Abstract
Background
Neural correlates of stress regulation via the hypothalamic-pituitary-adrenal (HPA) axis have been identified, but little is known about how these apply to real-world interpersonal stress contexts such as mother-infant interaction. We extended stress regulation research by examining maternal neural activation to infant cry related to HPA regulation with their infants.
Methods
Twenty-two primiparous mothers listened to their own 18-month infant's cry sound, unfamiliar infant cry, and control sound during fMRI scanning. Salivary cortisol was collected at four timepoints in a separate session involving the Strange Situation stressor. Cortisol trajectories were modeled using hierarchical linear modeling, and trajectory terms were used to predict neural response to own infant cry.
Results
Mothers who showed less HPA reactivity – indexed by trajectory curvature, rather than level – showed increased activation to their infant's cry relative to control sound across limbic/paralimbic and prefrontal circuits. These included periaqueductal gray, right insula, and bilateral orbitofrontal cortex, as well as anterior cingulate-medial prefrontal cortex. Activations overlapped to some extent with previous HPA regulation findings, and converged more extensively with circuits identified in other maternal response paradigms.
Conclusions
Maternal stress regulation involves both circuits found across stressor types (i.e., prefrontal) and areas unique to the mother-infant relationship (i.e., limbic/paralimbic). The shape of mothers' HPA response trajectory was more important than the level of such response in defining stress-related neural correlates. Future research should consider dimensions of the stress context and of physiological trajectories to define stress-regulatory circuits.
Keywords: maternal response, infant cry, stress, multilevel modeling, HPA, fMRI
Neuroimaging studies have yielded important insights into brain mechanisms stimulating and regulating stress responses. However, the predominant focus on cognitive tasks performed within an fMRI scanner leaves important questions about neural response circuits relevant to managing psychosocial stress in daily life (1). A stress context with important functional implications is the mother-infant relationship; mothers able to regulate their own stress in the face of infant distress can offer a sensitive response that, in turn, shapes the infant's developing self-regulation. Although parenting research outlines what an adaptive maternal response might look like, links from specific neural mechanisms to women's stress reactivity/regulation with their infants have yet to be defined. We sought to shed light on this issue by investigating patterns of neural response to infant cry associated with first-time mothers' hypothalamic-pituitary-adrenal (HPA) axis regulation during challenging interactions with their infants.
Parental neuroimaging research suggests that an ideal response to infant distress includes both emotional response (i.e., limbic/paralimbic) and prefrontal regulatory circuits (2). In particular, basic elements of mothering behavior appear to depend on brainstem (periaqueductal gray/PAG) and thalamic activity interacting with higher-order prefrontal signals (3). At the subjective level, mothers reporting stronger parental feelings have shown greater orbitofrontal cortex (OFC) activation to their infants across a variety of stimulus types and contexts (4,5). Additionally, mothers better prepared for parenting by positive early care experiences themselves have shown increased medial prefrontal (mPFC) activation to infant stimuli (6). Together, these studies suggest that mothers best equipped to parent respond to their infants with emotional resonance tempered by adequate top-down regulation.
Mothers must regulate their own physiological stress when confronted with infant distress in order to parent effectively. Individual differences in maternal HPA regulation, often assessed through salivary cortisol, have been related to various adjustment markers including parenting quality. Specifically, mothers with higher cortisol response show more negative intrusiveness and less synchrony during infant interactions, as well as more harsh parenting practices generally (7–9). These difficulties in parenting likely reflect greater stress on the mother's part when her infant becomes distressed, as suggested by the finding that low perceived power predicted mothers' cortisol reactivity to their infant's elevated vocal pitch (10). What is lacking in this literature is a link to central (neural) processing styles that give rise to such dysregulated responses.
A recent review of cortisol release to acute stress implicated prefrontal regions including OFC, mPFC, and anterior cingulate cortex (ACC), as well as brainstem areas, in the modulation of HPA response (11). This circuitry parallels the responses seen in parents to infant distress. Yet strong conclusions about neural correlates of maternal stress regulation cannot yet be made due to several limitations of previous research. First, most studies of brain-HPA associations have been confined to non-personal stressors, and none to our knowledge have involved parenting-specific stress. Second, most characterizations of HPA function involve cortisol levels during a particular stress phase and fail to describe regulation across an entire stress task, despite growing attention to the importance of reactivity/recovery dynamics for adjustment (12,13).
The current study was designed to address these gaps by testing associations between neural response to infant distress (cry sound) and HPA response during a potentially distressing interaction with their infants in first-time mothers. Although this study sample was initially recruited to test effects of depression on maternal brain response, the recognition that mothers differed on other meaningful dimension opened the way for the current investigation of potentially overlapping, but distinct, correlates related to stress. Based on existing brain-HPA research, we hypothesized mothers' prefrontal, limbic and paralimbic activity would predict HPA regulation, as reflected in lower/less reactive cortisol response profiles with their infants. We tested these hypotheses by determining associations between brain areas responsive to infant cry and mothers' cortisol response profiles elicited by separations and reunions during the Strange Situation.
Method
Participants
Primiparous mothers of 15–18-month-old infants were recruited from the Women Infants Children (WIC) program as part of a larger longitudinal study of mothers at risk for parenting problems (13). Mothers who indicated interest by responding to a flier were contacted for further screening. Eligible mothers gave informed consent according to procedures approved by the University of Oregon Institutional Review Board. All mothers who met initial study criteria agreed to participate; however, the final sample (n=22) represents the subset of those screened into the study (n=34) eligible to complete the study; reasons for discontinuation included new pregnancy (2), failure to complete a well baby visit (4) or lack of infant cry at the visit (5), and inability to scan due to size limitations (1). Mothers were selected based solely on timing of the 18-month well baby visit and diagnostic criteria described below; they did not differ systematically from mothers not included in the fMRI study on any demographic or psychological variables (see Table S1 in the Supplement).
The majority of mothers (77%) were Caucasian (14% African American, 9% Latina). Most had experienced a vaginal delivery (18% caesarian section). Although a substantial proportion (64%) had engaged in post-high school education, only 18% completed college. Mothers tended to be young (M age=24.1, SD=4.1) and low SES (32% reporting household income <$10,000 per year, 36% $20,000–$40,000, 32% >$40,000). A minority (36%) were married. Demographic differences were not associated with stress measures or neural response.
As noted, mothers were initially recruited to represent a range of mood-related psychopathology. Half (n=11) had been diagnosed with a Major Depressive Episode (MDE) during the perinatal period (pregnancy-6 months postpartum), and the other half had no diagnosed Axis I disorders. At the time of scanning, mothers in the depressed group still exhibited symptoms at the level of minor depression, but were no longer in a MDE. Depression-related differences in neural response are reported elsewhere (14).
Stimulus Collection and Presentation
Researchers attended participants' 18-month well-baby visits and recorded infant cry sound following injections. Twenty-one seconds from the beginning of the first cry expiration were selected for the cry stimulus and maximum amplitude set to 1 dB for the recording. In addition to participant-specific stimuli, cry sound from an unfamiliar infant was collected (using the same procedures) to be presented to all participants. The unfamiliar infant cry was similar in fundamental frequency (578Hz vs. 588Hz) and number of expirations (4 vs. 4.5) to the median for study mothers' infant cries (range 501–1764Hz, 2–12 expirations). A non-cry control sound was developed by editing a rising and falling tone to have a fundamental frequency within the range of normal infant cry (400–600 Hz [15]) and 1dB maximum amplitude. Fundamental frequencies of cry sounds and mothers' subjective ratings of the sounds (15) were unrelated to HPA or neural response.
Stress Measures
Mothers' HPA response profiles were measured during a laboratory session involving the Strange Situation (SS), a commonly used stressor for mother-infant dyads that involves a series of separations and reunions (16). The SS was completed within 1 week of the scanning session, and conducted at approximately the same time in the afternoon to minimize diurnal rhythm effects. During separations, mothers could continuously see/hear their infants' distress on a remote monitor. Four saliva samples were collected via sorbette over the course of the session, timed to account for the 15–20 minute lag in peak salivary cortisol. The first sample, collected within 5 minutes of arrival, indexed mothers' HPA activity leading up to the session; the second sample, collected directly after SS conclusion, indexed stress during the early portion of the task; the third sample, collected 20 minutes after the second separation, indexed peak stress; and the final sample, collected 30 minutes after the previous, indexed post-task recovery. Thus, the 4 samples capture the mother's HPA trajectory related to the laboratory stress task as a whole. Samples were frozen and shipped to Salimetrics where they were assayed for cortisol by enzyme immunoassay. The test used 25μl of saliva, lower limit of sensitivity .007μg/dl, range .007–3.0μg/dl, average intra-/inter-assay coefficients of variation <5%/10%. All 4 sample values were used to characterize mothers' HPA response profiles, as described below.
Mothers also completed self-report measures of parenting stress (17), competence (18), and depressive symptoms (19), which were used to contextualize HPA measures.
Scanning
MR imaging was carried out with a 3T Siemens Allegra 3 magnet. A standard birdcage coil was used to acquire data from the whole brain. Sessions began with a shimming routine to optimize signal-to-noise ratio, followed by a fast localizer scan (FISP) and Siemens Autoalign routine, then the 2 functional runs and anatomical scan.
Functional
T2*-weighted gradient echo sequence, 64×64 voxel matrix, TE=30ms, TR=2000ms, flip angle=80°, 32 contiguous slices thickness=4mm; 273 volumes containing whole brain and cerebellum per run.
Structural
T1-weighted 3D MP-RAGE sequence, TI=1100ms, TR=2500ms, TE=4.4ms, 176 transverse slices 1.0mm thick, 256×176 matrix FOV=256mm.
fMRI Task Protocol
The stimulus protocol was created as a block design with 3 conditions + rest: 1) own infant cry, 2) other infant cry, 3) control sound. Sound blocks were 23s (2s pause+21s sound), and rest blocks 21s. The order of presentation of the conditions within runs was counterbalanced within and across participants. Participants were instructed to listen to the sounds to allow the most natural range of response to cry. Sound was presented via earphones in the scanner, and a sound check carried out before each scan to ensure audibility. Each run contained 6 repetitions of each condition, and participants completed two 9-minute runs.
Functional MRI analysis
All neuroimaging data analysis was conducted using tools from the fMRIB Software Library (FSLv.4.1). Preprocessing steps included motion correction with MCFLIRT, nonbrain structure removal with BET, spatial smoothing using Gaussian kernel 5-mm FWHM, intensity normalization using grand mean scaling, and high-pass temporal filtering (sigma=65s). Within-subject time series data were analyzed using FILM with local autocorrelation correction, and boxcar models describing onset/offset of each sound stimulus were convolved with a double-gamma basis function. Functional data were registered to the participant's own high-resolution structural image (6 DOF) and to a standard brain (Montreal Neurological Institute template; 12 DOF) using FLIRT. Data were screened for excessive motion (>1mm) and artefacts. Within-participant and group-level functional data analyses were carried out using FEATv.5.98.
Data Analysis
A multistep analysis was used to identify neural activations associated with mothers' stress regulation with their infants. First, mothers' HPA response profiles were modeled by fitting quadratic growth curves to cortisol values across the SS session. This model was fitted using the hierarchical linear modeling (HLM) program in a larger sample (n=100) of primiparous mothers from which the current sample was drawn (13). This model yielded estimates of latent growth characteristics for each mother: 1) an intercept (cortisol level during peak separation stress), reflecting level of HPA response; 2) a slope (instantaneous rate of change during peak stress), reflecting the tendency to continue responding or to recover following separation stress, or timing of HPA response; and 3) a quadratic term (rate of cortisol acceleration/deceleration across the stress session), reflecting the overall trend toward a sharp, peaked response vs. a flatter, less reactive profile, or shape of HPA response. This approach focuses not on sample-wide increases/decreases from entry to post-stress samples, but rather on continuous individual differences in separable aspects of a response trajectory, which have in turn been associated with meaningful differences in adjustment (13,20,21). Whereas the first characteristic – cortisol level – has commonly been used to capture differences in HPA function, an index of regulation across an acute stressor has been lacking. One aim of this study was to determine whether cortisol response timing and/or shape, above and beyond the level, would predict meaningful differences in brain activity.
Next, we tested associations between mothers' HPA response profiles during the stress session and neural responses to their infants' cry. The current paper focuses on the contrast of parameter estimates (COPE) for own cry>control sound (the more subtle own cry>other cry contrast was tested but showed no associations with HPA). For each participant, the 3 explanatory variables (EVs for own infant cry, other infant cry, and control sound) were modeled explicitly; the rest condition was modeled by inserting zero for all 3 EVs. First-level COPE images were averaged across runs using fixed-effects analyses. These served as inputs to higher-level group analyses, conducted using FLAME to model random-effects components of mixed-effects variance.
Predictor variables based on cortisol measures (centered cortisol intercept, slope, and quadratic terms derived from the HLM analysis) were entered as predictors of mothers' neural activation to own cry>control sound. Both positive and negative covariation of cortisol terms with BOLD response were tested, and results were cluster-threshold corrected (cutoff Z=2.3, familywise error rate <.05 in whole-brain analyses). This analysis identified clusters of differential neural activation to own infant cry related to mothers' cortisol profiles with their infants. As an additional quality control step, mothers' BOLD signal change associated with own infant cry (>resting baseline) was plotted against cortisol values to confirm that differences in response were attributable to the own cry stimulus (not control sound), and correlations were not driven by influential outliers.
Results
Maternal HPA response profiles
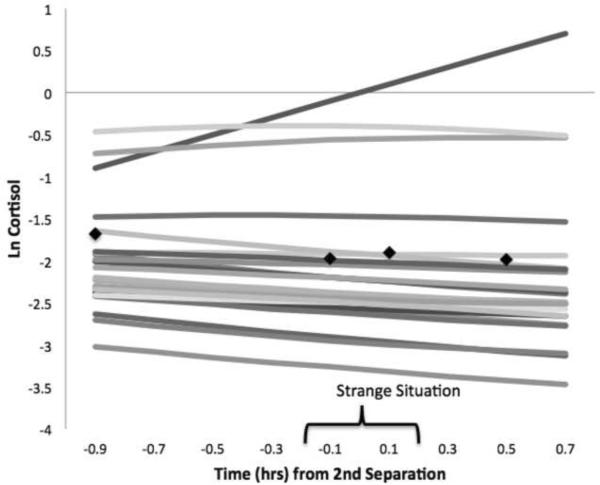
Based on HLM parameter estimates for cortisol intercept, slope, and quadratic terms, mothers as a group showed decreasing cortisol (negative slope) during the separation stress, but did not show reactivity to the SS overall (nonsignificant quadratic). At the same time, significant between-mother variability on all terms supported the investigation of individual differences in response trajectories. Correlations among terms revealed that mothers with the highest cortisol levels also tended to show ongoing response (tau correlation with slope=.73), and a more peaked overall trajectory (tau correlation with quadratic=−.86). This pattern was also reflective of the larger sample of mothers in which model fitting was performed. In other words, mothers' HPA response profiles varied from higher/more reactive to lower/nonreactive (Figure 1 displays fitted cortisol trajectories and mean observed values in the sample).
Figure 1.
Mothers' fitted cortisol trajectories during the Strange Situation session (mean observed values for each sampling point shown with black markers).
To better understand the meaning of these cortisol terms for mother-infant adjustment, correlations with both infant behaviors and mother self-report measures were tested. Infant behaviors during the SS – contact maintenance, resistance, avoidance, disorganization -- and resulting attachment classifications were unrelated to mothers' cortisol. The cortisol quadratic related positively to mothers' parenting competence (r=.57) and negatively to parenting stress (r=−.34) and current depressive symptoms (r=−.48), suggesting that increased cortisol reactivity marked poorer maternal functioning – but not necessarily a more distressed infant -- in this sample.
Neural activation related to HPA response terms
Tests of cortisol predictors revealed mothers' neural activation to their infant's cry related to both the timing and shape of their HPA response profiles (slope, quadratic), but not to the level (intercept). All significant covariation was in the positive (not inverse) direction, so that higher levels of activation to own infant cry related to a more extended, but less peaked, cortisol profile.
Although mothers as a group showed a negative cortisol slope, those with more positive slopes (indicating ongoing response) activated more strongly to their infant's cry in right anterior ventrolateral prefrontal cortex/frontal pole, as well as right superior parietal lobe and occipital pole (Table 1, upper panel).
Table 1.
Mothers' Neural Activation to Own Infant Cry > Control Sound Associated with HPA Response Parameters
| Covariate | Region | BA | L/R | X | Y | Z | Z Max | Volume (mm3) | p |
|---|---|---|---|---|---|---|---|---|---|
| 1. Cortisol - Intercept | (no activations) | ||||||||
| 2. Cortisol - Slope | Anterior vlPFC-Frontopolar Cortex | 46 | R | 48 | 50 | 6 | 4.04 | 8541 | .004 |
| Angular, Supramarginal Gyri | 39, 40 | R | 43 | −49 | 57 | 4.08 | 11405 | .0005 | |
| Occipital pole | 19 | L | −25 | −90 | 22 | 3.47 | 7765 | .007 | |
| 3. Cortisol - Quadratic | Midbrain-PAG, Cerebellum | L/R | −3 | −41 | −11 | 3.97 | 10758 | .0008 | |
| Posterior Insula to | R | 31 | −23 | 5 | 3.75 | 10242 | .001 | ||
| Striatum, Thalamus | R/L | ||||||||
| Lateral OFC-vlPFC to Anterior Insula | 11,47 | R | 40 | 44 | −15 | 4.19 | 8066 | .005 | |
| Lateral OFC to Anterior Insula | 47 | L | −30 | 13 | −17 | 3.71 | 6580 | .02 | |
| ACC | 24,32 | L/R | 0 | 27 | 20 | 3.62 | 6441 | .02 | |
| dmPFC | 9 | L/R | 8 | 57 | 25 | 3.91 | 5551 | .04 | |
| Supramarginal, Angular Gyri | 40,39 | L | −50 | −49 | 45 | 3.67 | 5571 | .04 |
Note. Clusters met thresholding criteria for whole-brain FWE < .05. Coordinates based on Montreal Neurological Institute template. BA=putative Brodmann's Area. vlPFC=ventrolateral prefrontal cortex; PAG=periaqueductal gray; OFC=orbitofrontal cortex; ACC=anterior cingulate cortex; dmPFC=dorsomedial prefrontal cortex.
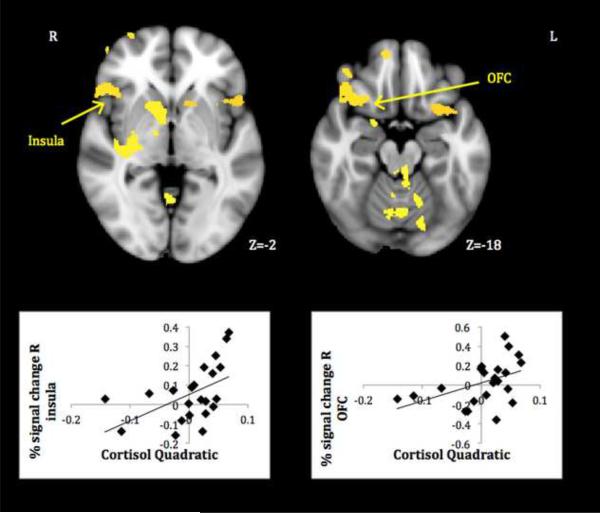
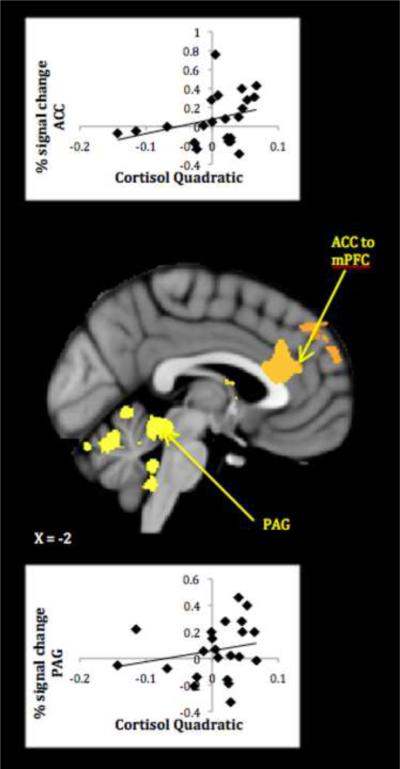
Mothers with more positive cortisol quadratic terms activated more strongly in a number of hypothesized limbic/paralimbic and medial prefrontal regions (Table 1, lower panel). As a check on the validity of cortisol quadratic-neural response associations, plots of average signal change within 3mm spheres centered on activation peaks to own infant cry>rest were created, and only those correlations undiminished by the removal of outlying data points are discussed further. These plots demonstrate that whereas less stress-reactive mothers were most likely to activate to their infant's cry, more stress-reactive mothers tended to show no change (activation or deactivation) to the cue.
Activations reliably related to cortisol quadratic terms included core limbic areas implicated in organizing and motivating maternal behavior -- PAG, thalamus and striatum – as well as areas involved in the experience and modulation of emotion -- insula and lateral OFC (see Figures 2–3). Less cortisol-reactive mothers also activated more in areas important for regulating responses to self-relevant input–ACC-mPFC. All in all, results showed that mothers who were more physiologically stressed during difficult interactions with their infants responded less, not more, to their infant's cry in key neural circuits. Controlling for mothers' concurrent depressive symptoms revealed the same pattern of activations, though somewhat reduced in size (see Table S2 in the Supplement).
Figure 2.
Mothers' neural response to own infant cry > control sound related to diminished HPA reactivity. (OFC=orbitofrontal cortex)
Figure 3.
Mothers' neural response to own infant cry > control sound related to diminished HPA reactivity. (PAG=periaqueductal gray).
Discussion
This study demonstrated links between the shape of mothers' HPA response profiles with their infants and neural activation to their infant's distress, confirming the involvement of prefrontal circuits implicated in prior stress research while also pointing to possible parenting-specific neural correlates of stress regulation. The current findings further support the utility of considering not just cortisol levels, but characteristics of an entire response trajectory, as well as distinct stress types/contexts, to better understand stress neurophysiology. Mothers who displayed a less reactive HPA profile with their infants--reflected not in cortisol levels themselves, but rather in a more positive quadratic estimate for the entire trajectory–showed heightened activation to their infant's cry across limbic/paralimbic and anterior cingulate circuits.1 Specific components of this response map onto previous stress and maternal adjustment findings (5,11), though their combination in this stress context is novel.
Activation of the paralimbic belt–anterior insula and OFC–has been found in previous studies of empathy (23,24) and mothers attuned to their distressed infant's needs (4). Our findings suggest that mothers who process this cue for a potentially stressful parenting context (i.e., where they must soothe their distressed infant) with greater empathic attunement2 are better prepared to regulate their stress response in the context itself. In fact, the areas detected here using an auditory task overlap substantially with those found using the visually presented maternal stressor reported by Noriuchi and colleagues, providing evidence for convergence across modalities. Although insula activity may also relate to negative emotional responses and/or poorer maternal preparation (26), the larger matrix of associations with parenting measures in this sample suggests such emotional resonance generally supported, rather than hindered, maternal competence. Current findings highlight a perceptual-emotional path from posterior insula (representing sensory attributes of salient events) to anterior insula (subjective awareness) and elaborated by OFC evaluation in less stress-reactive mothers. The ventrolateral PFC-lateral OFC may further serve to regulate negative emotional responses (27), titrating mothers' eventual subjective and physiological response to infant cues. Indeed, these patterns fit broadly with previous findings of insula deactivation in cortisol responders, and OFC activation in cortisol nonresponders, to the social-evaluative component of a stress task (1). These results suggest that when responding to a socially-relevant stressor, it is important to maintain sensitivity to what may be initially negative cues, but to be ready to evaluate and shape one's response to those cues as needed.
Having received the message communicated by their infants' cry, less stress-reactive mothers appeared better prepared to develop a thoughtful response to it, activating ACC-mPFC networks implicated in stress regulation across various contexts (11). In the present parenting context, these activations may further reflect mothers' elaboration of what their infant's message means to them and selection of appropriate response strategies (28). ACC activity has shown both positive and negative associations with acute cortisol output depending on whether basal or stress-reactive levels are considered, but it appears to play an important role in downregulating the system when a stressor is presented (29). Again, these results fit with previous finding of increased ACC activity in cortisol nonresponders, underlining its importance in overall stress regulation patterns (1,11). The specific region of ACC activity found here is dorsal to the region most directly involved in limbic regulation, but is likely to connect to a variety of PFC and subcortical regions involved in coordinating a comprehensive cognitive-behavioral response (30).
A final parenting-specific link in the chain of less stress-reactive neural activity involved midbrain areas known to mediate mothering behavior. The PAG serves an important role in orchestrating maternal behaviors across mammalian species (31) and may represent the end goal of a well regulated maternal response to infant cues. Having attended to and interpreted their infant's distress, mothers' ability to select and enact the right soothing behaviors may help explain a lower stress-reactive profile with their infants.
Although the explanation for these neural correlates of mothers' HPA profiles is necessarily preliminary, they fit broadly with what is known about stress regulation and expand what that means. On the one hand, prefrontal (as opposed to amygdala) dominance during stress conditions suggests a mother who feels in control of the situation and is able to modulate bottom-up stress reactivity (32). This is probably part of a feedback-feedforward process, with mothers who maintain prefrontal control of HPA reactivity better able to make parenting decisions, leading to better competence and perceived control. Our findings also parallel those of Pruessner and colleagues (33), in that prefrontal (OFC, ACC) involvement in stress appraisal dictates an overall HPA response style, not acute cortisol output at any particular time, to psychological stress. At the same time, these findings suggest that a “regulated” response to interpersonal stressors involves more than prefrontal control; empathic awareness and action may also be critical components.
Not surprisingly, the present findings showed some overlap with neural differences we had previously found associated with mood dysregulation (14), though these were by no means redundant. In particular, we found aspects of thalamostriatal activity reduced in both depressed mothers and those showing more reactive HPA profiles. This may represent the component of HPA dysregulation involved in clinical depression. We also found links among self-reported depressive symptoms, cortisol reactivity, and diminished medial prefrontal activity (Table S2 in the Supplement). This neuroendocrine profile helps explain the maintenance of mothers' mood problems, though unique cortisol-related activity after controlling for depressive symptoms suggests they cannot fully explain these associations. Interestingly, we had found insula and PAG activation in the non-depressed mothers (and not in depressed mothers) but nonsignificant differences across groups. Stress reactivity may be one of the relevant individual differences explaining well adjusted mothers' neurobehavioral responses missed by simply examining mood symptoms.
Limitations of the current study can be used to guide further investigation of parental stress. Although associations we found between cortisol and both mothers' reports of parenting quality and previously identified “maternal brain” activations suggest better vs. worse responses, our relatively global measures and the gap between fMRI and laboratory stress sessions leave important questions about sequences of response. Study designs linking mothers' neural response to infant cues to stress perceptions and simultaneous HPA activity, and ultimately to mother-infant behavioral exchanges, would help clarify what actually unfolds in more vs. less stress-resilient dyads. We were also limited in the range of infant-related stress, and associated HPA responses, we could observe. We were able to relate neural response to a more real-world stressor than is typical in such research, yet the selectivity of infant stress cues (cry sound without visual/narrative context) in the scanner and artificiality inherent in standard laboratory-based procedures (SS) limited ecological validity. Research examining mothers' responses to a broader spectrum of infant stress situations both in and out of the scanner would help define which response components are needed in which contexts. Finally, although our individual-differences approach to continuous HPA profile variability did not require significant sample-wide reactivity, the relatively mild HPA responses (compared to the larger group of mothers tested; see Table S1 in the Supplement) limited power for detecting reactivity-related differences. Assaying a greater range of response levels/shapes could uncover neural correlates we were unable to detect.
These limitations notwithstanding, the present study offers several novel points for stress research. First, neural response to stress may not predict cortisol levels themselves, but rather the overall shape of HPA response profiles, and further analysis using the latter could uncover brain-HPA associations missed by previous approaches. One way of capturing response shapes that avoids creating an artificial dichotomy or confounding with levels is to model latent growth terms, as illustrated here. Second, whereas some neural correlates of HPA regulation are likely consistent across stressor types/contexts (i.e., prefrontal networks), other circuits may be selectively involved in responding effectively to specific types of cognitive vs. interpersonal stress. A better understanding of the response appropriate for a given situation will yield insight into the causes of stress-related dysregulation and offer guidance for its alleviation.
Supplementary Material
Acknowledgments
Support provided by the National Science Foundation [0643393]; a National Institute of Mental Health postdoctoral fellowship [F32MH083462-02] to HL; and a pilot grant from the University of Oregon Brain Biology Machine Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Research conducted at the University of Oregon Dept. of Psychology
A preliminary version of these analyses based on a subset (n=8) of the full sample was presented in poster form as Putting stress to the test: Areas of brain activation to infant cry associated with new mothers' HPA activity at the International Society on Infant Studies in Baltimore, MD, March 2010.
All authors reported no biomedical financial interests or potential conflicts of interest.
In addition, mothers with more delayed/extended cortisol responses-reflected in more positive linear terms-showed increased activation in non-hypothesized frontopolar, parietal, and occipital regions. Activity in these regions, shown to be involved in attentional shifting and use of sensory information (22), may reflect more effortful processing of the cry cue that prolongs maternal stress; however, interpretation of these activations, which do not map well onto prior parenting/stress-related research, remains highly speculative. Therefore, we chose to focus our discussion on the quadratic-related activations.
References
- 1.Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, et al. Neural correlates of processing stressful information: An event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kober H, Feldman Barrett L, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional groupings and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: Mothers' response to infant's attachment behaviors. Biol Psychiatry. 2007;63:415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon I, Zagoory-Sharon O, Leckman JR, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol Behav. 2010;101:679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Martorell GA, Bugental DB. Maternal variations in stress reactivity: Implications for harsh parenting practices with very young children. J Fam Psychol. 2006;20:641–647. doi: 10.1037/0893-3200.20.4.641. [DOI] [PubMed] [Google Scholar]
- 9.Mills-Koonce WR, Propper C, Gariepy JL, Barnett M, Moore GA, Calkins S, Cox MJ. Psychophysiological correlates of parenting behavior in mothers of young children. Dev Psychobiol. 2009;51:650–661. doi: 10.1002/dev.20400. [DOI] [PubMed] [Google Scholar]
- 10.Lin EK, Bugental DB, Turek V, Martorell GA, Olster DH. Children's vocal properties as mobilizers of stress-related physiological responses in adults. Personality Social Psychol Bull. 2002;28:346–357. [Google Scholar]
- 11.Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 12.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Laurent HK, Ablow JC, Measelle J. Risky shifts: How the timing and course of mothers' depressive symptoms across the perinatal period shape their own and infant's stress response profiles. Dev Psychopathol. 2011;23:521–538. doi: 10.1017/S0954579411000083. [DOI] [PubMed] [Google Scholar]
- 14.Laurent HK, Ablow JC. A cry in the dark: Depressed mothers show reduced neural response to their own infant's cry. SCAN. 2011 doi: 10.1093/scan/nsq091. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeskind PS, Lester BM. Acoustic features and auditory perception of the cries of newborns with prenatal and perinatal complications. Child Dev. 1978;49:580–89. [PubMed] [Google Scholar]
- 16.Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- 17.Abidin R. Parenting Stress Index/Short Form. Pediatric Psychology; Charlottesville: 1990. [Google Scholar]
- 18.Conduct Problems Prevention Research Group Being a Parent. 1990 [Google Scholar]
- 19.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 20.Laurent HK, Powers SI. Emotion regulation in emerging adult couples: Temperament, attachment, and HPA response to conflict. Biol Psychol. 2007;76:61–71. doi: 10.1016/j.biopsycho.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples' attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. J Pers Soc Psychol. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- 22.Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal prefrontal cortex for attention shifting. Neuroimage. 2004;23:670–679. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natnl Acad Sci. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Decety J, Meyer M. From emotion resonance to empathic understanding: A social developmental neuroscience account. Dev Psychopathol. 2008;20:1053–1080. doi: 10.1017/S0954579408000503. [DOI] [PubMed] [Google Scholar]
- 25.Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27:731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- 26.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharm. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cog Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 28.Swain JE. The human parental brain: In vivo neuroimaging. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.10.017. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirtcliff EA, Vitacco MJ, Graf AR, Gostisha AJ, Merz JL, Zahn-Waxler C. Neurobiology of empathy and callousness: Implications for the development of antisocial behavior. Behav Sci Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Sukikara MH, Mota-Ortiz SR, Baldo MV, Felicio LF, Canteras NS. The periaqueductal gray and its potential role in maternal behavior inhibition in response to predatory threats. Behav Brain Res. 2010;209:226–233. doi: 10.1016/j.bbr.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 32.Arnsten AFT. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, et al. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;6:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.