Abstract
Objectives
Adverse conditions in Africa produce some of the highest rates of infant mortality in the world. Fetal growth restriction and preterm delivery are commonly regarded as major pathways through which conditions in the developing world affect infant survival. The aim of this article was to compare patterns of birthweight, preterm delivery, and perinatal mortality between black people in Tanzania and the USA.
Design
Registry-based study.
Settings
Referral hospital data from North Eastern Tanzania and US Vital Statistics.
Sample
Consisted of 14 444 singleton babies from a hospital-based registry (1999–2006) and 3 530 335 black singletons from US vital statistics (1995–2000).
Main outcome measures
birthweight, gestational age and perinatal mortality.
Methods
Restricting to babies born with at least 500g, we compared birthweight, gestational age, and perinatal mortality (stillbirths and deaths in the first week) in the two study populations.
Results
Perinatal mortality in the Tanzanian sample was 41/1000, compared with 10/100 among USA blacks. Tanzanian babies were slightly smaller on average (43g), but fewer were preterm (before 37 weeks) (10.0% vs 16.2%). Applying the USA weight-specific mortality rates to Tanzanian babies born at term suggested that birthweight does not play a role in their increased mortality relative to USA blacks.
Conclusions
Higher mortality independent of birthweight and preterm delivery for Tanzanian babies, suggests the need to address the contribution of other pathways that can further reduce the excess perinatal mortality.
Keywords: birthweight, developing country, fetal development, perinatal mortality, preterm birth
Introduction
Of the 25 countries in the world with the highest rates of neonatal mortality, 21 are on the African continent (1). Among these is Tanzania, where around 10% of all live-born babies die before their first birthday (1, 2) , a rate that is at least ten times higher than that of high resource countries. Major contributors to perinatal and neonatal mortality in Africa are thought to be infections, conditions related to immaturity, asphyxia and congenital malformations (1, 3, 4).
In high resource countries there has been considerable emphasis on the contribution of preterm delivery and low birthweight to infant mortality (5–7). Intrauterine growth restriction is regarded as an even bigger problem in developing countries (8), although there are few population-based data available. Some of the underlying causes of infant mortality in Africa (such as malaria or malnutrition) can reduce birthweight, but little is actually known about the degree to which the mortality due to such factors is mediated through fetal growth restriction or preterm delivery. We compared the patterns of birthweight, preterm delivery, and perinatal mortality between births in a hospital-based registry in Tanzania and comparable data for USA blacks.
Material and methods
We used data on singleton births born during 1999–2006 from a birth registry managed by the Kilimanjaro Christian Medical Centre (KCMC), a zonal hospital situated in Moshi Urban district of Kilimanjaro region, Tanzania. The KCMC, in addition to serving the local community, receives women referred for delivery from a large geographic area. Referrals account for approximately 20% of all deliveries. Women consented verbally to allowing interview information and birth outcome data to be recorded in the Birth Registry. The study was approved by the local and national ethics committee in Tanzania. We used publicly accessible vital statistics files to compile data for singleton black USA live births, stillbirths, and early neonatal deaths during 1995–2000.
There were 16 762 singleton births registered at the Tanzanian hospital during 1999–2006. We excluded a total of 136 births (1%) whose birthweight was either missing or below 500g. To minimize referral bias for Tanzanian births, we further excluded 2 182 babies (13%) born to mothers referred for medical reasons from outside Moshi Urban district.
The USA data consisted of 3 565 515 black singleton births born during 1995–2000. We excluded a total of 1% of births, including 662 births from parents with foreign residency, 6 716 births whose birthweight was unknown, 2 586 births whose birthweight was imputed, and 25 216 births with birthweight below 500g. The final analysis samples consisted of 14 444 Tanzanian and 3 530 335 US Black singleton births (including stillbirths).
Maternal sociodemographic characteristics collected included age, parity, marital status, level of education, and tobacco use during pregnancy. Level of education was categorized in terms of years of schooling, as “primary or lower” (up to seven years for Tanzania and up to eight years for the USA), “secondary” (8 to 12 years), and “post-secondary” (>12 years). Records also included baby’s sex, weight, gestational age, vital status at birth, and death during initial hospitalization. Birthweghts were grouped into 200g categories. Gestational age was calculated from the last menstrual period (LMP) and was recorded in number of completed weeks. Preterm birth was defined as delivery <37 completed weeks of gestation, and severe preterm birth as delivery <32 completed weeks. Stillbirth was defined as fetal death of a baby weighing at least 500g and early neonatal death as death of a live born baby occurring within seven days after birth.
The stillbirth rate (SBR) was computed as the proportion of stillbirths of all live and stillbirths (expressed as per 1000 births). Early neonatal mortality rate (ENMR) was calculated as the proportion of deaths to live births during the first week of life (expressed as per 1000 live births). Perinatal mortality rate (PNMR) was calculated as the proportion of stillbirths and early neonatal deaths among all live births and stillbirths (expressed as per 1000 births). Relative risk (RR) for perinatal mortality within levels of maternal sociodemographic characteristics and corresponding 95% confidence intervals (CI) were also computed.
The frequency distribution of birthweight is in essence normal (Gaussian), with an extended lower tail. For each country, the birthweight distribution was partitioned into its predominant distribution (essentially Gaussian and approximates the frequency distribution of term births), and the residual distribution, consisting of small births that fell outside the Gaussian distribution (and estimating the proportion of small preterm births) (7, 9). A web-based program (10) was used to estimate these parameters for each country.
Fetal growth restriction at the population level was expressed as an overall downward shift of birthweights at term (7). We assessed the contribution of smaller birth weights to perinatal mortality in Tanzania by applying the weight-specific mortality rates of USA black babies to Tanzanian babies.
Results
Perinatal mortality was 41.1 per 1000 births in the Tanzanian registry and 10.3 among blacks in the United States (RR = 4.0; 95% CI 3.7–4.3). Stillbirths constituted 70% of perinatal deaths in Tanzania and 67% in the USA. Two-thirds of Tanzanian mothers had no more than primary level education, compared with 3% of mothers in the USA (Table 1). The USA had a higher proportion of teenage births than Tanzania (15% vs. 5%) and a much higher proportion of unmarried mothers (69% vs. 10%).
Table I.
Maternal characteristics and mortality among Tanzanian (1999–2006) and Black US Babies (1995–2000)
| Tanzania (n=14 444) | United States* (n=3 530 335) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | % Babies | PNMR | RR (PNMR) (95% CI) |
SBR | ENMR | % Births | PNMR | RR (PNMR) (95% CI ) |
SBR | ENMR |
| Overall | 100.0 | 41.1 | 28.8 | 12.6 | 100.0 | 10.3 | 6.9 | 3.4 | ||
| Education level | ||||||||||
| Primary or lower | 65.6 | 44.2 | 1.33 (1.11, 1.62) | 32.1 | 12.5 | 2.9 | 10.6 | 1.34 (1.26, 1.43) | 6.7 | 3.9 |
| Secondary | 5.1 | 32.7 | 0.99 (0.65, 1.52) | 24.5 | 8.4 | 63.8 | 9.6 | 1.22 (1.19, 1.25) | 6.3 | 3.3 |
| Post-secondary | 29.3 | 33.0 | 1.00 | 19.7 | 13.6 | 33.3 | 7.9 | 1.00 | 5.0 | 2.9 |
| Missing n (%) | 45 (0.3) | 72730 (2.1) | ||||||||
| maternal age (yrs) | ||||||||||
| <19 | 5.4 | 36.0 | 1.01 (0.69, 1.50) | 20.6 | 15.8 | 15.1 | 10.9 | 1.19 (1.15, 1.23) | 7.3 | 3.6 |
| 19–25 | 38.0 | 35.6 | 1.00 | 23.5 | 12.4 | 43.0 | 9.1 | 1.00 | 6.0 | 3.1 |
| 26–30 | 28.6 | 41.4 | 1.16 (0.95, 1.42) | 29.0 | 12.8 | 21.1 | 10.3 | 1.13 (1.10, 1.16) | 6.9 | 3.5 |
| 30–35 | 18.4 | 47.1 | 1.33 (1.06, 1.65) | 35.1 | 12.5 | 13.9 | 11.3 | 1.24 (1.20, 1.28) | 7.6 | 3.8 |
| 36+ | 9.6 | 53.9 | 1.52 (1.17, 1.97) | 43.0 | 11.4 | 6.9 | 14.8 | 1.62 (1.57, 1.68) | 10.5 | 4.4 |
| Missing n (%) | 29 (0.2) | - | ||||||||
| Birth Order | ||||||||||
| 1 | 35.6 | 36.6 | 1.00 | 23.8 | 13.0 | 30.8 | 9.7 | 1.00 | 6.4 | 3.3 |
| 2 | 26.7 | 38.3 | 1.05 (0.85, 1.29) | 25.8 | 12.8 | 25.9 | 8.7 | 0.89 (0.87, 0.92) | 5.6 | 3.0 |
| 3 | 17.8 | 37.2 | 1.02 (0.80, 1.30) | 23.5 | 14.0 | 18.6 | 9.6 | 0.99 (0.96, 1.02) | 6.4 | 3.2 |
| 4+ | 19.9 | 55.3 | 1.51 (1.23, 1.86) | 46.2 | 9.5 | 24.7 | 12.4 | 1.28 (1.24, 1.31) | 8.4 | 4.0 |
| Missing n (%) | 24 (0.2) | 28980 (0.8) | ||||||||
| Marital Status | ||||||||||
| Married | 90.2 | 40.1 | 1.00 | 28.2 | 12.3 | 30.6 | 7.1 | 1.00 | 4.0 | 3.1 |
| Unmarried | 9.8 | 39.7 | 0.99 (0.75, 1.30) | 26.7 | 13.3 | 69.4 | 8.9 | 1.25 (1.22, 1.29) | 5.3 | 3.6 |
| Missing n (%) | 341 (2.4) | 8616 (0.2) | ||||||||
| Baby's sex | ||||||||||
| Male | 51.5 | 40.4 | 0.99 (0.84, 1.16) | 27.2 | 13.6 | 50.8 | 11.3 | 1.21 (1.19, 1.23) | 7.4 | 4.0 |
| Female | 48.5 | 40.8 | 1.00 | 30.1 | 11.0 | 49.2 | 9.3 | 1.00 | 6.4 | 2.9 |
| Missing n (%) | 107 (0.7) | - | ||||||||
| Tobacco use during pregnancy | ||||||||||
| Yes | 0.2 | - | - | - | - | 9.7 | 13.3 | 1.45 (1.40, 1.50) | 9.1 | 4.2 |
| No | 99.8 | 40.3 | - | 28.0 | 12.7 | 90.3 | 9.2 | 1.00 | 5.9 | 3.3 |
| Missing n (%) | 63 (0.4) | 345882 (9.8) | ||||||||
Source: US National Center for Health Statistics (1995–2000)
Table 1 presents perinatal mortality rates by maternal social characteristics, smoking during pregnancy, and infant characteristics. In both countries, women with low maternal education (primary or lower) had about 30% higher perinatal mortality than women with post-secondary education. Maternal age was a predictor of mortality in both countries. Both countries showed a similar gradient of risk starting with mothers ages 26–30 and rising with older ages. Only in the USA was risk elevated among younger mothers (<19 years).
In the USA, unmarried mothers had higher perinatal mortality, while the rates were about the same for married and unmarried women in Tanzania. Tanzanian boys had a slightly lower perinatal mortality than girls, in contrast to the excess male mortality seen in the USA. Tobacco use during pregnancy was associated with a 45% increase in mortality in USA births. In Tanzania, too few mothers reported smoking during pregnancy (0.2%) to estimate their risk.
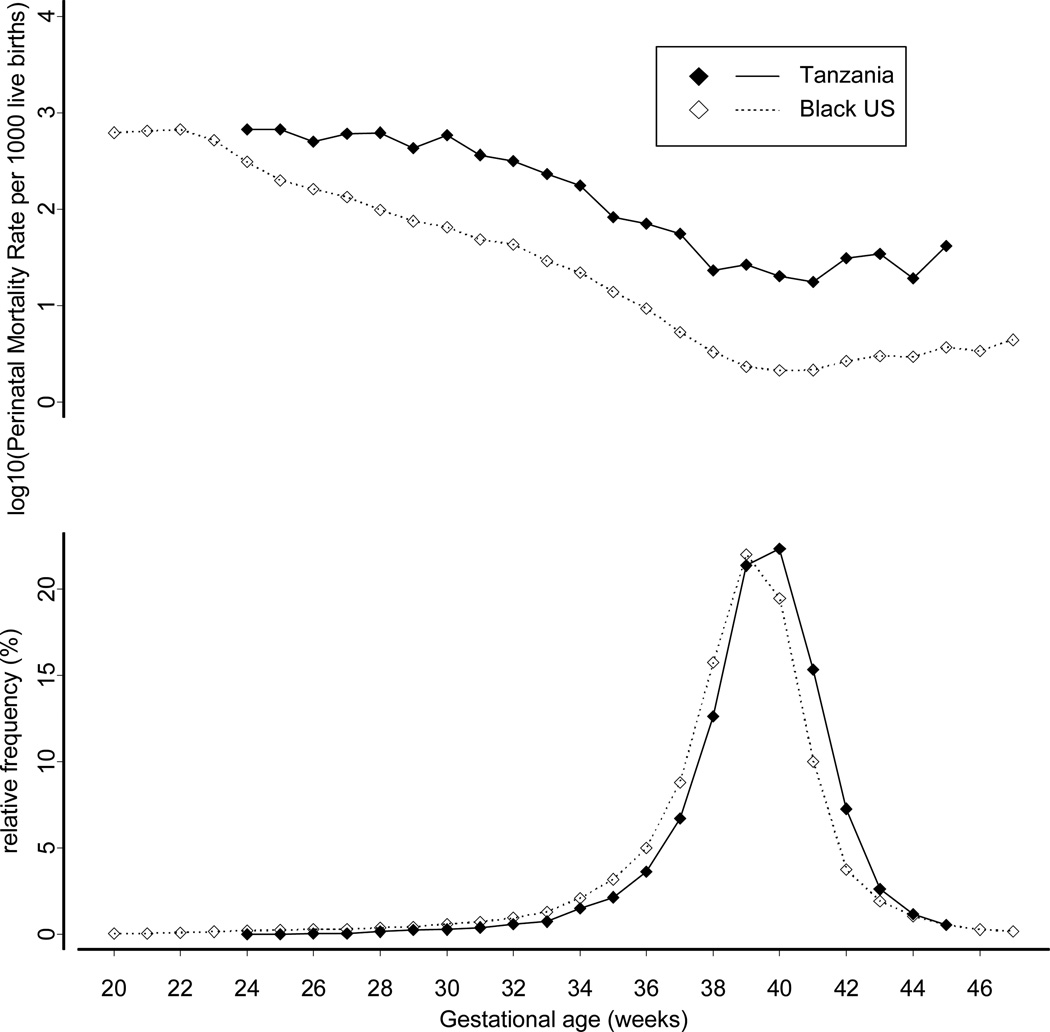
Table 2 shows the rates of preterm delivery in Tanzania and the United States. Black women in the USA had a higher rate of preterm births, with 2.6 times as many early preterm births (<32 weeks) as in Tanzania. The associations of preterm risk with known risk factors were similar in the two countries. In both Tanzania and the USA, the risk of preterm was highest among young mothers, the unmarried, those with highest parity, and those with low education. We then plotted the complete distribution of gestational age at birth in Tanzania and the US (Figure 1). The distribution of gestational age was shifted toward later deliveries (about one week) in Tanzania.
Table II.
Maternal Characteristics and preterm birth among Tanzanian (1999–2006) and Black US Babies (1995–2000)
| Tanzania (n=14 444) | United States* (n=3 530 335) | |||||
|---|---|---|---|---|---|---|
| Characteristic | % Babies | % preterm |
% severe preterm |
% Babies | % preterm |
% severe preterm |
| Overall | 100 | 10.0 | 1.4 | 100 | 16.2 | 3.6 |
| Education level | ||||||
| Primary or lower | 65.6 | 11.0 | 1.5 | 2.9 | 19.5 | 4.6 |
| Secondary | 5.1 | 10.2 | 1.5 | 63.8 | 17.0 | 3.7 |
| Post-secondary | 29.3 | 7.3 | 0.9 | 33.3 | 13.9 | 3.1 |
| Missing n (%) | 45 (0.3) | 72730 (2.1) | ||||
| maternal age (years) | ||||||
| <19 | 5.4 | 14.8 | 1.8 | 15.1 | 18.5 | 4.3 |
| 19–25 | 38.0 | 9.6 | 1.0 | 43.0 | 15.1 | 3.2 |
| 26–30 | 28.6 | 9.1 | 1.3 | 21.1 | 15.2 | 3.4 |
| 30–35 | 18.4 | 10.2 | 1.8 | 13.9 | 16.9 | 4.0 |
| 36+ | 9.6 | 10.4 | 1.1 | 6.9 | 19.8 | 4.7 |
| Missing n (%) | 29 (0.2) | - | ||||
| Birth Order | ||||||
| 1st | 35.6 | 8.8 | 0.9 | 30.8 | 15.2 | 3.5 |
| 2nd | 26.7 | 9.2 | 1.2 | 25.9 | 14.9 | 3.2 |
| 3rd | 17.8 | 9.8 | 1.3 | 18.6 | 15.8 | 3.3 |
| 4th or higher | 19.9 | 12.8 | 2.1 | 24.7 | 18.9 | 4.4 |
| Missing n (%) | 24 (0.2) | 28980 (0.8) | ||||
| Marital Status | ||||||
| Married | 90.2 | 9.5 | 1.2 | 30.6 | 13.6 | 2.8 |
| Unmarried | 9.8 | 12.0 | 1.8 | 69.4 | 17.2 | 3.9 |
| Missing n (%) | 107 (0.7) | - | ||||
| Baby's sex | ||||||
| Male | 51.5 | 10.0 | 1.2 | 50.8 | 16.4 | 3.7 |
| Female | 48.5 | 9.7 | 1.4 | 49.2 | 15.9 | 3.5 |
| Missing n (%) | 341 (2.4) | - | ||||
| Tobacco use during pregnancy | ||||||
| Yes | 0.2 | 4.6 | 0.0 | 9.7 | 21.9 | 5.3 |
| No | 99.8 | 9.9 | 1.3 | 90.3 | 15.7 | 3.4 |
| Missing n (%) | 63 (0.4) | 345882 (9.8) | ||||
Source: US National Center for Health Statistics (1995–2000)
Figure 1.
Gestational Age at birth and Gestational age-specific Mortality Distributions for Tanzanian (1999–2006) and Black US (1995–2000) babies
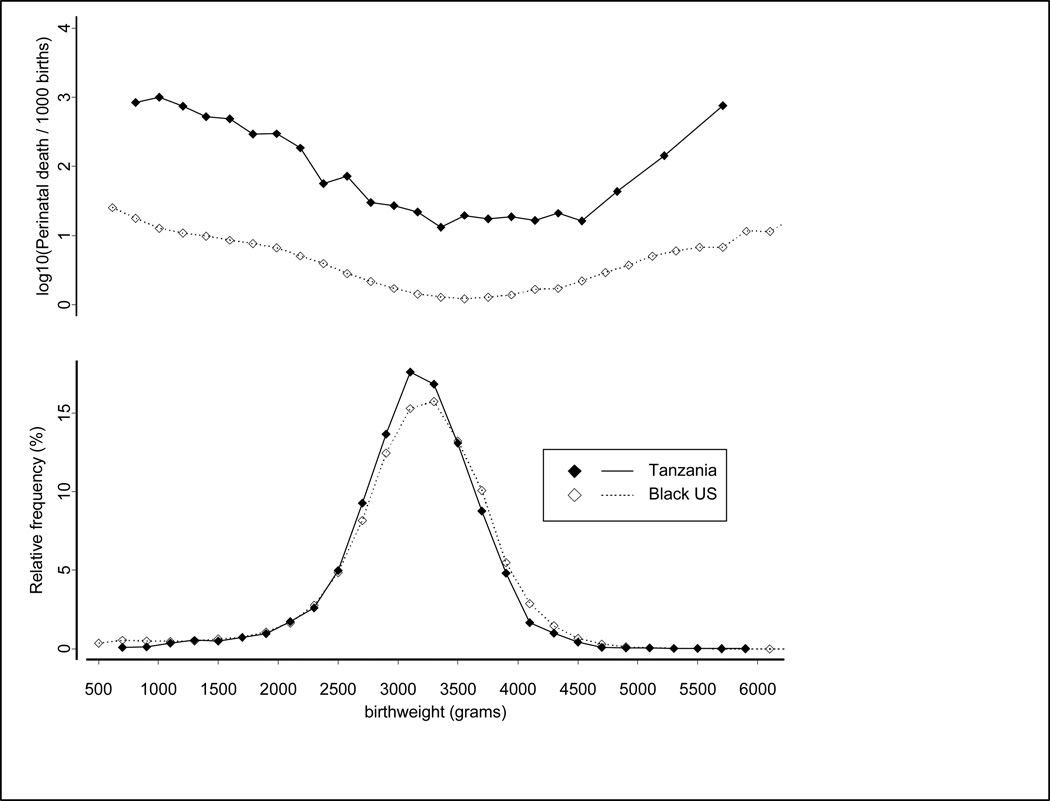
Despite their longer gestational age, Tanzanian babies were slightly smaller on average (Figure 2). The mean birthweight in the predominant (Gaussian) distribution of birthweights was 3190g for the Tanzanian babies (SD 453g) and 3230g for the USA (SD 492g). Even though Tanzanian babies were smaller, fewer Tanzanian births had the lowest birthweights. The proportion of small births falling outside the Gaussian distribution (residual) was 3.7% in Tanzania and 4.9% in the United States.
Figure 2.
Birth weight and Birth weight-specific Mortality Distributions for Tanzanian (1999–2006) and Black US (1995–2000) babies.
Applying the USA birthweight-specific mortality rates to Tanzanian births produced a standardized mortality rate of 5.9/1000 for Tanzanian babies compared with the observed mortality rate of 10.3/1000 for USA black babies. This advantage for Tanzanian babies after standardization was unexpected. We conjectured that the advantage was due to the smaller proportion of small and preterm babies in the Tanzanian sample. We confirmed this by excluding all preterm babies in both samples, and repeating the standardization procedure. Applying the USA weight-specific mortality rates to both populations, the standardized mortality rate in Tanzania term births was 3.1/1000 births compared with 2.8/1000 in the US.
Discussion
Babies in a hospital-based registry in Tanzania had four times high rates of perinatal mortality compared to black babies in the USA. However, we found no evidence that this excess risk was due to differences in either birthweight or preterm delivery. Rates of preterm delivery were actually lower than for USA black women. Birthweights in Tanzania were slightly smaller, but standardization showed that this difference in birthweight made virtually no contribution to the higher mortality rates in Tanzania.
Previous studies have compared babies of African mothers who immigrated with natives of the country to which they immigrated (11–14). In Europe African mothers deliver heavier babies than native-born Europeans, however, USA babies of immigrant African mothers were lighter than white Americans but heavier than black Americans. Immigrants are often a selected group, possibly healthier and more well-to-do than those who remain behind. After some period of time, immigrants may be less likely to experience birth outcomes that reflect the conditions prevailing in their home country. There is a well-recognized heterogeneity among both Africans and black Americans (15, 16), and we considered USA black women being the best available comparison group because they represent a population of African origin within the health care system of a developed country.
The Tanzanian registry is based on high-quality data from over 14 000 singleton babies born in a hospital. The comparison is a population-based sample of USA black babies using vital statistics data. Despite differences in study populations and methods of data collection, there were strong parallels in risk factors for perinatal mortality (Table 1). However, single mothers in Tanzania showed no evidence of the increased risk seen in the USA. Also, the excess risk for female babies in Tanzania is not consistent with developed countries, where higher risk is among males.
Registries based at a medical center are less complete than population-based registries. Population-based birth data are, unfortunately, extremely difficult to collect in the developing world. We considered the following limitations of our registry data. Some deaths during the first week were presumably unrecorded among babies discharged before one week, causing perinatal mortality to be somewhat underestimated. Referral bias can occur with hospital-based registries, but we removed all women whose babies were medically referred, who were also living outside the hospital catchment area. The rate of preterm delivery was unexpectedly low in this poor African setting, perhaps because gestational age in Tanzania is based entirely on LMP and often uncertain (17). In the USA, 9% of Black babies had gestational age imputed by other methods including ultrasound (18), however, excluding US births with imputed gestation age had only trivial effects on the results (data not shown). Around 30% of deliveries occur at home in the Kilimanjaro region (19) and preterm babies might be selectively missed, however, local midwives and obstetricians didn’t think women with preterm labor would be less likely to deliver in hospital. We further restricted the analysis to the 6 368 mothers living close to the hospital and found no higher rate of preterm in this group. Also restricting the analysis to the youngest women with the lowest education, preterm was still less in the Tanzanian sample than in the US (14% vs. 22%). A recent article by Fawzi et al. reported a much higher preterm rate (17%) in a cohort of Tanzanian women living in Dar es Salaam (20) .That cohort included twins and other multiple deliveries, which could explain a part of the difference. In summary, while we cannot find a reason for bias in our ascertainment of babies born preterm, we cannot exclude the possibility of bias.
The observed excess of preterm babies among USA blacks compared with our Tanzanian sample might reflect a real difference. We considered the possibility that caesarean section or induction of labor may be more common in the USA, but this does not seem to be so. The proportion of medically-indicated preterm birth was 5.6% among USA blacks (21) and 5.1% in the Tanzanian sample. Maternal smoking during pregnancy was far higher among black Americans than among the Tanzanians. A low rate of maternal smoking in Tanzania has been confirmed by others (19). There is considerable concern in the US over the high rate of preterm among Blacks (26). Unmeasured risk factors unique to US Blacks might include stress and related conditions that accompany life as a racial minority.
Perinatal mortality was very high in the Tanzanian sample, despite the fact that mothers received hospital-based obstetric care. Conditions contributing to perinatal mortality in Africa include infectious diseases, poor access to routine health care, and social, nutritional and material deprivation. In the Tanzania sample, the prevalence of maternal HIV-infection was 7.4%, and 20% of mothers reported malaria infection during pregnancy; however, perinatal mortality among offspring was similar for mothers with and without malaria. The prevalence in Tanzania of gynecological disease, pre-enclampsia and bleeding during pregnancy were 0.4, 3.7 and 1.3 percent respectively, similar to USA blacks (18).
Regardless of what selection might have affected entry into the Tanzanian registry, it is clear that the high rate of perinatal mortality was not due to excesses in either of babies born preterm or born with fetal growth restriction, compared with USA blacks. The rate of preterm delivery was relatively low in the Tanzanian sample, and confirmed in the analysis of the residual distribution of birth weights. We standardized for birthweight in order to take into full account the differences in birthweight distributions. Standardizing has little meaning if birthweight is not causally related to mortality (7). Still, many regard intrauterine growth restriction as a major contributor to the high mortality rates of developing countries (8). After standardization by birth weights we could not find any evidence that fetal growth restriction might contribute to the excess mortality in the Tanzanian sample.
In conclusion, the factors responsible for the excess perinatal mortality in our Tanzanian sample apparently work through pathways other than impaired fetal growth or preterm delivery. Rather than promoting fetal growth, public health interventions should focus on the clinical and social changes that can directly reduce perinatal mortality.
Acknowledgements
Funding for this work was provided by the Norwegian Council for Higher Education’s Program for Development Research (NUFU) with funds allocated to the Centre for International Health at the University of Bergen under the Health Systems Research and Health Promotion in relation to Reproductive Health in Tanzania project of which the Medical Birth Registry at Kilimanjaro Christian Medical Center (KCMC) is one element.
The authors thank mothers who agreed to participate in the birth registry study and the key personnel at KCMC who made a great contribution to the study. The authors also thank the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences who also partially supported this research.
Abbreviations
- KCMC
Kilimanjaro Christian Medical Centre
- USA
United States of America
- LMP
Last Menstrual Period
- SBR
Stillbirth Rate
- ENMR
Early Neonatal Mortality Rate
- PNMR
Perinatal Mortality Rate
- RR
Relative Risk
- SD
Standard Deviation
- CI
Confidence Interval
Footnotes
Competing interests: None declared.
References
- 1.The World Health Report. Make every mother and child count. Geneva: World Health Organization; 2005. pp. 1–243. [Google Scholar]
- 2.Neonatal and Perinatal Mortality. Country, Regional and Global Estimates. Geneva: World Health Organization; 2006. [Google Scholar]
- 3.Kilonzo A, Kouletio M, Whitehead SJ, Curtis KM, McCarthy BJ. Improving surveillance for maternal and perinatal health in 2 districts of rural Tanzania. Am J Public Health. 2001;91:1636–1640. doi: 10.2105/ajph.91.10.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinderaker SG, Olsen BE, Bergsjo PB, Gasheka P, Lie RT, Havnen J, et al. Avoidable stillbirths and neonatal deaths in rural Tanzania. Bjog. 2003;110:616–623. [PubMed] [Google Scholar]
- 5.Buekens P, Wilcox AJ, Kiely J, Masuy-Stroobant G. Birthweight, preterm births and neonatal mortality in Belgium and the United States. Paediatr Perinat Epidemiol. 1995;9:273–280. doi: 10.1111/j.1365-3016.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox A, Skjaerven R, Buekens P, Kiely J. Birth weight and perinatal mortality. A comparison of the United States and Norway. Jama. 1995;273:709–711. [PubMed] [Google Scholar]
- 7.Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001;30:1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 8.de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998;52(Suppl 1):S5–S15. [PubMed] [Google Scholar]
- 9.Wilcox AJ, Russell IT. Birthweight and perinatal mortality: I. On the frequency distribution of birthweight. Int J Epidemiol. 1983;12:314–318. doi: 10.1093/ije/12.3.314. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox A. Analysis of Birth weight and Infant mortality: Analysis program. 2000 [Google Scholar]
- 11.Vangen S, Stoltenberg C, Skjaerven R, Magnus P, Harris JR, Stray-Pedersen B. The heavier the better? Birthweight and perinatal mortality in different ethnic groups. Int J Epidemiol. 2002;31:654–660. doi: 10.1093/ije/31.3.654. [DOI] [PubMed] [Google Scholar]
- 12.Buekens P, Masuy-Stroobant G, Delvaux T. High birthweights among infants of north African immigrants in Belgium. Am J Public Health. 1998;88:808–811. doi: 10.2105/ajph.88.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding S, Santana P, Cruickshank JK, Boroujerdi M. Birth weights of black African babies of migrant and nonmigrant mothers compared with those of babies of European mothers in Portugal. Ann Epidemiol. 2006;16:572–579. doi: 10.1016/j.annepidem.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.David RJ, Collins JW., Jr Differing birth weight among infants of U.S.-born blacks, African-born blacks, and U.S.-born whites. N Engl J Med. 1997;337:1209–1214. doi: 10.1056/NEJM199710233371706. [DOI] [PubMed] [Google Scholar]
- 15.Tishkoff S, Williams S. Genetic analysis of African populations: Human evolution and complex disease. Genetics. 2002;3:611–621. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- 16.Howard DL, Marshall SS, Kaufman JS, Savitz DA. Variations in low birth weight and preterm delivery among blacks in relation to ancestry and nativity: New York City, 1998–2002. Pediatrics. 2006;118:e1399–e1405. doi: 10.1542/peds.2006-0665. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, McLean FH, Boyd ME, Usher RH. The validity of gestational age estimation by menstrual dating in term, preterm and postterm gestations. JAMA. 1988;260:3306–3308. [PubMed] [Google Scholar]
- 18.Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM. Births: final data for 2000. Natl Vital Stat Rep. 2002;50:1–101. [PubMed] [Google Scholar]
- 19.DHS Final Report Tanzania. Maternal and Child Health: National Bureau of Statistics Tanzania. 2004. pp. 131–162. [Google Scholar]
- 20.Fawzi WW, Msamanga GI, Urassa W, Hertzmark E, Petraro P, Willett WC, et al. Vitamins and perinatal outcomes among HIV-negative women in Tanzania. N Engl J Med. 2007;356:1423–1431. doi: 10.1056/NEJMoa064868. [DOI] [PubMed] [Google Scholar]
- 21.Ananth CV, Joseph KS, Oyelese Y, Demissie K, Vintzileos AM. Trends in preterm birth and perinatal mortality among singletons: United States, 1989 through 2000. Obstet Gynecol. 2005;105:1084–1091. doi: 10.1097/01.AOG.0000158124.96300.c7. [DOI] [PubMed] [Google Scholar]
- 22.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 23.Ananth CV, Savitz DA, Luther ER. Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol. 1996;144:881–889. doi: 10.1093/oxfordjournals.aje.a009022. [DOI] [PubMed] [Google Scholar]
- 24.Higgins S. Smoking in pregnancy. Curr Opin Obstet Gynecol. 2002;14:145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen JT, Thulstrup AM, Larsen H, Moller M, Sorensen HT. Smoking, sex of the offspring, and risk of placental abruption, placenta previa, and preeclampsia: a population-based cohort study. Acta Obstet Gynecol Scand. 2001;80:894–898. doi: 10.1034/j.1600-0412.2001.801005.x. [DOI] [PubMed] [Google Scholar]
- 26.Hogue CJ, Hargraves MA. Preterm birth in the African-American community. Semin Perinatol. 1995;19:255–262. doi: 10.1016/s0146-0005(05)80039-4. [DOI] [PubMed] [Google Scholar]
- 27.Habib NA, Daltveit AK, Bergsjo P, Shao J, Oneko O, Lie RT. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. BJOG. 2008;115:616–624. doi: 10.1111/j.1471-0528.2008.01672.x. [DOI] [PubMed] [Google Scholar]