Abstract
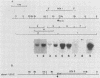
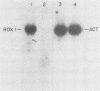
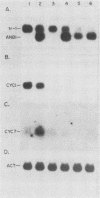
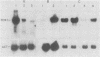
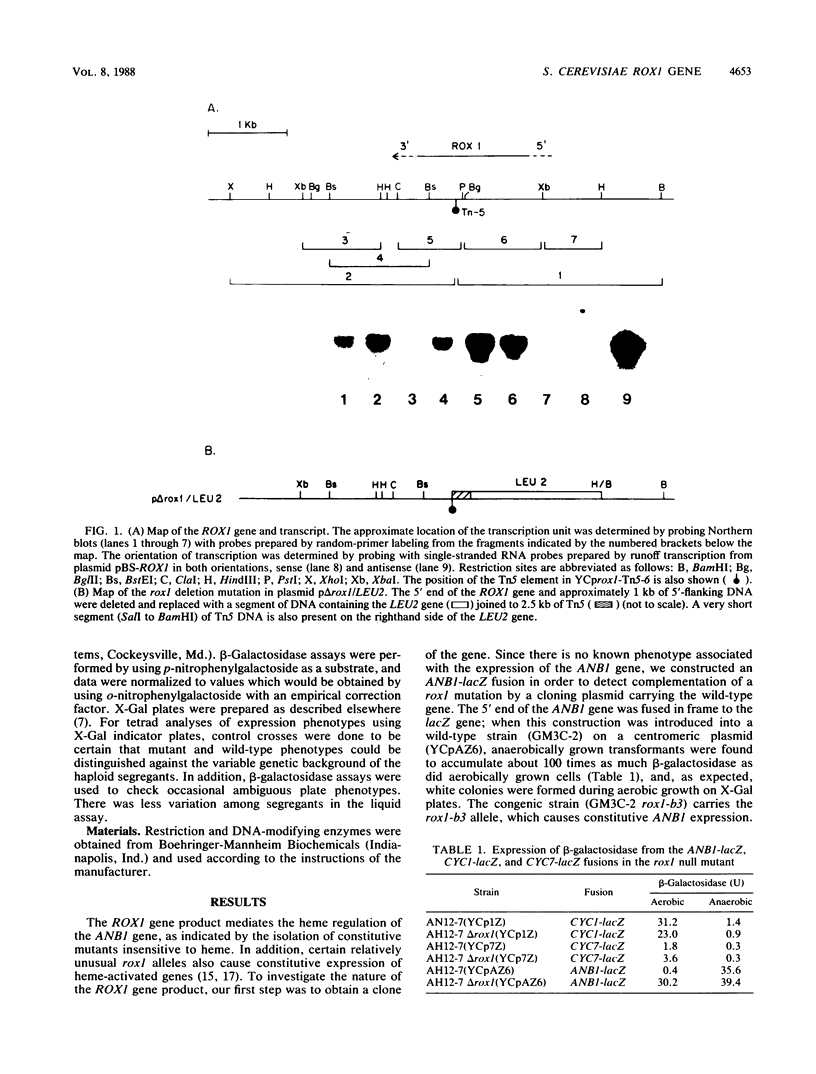
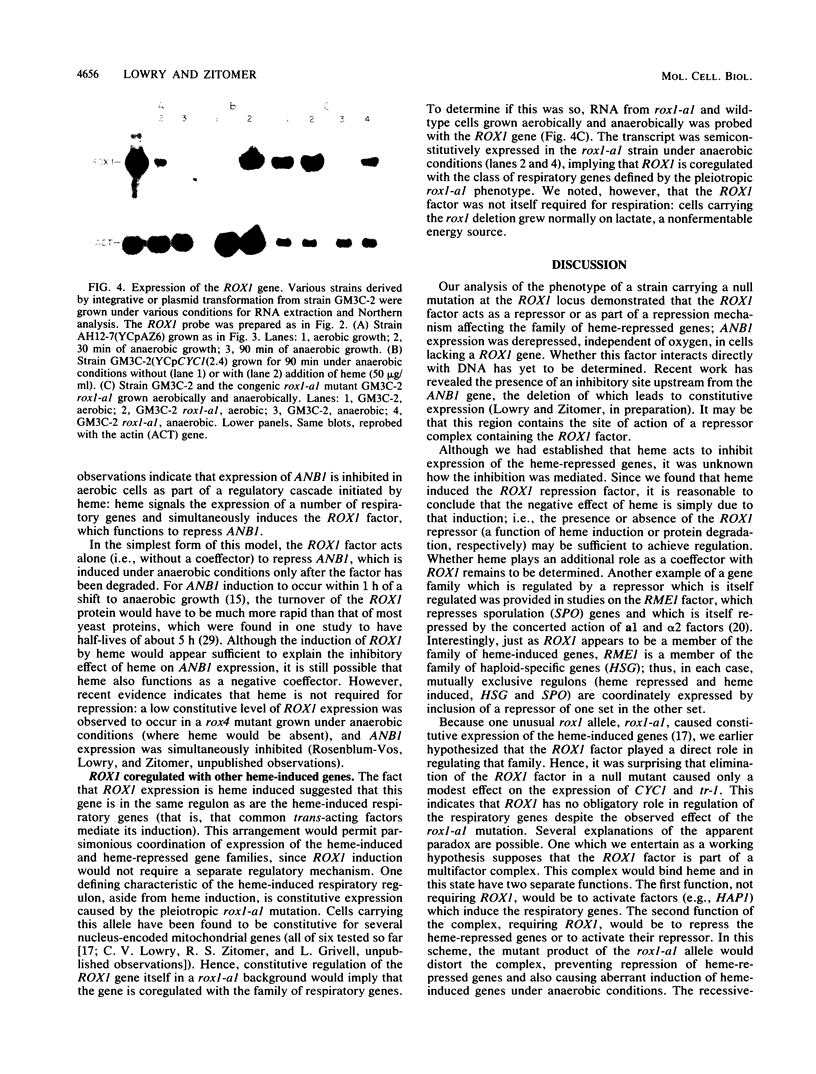
The ROX1 gene encodes a product implicated in the regulation of heme-repressed and heme-induced genes in Saccharomyces cerevisiae. The gene has been cloned and shown to code for a 1.4-kilobase transcript. The cloned gene was used to construct a null mutant to determine the role of ROX1 in regulating the expression of several heme-regulated genes. Constitutive expression of ANB1 (a heme-repressed gene) was observed in the null strain, indicating that ROX1 codes for a repressor or a facilitator of repression. Enhancement of expression of CYC7 in the null strain indicated that the ROX1 factor is required for repression of CYC7 to its normal low level of expression, consistent with evidence that CYC7 has a hybrid heme-induced, heme-repressed regulatory mechanism. The null mutation had only a slight negative effect on expression of the heme-induced genes CYC1 and tr-1 (a heme-induced homolog of ANB1), suggesting that the ROX1 factor is not directly involved in their regulation despite the existence of an unusual rox1 mutation (rox1-a1) causing constitutive expression of this group. The respiratory competence of the null mutant indicates that ROX1 is not a respiratory factor. ROX1 expression was found to be induced by heme, indicating that the heme repression of ANB1 and its family is the result of a cascade in which heme induces a repression factor which keeps the family of heme-repressed genes inactive during aerobic growth. The rox1-a1 allele had earlier been shown to cause constitutive expression of the family of heme-induced respiratory genes. This allele was found to cause constitutive expression of the ROX1 transcript itself, indicating that ROX1 is in the major heme-induced regulon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Burland T. G., Paul E. C., Oetliker M., Dove W. F. A gene encoding the major beta tubulin of the mitotic spindle in Physarum polycephalum plasmodia. Mol Cell Biol. 1988 Mar;8(3):1275–1281. doi: 10.1128/mcb.8.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavilier L., Péré-Aubert G., Somlo M., Slonimski P. P. Réseau d'interactions entre des génes non liés : régulation synergique ou antagoniste de la synthèse de l'iso-1-cytochrome c, de l'iso-2-cytochrome c et du cytochrome b2. Biochimie. 1976;58(1-2):155–172. doi: 10.1016/s0300-9084(76)80366-5. [DOI] [PubMed] [Google Scholar]
- Clyman J., Cunningham R. P. Escherichia coli K-12 mutants in which viability is dependent on recA function. J Bacteriol. 1987 Sep;169(9):4203–4210. doi: 10.1128/jb.169.9.4203-4210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Guarente L., Lalonde B., Gifford P., Alani E. Distinctly regulated tandem upstream activation sites mediate catabolite repression of the CYC1 gene of S. cerevisiae. Cell. 1984 Feb;36(2):503–511. doi: 10.1016/0092-8674(84)90243-5. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Healy A. M., Helser T. L., Zitomer R. S. Sequences required for transcriptional initiation of the Saccharomyces cerevisiae CYC7 genes. Mol Cell Biol. 1987 Oct;7(10):3785–3791. doi: 10.1128/mcb.7.10.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Lieber R. H. Negative regulation of the Saccharomyces cerevisiae ANB1 gene by heme, as mediated by the ROX1 gene product. Mol Cell Biol. 1986 Dec;6(12):4145–4148. doi: 10.1128/mcb.6.12.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Weiss J. L., Walthall D. A., Zitomer R. S. Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc Natl Acad Sci U S A. 1983 Jan;80(1):151–155. doi: 10.1073/pnas.80.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Zitomer R. S. Oxygen regulation of anaerobic and aerobic genes mediated by a common factor in yeast. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6129–6133. doi: 10.1073/pnas.81.19.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matner R. R., Sherman F. Differential accumulation of two apo-iso-cytochromes c in processing mutants of yeast. J Biol Chem. 1982 Aug 25;257(16):9811–9821. [PubMed] [Google Scholar]
- Mitchell A. P., Herskowitz I. Activation of meiosis and sporulation by repression of the RME1 product in yeast. 1986 Feb 27-Mar 5Nature. 319(6056):738–742. doi: 10.1038/319738a0. [DOI] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer K., Arcangioli B., Guarente L. Yeast HAP1 activator competes with the factor RC2 for binding to the upstream activation site UAS1 of the CYC1 gene. Cell. 1987 Apr 10;49(1):9–18. doi: 10.1016/0092-8674(87)90750-1. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Prezant T., Guarente L. Yeast HAP1 activator binds to two upstream activation sites of different sequence. Cell. 1987 Apr 10;49(1):19–27. doi: 10.1016/0092-8674(87)90751-3. [DOI] [PubMed] [Google Scholar]
- Richter K., Ammerer G., Hartter E., Ruis H. The effect of delta-aminolevulinate on catalase T-messenger RNA levels in delta-aminolevulinate synthase-defective mutants of Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 10;255(17):8019–8022. [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Control of vacuole permeability and protein degradation by the cell cycle arrest signal in Saccharomyces cerevisiae. J Bacteriol. 1978 Oct;136(1):234–246. doi: 10.1128/jb.136.1.234-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdière J., Creusot F., Guarente L., Slonimski P. P. The overproducing CYP1 and the underproducing hap1 mutations are alleles of the same gene which regulates in trans the expression of the structural genes encoding iso-cytochromes c. Curr Genet. 1986;10(5):339–342. doi: 10.1007/BF00418404. [DOI] [PubMed] [Google Scholar]
- Wright C. F., Zitomer R. S. A positive regulatory site and a negative regulatory site control the expression of the Saccharomyces cerevisiae CYC7 gene. Mol Cell Biol. 1984 Oct;4(10):2023–2030. doi: 10.1128/mcb.4.10.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. F., Zitomer R. S. Point mutations implicate repeated sequences as essential elements of the CYC7 negative upstream site in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Nov;5(11):2951–2958. doi: 10.1128/mcb.5.11.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zagorec M., Labbe-Bois R. Negative control of yeast coproporphyrinogen oxidase synthesis by heme and oxygen. J Biol Chem. 1986 Feb 25;261(6):2506–2509. [PubMed] [Google Scholar]
- Zitomer R. S., Hall B. D. Yeast cytochrome c messenger RNA. In vitro translation and specific immunoprecipitation of the CYC1 gene product. J Biol Chem. 1976 Oct 25;251(20):6320–6326. [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Sellers J. W., McCarter D. W., Hastings G. A., Wick P., Lowry C. V. Elements involved in oxygen regulation of the Saccharomyces cerevisiae CYC7 gene. Mol Cell Biol. 1987 Jun;7(6):2212–2220. doi: 10.1128/mcb.7.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]