic whole-body plethysmograph was used to measure specific airway resistance (sRaw) in unrestrained rats. The system consists of a plethysmograph chamber that operates as a resonating cavity. The acoustic pressure inside the cavity is determined by the cavity geometry. When the system is excited near the resonant frequency, the acoustic pressure amplitude in the plethysmograph is modulated in direct proportion to the change in volume of the animal. A screen placed across the outside end of the plethysmograph nozzle acts as a pneumotacograph to measure air flow. The pressure drop across the screen is measured with a pressure transducer. The system, therefore, allows the measurement of two independent flows (or pressure) from which sRaw can be estimated (Reynolds et al., 2008). These measurements are comparable with those obtained with traditional double-chamber plethysmographs, overcoming the issue of single-chamber plethysmographs, which produce an empirical index of airway resistance called Penh, the accuracy of which has been questioned (Sly et al., 2005). After measuring baseline sRaw, saline or methacholine at increasing concentrations of 2.5, 5, and 10 mg/ml was nebulized into the chamber for 3 min. After each challenge, sRaw was recorded for 3 min. Mean sRaw was calculated for either saline or methacholine for each animal over 3 min. This procedure was carried out for all experimental groups 1 h after the end of the last exposure.
Bronchoalveolar Lavage (BAL)
In a different set of weanling rats, collection of bronchoalveolar lavage (BAL) fluid was performed 1 h after the end of the last exposure. BAL was carried out according to the same protocol described earlier. Briefly, the animals were anesthetized by ip injection with sodium pentobarbital (50 mg/kg). A small plastic catheter attached to a syringe was inserted into the trachea and secured with a proximal and distal suture. The lavages were performed by instilling and withdrawing 28 ml/kg of saline into the airways and repeating the procedure thrice. At the end of the procedure the animals were euthanized by an additional ip injection of sodium pentobarbital (50 mg/kg) followed by exanguination as per the “AVMA Guidelines on Euthanasia,” June 2007. Total cell count in BAL fluid was performed with a hemocytometer and expressed as total cells per milliliter of fluid, and a microscope slide for differential count was prepared using a cyto-centrifuge at 9500 × g for 5 min. The slides were fixed by air-drying and then stained with the Giemsa-Wright method. Cells were identified based on size and morphological features, and we avoided any cell that had evidence of artifact or lysis. Differential cell count was performed on 200 cells to determine the number of polymorphonuclear leukocytes (PMNs), lymphocytes, and macrophages. Slides were coded and reviewed separately by two pathologists, who were blinded with regard to the experimental conditions. T cells and their subpopulations (CD4+/CD8+), B cells, and macrophages were measured by flow cytometry according to our standard protocol. Briefly, BAL fluid was centrifuged at 800 × g for 8 min at 4°C. The supernatant was frozen for subsequent analysis. Cell pellets were resuspended in 2 ml of room-temperature 1 × BD Pharmingen lysis buffer and incubated at room temperature for 3 min. Ten milliliters phosphate-buffered saline (PBS) was added and cells were pelleted as already described. Pellets were resuspended in 0.5 ml PBS and cell counts were done. Cells were blocked with Fc receptor antibody (BD number 550270) at 1 μg/106 cells for 30 min on ice. CD45 (BD number 554876) or isotype control (BD number 550615) was added at 1 μg/106 cells to appropriate tubes and incubated on ice for 30 min. Cells were washed with PBS twice, and after the second wash fluorochrome antibodies were added at 1 μg/106 cells and streptavidin APC Cy-7 at 0.5 μg/106 cells. Cells were incubated on ice in the dark for 30 min, then washed twice with PBS. After the second wash the pellets were resuspended on 0.5 ml of 0.4% paraformaldehyde and incubated overnight at 4°C in the dark. Antibodies used were CD3 (BD number 557030), CD4 (BD number 554839), CD8a (BD number 559976), mouse immunoglobulin (Ig) M isotype control (BD number 550883), mouse IgG2a isotype control (BD number 553458), and mouse IgG1 isotype control (BD number 550617). Data were acquired and analyzed using FACSAria (BD Biosciences, San Jose, CA).
The rationale for measuring T-cell population in BAL is due to the fact that neurotrophins were consistently found to exert an additional effect on immune responses (Nockher and Renz, 2006a; 2006b).
Multiplexed Cytokine/Chemokine Analysis
An aliquot of the frozen BAL fluid was used for simultaneous measurement of multiple inflammation relevant airway cytokines and chemokines. A panel of 24 cytokines and chemokines, including Eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), GRO/KC, interferon (IFN)-γ, interleukin (IL)-10, IL-12 (p70), IL-13, IL-17, IL-18, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IP-10, leptin, monocyte chemotactic protein-1 (MCP-1), macrophage inflammory protein (MIP)-1α, RANTES, tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF), was simultaneously measured in BAL fluid using a Milliplex MAP rat cytokine/chemokine panel (Millipore; Billerica, MA) according to manufacturer's specifications. The analysis was performed using Luminex 200 System and xMAP technology for multiplexed quantification (Luminex, Austin, TX).
Statistical Analysis
Measurements of specific airway resistance (sRaw) were analyzed using a one-way analysis of variance (ANOVA) for repeated measures with post hoc Fisher correction to identify significant pairs. All other data were analyzed using paired and unpaired t-tests when appropriate (Sigmastat 2.0 for Windows, SPSS, Inc., Chicago). Values in the text and figures are presented as mean ± SE. Differences having a p value <.05 were considered significant.
RESULTS
Neurotrophin and Neurotrophin Receptor Expression
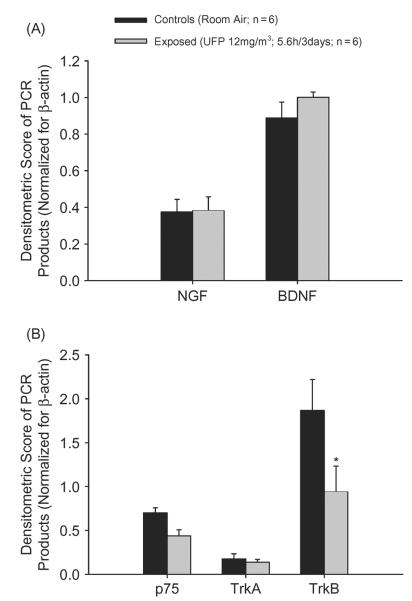
In adult rats, RT-PCR analysis of lung tissues mRNA encoding for NGF and BDNF did not reveal any significant difference in exposed animals compared to controls. The computerized densitometric analysis of the intensity of the bands for the target genes normalized for the standard reference gene β-actin is shown in Figure 3A. The same analysis conducted for the high- and low-affinity NGF receptors, TrKa and p75, respectively, confirmed a lack of effect in treated animals compared to controls (Figure 3B). However, the expression of the BDNF receptor, TrKb, showed a twofold significant decrease in animals exposed to nanoparticles compared to controls (Figure 3B).
FIGURE 3.
(A) In adult rats, NGF and BDNF expression is unchanged following exposure to TiO2 nanoparticulate. (B) Consistent with their agonists, the expression of neurotrophin receptors was unaffected except for a decreased expression of the BDNF receptor, TrKb, which may be due to a physiologically diminished activity of neurotrophins associated with age. Data were generated by densitometric analysis of the intensity of the target gene bands normalized for the standard reference gene, β-actin, and are expressed as mean ± SE (n = 6 for both groups; asterisk indicates signficant at p < .05).
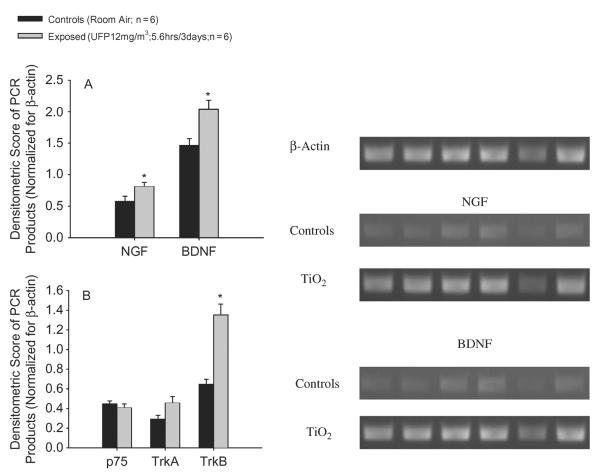
In weanling rats, exposure to TiO2 nanoparticles produced a 1.4-fold increase in NGF and BDNF lung expression compared to controls (Figure 4A). This effect was associated with a twofold rise in the lung expression of the BDNF receptor, TrKb, whereas the expression of NGF high- and low-affinity receptors (TrKa and p75), respectively, did not reveal any significant change in treated animals compared to controls (Figure 4B). The intensity of the bands for the target genes was analyzed by computer densitometry and normalized for the standard reference gene, β-actin, as previously described. A representative gel image is also shown in Figure 4.
FIGURE 4.
(A) In weanling rats, exposure to TiO2 nanoparticles upregulates NGF and BDNF expression in lung homogenates. This effect is associated with a significant increase of the high-affinity receptor for BDNF, TrKb. The high-affinity receptor for NGF, TrKa, is also upregulated, although the magnitude of the change is not enough to reach statistical significance, whereas the expression of the NGF low-affinity receptor, p75, is unaffected (B). The intensity of the bands for the target genes was analyzed by computer densitometry and normalized for the standard reference gene, β-actin, as previously described. A representative gel image of the NGF and BDNF bands is also shown. Data are expressed as mean ± SE (n = 6 for both groups; asterisk indicates signficant at p < .05).
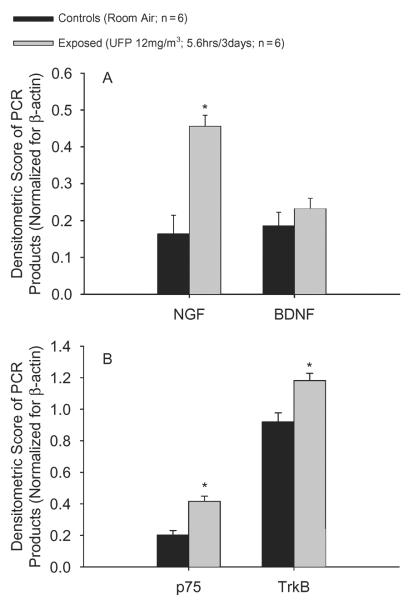
In newborn rats, the same exposure regimen resulted in a 2.8-fold increase in NGF expression compared to age-matched controls, whereas the expression of BDNF was unaffected (Figure 5A). This effect was associated with a twofold rise in NGF low-affinity receptor, p75, and a 1.3-fold increase of BDNF receptor, TrKb, in treated animals compared to controls. In contrast, TrkA mRNA transcripts were undetectable (Figure 5B).
FIGURE 5.
(A) Newborn rats show increased lung NGF expression following exposure to TiO2 nanoparticles compared to their age-matched controls. This effect appears to be of a higher magnitude compared to weanlings exposed to the same level of inhaled particulate. BDNF expression was unaffected. (B) Consistent with its agonist, the NGF low-affinity receptor, p75, is upregulated in the lungs of newborn animals exposed to TiO2 nanoparticles compared to control, whereas for the NGF high-affinity receptor, TrKa, levels are undetectable. TrKb expression is slightly yet significantly increased despite the lack of response of its agonist, BDNF, indicating a possible increase of TrKb-mediated BDNF activity. Data shown in figure are obtained by densitometric analysis of the target genes band as previously described and are expressed as mean ± SE (n = 6; asterisk indicates signficant at p < .05).
Histopathology
The analysis of photomicrographs of hematoxylin and eosin (H&E)-stained sections from the lung of weanling rats did not reveal any evidence of acute or chronic inflammatory changes and/or the presence of any acute or chronic cell infiltrates in TiO2-exposed animals compared to age-matched controls, despite the fact that tissue neurotrophins were significantly upregulated (Figure 6, upper panel). In adult rats the absence of exposure-related histopathological changes is consistent with the absence of any significant changes in neurotrophin expression (Figure 6, lower panel).
FIGURE 6.
Photomicrographs of hematoxylin and eosin-stained sections from the lung of weanling rats (A) sacrificed after the last day of exposure to air or TiO2 nanoparticles show the absence of any significant pathological changes compared to age-matched controls, despite the fact that tissue neurotrophins were significantly upregulated. In adult rats (B), the absence of exposure-related histopathological changes is consistent with the absence of any significant changes in neurotrophin expression (magnification 20×; micrographs are representative of 3 rats per group).
Airway Mechanics
Weanling rats (2 wk old) exposed to TiO2 nanoparticles exhibited significantly higher baseline specific airway resistance (sRaw) compared to their age-matched controls 1 h after the end of the last exposure (sRaw: 1.1 ± 0.08 cm H2O-s vs. 0.9 ± 0.03 cm H2O-s for exposed and control animals, respectively; n = 5–6; Figure 7A). In exposed animals, inhalational challenge with 10 mg/ml methacholine produced a significantly higher rise in sRaw compared to control animals (2.3 ± 0.2 cm H2O-se vs. 1.4 ± 0.1 cm H2O-s, respectively; values are expressed as peak sRaw changes; n = 5–6; Figure 7B). The rationale for performing airway measurements only on weanling rats is based on the absence of any significant changes in the molecular responses following particle exposure in adult animals and on the technical limitations of the system, which do not allow for a reliable measurement of pulmonary parameters in newborn rats due to their very small size.
FIGURE 7.
(A) In weanling rats, baseline specific airway resistance (sRaw; cm H2O-s) is increased in animals exposed to TiO2 compared to their age-matched controls 1 h after the end of the last exposure. In the same animals, the airway response to increasing concentration of inhaled methacholine is increased in exposed rats compared to controls (B). Data are expressed as mean ± SE (n = 5–6; asterisk indicates significant at p < 0.05 and double asterisk indicates signficant at p < 0.01).
BAL
In exposed weanling animals, the total number of cells recruited in the airways per ml of BAL fluid showed a quantitative increase compared to age-matched controls, although this trend did not reach statistical significance (3.1 × 106 ± 4.9 × 105 vs. 2.7 ± 106 ± 2.9 ± 5, respectively; n = 6 for both groups; data not shown). Accordingly, no statistical significant differences in the differential count for PMN, lymphocytes, and macrophages were observed (data not shown). Flow cytometric analysis of BAL fluid also revealed that T cells and their subpopulations, CD4+/CD8+, as well as B cells, and macrophages were not significantly affected by nanoparticles exposure as compared to control weanling animals (data not shown).
Multiplexed Quantification of BAL Fluid Cytokines
The levels of 24 cytokines and chemokines were examined in the BAL fluid of control and exposed weanling animals. Eleven of these were detectable and one, GRO/KC, was found to be significantly elevated in the lavage fluid of weanling animals exposed to TiO2 compared to age-matched controls (0.6 ± 0.08 pg/μg protein vs. 0.3 ± 0.07 pg/μg protein, respectively; Figure 8). GRO/KC is a chemokine belonging to the CXC family and was shown to be the rat equivalent of human IL-8 (Verri et al., 2006). The list of all detected markers and their concentrations in the BAL fluid of the different experimental groups is shown in Table 2.
FIGURE 8.

The analysis of BAL fluid by multiplexed quantification using Luminex 200 System and xMAP technology revealed the presence of increased concentrations of the cytokine GRO/KC (rat equivalent of human IL-8) in the airways of exposed weanling animals compared to their age-matched controls. Data are expressed as picograms of analyte per microgram of total protein and are mean ± SE (n = 6 for both groups; asterisk indicates signficant at p < .05).
TABLE 2.
Cytokine and Chemokine Levels (pg/μg Total Protein) in BAL Fluid of Control and TiO2-Exposed Weanling Animals
| Substance | Controls (n = 6) | TiO2 (n = 6) |
|---|---|---|
| Eotaxin | 0.03 ± 0.006 | 0.04 ± 0.007 |
| GRO/KC | 0.3 ± 0.07 | 0.6 ± 0.08a |
| IL-17 | 0.013 ± 0.003 | 0.018 ± 0.003 |
| IL-18 | 0.4 ± 0.04 | 0.5 ± 0.07 |
| IL-1β | 0.04 ± 0.009 | 0.03 ± 0.01 |
| IL-4 | 0.05 ± 0.009 | 0.07 ± 0.01 |
| Leptin | 0.1 ± 0.02 | 0.1 ± 0.02 |
| MCP-1 | 0.04 ± 0.01 | 0.03 ± 0.01 |
| RANTES | 0.03 ± 0.004 | 0.02 ± 0.008 |
| TNF-α | 0.05 ± 0.004 | 0.048 ± 0.02 |
| VEGF | 0.3 ± 0.04 | 0.4 ± 0.08 |
Significant at p < .05 vs. controls.
DISCUSSION
The results of this study show for the first time that exposure of newborn and weanling rats to TiO2 nanoparticulate influences the expression of lung neurotrophins, key regulatory elements of neuronal development and responsiveness that play a critical role in the pathophysiology of childhood asthma. This effect is associated with the development of airway hyperreactivity (AHR) and mild airway inflammation in younger rats (2 wk old). In contrast, inhalation exposure to nanoparticles did not markedly influence these parameters in older adult animals, supporting the presence of a critical window of vulnerability in earlier stages of lung development compared to later in life as already suggested in the literature (Hu et al., 2002; Piedimonte, 2002a; Piedimonte et al., 2004). These findings, in turn, may represent a potentially relevant clinical factor indicating the importance of early influences of nanoparticles on the developing airways and their related impact on the development of airway inflammation and eventually bronchial asthma.
Environmental pollution deriving from vehicle emission and industrial processes has consistently been shown to affect the incidence of respiratory diseases (Peters et al., 2004; Mossman et al., 2007; Bedeschi et al., 2007). However, nanoparticles may pose a greater risk in terms of their effects on the respiratory and cardiovascular system due to their small size and large particle number and surface area per unit mass, which, in turn, determines the overall distribution in lung and distal organs, and may also provide a more efficient transport mechanism for other toxic compounds associated with or adhering to the surface of the nanoparticles (Mossman et al., 2007). In this study, nano-sized titanium dioxide was used, since it is a useful prototypical material for the study of the mechanisms of nanoparticle effects due to its assumed immunologically “inert” nature, which allows examination of the effects of particle size without confounding factors, such as high toxicity (Oberdorster et al., 2005). Nano-sized TiO2 is used extensively in several industrial processes and therefore is commonly found in products such as photocatalysts to clean air and water, antibacterial agents on glass and steel, and as components of many cosmetics and sunscreens (Gwinn & Vallyathan, 2006; Maynard et al., 2006; Grassian et al., 2007). Exposure to TiO2 nanoparticles produced airway inflammation, alveolar macrophage recruitment, and activation of various growth factors and chemokines (de Haar et al., 2006; Shieh et al., 2006). This collective evidence suggests also that nanoparticle-induced airway inflammation may predispose and/or amplify the airway responses to other environmental pollutants. Lambert et al. (2003) showed that ultrafine particles enhance RSV-induced airway reactivity in a mouse model of RSV infection. Shvedova et al. (2008) demonstrated an increased susceptibility to bacterial infection following exposure to carbon nanotubes in mice. These investigators, however, focused mainly on cytokine and chemokines expression and production of reactive oxygen species (ROS) as factors in the pathophysiology of inflammatory airway diseases. Our study focused on the neuroinflammatory pathways leading to abnormal airway responses and the impact of TiO2 nanoparticles exposure on their regulation. In particular, the effects of this environmental exposure on the neurotrophin-mediated regulation of the subepithelial neurosensory network were investigated. The neurogenic-mediated control of airway responses, which was described in detail in the introduction, is a critical mechanism in the regulation of airway functions and the development of asthma during childhood (Nassenstein et al., 2006). In order to confirm the relevance of nanoparticle-induced disruption of airway neurotrophin expression, the physiological effects associated with these mechanisms were also studied.
An important finding of our study was that the TiO2-induced airway responses are age dependent. Adult rats exposed to the same exposure conditions as younger animals did not show any significant changes in the expression of the airway neurotrophins NGF and BDNF or their receptors, TrKa, p75, and TrKb. These age-related differences have already been shown to play a critical role in determining the susceptibility of the developing airways to other inhaled pathogens (Piedimonte, 2001; Hu et al., 2002; Piedimonte et al., 2004), and, although the magnitude of the particle-induced airway responses in our study was not as dramatic as with more toxic inhalants, data suggest the possibility that this type of environmental exposure may render the lung more sensitive to secondary insults present in the environment, including respiratory viruses (Lambert et al., 2003; Mossman et al., 2007; Shvedova et al., 2008). The disruption of neurotrophin-related airway responses was associated to AHR as demonstrated by the presence of a higher baseline specific airway resistance in exposed animals compared to their age-matched controls and by the enhanced response to methacholine challenge in treated animals 1 h after the last exposure. In addition, the analysis of inflammation-relevant cytokines and chemokines in the airways of exposed and control rats showed increased levels of the chemokine GRO/KC in animals exposed to TiO2 nanoparticles compared to age-matched controls exposed to room air. GRO/KC is best known for its role in neutrophil chemotaxis and degranulation early during inflammation. In this regard its effects are similar to those of other CXC-family cytokines including interleukin-8 (IL-8; CXCL8) in humans (Verri et al., 2006). Our in vivo data are consistent with previous observations on cultured human epithelial cells showing that exposure to nanosized particles of TiO2 triggers a pro-inflammatory response (Singh et al., 2007), as well as several controlled human exposure studies indicating the capability of environmental pollutants to induce an acute inflammatory response in human airways (Krishna et al., 1998). In addition, IL-8 was found to enhance airway responses to increasing concentrations of histamine in guinea pigs (Qi et al., 1999). However, in our study this effect was not associated with a significant influx of inflammatory cells such as polymorphonuclear leucocytes (PMN) into the airways. These findings are consistent with the lack of any inflammatory cell infiltrates in lung tissue of exposed and control animals and are in agreement with Geiser et al. (2008), who showed no marked increase in inflammatory cells recruitment in the airways of animals exposed to inhaled TiO2 nanoparticles compared to controls. An interesting and potentially relevant finding in our study is the simultaneous upregulation of airway neurotrophins and the pro-inflammatory cytokine IL-8. Since early evidence suggests the lack of a direct involvement between sensory neuropeptides and IL-8 in the induction of AHR (Fujimura et al., 1996), our data suggest that acute exposure to nanoparticles may result in the activation of different pathways leading to the development of heightened airway responsiveness, a critical feature of asthma. Nonetheless, further studies are needed to elucidate this mechanism.
Nanosized particles of TiO2 have consistently shown the capacity to elicit toxic and inflammatory effects in several experimental studies (Singh et al., 2007). Further, the constant and unavoidable exposure to respiratory viruses, such as RSV, and the fact that these types of environmental exposures cannot be separated make understanding their potential pathophysiological relationship critical. Our data, although preliminary, provide a strong argument for a significant role of nanoparticle exposure as a potential risk factor for the development of abnormal airway responses. Previous studies on the mechanism of neurotrophin-driven, RSV-induced airway responses and their critical role in the early development of airway disease (Bonini et al., 1996; Braun et al., 1998; 1999; Piedimonte, 2002b; 2002c) provide a strong rationale for studying the effects of nanoparticles in early stages of development. Our results are novel because they shed some light onto the subtle effects of particulate exposure and suggest their importance in the study of pathophysiological mechanisms in early life factors leading to the development of airway diseases.
In conclusion, understanding the airway responses to inhaled nanoparticles and the extent to which they affect the development of a particular disease is of paramount importance to identify targets for therapeutic intervention. Our focus on the early changes in the developing airways is, therefore, critical because it may lead to new preventative and/or therapeutic measures in the treatment of chronic inflammatory airway diseases, and identification of new risk factors for their development. Further studies on the concomitant and/or sequential impact of environmental pathogens such as viruses and ambient pollutants such as nanomaterials, including carbon nanotubes, diesel exhaust particles (DEP), and, to a lesser extent, quantum dots, are needed.
Acknowledgments
Supported in part by NIH/NHLBI HL-61007, and by M.S. WVU startup funds (12.490001520.71222272.5014701.152). The authors thank Jared Cumpston, Amy Cumpston, Donny Leonard, Vic Robinson, and Samuel Stone for their expert technical assistance in this study, and Dr. Kathleen Brundage for her expert assistance with flow cytometry experiments at the West Virginia University Flow Cytometry Core Facility (supported in part by NIH grants RR016440 and RR020866).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, Boumghar A, Forastiere F, Forsberg B, Touloumi G, Schwartz J, Katsouyanni K. Acute effects of particulate air pollution on respiratory admissions: Results from APHEA 2 project. Air Pollution and Health: A European Approach. Am. J. Respir. Crit. Care Med. 2001;164:1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- Bedeschi E, Campari C, Candela S, Collini G, Caranci N, Frasca G, Galassi C, Francesca G, Vigotti MA. Urban air pollution and respiratory emergency visits at pediatric unit, Reggio Emilia, Italy. J. Toxicol. Environ. Health A. 2007;70:261–265. doi: 10.1080/15287390600884784. [DOI] [PubMed] [Google Scholar]
- BeruBe K, Balharry D, Sexton K, Koshy L, Jones T. Combustion-derived nanoparticles: Mechanisms of pulmonary toxicity. Clin. Exp. Pharmacol. Physiol. 2007;34:1044–1050. doi: 10.1111/j.1440-1681.2007.04733.x. [DOI] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl. Acad. Sci. USA. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Appel E, Baruch R, Herz U, Botchkarev V, Paus R, Brodie C, Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Lewin GR, Virchow JC, Renz H. Neurotrophins: A link between airway inflammation and airway smooth muscle contractility in asthma? Int. Arch. Allergy Immunol. 1999;118:163–165. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- Brown JS, Wilson WE, Grant LD. Dosimetric comparisons of particle deposition and retention in rats and humans. Inhal. Toxicol. 2005;17:355–385. doi: 10.1080/08958370590929475. [DOI] [PubMed] [Google Scholar]
- Cade A, Brownlee KG, Conway SP, Haigh D, Short A, Brown J, Dassu D, Mason SA, Phillips A, Eglin R, Graham M, Chetcuti A, Chatrah M, Hudson N, Thomas A, Chetcuti PA. Randomised placebo controlled trial of nebulised corticosteroids in acute respiratory syncytial viral bronchiolitis. Arch. Dis. Child. 2000;82:126–130. doi: 10.1136/adc.82.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat .Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen B, Frazer D, Stone S, Schwegler-Berry D, Cumpston J, McKinney W, Linsley W, Frazer A, Donlin M, Vandestouwe K, Castranova V, Nurkiewicz T. Development of a small inhalation system for rodent exposure to fine and ultrafine titanium dioxide aerosols; St. Paul, MN: 7th International Aerosol Conference; 2006. [Google Scholar]
- de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin. Exp. Allergy. 2006;36:1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: Mortality among residents of 90 cities. J. Toxicol. Environ. Health A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Gilmour MI, MacNee W. Asthma and PM10. Respir. Res. 2000;1:12–15. doi: 10.1186/rr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GF, Everard ML, Marsh MJ, Milner AD. Randomised controlled trial of budesonide for the prevention of post-bronchiolitis wheezing. Arch. Dis. Child. 1999;80:343–347. doi: 10.1136/adc.80.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura M, Tsujiura M, Nomura M, Mizuguchi M, Matsuda T, Matsushima K. Sensory neuropeptides are not directly involved in bronchial hyperresponsiveness induced by interleukin-8 in guinea-pigs in vivo. Clin. Exp. Allergy. 1996;26:357–362. [PubMed] [Google Scholar]
- Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. The role of macrophages in the clearance of inhaled ultrafine titanium dioxide particles. Am. J. Respir. Cell Mol. Biol. 2008;38:371–376. doi: 10.1165/rcmb.2007-0138OC. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Xia M, Ingram DA, Kishiyama JL, Kaltreider HB, Byrd PK, Ichikawa S, Sreedharan SP. Neuropeptide signaling of lymphocytes in immunological responses. Int. Arch. Allergy Immunol. 1995;107:202–204. doi: 10.1159/000236977. [DOI] [PubMed] [Google Scholar]
- Grassian VH, O'Shaughnessy T, Adamcakova-Dodd PA, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn MR, Vallyathan V. Nanoparticles: Health effects—Pros and cons. Environ. Health Perspect. 2006;114:1818–1825. doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L494–L502. doi: 10.1152/ajplung.00414.2001. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichard LF. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004;21:344–353. doi: 10.1023/b:pham.0000016249.52831.a5. [DOI] [PubMed] [Google Scholar]
- Karr CJ, Rudra CB, Miller KA, Gould TR, Larson T, Sathyanarayana S, Koenig JQ. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ. Res. 2009;109:321–327. doi: 10.1016/j.envres.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Parada LF. The molecular basis for understanding neurotrophins and their relevance to neurologic disease. Arch. Neurol. 2000;57:654–657. doi: 10.1001/archneur.57.5.654. [DOI] [PubMed] [Google Scholar]
- Krewski D, Burnett R, Jerrett M, Pope CA, Rainham D, Calle E, Thurston G, Thun M. Mortality and long-term exposure to ambient air pollution: Ongoing analyses based on the American Cancer Society cohort. J. Toxicol. Environ. Health A. 2005;68:1093–1109. doi: 10.1080/15287390590935941. [DOI] [PubMed] [Google Scholar]
- Krewski D, Rainham D. Ambient air pollution and population health: overview. J. Toxicol. Environ. Health A. 2007;70:275–283. doi: 10.1080/15287390600884859. [DOI] [PubMed] [Google Scholar]
- Kreyling W. Deposition, retention, and clearance of ultrafine particles. BIA-Workshop, Ultrafine Aerosols at Workplaces; Neuherberg/Munich: Germany: 2003. [Google Scholar]
- Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, Oberdorster G, Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Krishna MT, Chauhan AJ, Frew AJ, Holgate ST. Toxicological mechanisms underlying oxidant pollutant-induced airway injury. Rev. Environ. Health. 1998;13:59–7. [PubMed] [Google Scholar]
- Lambert AL, Mangum JB, DeLorme MP, Everitt JI. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicol. Sci. 2003;72:339–346. doi: 10.1093/toxsci/kfg032. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor35years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu. Rev. Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337:362–364. doi: 10.1038/337362a0. [DOI] [PubMed] [Google Scholar]
- Maggi CA. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- Marple VA, Liu BY, Rubow KL. A dust generator for laboratory use. Am Ind Hyg Assoc J. 1978;39:26–32. doi: 10.1080/0002889778507709. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Moller W, Felten K, Sommerer K, Scheuch G, Meyer G, Meyer P, Haussinger K, Kreyling WG. Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 2008;177:426–432. doi: 10.1164/rccm.200602-301OC. [DOI] [PubMed] [Google Scholar]
- Mossman BT, Borm PJ, Castranova V, Costa DL, Donaldson K, Kleeberger SR. Mechanisms of action of inhaled fibers, particles and nanoparticles in lung and cardiovascular diseases. Particle Fibre Toxicol. 2007;4:4. doi: 10.1186/1743-8977-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Schulte-Herbruggen O, Renz H, Braun A. Nerve growth factor: The central hub in the development of allergic asthma? Eur. J. Pharmacol. 2006a;533:195–206. doi: 10.1016/j.ejphar.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins and asthma: novel insight into neuroimmune interaction. J. Allergy Clin. Immunol. 2006b;117:67–71. doi: 10.1016/j.jaci.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Nockher WA, Renz H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006;117:583–589. doi: 10.1016/j.jaci.2005.11.049. [DOI] [PubMed] [Google Scholar]
- Oberdorster G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Particle Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N. Engl. J. Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. Neural mechanisms of respiratory syncytial virus-induced inflammation and prevention of respiratory syncytial virus sequelae. Am. J. Respir. Crit. Care Med. 2001;163:S18–S21. doi: 10.1164/ajrccm.163.supplement_1.2011113. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. The association between respiratory syncytial virus infection and reactive airway disease. Respir. Med. 2002b;96(suppl. B):S25–S29. [PubMed] [Google Scholar]
- Piedimonte G. Origins of reactive airways disease in early life: Do viral infections play a role? Acta Paediatr. Suppl. 2002a;91:6–11. doi: 10.1111/j.1651-2227.2002.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus-reactive airway disease link. Respir. Res. 2002c;3(suppl. 1):S21–S25. doi: 10.1186/rr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedimonte G. Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2003;22:S66–S74. doi: 10.1097/01.inf.0000053888.67311.1d. discussion S74-S75. [DOI] [PubMed] [Google Scholar]
- Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatr. Res. 2004;55:657–665. doi: 10.1203/01.PDR.0000112244.72924.26. [DOI] [PubMed] [Google Scholar]
- Qi H, Lu K, Wang W. The relationship between interleukin-8 and airway hyperresponsiveness in guinea pigs. Chin. Med. J. (Engl.) 1999;112:985–987. [PubMed] [Google Scholar]
- Reynolds JS, Johnson VJ, Frazer DG. Unrestrained acoustic plethysmograph for measuring specific airway resistance in mice. J. Appl. Physiol. 2008;105:711–717. doi: 10.1152/japplphysiol.00949.2007. [DOI] [PubMed] [Google Scholar]
- Richter H, Sheddon P. Early nebulized budesonide in the treatment of bronchiolitis and the prevention of postbronchiolitic wheezing. J. Pediatr. 1998;132:849–853. doi: 10.1016/s0022-3476(98)70316-6. [DOI] [PubMed] [Google Scholar]
- Shieh KJ, Li M, Lee YH, Sheu SD, Liu YT, Wang YC. Antibacterial performance of photocatalyst thin film fabricated by defection effect in visible light. Nanomedicine. 2006;2:121–126. doi: 10.1016/j.nano.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Fabisiak JP, Kisin ER, Murray AR, Roberts JR, Tyurina YY, Antonini JM, Feng WH, Kommineni C, Reynolds J, Barchowsky A, Castranova V, Kagan VE. Sequential exposure to carbon nanotubes and bacteria enhances pulmonary inflammation and infectivity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;38:579–590. doi: 10.1165/rcmb.2007-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Shi T, Duffin R, Albrecht C, van Berlo D, Hohr D, Fubini B, Martra G, Fenoglio I, Borm PJ, Schins RP. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: Role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007;222:141–151. doi: 10.1016/j.taap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Sly PD, Turner DJ, Collins RA, Hantos Z. Penh is not a validated technique for measuring airway function in mice. Am. J. Respir. Crit. Care Med. 2005;172:256. doi: 10.1164/ajrccm.172.2.954. [DOI] [PubMed] [Google Scholar]
- Verri WA, Jr., Cunha TM, Parada CA, Poole S, Cunha FQ, S. H. Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol. Ther. 2006;112:116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- von Klot S, Wolke G, Tuch T, Heinrich J, Dockery DW, Schwartz J, Kreyling WG, Wichmann HE, Peters A. Increased asthma medication use in association with ambient fine and ultrafine particles. Eur. Respir. J. 2002;20:691–702. doi: 10.1183/09031936.02.01402001. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Webb TR, Reed KL, Frerichs S, Sayes CM. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology. 2007;230:90–104. doi: 10.1016/j.tox.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Wong GW, Lai CK. Outdoor air pollution and asthma. Curr. Opin. Pulmon. Med. 2004;10:62–66. doi: 10.1097/00063198-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kusaka Y, Zhu X, Sato K, Mo Y, Kluz T, Donaldson K. Comparative toxicity of standard nickel and ultrafine nickel in lung after intratracheal instillation. J. Occup. Health. 2003;45:23–30. doi: 10.1539/joh.45.23. [DOI] [PubMed] [Google Scholar]
- Zhou YM, Zhong CY, Kennedy IM, Leppert VJ, Pinkerton KE. Oxidative stress and NFkappaB activation in the lungs of rats: A synergistic interaction between soot and iron particles. Toxicol. Appl. Pharmacol. 2003;190:157–169. doi: 10.1016/s0041-008x(03)00157-1. [DOI] [PubMed] [Google Scholar]