Abstract
Objective
Despite the extended overnight fast, paradoxically, people are typically not ravenous in the morning and breakfast is typically the smallest meal of the day. Here we assessed whether this paradox could be explained by an endogenous circadian influence on appetite with a morning trough, while controlling for sleep/wake and fasting/feeding effects.
Design and Methods
We studied 12 healthy non-obese adults (6 male; age, 20–42 year) throughout a 13-day laboratory protocol that balanced all behaviors, including eucaloric meals and sleep periods, evenly across the endogenous circadian cycle. Participants rated their appetite and food preferences by visual analog scales.
Results
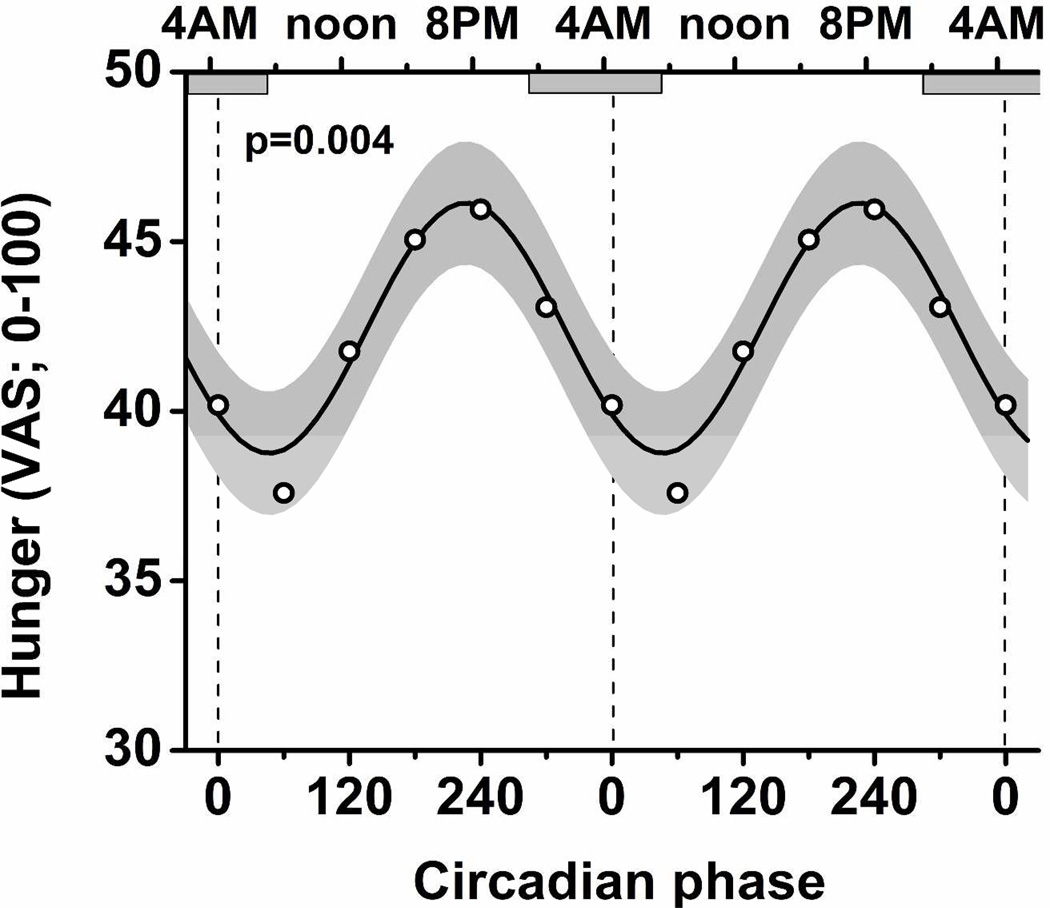
There was a large endogenous circadian rhythm in hunger, with the trough in the biological morning (8 AM) and peak in the biological evening (8 PM; peak-to-trough amplitude=17%; P=0.004). Similarly phased significant endogenous circadian rhythms were present in appetite for sweets, salty and starchy foods, fruits, meats/poultry, food overall, and for estimates of how much food participants could eat (amplitudes 14–25%; all P < 0.05).
Conclusions
In people who sleep at night, the intrinsic circadian evening peak in appetite may promote larger meals before the fasting period necessitated by sleep. Furthermore, the circadian decline in hunger across the night would theoretically counteract the fasting-induced hunger increase that could otherwise disrupt sleep.
Keywords: Appetite, Circadian, Energy Balance, Hunger, Metabolism
Introduction
In addition to what we eat, there is evidence of the importance of when we eat in the development of obesity (1, 2, 18). Food intake does not appear to be simply regulated by a homeostatic energy balance mechanism. Otherwise, appetite would be greatest in the morning following the extended overnight fast, whereas humans typically are not hungriest in the morning, breakfast is usually the smallest meal of the day—accounting for only 16–18% of the daily calories, and many people skip breakfast altogether (3). Indeed, in Western cultures, dinner is the largest meal of the day (35–36%) (3). This paradox could be explained by: (a) a large meal in the evening and decreased energy expenditure during sleep compensating for the prolonged overnight fast; (b) sleep itself inhibiting appetite, with this inhibition carrying over into the morning wakefulness period; and/or (c) an endogenous circadian influence on appetite that suppresses hunger during the morning. To examine this third possibility that the internal circadian clock regulates hunger, we assessed the sensation of hunger and appetite in healthy participants throughout a 13-day in-laboratory protocol that controlled for the timing of meals and sleep.
Methods and Procedures
Aspects of this study testing different hypotheses have been published (4–7). Twelve healthy adults (6 male; age, 20–42 y; BMI, 19.9–29.6 kg/m2) completed a 13-day in-laboratory protocol. All subjects gave written informed consent and the studies were approved by Partners Human Research Committee. Participants maintained a fixed sleep/wake schedule with 8 h in bed for 2–3 weeks at home then adapted to the laboratory environment for two days and two nights while maintaining the same 8-hour sleep schedule. Thereafter, all behavioral and environmental factors that can potentially affect appetite or the circadian cycle (e.g., meals, sleep, activity, posture, room temperature, light) were controlled while hunger and appetite were measured across the circadian cycle. This was achieved by having participants complete a 240-hour ‘forced desynchrony’ (FD) protocol in which the daily behaviors, sleep and meals occurred across all circadian phases (12 recurring 20-hour behavioral cycles, maintaining a 2:1 wake/sleep ratio), with constant dim light (<4 lux) to avoid resetting the phase of the circadian system. During wake periods, subjects used computerized, 100-mm visual analog scales (VAS; with “not at all” and “very much”/”extremely”, defining the extremes) to rate hunger, appetite and food preferences at 5 fixed times within each of wake periods (totaling 60 times across the FD protocol for each subject): 1 hour 10 minute, 6 hour 30 minute, 8 hour 30minute, 10 hour 30 minute, and 12 hour 20 minute after each scheduled waketime. Meals were eucaloric (based on a Harris Benedict formula with an activity factor of 1.4 and subjects were required to consume all their food). Meals were and given 1 hour 25 minute [breakfast], 6 hour 45 minute [lunch], 10 hour 45 minute [dinner] and 12 hour 10 minute [snack] after each scheduled waketime in the FD protocol. Macronutrient distribution for each 20-h day was 60% carbohydrate, 24% fat and 16% protein. The actual group-average total energy intake was 2,071 kcal per 20-hour, with 29% for breakfast, 29% for lunch, 31% for dinner and 11% for snack. Subjects were instructed to rate their hunger and appetite measures at that moment and to respond without concern for calories, fat or a 'healthy diet'. One subject was excluded from analysis due to incorrect use of the VAS. Core body temperature (CBT) was measured continuously throughout the protocol via rectal thermistors and used to assign circadian phases of all measurements (0–359°) according to the time from the fitted CBT minimum (0°) and the individual’s circadian period (4). Circadian effects were assessed by using mixed model cosinor analyses including the fundamental component of the circadian cycle (~24 hour) and the time since scheduled awakening as fixed factors, and the time since the start of the FD protocol as covariate to correct for any linear changes across the FD. Statistical significance was set at P<0.05.
Results
There was a significant endogenous circadian rhythm in hunger, with a trough at 50° (equivalent to 7:50 AM in these subjects) and a peak at 230° (7:50 PM; P=0.004; 17% peak-to-trough difference as percentage of mean; Figure 1). This effect was independent of the duration of time since scheduled awaking, and therefore independent of the duration of time since each meal (i.e., no significant interaction between circadian phase and time since wake P=0.34). Similarly-phased significant endogenous circadian rhythms were present in appetite for sweet, salty and starchy foods, fruits, meats/poultry, food overall, and for estimates of how much food participants could eat (peak-to-trough differences 14–25% as percentages of means; troughs ranged from 40°–60° [7:10–8:30 AM); P always <0.05: Supplementary Figure 1). The self-rating of nausea showed the strongest circadian rhythm with a peak at 20° (5:50 AM) and a trough at 200° (5:50 PM; P<0.0001; 105% peak-to-trough difference as percentage of mean; Supplementary Figure 1). Of interest, the desire to eat vegetables or consume dairy products, and the sensation of fullness had no significant circadian rhythms (not shown). The endogenous circadian rhythms in hunger and appetite were not due to changes in caloric intake; for actual consumed calories (Supplemental Figure 2), there was no significant circadian rhythm (P=0.98) and no significant interaction between circadian phase with the meals (breakfast, lunch, dinner, snack; P=0.52).
Figure 1. Endogenous circadian rhythm in hunger.
Cosinor model is shown as mean (black line) ± 95% confidence interval (gray area). In addition, averaged data are shown grouped into six 60°(~4 h) bins (open circles). The model and data points are ‘double-plotted’ to better portray circadian rhythmicity. Bottom X-axis, circadian phase; top X-axis, corresponding clocktime; horizontal gray boxes, average habitual scheduled sleep episode; P-value, statistical significance of a circadian rhythm in hunger.
Discussion
Our study has uncovered a large endogenous circadian rhythm in hunger, independent of time since waking up and time since prior meals, and independent of calories consumed. The circadian peak in hunger occurred in the biological evening, corresponding to ~8 PM, and the circadian trough in hunger occurred at ~8 AM. These observations may help explain the commonly observed daily rhythm in food intake in the Western world, with paradoxically limited intake after an overnight fast and the largest meal in the evening (3). The similar rhythms in appetite for sweet, salty, and starchy foods, meats/poultry and fruits supports this central observation. The lack of significant circadian rhythms in the desire to eat vegetables suggests that the circadian system particularly regulates the desire for high-energy foods.
Limitations of the study included the absence of hormonal markers of hunger and appetite, and the relatively small sample size. In addition, we studied healthy subjects and it is possible that individuals with obesity, diabetes, or shift workers may have different circadian rhythms.
The regulation of hunger shows apparent homology with the regulation of sleep as described by a two-process model in which the maximal circadian sleep drive at the end of the night compensates for the decreasing homeostatic sleep drive, enabling a consolidated 8-hour sleep episode (8). In a similar way, the circadian minimum hunger drive at the end of the night may partially compensate for the homeostatic hunger drive increasing across the overnight fast. Whether the circadian trough in hunger concurrent with the end of the typical overnight fast also enables consolidated sleep warrants further investigation. The circadian rhythm in nausea peaking at 6 AM, towards the end of the usual sleep episode in these subjects, may be related to the increased prevalence of gastrointestinal problems reported by shift workers who remain awake at that time and who are subjected to misalignment between the circadian system and the fasting/feeding cycle (9).
Neural and hormonal mechanisms are likely involved in driving the circadian rhythm in hunger and appetite. The central circadian pacemaker located in the suprachiasmatic nucleus influences hypothalamic nuclei important in weight regulation such as the arcuate nucleus, dorsomedial nucleus of the hypothalamus and lateral hypothalamus and possibly relevant endocrine factors such as leptin, ghrelin, glucagon-like-peptide (GLP)-1, cholecystokinin (CCK), peptide YY (PYY), and insulin (9). It is currently unknown whether there is a true endogenous circadian rhythm in ghrelin independent of behaviors including sleep and feeding, but there are circadian oscillators in the stomach, and ghrelin continues to oscillate under fasted conditions in mice and humans (10, 11), with the trough in humans at ~8 AM, potentially related to the endogenous circadian trough in hunger revealed in our study. There is currently no evidence for or against endogenous circadian rhythms in GLP-1, CCK or PYY, and no evidence for a circadian rhythms, independent of the sleep/wake and fasting/feeding cycle, in insulin or average leptin under FD conditions (12).
There are additional potential factors that may help explain a relatively reduced hunger in the morning despite an overnight fast. Although such factors were fully controlled in our protocol, in normal life they could summate with the endogenous circadian effect we uncovered in the laboratory. First, sleep itself may inhibit appetite with such inhibition carrying over into the morning wakefulness period, e.g., via sleep induced release of leptin, an anorexigenic signal (13), although sleep also stimulates orexigenic ghrelin (14). In night eating syndrome (NES), characterized by evening and night hyperphagia, morning anorexia, and insomnia, the nocturnal rise in leptin is blunted and may cause increased hunger and food intake at night (15). Whether effects of the endogenous circadian system and/or of sleep on hunger are changed in NES is currently unknown. Second, a traditionally large meal in the evening and decreased energy expenditure during sleep could more than compensate for the prolonged overnight fast preventing a relative negative energy balance in the morning. However, the low leptin levels shortly after awakening in the morning could signal a negative energy balance and thus does not support this hypothesis (16).
The time of meals has changed considerably across history, and differs by culture, work/school/family schedules, and individual preference (17). For example in Mediterranean and Latin American countries, lunch—often followed by a siesta, well-suited for the hot afternoon—is typically the largest meal of the day. While this would seem very different from the Western pattern with the dinner as largest meal, Mediterranean meals are typically eaten at a later time of day, e.g., with lunches occurring around 3 PM, instead of noon, and dinners around 9 or 10 PM (18). Thus, despite the different time periods between meals, the majority of the caloric intake still occurs at the end of the wake episode. Whether habitual earlier meal times result in an advanced circadian rhythm in hunger and appetite deserves further study.
The endogenous circadian rhythm in hunger that peaks in the biological evening may have evolutionary advantage in times of food shortage as eating the largest meals in the evening may aid energy storage overnight during sleep. However, the tendency to eat the largest meals in the evening when high energy foods are plentiful could contribute to the rising prevalence of obesity in western countries (1, 2, 18). Moreover, shift workers may be particularly vulnerable to this tendency as they remain awake longer during the times of highest circadian appetite, especially for sweet, salty and starchy foods.
Supplementary Material
What is already known about this subject?
-
-
Despite the extended overnight fast, paradoxically, people typically are not ravenous in the morning and breakfast is typically the smallest meal of the day.
-
-
In recent years, the circadian system has been demonstrated to be intricately related to the regulation of metabolism.
-
-
Whether the circadian system regulates hunger and appetite separate from the sleep/wake and fasting/feeding rhythm is a key unanswered question.
What does this study add?
-
-
This study demonstrates that the circadian system regulates hunger and appetite separate from the sleep/wake and fasting/feeding rhythms in humans.
-
-
The endogenous circadian troughs in hunger and appetite occurs in the morning, close to the habitual time of awakening, and may facilitate the typical overnight fast.
-
-
The endogenous circadian peaks in hunger and appetite occur in the evening, and may promote larger meals in preparation for the overnight fast.
Acknowledgement
This research was supported by NIH-R01-HL76409 and NIH-K24 HL076446 to SAS, NCRR GCRC M01 RR02635; NIH-P30-HL101299 and NIH-R01-HL094806 in support of FAJLS; National Space Biomedical Research Institute through NASA NCC 9-58 in support of CJM.
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity. 2009;17(11):2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19(7):1374–1381. doi: 10.1038/oby.2011.100. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Agriculture ARS, Beltsville Human Nutrition Research Center, Food Surveys Research Group & US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. What We Eat in America, NHANES 2009–2010. 2012 Available: www.ars.usda.gov/ba/bhnrc/fsrg.
- 4.Scheer FA, et al. Impact of the human circadian system, exercise and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107(47):20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu K, Scheer FA, Laker M, Smales C, Shea SA. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011;123:961–970. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE. 2011;6(9):e24549. doi: 10.1371/journal.pone.0024549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166(1):63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 9.Kalsbeek A, et al. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011;585(10):1412–1426. doi: 10.1016/j.febslet.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009;106(32):13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espelund U, et al. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. J Clin Endocrinol Metab. 2005;90(2):741–746. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- 12.Scheer F, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J. Clin. Endocrinol. Metab. 1998;83(6):1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 14.Dzaja A, et al. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286(6):E963–E967. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- 15.Birketvedt GS, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA. 1999;282(7):657–663. doi: 10.1001/jama.282.7.657. [DOI] [PubMed] [Google Scholar]
- 16.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100(7):1882–1887. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan S. History Magazine. Niagara Falls, NY: Edward Zapletal; 2001. What Time is Dinner? [Google Scholar]
- 18.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2012.229. [published online ahead of print Jan 29, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.