Abstract
The last several decades have witnessed dramatic advances in unfolding the diversity and commonality of oceanic diazotrophs and their N2-fixing potential. More recently, substantial progress in diazotrophic cell biology has provided a wealth of information on processes and mechanisms involved. The substantial contribution by the diazotrophic cyanobacterial genus Trichodesmium to the nitrogen influx of the global marine ecosystem is by now undisputable and of paramount ecological importance, while the underlying cellular and molecular regulatory physiology has only recently started to unfold. Here, we explore and summarize current knowledge, related to the optimization of its diazotrophic capacity, from genomics to ecophysiological processes, via, for example, cellular differentiation (diazocytes) and temporal regulations, and suggest cellular research avenues that now ought to be explored.
Keywords: cyanobacteria, N2 fixation, genome evolution, diazocytes, adaptation, nutrient stress
Introduction
Balancing of nitrogen (N) inputs and exports in global oceans requires substantial biogenic fixation of atmospheric nitrogen (N2). In this context, planktonic colony-forming cyanobacteria of the genus Trichodesmium are recognized as major players. Representatives within the genus have consistently been shown to be stable components of tropical and subtropical segments of the Atlantic, Pacific, and Indian Oceans where they may form enormous surface accumulations (‘blooms’) visible to the naked eye (see Capone & Carpenter, 1982; Capone et al., 1998; Karl et al., 2002; Tyrrell et al., 2003; Davis & McGillicuddy, 2006; Westberry & Siegel, 2006; Carpenter & Capone, 2008). Trichodesmium contributes to sustaining marine life via active release of key nutrients, for example carbon and nitrogen, and upon death and decay, hence making this fully photoautotrophic genus a vital player in the biogeochemical cycling of basic elements in contemporary oceans (Carpenter & Capone, 2008). The global input via N2 fixation by Trichodesmium was initially estimated to amount to about 5 Tg N annually by Capone & Carpenter (1982), an estimate that by now has risen to about 60–80 Tg N annually (Capone et al., 1997; Mahaffey et al. 2005; Westberry & Siegel, 2006; Carpenter & Capone, 2008), which makes up a substantial part of the current estimate of global marine N2 fixation, 100–200 Tg N annually (Karl et al., 2002).
As the N2-fixing enzyme, nitrogenase, encoded by the nifHDK genes, is rapidly inactivated by O2, diazotrophic cyanobacteria either fix N2 at night (to avoid photosynthetically evolved oxygen) or differentiate a thick-walled, photosystem II-deficient heterocystous cell type to specifically sustain daytime N2 fixation (Kumar et al., 2010). Members of the genus Trichodesmium fix N2 exclusively in the light (Dugdale et al., 1961; Capone et al., 1997), although the genus is affiliated to Section III filamentous cyanobacteria that are unable to form heterocysts and therefore expected to fix N2 (primarily) during the dark phase (see Bergman et al., 1997). Knowledge has expanded dramatically in regard to the diazotrophic physiology and molecular biology of Trichodesmium, but there are still gaps related to its unique cell biology and overall behavior in an ecophysiological context. We here summarize our current knowledge by highlighting its diazotrophic peculiarities from various perspectives.
Speciation
Trichodesmium erythraeum was named by Ehrenberg in 1830 after observing blooms that discolored the water at the Bay of Tor in the Red Sea (Ehrenberg, 1830). Jules Verne (1869) in'20 000 leagues under the sea' also mentions blooms in this Bay (Box 1). Two other species, T. thiebautii and T. hildebrandtii, were named by Gomont (1892), and Wille (1904) later described another three species, T. contortum, T. tenue, and T. radians. These species were re-examined in 1995 (Janson et al., 1995) using specimens from the Indian Ocean, Caribbean, and Sargasso Seas. Ultrastructural arrangement of gas vesicles and glycogen clusters (carbon storage) were used as primary markers and separated the species into two clades: (i) T. tenue and T. erythraeum and (ii) T. thiebautii, T. hildebrandtii, and T. contortum. In 2001, a close relationship between Trichodesmium spp. (Lundgren et al., 2001) and marine cyanobacterial members of the genus Katagnymene (K. pelagica and K. spiralis; Lemmermann, 1900) was discovered using phylogenetic analysis of nifH gene sequences. Using the more variable fragment of the hetR gene, and a few other genetic markers, as targets revealed that the two Katagnymene species in fact cluster within one of the two Trichodesmium clades (Orcutt et al., 2002; Lundgren et al., 2005). Despite the morphological differences, K. pelagica and K. spiralis were, in addition, found to be the same species (Lundgren et al., 2005). Examining 21 cultivated isolates of Trichodesmium/Katagnymene, using genetic and morphological markers, Hynes et al. (2012) verified the existence of two Trichodesmium clades and suggested that these may inhabit different ecological niches, based on different pigment characteristics.
Box 1
From Jules Verne, ‘20,000 leagues under the sea’ [translated by Lewis Mercier, (Verne, 1872)]:
‘Here it is, M. Aronnax. According to my idea, we must see in this appellation of the Red Sea a translation of the Hebrew word 'Edom’; and if the ancients gave it that name, it was on account of the particular colour of its waters.' ‘But up to this time I have seen nothing but transparent waves and without any particular colour.’ ‘Very likely; but as we advance to the bottom of the gulf, you will see this singular appearance. I remember seeing the Bay of Tor entirely red, like a sea of blood.’ ‘And you attribute this colour to the presence of a microscopic seaweed?’ ‘Yes.’ ‘So, Captain Nemo, it is not the first time you have overrun the Red Sea on board the Nautilus?’ ‘No, sir.’
However, a full revision of the genera Trichodesmium and Katagnymene is now warranted, as the latter also includes several freshwater species (T. iwanoffianum Nygaard, T. lacustre Klebahn, K. accurata Geitler, K. mucigera Compére and K. spirulinoides An; see Komárek & Anagnostidis, 2005), one of which, in addition, represents the ‘type strain’ of the genus Katagnymene. Sequencing of additional genomes within the Trichodesmuim genus is also needed if we are to fully comprehend the taxonomy and phylogeny of the genus.
An expanding and flexible genome
Trichodesmium erythraeum IMS101 (from now on Trichodesmium IMS101) was one of the first strains isolated into axenic cultures (Prufert-Bebout et al., 1993) and still represents the only sequenced genome within the genus (http://genome.jgi-psf.org/trier/trier.home.html). The genome, which comprises 7.75 Mbp, is among the larger cyanobacterial genomes sequenced to date (Fig. 1a). A recent phylogenetic survey of 58 sequenced cyanobacterial genomes, based on a concatenated alignment of 285 protein orthologs, verified that Trichodesmium IMS101 is affiliated to a lineage composed of other filamentous nonheterocystous species within Oscillatoriales (Fig. 1b; Larsson et al., 2011): the marine Lyngbya sp. PCC 8106 and two species within Arthrospira (A. platensis and A. maxima; previously denoted Spirulina). These are all ecologically successful and widespread inhabitants of marine waters and alkaline lakes, respectively. Recent analyses based on the 16S rRNA gene sequence give a similar clustering of Trichodesmium IMS101 (Schirrmeister et al., 2011). Larsson et al. (2011) also showed that the capacity to fix N2 within this Trichodesmium clade was lost in A. platensis and A. maxima, as also verified by Latysheva et al. (2012), while retained in the deeper-branching Trichodesmium and Lyngbya sp. PCC 8106. Interestingly, these four lineages all possess hetR (Larsson et al., 2011), a gene encoding a protease with a key function in cell differentiation (N2-fixing heterocysts and resting akinetes; see Kumar et al., 2010), although they all lack these developmental capacities. Additionally, comparative genomic analyses show that several other gene orthologs involved in heterocyst differentiation are present in the Trichodesmium IMS101 genome (e.g. hetCF, patB) while others, not unexpectedly, are missing such as those involved in the deposition of the heterocyst outer envelope (e.g. hglCDE, hepB) (Table S1; El-Shehawy et al., 2003; Larsson et al., 2011). However, orthologous genes are not always functionally equivalent. For instance, the sepJ gene of Trichodesmium is missing a vital domain essential for filament integrity under nitrogen deprivation, although it fully complements a sepJ deletion mutant of Nostoc sp. PCC7120 when grown in the presence of combined nitrogen (Mariscal et al., 2011). The nif gene operon of Trichodesmium IMS101 is conserved in a manner typical of some heterocystous cyanobacteria, although Trichodesmium lacks the large DNA insertion element present in the structural nifD gene of several of the heterocystous cyanobacteria, as well as the intergenic region between nifB and nifVZT/cysE (Fig. S1). These findings strengthen an evolutionary relationship between the genus Trichodesmium and the heterocystous clade, whereas distinct differences are also apparent, relationships now worth examining in greater detail.
Fig. 1.
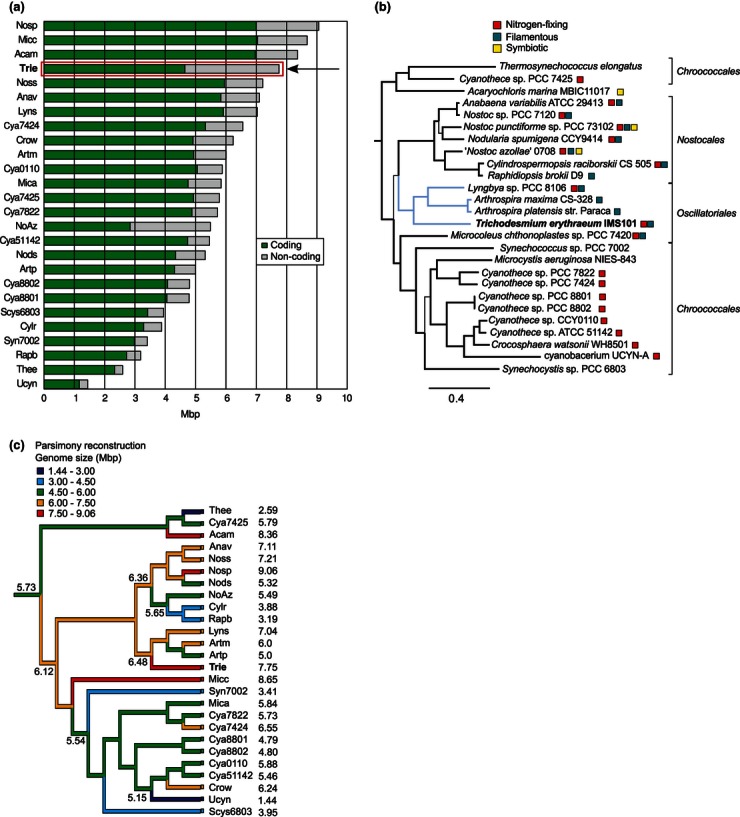
Phylogeny and genome properties of Trichodesmium IMS101. (a) Genome sizes and proportions of coding and noncoding nucleotides in genomes of organisms included in (b) and (c). Genomes are sorted by total size. The genome of Trichodesmium IMS101 is indicated by an arrow. (b) Maximum-likelihood phylogenetic tree based on a concatenated alignment of 285 single-copy orthologs. The tree is a subtree of a larger phylogeny of 58 cyanobacteria (see Larsson et al., 2011). Specific phenotypes for cyanobacteria are shown by the colored boxes next to the tip labels. The clade containing Trichodesmium (order Oscillatoriales) is highlighted with blue branches. Thick and thin branches indicate bootstrap support values (200 replicates) of 100 and between 58 and 84, respectively. Bar, 0.4 expected substitutions per site. (c) Ancestral genome sizes (reconstructed by parsimony) in the phylogeny from b. Organism names are abbreviated (see below for full names). Contemporary genome sizes (Mbp) are shown in the right margin and at specific nodes in the tree. Organism abbreviations are as follows: Acam = Acaryochloris marina MBIC11017, Anav = Anabaena variabilis ATCC29413, Artm = Arthrospira maxima CS328, Artp = Arthrospira platensis str. Paraca, Crow = Crocosphaera watsonii WH8501, Cya0110 = Cyanothece sp. CCY0110, Cya51142 = Cyanothece sp. ATCC51142, Cya7424 = Cyanothece sp. PCC7424, Cya7425 = Cyanothece sp. PCC7425, Cya7822 = Cyanothece sp. PCC7822, Cya8801 = Cyanothece sp. PCC8801, Cya8802 = Cyanothece sp. PCC 8802, Cylr = Cylindrospermopsis raciborskii CS505, Lyns = Lyngbya sp. PCC 8106, Mica = Microcystis aeruginosa NIES 843, Micc = Microcoleus chthonoplastes PCC7420, NoAz = ‘Nostoc azollae’ 0708, Nods = Nodularia spumigena CCY9414, Nosp = Nostoc punctiforme PCC73102, Noss = Nostoc sp. PCC7120, Rapb = Raphidiopsis brookii D9, Scys6803 = Synechocystis sp. PCC6803, Syn7002 = Synechococcus sp. PCC7002, Thee = Thermosynechococcus elongatus, Trie = Trichodesmium erythraeum IMS101, Ucyn = cyanobacterium UCYN-A. The figures are adapted from Larsson et al. (2011) with the author's permission.
Another notable feature of the Trichodesmium IMS101 genome is its comparatively low coding capacity (Larsson et al., 2011). With c. 40% noncoding DNA, it holds one of the lowest coding percentages among all to date sequenced genomes of cyanobacteria (Fig. 1a) and other bacteria (Hou & Lin, 2009). The intergenic sequences within the Trichodesmium IMS101 genome (459-bp median length) are also relatively large for cyanobacteria (14.5- to 231-bp median intergenic length in 39 other finished cyanobacterial genomes). The reason for these large and presumably noncoding intergenic spacers in the Trichodesmium IMS101 genome is unknown. It is, however, interesting to note that among the 58 genomes examined, the genome of Trichodesmium IMS101 is one of a few in which the genome is currently expanding in size, as is also the case for the genomes of the limnic Microcystis aeruginosa NIES 843 and the marine Acaryochloris marina MBIC11017 (Fig. 1c; Larsson et al., 2011). This suggests that the genome of Trichodesmium IMS101 is in an expanding dynamic state, in contrast to the shrinking genomes of the unicellular marine genera Synechococcus and Prochlorococcus (with genomes < 2 Mbp; Palenik et al., 2006; Kettler et al., 2007), genera which to a large extent share the same tropical/subtropical marine habitat as Trichodesmium. Based on these data, it is suggested that different strategies are used to cope with the various constraints enforced by these oligotrophic oceans (Larsson et al., 2011). Trichodesmium may use a strategy to flexibly adapt by incorporating functions and capacities when needed (e.g. via horizontal gene transfer, HGT) and maintain gene duplications (in-paralogs) affecting c. 10% of all genes in the Trichodesmium IMS101 genome, as a mechanism to promote genome expansion and organismal adaptations (Swingley et al., 2008; Treangen & Rocha, 2011). One example of HGT in Trichodesmium IMS101 is the acquisition of long eukaryotic triglyceride collagen protein fibers that may sustain Trichodesmium colony formation (Layton et al., 2008). Paralogs and horizontally gained genes that do not provide a fitness advantage will undergo inactivation (sequence divergence and loss of function) and eventually be lost. Indeed, 21% of in-paralogs in Trichodesmium IMS101 appear to have been subject to inactivation and are now present only as pseudogenes within the genome (Larsson et al., 2011), thereby contributing to the abundance of noncoding nucleotides (Fig. 1a). Considering that bacterial genomes are subject to a deletion bias (Mira et al., 2001), the large noncoding proportion of the Trichodesmium IMS101 genome which cannot be attributed to remnants of previously functional genes (pseudogenes) is enigmatic. However, it is possible that at least parts of these intergenic regions contain as yet nonannotated genes or small RNAs (Hewson et al., 2009; Shi et al., 2009). It appears that the unicellular marine cyanobacteria with reduced genomes (e.g. Prochlorococcus spp. in Clade II) use an opposite strategy to compete for life-space and survival, that is maintain a large surface-to-volume ratio and fewer genes, a strategy recently characterized as ‘cryptic escape’ (Yooseph et al., 2010).
The diazocytes – separation in space
A reduced oxygen environment is a prerequisite for effective N2 fixation activity in Trichodesmium as in all other bacteria. Both transcription of nif genes and biosynthesis of the nitrogenase enzyme complex have been found to be sensitive to oxygen inactivation (Zehr et al., 1997; Staal et al., 2007). Ever since the pioneering studies by Dugdale and co-workers (Dugdale et al., 1961), a compelling research area has therefore been to elucidate how Trichodesmium reconciles oxygenic photosynthesis and oxygenophobic N2 fixation within its ‘heterocyst-free’ physiology. Initially, N2 fixation was proposed to be limited to low-oxygen or anaerobic regions in the center of Trichodesmium colonies (Paerl & Bebaut, 1988). However, later, it became apparent that colony formation was not a prerequisite as ‘free’ trichomes, the dominant form in Trichodesmium laboratory cultures, are also able to fix N2. Attention then switched to the structural differences along the Trichodesmium trichomes that early on were observed using light microscopy, recognized as ‘nongranulated’ or ‘lighter’ cell regions in parts of the trichomes (Carpenter & Price, 1976; Bryceson & Fay, 1981; Li & Lee, 1990), in both single trichomes, and colony-associated trichomes. This cellular arrangement occurs in both natural populations and cultures of Trichodesmium IMS101 (Lin et al., 1998; El-Shehawy et al., 2003). On average, ∼ 15% of the total cell population of the trichomes may be described as less granulated or lighter (more transparent). These cells are arranged in strings or ‘zones’ composed of ∼ 2–30 cells, but are not always obvious in LM (Fig. 2a). However, when stained (e.g. with Lugol's solution; Fig. 2c), each trichome harbors typically 1–2 such zones per trichome, but up to four zones have been observed in longer trichomes (El-Shehawy et al., 2003). The nongranulated appearance is caused by a diminished number and/or size of subcellular structures such as cyanophycin granules, gas vacuoles, and polyphosphate granules (Fig. 2c and e), while additional membranes are synthesized (Fredriksson & Bergman, 1997).
Fig. 2.
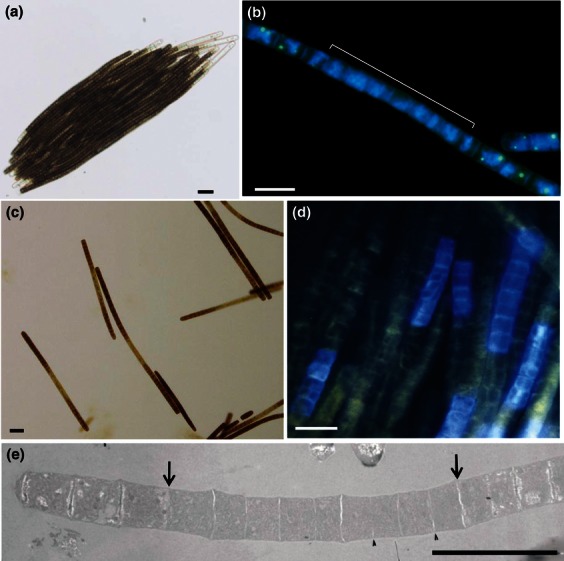
Morphological characteristics of Trichodesmium trichomes, with emphasis on cell differentiation and their nitrogenase containing cell type, the diazocytes. (a) A light micrograph depicting a dark pigmented colony consisting of longitudinally arranged trichomes of a newly isolated strain, T. erythraeum TNZ0801. Scale bar, 25 μm. (b) The DNA distribution in cells of a Trichodesmium IMS101 trichome visualized after staining with the dye 4′,6-diamidino-2-phenylindole (DAPI), fluorescing blue. Note the DNA presence in all cells, the centrally located diazocyte-like zone (marked) being recognized as they are devoid of the yellow/green fluorescent granules representing polyphosphate storage. Scale bar, 20 μm. (c) Trichomes of Trichodesmium IMS101 stained with Lugol's solution. Note several lighter-stained central diazocyte zones, in which catabolic carbon metabolism has degraded the Lugol-stainable stored carbon supplies. Scale bar 20 μm. (d) Fluorescence in situ immunolocalization of NifH into groups of adjacent cells, diazocytes, in central areas of intact trichomes of Trichodesmium IMS101. The NifH protein is detected as a blue fluorescence due to a secondary anti-NifH-antibody coupled to a blue-fluorescing chromophore. Scale bar, 10 μm. (e) Transmission electron micrograph depicting a longitudinally sectioned trichome of Trichodesmium IMS101. Note the more homogenous zone of cells, representing diazocytes between the arrows. Arrowheads point to ongoing cell division (the formation of division septa) in two of the diazocytes. Scale bar, 20 μm.
The ‘lighter’ cells were in 1991 (Bergman & Carpenter, 1991) proven to be the nitrogenase enzyme containing cells in Trichodesmium (Fig. 2d). The existence of a ‘spatial’ nitrogenase sequestration mechanism in a nonheterocystous cyanobacterium was thereby proven. The cells were subsequently termed diazocytes: di (two) azo (nitrogen) cyte (cell) (Fredriksson & Bergman, 1997). The exclusive localization of nitrogenase in diazocytes was corroborated by immuno-TEM (sectioned trichomes) and immuno-LM (whole-mount intact trichomes; Fig. 2d) analyses of both cultured and natural populations from the Indian, Pacific, and Atlantic Oceans, using a battery of antibodies (Bergman & Carpenter, 1991; Bergman et al., 1993; Janson et al., 1994; Fredriksson & Bergman, 1997; Berman-Frank et al., 2001b), including also one monoclonal anti-Trichodesmium IMS101-NifH antibody (targeting the smaller Fe protein subunit; Zehr et al., 1990; Bergman et al., 1993). The frequency of the diazocytes is lower at dawn and increased toward noon and is negatively regulated by the presence of combined nitrogen (Fredriksson & Bergman, 1995; Lin et al., 1998; Sandh et al., 2009, 11). Indeed, theoretical models suggest that a spatial separation of processes (such as nitrogen fixation and photosynthesis) favors biomass production compared to temporal separation (Rosetti & Bagheri, 2012) as, for instance, in unicellular cyanobacteria (Bergman et al., 1997). As a few other studies have suggested that the nitrogenase enzyme is present in all cells within the trichomes (Paerl et al., 1989; Ohki, 2008; Orcutt et al., 2009), a variation in cellular localization may exist depending on different environmental conditions or species examined. Using 15N and Nano-SIMS, Finzi-Hart et al. (2009) observed that the fixed N is rapidly distributed into the majority of cells along Trichodesmium trichomes, although cells in the center showed a lower 15N label, a pattern that may suggest a zone of diazocytes. A lower 15N label is also typical for heterocysts analyzed by Nano-SIMS due to a most rapid transfer of fixed nitrogen out of these cells (Popa et al., 2007; Ploug et al., 2010).
It was recently shown that, as for the differentiation of heterocysts, removal of combined nitrogen from the growth medium is the sole and sufficient mean needed to elicit the development of the centrally located nitrogenase containing diazocytes (Fig. 2d) in Trichodesmium IMS101 (Sandh et al., 2012). The fact that the development of diazocytes takes between 8–27 h and that changes in cellular ultrastructure precede the expression of the nitrogenase enzyme (Sandh et al., 2012) strongly argues for a genetically based developmental background. Phylogenetically, the nonheterocystous Trichodesmium clade is a sister group to the heterocystous clade (Fig. 1b) and Trichodesmium shares several genomic and behavioral features with this clade (see above, below and Table S1). For instance, some heterocystous cyanobacteria (Anabaena sp.) first develop strings or subsets of adjacent proheterocysts upon nitrogen deprivation, while only the central cell develops into a mature heterocyst and the other regresses into vegetative cells (Wilcox et al., 1975). The similarity of the patterning of proheterocysts and diazocytes hints that several early regulatory elements may be shared in the nitrogen-regulated pathways of heterocystous genera and Trichodesmium. Diazocytes may during evolution have ‘frozen’ at this more minimalistic initial stage (Fig. 3), as a full differentiation is disadvantageous for the conditions offered in oceans (Staal et al., 2003; : Stal, 2009). In contrast to heterocysts, the diazocytes are not terminally differentiated cells and retain their ability to divide (Fig. 2e; Fredriksson & Bergman, 1995), which may also contribute to their more flexible life style and allow the diazocytes (Trichodesmium) to more easily/rapidly adapt to prevailing conditions. To what extent Trichodesmium and heterocystous cyanobacteria share additional regulatory mechanisms that govern nitrogen deprivation signaling and pattern formation is now of great interest to be resolved.
Fig. 3.
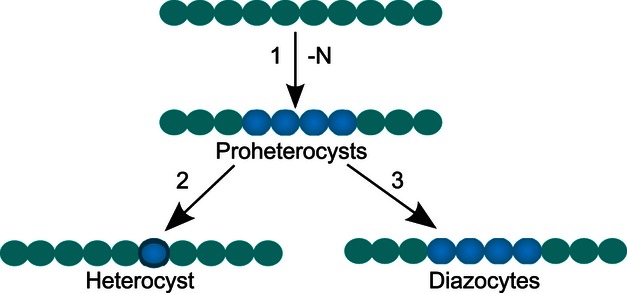
A potential evolutionary scenarium for the development of the diazocytic phenotype in Trichodesmium. (1) An ancient nonheterocystous filamentous cyanobacterium, a forerunner of Nostocales and Oscillatoriales (Fig. 1b and c), under nitrogen deprivation conditions develop strings of proheterocysts (as in Anabaena-type spp.; Wilcox et al., 1975). (2) The majority of the proheterocysts revert back into vegetative cells, while one continus the evolution into a thick-walled heterocyst, the dominating nitrogen-fixing phenotype in limnic and terrestrial ecosystems today. (3) In oceans, this proheterocystous phenotype may have been retained and evolved into the strings of diazocytes we see in Trichodesmium spp. today, while the closest relatives (Fig. 1b) either fix nitrogen in the dark (Lyngbya spp.; e.g. Lundgren et al., 2003) or have lost the capacity to fix nitrogen (Arthrospira spp.; Larsson et al., 2011; Latysheva et al., 2012).
Diazotrophy – separation in time
The timing of the N2 fixation physiology of Trichodesmium is governed by the circadian clock (Chen et al., 1996, 1998; Dong & Golden, 2008). This regulates the transcription of the nif genes, the daily de novo synthesis of the subunits of the nitrogenase enzyme (NifHDK), a post-translational modification of NifH (Capone et al., 1990; Zehr et al., 1993; Chen et al., 1996), and the supply of appropriate levels of energy and reducing equivalents necessary for N2 fixation activity (Staal et al., 2007).
Hence, it appears that Trichodesmium spp. not only separates N2 fixation physically from net oxygen evolution via the development of a special cell type (diazocytes), although this may be the major protective mechanism, but also separates these two incompatible processes temporarily (Berman-Frank et al., 2001b), although in a more subtle way than in other nonheterocystous cyanobacteria (Bergman et al., 1997). For instance, in contrast to in the latter and in concert with heterocystous cyanobacteria, nitrogenase activity in Trichodesmium operates within the day/light phase of the diel cycle, however, at a period around mid-day when the oxygen production is lowered and oxygen-scavenging mechanisms enhanced (respiration/Mehler reaction). This ‘mid-day depression’ in photosynthetic oxygen evolution is manifested as a lower quantum yield (∼ 50%) and a low or negative net O2 evolution (Berman-Frank et al., 2001b). Chlorophyll fluorescence kinetic microscopy at the single-cell level has also revealed flexible temporal and spatial switching between high fluorescence states (within a row of cells) and recovery states during subsequent non-N2-fixing periods in Trichodesmium IMS101 (Küpper et al., 2004). This rapid sequential switching within type I cells (functional PSII activity and enhanced Mehler reaction; potentially being the diazocytes) would allow diazotrophy even in cells lacking thick cell walls, may be orchestrated by rearrangements of the phycobilisomes between PSI and PSII (Küpper et al., 2009; Andresen et al., 2010), and may constitute the part of Trichodesmium's nitrogenase-protecting mechanism.
The increase in Mehler reaction (Kana, 1993; Milligan et al., 2007) and dark respiratory activity during the diazotrophic periods (Carpenter & Roenneberg, 1995; Kranz et al., 2009) is supported by the enhanced levels of the respiratory enzyme cytochrome c oxidase detected using proteomic analyses on N2-fixing cultures of Trichodesmium IMS101 (Sandh et al., 2011) and the enhanced levels of this enzyme specifically in the diazocytes, detected using immunogold localization in natural Trichodesmium populations (Bergman et al., 1993). In addition, Bryceson & Fay (1981) described zones of cells with higher tetrazolium salt depositions within specific areas of the trichomes, which support a reducing environment within the diazocytes. Although the cellular localization is not yet resolved, recent reports on novel carotenoids with strong antioxidant activity in Trichodesmium (Kelman et al., 2009) are also interesting in this context and worth examining further.
In addition to a lower net oxygen evolution, the CO2 fixation is also lowered at mid-day (Berman-Frank et al., 2001b) as recently verified using Nano-SIMS (Finzi-Hart et al., 2009). Earlier 14C labeling experiments also demonstrated a lower CO2 incorporation in central parts of the trichomes (Paerl, 1996). The decreased CO2 fixation impacts the subcellular storage of carbon (glycogen) and the general carbon metabolism. For instance, carbohydrate storages are degrading (Fig. 2c; El-Shehawy et al., 2003; Sandh et al., 2011), and recent proteomic analysis verifies a shift toward a catabolic carbon metabolism under diazotrophy, that is, a down-regulation of enzymes involved in glycogen storage and an up-regulation of enzymes involved in central carbon metabolism (Sandh et al., 2011). Diazotrophic conditions also led to enhanced levels of proteins involved in the biosynthesis of reducing equivalents (NADPH; through the oxidative pentose phosphate pathway) and the generation of a micro-oxic environment, consistent with increased respiratory activities (Sandh et al., 2011) and oxygen levels at mid-day.
Trichodesmium IMS101 also practices a light/dark (day/night) separation of other basic cellular processes. While the highly energy-demanding processes, such as CO2 fixation and diazotrophy, take place in light/day phase, cell division and diazocyte development are more pronounced in the dark/night phase (Chen et al., 1999; Sandh et al., 2009). Temporal separation of similar processes on a diel basis has previously been observed in marine unicellular cyanobacteria (Holtzendorff et al., 2001, 2002; Stockel et al., 2008; Shi et al., 2010). The circadian clock governs many of these processes and is entrained by the cellular ATP/ADP ratio, which in turn is governed by photosynthesis (Rust et al., 2011). Taken together, current data suggest that besides the development of diazocytes, some more subtle physiological mechanisms may act in concert to optimize diazotrophy in Trichodesmium.
Adaptation to nutrient stress
The nitrogen fixed in Trichodesmium is, as in other cyanobacteria, assimilated via the glutamine synthetase–glutamate synthase (GS-GOGAT) pathway, and as in heterocysts, the GS protein levels are higher in the diazocytes (Carpenter et al., 1992) to prevent feedback inhibition of the nitrogenase activity by the accumulation of the ammonia produced. Likewise, externally administrated sources of nitrogen negatively affect the expression of the nif genes, the synthesis of the nitrogenase enzyme, the nitrogenase activity, and diazocyte abundance in Trichodesmium (Ohki et al., 1991; Lin et al., 1998; Mulholland et al., 2001; El-Shehawy et al., 2003; Holl & Montoya, 2005; Sandh et al., 2011). As in other cyanobacteria (Herrero et al., 2004), when subject to N deprivation, there is a significant upshift in the cellular C : N ratio in Trichodesmium (Kranz et al., 2009), which may be a signal for enhanced transcription by the transcription factor NtcA (Table S1) of N-regulated genes. However, Trichodesmium appears to be flexible in this context, being able to fix N2 in the presence of low concentrations of dissolved inorganic and organic nitrogen (Holl & Montoya, 2005), and the transcript of ntcA is not exclusively regulated by the availability of, for example, ammonium (Post et al., 2012), which suggests that our knowledge in this area is still limited.
Phosphorus and iron are critical nutrients restricting growth and N2 fixation in today's oceans (Sanudo-Wilhelmy et al., 2001; Mills et al., 2004; Sohm et al., 2008; Moore et al., 2009). To overcome P limitations in oligotrophic waters, the Trichodesmium colonies migrate vertically in the water column to scavenge P and other nutrients using a buoyancy-regulating mechanism. This is provided by the pronounced gas vacuoles of Trichodesmium, which can withstand pressures down to depth of about 100–200 m (the highest known; Kromkamp & Walsby, 1992). In the upper euphotic zone, the colonies capture and store carbon and nitrogen (as glycogen and cyanophycin granules; Romans et al., 1994), and with this ‘ballast’, the colonies sink into deeper waters where P species may be acquired (Romans et al., 1994; Villareal & Carpenter, 2003; White et al., 2006b; Hewson et al., 2009). As the cellular ballast is metabolized in the deeper darker waters, the subsequently lighter colonies return to the euphotic zone to again capture light energy.
Trichodesmium is also known to adjust to periods of low P bioavailability by adopting high cellular N : P ratios (White et al., 2006a) in part by a substitution of its phospholipids by non-P membrane lipids (Van Mooy et al., 2009). Phosphorus uptake is also maximized via the uptake of both inorganic and organic phosphorous species (Stihl et al., 2001; Fu et al., 2005; Dyhrman et al., 2006; Orchard et al., 2009; Beversdorf et al., 2010; White et al., 2010). Enzymes hydrolyzing phosphoesters (alkaline phosphatase; Stihl et al., 2001; Orchard et al., 2009) and phosphonates (e.g. phosphonate hydrolase; Dyhrman et al., 2006) to yield phosphate have been identified in Trichodesmium. However, the globally widespread T. thiebautii lacks one of the alkaline phosphatase–encoding genes, phoA (Orchard et al., 2003). It has been shown that colonial alkaline phosphatase activities in Trichodesmium may rather be further enhanced by quorum-sensing signals (acylated homoserine lactones) released from colony-associated microorganisms (Van Mooy et al., 2012). The various phosphate pools available can be utilized either individually or in combination to sustain growth and N2 fixation (White et al., 2010). Any ‘luxury’ uptake of phosphate is stored in subcellular structures (polyphosphate granules), which are common in natural Trichodesmium populations (Romans et al., 1994). As increased polyphosphate storages have been found in phosphate-starved cells (Orchard et al., 2010), the regulation of these subcellular structures in Trichodesmium is enigmatic. The variability among Trichodesmium species in relation to P-uptake genes (Orchard et al., 2003) also raises the question of niche differentiation and calls for further investigation.
Iron is a pivotal cofactor in a number of cellular processes, such as photosynthesis, N2 fixation (in nitrogenase), and oxygen scavenging (Kustka et al., 2003; Shi et al., 2007). The high iron content of Trichodesmium cells suggests an efficient uptake and detainment capacity of iron and may even make Trichodesmium colonies ecologically valuable sources of this otherwise poorly soluble element in oligotrophic oceans (Kustka et al., 2003; Whittaker et al., 2011). No obvious genes in the Trichodesmium genome seem to encode for siderophores (Chappell & Webb, 2010), while the genes for transporters of siderophore-bound Fe3+ and Fe2+ and for enzymes related to cellular storage of iron are present (Castruita et al., 2006; Chappell & Webb, 2010). However, the involvement of siderophores in enhancing the uptake of low iron concentrations in Trichodesmium colonies has been reported (Achilles et al., 2003). The current hypothesis is that these siderophores are synthesized by associated microorganisms that indirectly facilitate the uptake of iron by Trichodesmium (Achilles et al., 2003). On the other hand, a recent study showed that such siderophore-bound iron is rather consumed by the bacteria than by the Trichodesmium cells (Roe et al., 2011). Another option is that the Trichodesmium colony formation per se facilitates the capture of enough particulate iron from, for example, eolian dust depositions to feed the colonies (Rubin et al., 2011).
Iron depletion is known to lead to a decrease in the frequency of diazocytes (Berman-Frank et al., 2001a; Küpper et al., 2008) and to a down-regulation of N2 fixation, while photosynthetic capacities are maintained (Shi et al., 2007; Brown et al., 2008; Küpper et al., 2008). Iron limitation may also elicit a switch in the phycobiliproteins being used (Küpper et al., 2008). Other cyanobacteria have also been shown to replace ferredoxin with the iron-free flavodoxin (Sandmann et al., 1990). Monitoring isiB transcription, encoding a flavodoxin, has suggested that Trichodesmium populations in the Atlantic Ocean are rarely, or not at all, iron limited, while those in the Pacific Ocean are (Chappell et al., 2012). However, the expression of the two fld genes, encoding flavodoxins, in Trichodesmium IMS101 is also regulated by N availability and growth stage (Lin et al., 2009; Chappell & Webb, 2010; Sandh et al., 2011), and their proposed use as iron limitation ‘markers’ may be questioned. Rather, the enhanced idiA transcription and IdiA levels noted under iron limitation may be a more suitable marker for iron stress in Trichodesmium (Webb et al., 2001; Chappell & Webb, 2010). As a strong up-regulation of Dps, yet another protein related to iron acquisition, was observed on the transfer of Trichodesmium to diazotrophic conditions (N stress), the role of Dps and other proteins related to iron acquisition and metabolism now also needs attention.
Impact on the ecosystem
The Trichodesmium abundance is roughly limited to waters warmer than 20 °C, and temperature tolerance for growth and N2 fixation in cultured strains of Trichodesmium (T. erythraeum IMS101, T. erythraeum GBRTRLI101 and T. tenue H94) ranges from 20 to 34 °C, with optimal temperatures being 24–30 °C depending on species and other growth conditions (Breitbarth et al., 2007; Chappell & Webb, 2010). However, Trichodesmium-like cyanobacteria (nifH and hetR phylogenies) were recently reported in Arctic waters suggesting wider temperature limits (Díez et al., 2012). Indeed, the very spotty nature of surface blooms of Trichodesmium does not represent the entire population in the ecosystem monitored and illustrates the difficulty in estimating the full global distribution of Trichodesmium via bloom registrations. In spite of this limitation, monitoring such blooms via remote sensing (via the SeaWiFS satellite; Subramaniam et al., 2002) verified that Trichodesmium blooms occur roughly between 20˚N and 20˚S in the eastern Pacific Ocean and that patches may occur even toward 40˚N and 40˚S in the Atlantic and the western Pacific and Indian Oceans (1998 and 2003; Westberry & Siegel, 2006).
Besides Trichodesmium, numerous unicellular cyanobacteria share the same marine aquatic environment, notably the small-celled non-N2-fixing genera Prochlorococcus and Synechococcus (cell diameter ∼ 1 μm, genome sizes of ∼ 2 Mbp; Partensky et al., 1999; Scanlan et al., 2009), but also several N2-fixing unicellular cyanobacteria (Zehr et al., 2001; Montoya et al., 2004; Moisander et al., 2010). Among the latter are representatives of the marine genera Cyanothece, Crocosphaera, and N2-fixing cyanobacteria of the ‘group A’ nifH phylotype (e.g. UCYN-A). These diazotrophic unicellular cyanobacteria may show a broader temperature tolerance (15–30 °C) than Trichodesmium, and some have been recovered from waters with detectable nitrate concentrations (Langlois et al., 2005). Observed community shifts from filamentous cyanobacteria in surface waters to unicellular cyanobacteria and/or heterotrophic bacteria in deeper waters (Langlois et al., 2005) may also suggest different ecological niche occupancies. Although estimates of the relative contribution to the total biogenic N2 fixation in oceans by unicellular cyanobacteria (and heterotrophic bacteria) are increasing (Halm et al., 2012; Sohm et al., 2011a,b; Turk et al., 2011; Zehr & Kudela, 2011), the role of Trichodesmium as a C and N source in the world's oceans is still profound. For instance, Trichodesmium may account for up to 50% of the nifH genes in the North Atlantic Ocean (0°N – 42°N and 67°W – 13°W; Langlois et al., 2008), and Trichodesmium/Katagnymene represent up to 106 nifH genes per liter, while the unicellular cyanobacteria were represented by 105 nifH genes per liter and proteobacteria by 104 nifH genes per liter (Rijkenberg et al., 2011). Tyrrell et al. (2003) reported an even higher colony abundance of Trichodesmium in the tropical Atlantic Ocean (0–15oN and 20oW), and a Trichodesmium surface bloom covered about 100 000 km2 in the Arabian Sea (Capone et al., 1998).
For still unknown reasons, large segments of the Trichodesmium population are suddenly trapped at the surface forming easily observed pigmented layers of dying and decomposing cells (‘blooms’) (Capone et al., 1998). Such decomposing blooms function as gigantic ‘fertilizer heaps’ releasing large quantities of carbon, nitrogen, and other nutrients for the benefit of nondiazotrophic and heterotrophic biota in the surrounding water bodies. The cause of this destructive ‘bloom’ phenomenon is unknown, while the involvement of viral infections (Hewson et al., 2004) and/or autocatalyzed cell death processes (Berman-Frank et al., 2004) have been proposed, but the question is still open. The nitrogen fixed by Trichodesmium may, in addition, enter marine food webs via grazing by tunicates, copepods, and fish (Roman, 1978; Bryceson, 1980; Carpenter, 1983; Oneil & Roman, 1994; Eberl & Carpenter, 2007). Nitrogen isotope ratios in zooplankton in the North Atlantic Ocean strongly indicate that N2 fixation is a major source of nitrogen for the marine zooplankton community (Montoya et al., 2002). This is at the same time unexpected, as the Trichodesmium toxin production has been inferred as a predator-deterring function (Layton et al., 2008) as well as a cause of death of several eukaryotic organisms, notably the copepod Acartia tonsa (Guo & Tester, 1994), several species of fish (Endean et al., 1993), and pearl oysters (Negri et al., 2004). Trichodesmium is now known to release different secondary metabolites, such as toxins, including the lipophilic chlorinated trichotoxin (Schock et al., 2011), the palytoxin causing clupeotoxism in humans via fish (Kerbrat et al., 2011), and the neurotoxin β-N-methylamino-L-alanine (Cox et al., 2005), the latter also found in diazotrophic bloom-forming cyanobacteria in the Baltic Sea (Jonasson et al., 2010). The ecological function of these toxins is still unknown.
Yet another factor that may play a role besides the mere cell number of an organism is their cell size/cell volume. As illustrated in Fig. 4, the size of Trichodesmium cells (approximate sizes given) makes the cellular volumes of this organism ‘gigantic’ compared to, for instance, cells of the unicellular genus Prochlorococcus and the diazotrophic genus Croccosphaera. As about 10–20 cells in each Trichodesmium filament are diazocytes (filled with NifH; Fig. 2b) and a large fraction of the nitrogen fixed may be released (as dissolved organic nitrogen or ammonium) from actively growing Trichodesmium populations (Capone et al., 1994; Glibert & Bronk, 1994; Mulholland & Capone, 2001; Mulholland et al., 2004; Mulholland, 2007), each Trichodesmium cell and filament may be viewed as a highly important source of new nitrogen for all nondiazotrophic small cells (unicellular cyanobacteria and bacteria) when sharing similar N-depleted aquatic marine environments.
Fig. 4.
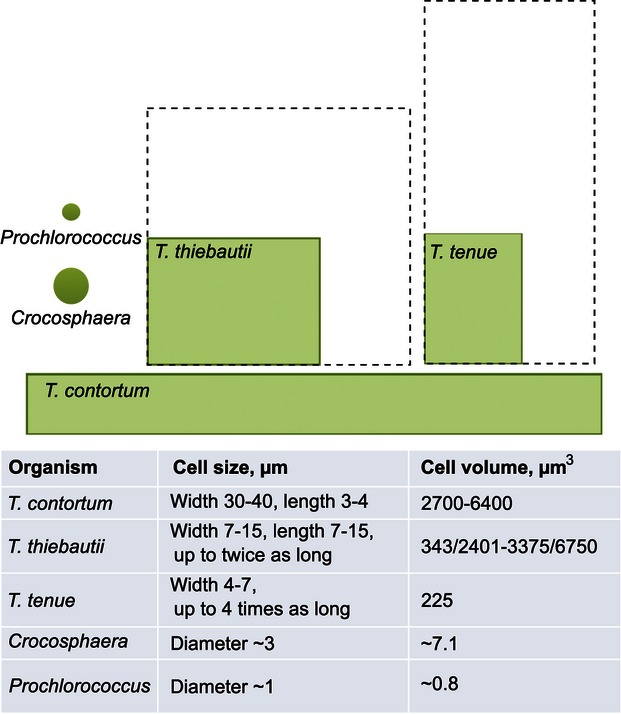
Schematic illustration of approximate cell size differences of Trichodesmium species and some unicellular cyanobacteria. The approximate cell sizes and volumes of different species of Trichodesmium are compared to the cell volumes of representatives of the nondiazotrophic but ubiquitous unicellular cyanobacterial genus Prochlorococcus and the unicellular diazotrophic genus Croccosphaera. Note the many-fold larger volume of the Trichodesmium cells. Hatched line shows maximum cell sizes. Trichodesmium cell sizes are according to Janson et al. (1995).
Trichodesmium in future scenarios
The global importance of Trichodesmium in oceanic biogeochemistry has triggered numerous studies mimicking future global warming scenarios. As mentioned above, the optimum temperature range for growth and nitrogen fixation of Trichodesmium is 24–30 °C (Breitbarth et al., 2007; Chappell & Webb, 2010), but Trichodesmium also survives lower temperatures and darkness (White et al., 2006b; Breitbarth et al., 2007). The ability to live at lower temperatures and in darkness is essential for their vertical migrations (White et al., 2006b) and may explain the occurrence of Trichodesmium in temperate (see LaRoche & Breitbarth, 2005) and potentially in cooler (Díez et al., 2012) waters. Global warming will lead to increased stratification resulting in shallower mixed layers and increased irradiance. High light intensity (up to 1000 μE) stimulates growth, diazocyte abundance, and N2 fixation in Trichodesmium (Andresen et al., 2010; Kranz et al., 2010; Levitan et al., 2010) and provokes changes in pigment composition (Andresen et al., 2010), and correlates with increased O2 evolution and CO2 fixation (Kranz et al., 2010). Higher light intensities also cause faster protein turnover (Andresen et al., 2010) and an increase in RuBisC/O:PSII ratio (Brown et al., 2008). The down-regulation in O2 production via PSII correlates with the earlier noted peaks in N2 fixation at noon (Berman-Frank et al., 2001b). Increased pCO2 levels will not only stimulate CO2 fixation, but also stimulate N2 fixation (dependent on carbon skeletons for sequestration of the ammonium produced) and growth in Trichodesmium (Hutchins et al., 2007; Levitan et al., 2007, 2010; Ramos et al., 2007; Kranz et al., 2009, 2010). One mechanism may be energy relocation from the costly carbon-concentrating mechanism (CCM; Badger et al., 2006; Kranz et al., 2011) toward CO2 and N2 fixation (Levitan et al., 2007; Kranz et al., 2011). This may in turn increase the release of the newly fixed N into the surrounding water body, thereby enhancing primary production of other organisms (Hutchins et al., 2007). Hence, in a scenario of increased temperatures and CO2 concentrations in the world's oceans, the abundance of surface blooms of Trichodesmium is expected to increase (Breitbarth et al., 2007; Hutchins et al., 2007; Levitan et al., 2007), unless other unforeseen natural factors provoke the opposite reaction. For instance, element colimitations, rather than single-element limitations, may regulate the growth and N2 fixation of natural Trichodesmium populations (Mills et al., 2004; Hutchins et al., 2007). Also, Garcia et al. (2011) have shown that the positive effect of higher CO2 concentrations (on, e.g., N2 fixation) is primarily seen at lower light intensities, and Rijkenberg et al. (2011) stress that the stimulations expected may be counteracted by decreased supply of nutrients from deeper waters as a consequence of enhanced stratification.
Conclusions and future outlooks
In 1968, Fay et al. (1968) proposed that the reducing conditions in the cyanobacterial cell type recognized as ‘heterocysts’ could be the site for N2 fixation. In 1973, Fleming & Haselkorn (1973) were the first to isolate nitrogenase from such heterocysts. Hence, heterocysts have during four decades acted as the ‘consensus model’ for successful light-driven N2 fixation in cyanobacteria. In 1991, Bergman and Carpenter were the first to show that nitrogenase is localized in subsets or short strings of cells (diazocyte) in Trichodesmium. These are now recognized as a prerequisite for the light-driven N2 fixation in Trichodesmium. This developmental mechanism is combined with mechanisms temporarily lowering oxygen evolution and orchestrating energy-competing processes in a multifaceted fashion. Hence, Trichodesmium is ‘second-to-none’ among potent daytime N2 fixers, as is in particular evidenced by its great global ecological impact. However, many questions still remain. These include identification of genes/proteins that underpin the development of the diazocytes and their regulation, including mechanisms involved in the protection of the oxygen-sensitive nitrogenase. Because freshwater species exist within the (former) genus Katagnymene (see e.g. Komárek & Anagnostidis, 2005), an intriguing question is whether these are capable of developing diazocytes. Sequencing additional genomes within this globally important genus will allow comparative genomic analyses and potentially shed light on, for example, its uniquely low DNA coding proportion and the significance of its apparently expanding genome. Besides genomic analyses, other ‘omics’ and ‘meta-omics’ approaches (transcriptomics, proteomics, and metabolomics) need to be introduced if we are to comprehend the unique N2-fixing physiology of Trichodesmium at all organization levels. ‘Meta-omics’ may provide more accurate information pertaining to the genetic diversity and the role of Trichodesmium and associated microorganisms than cultures, which often represents a minor part of the total species radiation. Such data may also reveal mutual and potentially life-sustaining interplays between Trichodesmium and the numerous associated microorganisms and open exciting research avenues into microbial evolution and marine microbial interphylum interactions, some potentially of a symbiotic nature. Focus should also be given to proteins that are highly up-regulated under N2-fixing conditions (Hewson et al., 2009; Sandh et al., 2011). Another area in need of exploration is the identification of adaptive ecological strategies, and possible niche differentiations, used by natural Trichodesmium populations. Finally, evolutionary aspects related to the uniquely different diazotrophic behavior of the genus Trichodesmium and to its placement in the evolution of affiliated cyanobacteria with unusual diazotrophic behavior are yet other compelling research areas to explore.
Acknowledgments
Financial supports from the Swedish Research Council, The Swedish Foundation for International Cooperation in Research and Higher Education (STINT), the SIDA/SAREC and Knut and Alice Wallenberg Foundation (to BB), and the US National Science Foundation (grants OCE-0934035 to EJC and EF-0629624 to SL) are gratefully acknowledged.
Statement
Re-use of this article is permitted in accordance with the Terms and Conditions set out at http://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. The Trichodesmium nif region.
Table S1. Gene orthologs related to heterocyst differentiation.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Achilles KM, Church TM, Wilhelm SW, Luther GW, Hutchins DA. Bioavailability of iron to Trichodesmium colonies in the western subtropical Atlantic Ocean. Limnol Oceanogr. 2003;48:2250–2255. [Google Scholar]
- Andresen E, Lohscheider J, Setlikova E, Adamska I, Simek M, Küpper H. Acclimation of Trichodesmium erythraeum ISM101 to high and low irradiance analysed on the physiological, biophysical and biochemical level. New Phytol. 2010;185:173–188. doi: 10.1111/j.1469-8137.2009.03068.x. [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD, Long BM, Woodger FJ. The environmental plasticity and ecological genomics of the cyanobacterial CO2 concentrating mechanism. J Exp Bot. 2006;57:249–265. doi: 10.1093/jxb/eri286. [DOI] [PubMed] [Google Scholar]
- Bergman B, Carpenter EJ. Nitrogenase confined to randomly distributed trichomes in the marine cyanobacterium Trichodesmium-Thiebautii. J Phycol. 1991;27:158–165. [Google Scholar]
- Bergman B, Siddiqui PJ, Carpenter EJ, Peschek GA. Cytochrome oxidase: subcellular distribution and relationship to nitrogenase expression in the nonheterocystous marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1993;59:3239–3244. doi: 10.1128/aem.59.10.3239-3244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman B, Gallon JR, Rai AN, Stal LJ. N-2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev. 1997;19:139–185. [Google Scholar]
- Berman-Frank I, Cullen JT, Shaked Y, Sherrell RM, Falkowski PG. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol Oceanogr. 2001a;46:1249–1260. [Google Scholar]
- Berman-Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, Falkowski P. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 2001b;294:1534–1537. doi: 10.1126/science.1064082. [DOI] [PubMed] [Google Scholar]
- Berman-Frank I, Bidle KD, Haramaty L, Falkowski PG. The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol Oceanogr. 2004;49:997–1005. [Google Scholar]
- Beversdorf LJ, White AE, Bjorkman KM, Letelier RM, Karl DM. Phosphonate metabolism of Trichodesmium IMS101 and the production of greenhouse gases. Limnol Oceanogr. 2010;55:1768–1778. [Google Scholar]
- Breitbarth E, Oschlies A, LaRoche J. Physiological constraints on the global distribution of Trichodesmium – effect of temperature on diazotrophy. Biogeosciences. 2007;4:53–61. [Google Scholar]
- Brown CM, MacKinnon JD, Cockshutt AM, Villareal TA, Campbell DA. Flux capacities and acclimation costs in Trichodesmium from the Gulf of Mexico. Mar Biol. 2008;154:413–422. [Google Scholar]
- Bryceson I. 1980. Nitrogen fixation and the autecology of Oscillatoria erythraea (Ehrenberg) Kuetzing, a planktonic cyanophyte from the coastal waters of Tanzania: a preliminary investigation. Paper presented at Symposium on the coastal and marine environment of the Red Sea, Gulf of Aiden and tropical western Indian Ocean in Kartoum, Sudan.
- Bryceson I, Fay P. Nitrogen-fixation in OscillatoriaTrichodesmiumErythraea in relation to bundle formation and trichome differentiation. Mar Biol. 1981;61:159–166. [Google Scholar]
- Capone DG, Carpenter EJ. Nitrogen fixation in the marine environment. Science. 1982;217:1140–1142. doi: 10.1126/science.217.4565.1140. [DOI] [PubMed] [Google Scholar]
- Capone DG, O'Neil JM, Zehr J, Carpenter EJ. Basis for diel variation in nitrogenase activity in the marine planktonic cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1990;56:3532–3536. doi: 10.1128/aem.56.11.3532-3536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone DG, Ferrier MD, Carpenter EJ. Amino acid cycling in colonies of the planktonic marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol. 1994;60:3989–3995. doi: 10.1128/aem.60.11.3989-3995.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. Trichodesmium, a globally significant marine cyanobacterium. Science. 1997;276:1221–1229. [Google Scholar]
- Capone DG, Subramaniam A, Montoya JP, Voss M, Humborg C, Johansen AM, Siefert RL, Carpenter EJ. An extensive bloom of the N(2)-fixing cyanobacterium Trichodesmium erythraeum in the central Arabian Sea. Mar Ecol Prog Ser. 1998;172:281–292. [Google Scholar]
- Carpenter EJ. Physiology and ecology of marine planktonic oscillatoria (Trichodesmium. Mar Biol Lett. 1983;4:69–85. [Google Scholar]
- Carpenter EJ, Capone DG. Nitrogen fixation in the marine environment. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ, editors. Nitrogen in the Marine Environment. 2nd edn. San Diego, CA: Academic Press/Elsevier; 2008. pp. 141–198. [Google Scholar]
- Carpenter EJ, Price CC. Marine OscillatoriaTrichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976;191:1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Carpenter EJ, Roenneberg T. The marine planktonic cyanobacteria Trichodesmium Spp – photosynthetic rate measurements in the Sw Atlantic-Ocean. Mar Ecol Prog Ser. 1995;118:267–273. [Google Scholar]
- Carpenter EJ, Bergman B, Dawson R, Siddiqui PJ, Soderback E, Capone DG. Glutamine synthetase and nitrogen cycling in colonies of the marine diazotrophic cyanobacteria Trichodesmium spp. Appl Environ Microbiol. 1992;58:3122–3129. doi: 10.1128/aem.58.9.3122-3129.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castruita M, Saito M, Schottel PC, Elmegreen LA, Myneni S, Stiefel EI, Morel FMM. Overexpression and characterization of an iron storage and DNA-binding Dps protein from Trichodesmium erythraeum. Appl Environ Microbiol. 2006;72:2918–2924. doi: 10.1128/AEM.72.4.2918-2924.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PD, Webb EA. A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium. Environ Microbiol. 2010;12:13–27. doi: 10.1111/j.1462-2920.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- Chappell PD, Moffett JW, Hynes AM, Webb EA. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J. 2012;6:1728–1739. doi: 10.1038/ismej.2012.13. doi: 10.1038/ismej.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YB, Zehr JP, Mellon M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp IMS 101 in defined media: evidence for a circadian rhythm. J Phycol. 1996;32:916–923. [Google Scholar]
- Chen YB, Dominic B, Mellon MT, Zehr JP. Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101. J Bacteriol. 1998;180:3598–3605. doi: 10.1128/jb.180.14.3598-3605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YB, Dominic B, Zani S, Mellon MT, Zehr JP. Expression of photosynthesis genes in relation to nitrogen fixation in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101. Plant Mol Biol. 1999;41:89–104. doi: 10.1023/a:1006231805030. [DOI] [PubMed] [Google Scholar]
- Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. P Natl Acad Sci USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CS, McGillicuddy DJ. Transatlantic abundance of the N(2)-fixing colonial cyanobacterium Trichodesmium. Science. 2006;312:1517–1520. doi: 10.1126/science.1123570. [DOI] [PubMed] [Google Scholar]
- Díez B, Bergman B, Pedrós-Alió C, Antó M, Snoeijs P. High cyanobacterial nifH gene diversity in Arctic seawater and sea ice brine. Environ Microbiol Rep. 2012;4:360–366. doi: 10.1111/j.1758-2229.2012.00343.x. doi: 10.1111/j.1758-2229.2012.00343.x. [DOI] [PubMed] [Google Scholar]
- Dong G, Golden SS. How a cyanobacterium tells time. Curr Opin Microbiol. 2008;11:541–546. doi: 10.1016/j.mib.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugdale RC, Menzel DW, Ryther JH. Nitrogen fixation in the Sargasso Sea. Deep-Sea Res. 1961;7:297–300. [Google Scholar]
- Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB, Webb EA. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature. 2006;439:68–71. doi: 10.1038/nature04203. [DOI] [PubMed] [Google Scholar]
- Eberl R, Carpenter EJ. Association of the copepod Macrosetella gracilis with the cyanobacterium Trichodesmium spp. in the North Pacific Gyre. Mar Ecol Prog Ser. 2007;333:205–212. [Google Scholar]
- Ehrenberg EO. Neue Beobachtungen über blauartige Erscheinungen in Aegypten und Siberien nebst einer Ubersicht und Kritik der früher bekannten. Ann Phys Chem. 1830;18:477–514. [Google Scholar]
- El-Shehawy R, Lugomela C, Ernst A, Bergman B. Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum. Microbiology. 2003;149:1139–1146. doi: 10.1099/mic.0.26170-0. [DOI] [PubMed] [Google Scholar]
- Endean R, Monks SA, Griffith JK, Llewellyn LE. Apparent relationships between toxins elaborated by the cyanobacterium Trichodesmium erythraeum and those present in the flesh of the narrow-barred Spanish mackerel Scomberomorus commersoni. Toxicon. 1993;31:1155–1165. doi: 10.1016/0041-0101(93)90131-2. [DOI] [PubMed] [Google Scholar]
- Fay P, Stewart WDP, Walsby AE, Fogg GE. Is the heterocyst the site of nitrogen fixation in blue-green algae? Nature. 1968;220:810–812. doi: 10.1038/220810b0. [DOI] [PubMed] [Google Scholar]
- Finzi-Hart JA, Pett-Ridge J, Weber PK, Popa R, Fallon SJ, Gunderson T, Hutcheon ID, Nealson KH, Capone DG. Fixation and fate of C and N in the cyanobacterium Trichodesmium using nanometer-scale secondary ion mass spectrometry. P Natl Acad Sci USA. 2009;106:6345–6350. doi: 10.1073/pnas.0810547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H, Haselkorn R. Differentiation in Nostoc muscorum: nitrogenase is synthesized in heterocysts. P Natl Acad Sci USA. 1973;70:2727–2731. doi: 10.1073/pnas.70.10.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson C, Bergman B. Nitrogenase quantity varies diurnally in a subset of cells within colonies of the nonheterocystous cyanobacteria Trichodesmium Spp. Microbiology. 1995;141:2471–2478. [Google Scholar]
- Fredriksson C, Bergman B. Ultrastructural characterisation of cells specialised for nitrogen fixation in a non-heterocystous cyanobacterium, Trichodesmium spp. Protoplasma. 1997;197:76–85. [Google Scholar]
- Fu FX, Zhang YH, Bell PRF, Hutchins DA. Phosphate uptake and growth kinetics of Trichodesmium (Cyanobacteria) isolates from the North Atlantic Ocean and the Great Barrier Reef, Australia. J Phycol. 2005;41:62–73. [Google Scholar]
- Garcia NS, Fu F-X, Breene CL, Bernhardt PW, Mulholland MR, Sohm JA, Hutchins DA. Interactive effects of irradiance and CO2 on CO2 fixation and N2 fixation in the diazotroph Trichodesmium erythraeum (Cyanobacteria) J Phycol. 2011;47:1292–1303. doi: 10.1111/j.1529-8817.2011.01078.x. [DOI] [PubMed] [Google Scholar]
- Glibert PM, Bronk DA. Release of dissolved organic nitrogen by marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol. 1994;60:3996–4000. doi: 10.1128/aem.60.11.3996-4000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomont M. Monographie des oscillariees (Nostocacees Homocystees) I and II. Ann Sci Nat Bot Ser. 1892;7(15):263–368. pls. 1–14; 16:91–264, pls.1–7. [Google Scholar]
- Guo C, Tester PA. Toxic effect of the bloom-forming Trichodesmium sp. (Cyanophyta) to the copepod Acartia tonsa. Nat Toxins. 1994;2:222–227. doi: 10.1002/nt.2620020411. [DOI] [PubMed] [Google Scholar]
- Halm H, Lam P, Ferdelman TG, Lavik G, Dittmar T, LaRoche J, D'Hondt S, Kuypers MM. Heterotrophic organisms dominate nitrogen fixation in the South Pacific Gyre. ISME J. 2012;6:1238–1249. doi: 10.1038/ismej.2011.182. doi: 10.1038/ismej.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A, Muro-Pastor AM, Valladares A, Flores E. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol Rev. 2004;28:469–487. doi: 10.1016/j.femsre.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Hewson I, Govil SR, Capone DG, Carpenter EJ, Fuhrman JA. Evidence of Trichodesmium viral lysis and potential significance for biogeochemical cycling in the oligotrophic ocean. Aquat Microb Ecol. 2004;36:1–8. [Google Scholar]
- Hewson I, Poretsky RS, Dyhrman ST, Zielinski B, White AE, Tripp HJ, Montoya JP, Zehr JP. Microbial community gene expression within colonies of the diazotroph, Trichodesmium, from the Southwest Pacific Ocean. ISME J. 2009;3:1286–1300. doi: 10.1038/ismej.2009.75. [DOI] [PubMed] [Google Scholar]
- Holl CM, Montoya JP. Interactions between nitrate uptake and nitrogen fixation in continuous cultures of the marine diazotroph Trichodesmium (Cyanobacteria) J Phycol. 2005;41:1178–1183. [Google Scholar]
- Holtzendorff J, Partensky F, Jacquet S, Bruyant F, Marie D, Garczarek L, Mary I, Vaulot D, Hess WR. Diel expression of cell cycle-related genes in synchronized cultures of Prochlorococcus sp. strain PCC 9511. J Bacteriol. 2001;183:915–920. doi: 10.1128/JB.183.3.915-920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzendorff J, Marie D, Post AF, Partensky F, Rivlin A, Hess WR. Synchronized expression of ftsZ in natural Prochlorococcus populations of the Red Sea. Environ Microbiol. 2002;4:644–653. doi: 10.1046/j.1462-2920.2002.00347.x. [DOI] [PubMed] [Google Scholar]
- Hou Y, Lin S. Distinct gene number-genome size relationships for eukaryotes and non-eukaryotes: gene content estimation for dinoflagellate genomes. PLoS ONE. 2009;4:e6978. doi: 10.1371/journal.pone.0006978. doi: 10.1371/journal.pone.0006978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins DA, Fu FX, Zhang Y, Warner ME, Feng Y, Portune K, Bernhardt PW, Mulholland MR. CO2 control of Trichodesmium N-2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnol Oceanogr. 2007;52:1293–1304. [Google Scholar]
- Hynes AM, Webb EA, Doney SC, Waterbury JB. Comparison of cultured Trichodesmium (Cyanophyceae) with species characterized from the field. J Phycol. 2012;48:196–210. doi: 10.1111/j.1529-8817.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- Janson S, Carpenter EJ, Bergman B. Compartmentalization of nitrogenase in a nonheterocystous cyanobacterium – Trichodesmium contortum. FEMS Microbiol Lett. 1994;118:9–14. [Google Scholar]
- Janson S, Siddiqui PJA, Walsby AE, Romans KM, Carpenter EJ, Bergman B. Cytomorphological characterization of the planktonic diazotrophic cyanobacteria Trichodesmium Spp from the Indian-Ocean and Caribbean and Sargasso Seas. J Phycol. 1995;31:463–477. [Google Scholar]
- Jonasson S, Eriksson J, Berntzon L, Spácil Z, Ilag LL, Ronnevi LO, Rasmussen U, Bergman B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. P Natl Acad Sci USA. 2010;107:9252–9257. doi: 10.1073/pnas.0914417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana TM. Rapid oxygen cycling in Trichodesmium-Thiebautii. Limnol Oceanogr. 1993;38:18–24. [Google Scholar]
- Karl D, Michaels A, Bergman B, Capone D, Carpenter E, Letelier R, Lipschultz F, Paerl H, Sigman D, Stal L. Dinitrogen fixation in the world's oceans. Biogeochemistry. 2002;57/58:47–98. [Google Scholar]
- Kelman D, Ben-Amotz A, Berman-Frank I. Carotenoids provide the major antioxidant defence in the globally significant N2-fixing marine cyanobacterium Trichodesmium. Environ Microbiol. 2009;11:1897–1908. doi: 10.1111/j.1462-2920.2009.01913.x. [DOI] [PubMed] [Google Scholar]
- Kerbrat AS, Amzil Z, Pawlowiez R, Golubic S, Sibat M, Darius HT, Chinain M, Laurent D. First evidence of palytoxin and 42-hydroxy-palytoxin in the marine cyanobacterium Trichodesmium. Mar Drugs. 2011;9:543–560. doi: 10.3390/md9040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler GC, Martiny AC, Huang K, et al. Patterns and implications of gene gain and loss in the evolution of Prochlorococcus. PLoS Genet. 2007;3:e231. doi: 10.1371/journal.pgen.0030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komárek J, Anagnostidis K. Cyanoproparyota: 2. Teil/2nd Part: Oscillatoriales. Vol. 2. München: Elsevier Spektrum Akademischer Verlag; 2005. [Google Scholar]
- Kranz SA, Sultemeyer D, Richter KU, Rost B. Carbon acquisition by Trichodesmium: the effect of pCO(2) and diurnal changes. Limnol Oceanogr. 2009;54:548–559. [Google Scholar]
- Kranz SA, Levitan O, Richter KU, Prasil O, Berman-Frank I, Rost B. Combined effects of CO(2) and light on the N(2)-fixing cyanobacterium Trichodesmium IMS101: physiological responses. Plant Physiol. 2010;154:334–345. doi: 10.1104/pp.110.159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz SA, Eichner M, Rost B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth Res. 2011;109:73–84. doi: 10.1007/s11120-010-9611-3. [DOI] [PubMed] [Google Scholar]
- Kromkamp J, Walsby AE. Buoyancy regulation vertical migration of Trichodesmium: a computer model prediction. In: Carpenter EJ, Capone DG, Rueter JG, editors. Marine pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs. Norwell, MA: Kluwer Academic Publishers; 1992. pp. 219–237. [Google Scholar]
- Kumar K, Mella-Herrera RA, Golden JW. Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol. 2010;2:a000315. doi: 10.1101/cshperspect.a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Ferimazova N, Setlik I, Berman-Frank I. Traffic lights in Trichodesmium. Regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiol. 2004;135:2120–2133. doi: 10.1104/pp.104.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpper H, Setlik I, Seibert S, Prásil O, Setlikova E, Strittmatter M, Levitan O, Lohscheider J, Adamska I, Berman-Frank I. Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. New Phytol. 2008;179:784–798. doi: 10.1111/j.1469-8137.2008.02497.x. [DOI] [PubMed] [Google Scholar]
- Küpper H, Andresen E, Wiegert S, Simek M, Leitenmaier B, Setlik I. Reversible coupling of individual phycobiliprotein isoforms during state transitions in the cyanobacterium Trichodesmium analysed by single-cell fluorescence kinetic measurements. BBA-Bioenergetics. 2009;1787:155–167. doi: 10.1016/j.bbabio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Kustka AB, Sanudo-Wilhelmy SA, Carpenter EJ, Capone D, Burns J, Sunda WG. Iron requirements for dinitrogen- and ammonium-supported growth in cultures of Trichodesmium (IMS 101): comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnol Oceanogr. 2003;48:1869–1884. [Google Scholar]
- Langlois RJ, LaRoche J, Raab PA. Diazotrophic diversity and distribution in the tropical and subtropical Atlantic ocean. Appl Environ Microbiol. 2005;71:7910–7919. doi: 10.1128/AEM.71.12.7910-7919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois RJ, Hummer D, LaRoche J. Abundances and distributions of the dominant nifH phylotypes in the Northern Atlantic Ocean. Appl Environ Microbiol. 2008;74:1922–1931. doi: 10.1128/AEM.01720-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRoche J, Breitbarth E. Importance of the diazotrophs as a source of new nitrogen in the ocean. J Sea Res. 2005;53:67–91. [Google Scholar]
- Larsson J, Nylander JA, Bergman B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol Biol. 2011;11:187. doi: 10.1186/1471-2148-11-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latysheva N, Junker VL, Palmer WJ, Codd GA, Barker D. The evolution of nitrogen fixation in cyanobacteria. Bioinformatics. 2012;28:603–606. doi: 10.1093/bioinformatics/bts008. [DOI] [PubMed] [Google Scholar]
- Layton BE, D'Souza AJ, Dampier W, Zeiger A, Sabur A, Jean-Charles J. Collagen's triglycine repeat number and phylogeny suggest an interdomain transfer event from a devonian or silurian organism into Trichodesmium erythraeum. J Mol Evol. 2008;66:539–554. doi: 10.1007/s00239-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmermann E. Ergebnisse einer Reise nach dem Pacific. Abh Natwiss Ver Brem. 1900;16:313–406. [Google Scholar]
- Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, Klepetar J, Prasil O, Berman-Frank I. Elevated CO(2) enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob Change Biol. 2007;13:531–538. [Google Scholar]
- Levitan O, Sudhaus S, LaRoche J, Berman-Frank I. The influence of pCO(2) and temperature on gene expression of carbon and nitrogen pathways in Trichodesmium IMS101. PLoS ONE. 2010;5:e15104. doi: 10.1371/journal.pone.0015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Lee M. Cellular-differentiation in the trichome of Trichodesmium-Thiebautii (Cyanophyta) Bot Mar. 1990;33:347–353. [Google Scholar]
- Lin S, Henze S, Lundgren P, Bergman B, Carpenter EJ. Whole-cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Appl Environ Microbiol. 1998;64:3052–3058. doi: 10.1128/aem.64.8.3052-3058.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Sandh G, Zhang H, Cheng J, Perkins K, Carpenter EJ, Bergman B. Two flavodoxin genes in Trichodesmium (Oscillatoriales, Cyanophyceae): remarkable sequence divergence and possible functional diversification. J Exp Mar Biol Ecol. 2009;371:93–101. [Google Scholar]
- Lundgren P, Soderback E, Singer A, Carpenter EJ, Bergman B. Katagnymene: characterization of a novel marine diazotroph. J Phycol. 2001;37:1052–1062. [Google Scholar]
- Lundgren P, Bauer K, Lugomela C, Söderbäck E, Bergman B. Reevaluation of the nitrogen fixation behavior in the marine non-heterocystous cyanobacterium Lyngbya Majuscula. J Phycol. 2003;39:310–314. [Google Scholar]
- Lundgren P, Janson S, Jonasson S, Singer A, Bergman B. Unveiling of novel radiations within Trichodesmium cluster by hetR gene sequence analysis. Appl Environ Microbiol. 2005;71:190–196. doi: 10.1128/AEM.71.1.190-196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey C, Michaels AF, Capone DG. The conundrum of marine N2 fixation. Am J Sci. 2005;305:546–595. [Google Scholar]
- Mariscal V, Herrero A, Nenninger A, Mullineaux CW, Flores E. Functional dissection of the three-domain SepJ protein joining the cells in cyanobacterial trichomes. Mol Microbiol. 2011;79:1077–1088. doi: 10.1111/j.1365-2958.2010.07508.x. [DOI] [PubMed] [Google Scholar]
- Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J Phycol. 2007;43:845–852. [Google Scholar]
- Mills MM, Ridame C, Davey M, La Roche J, Geider RJ. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature. 2004;429:292–294. doi: 10.1038/nature02550. [DOI] [PubMed] [Google Scholar]
- Mira A, Ochman H, Moran NA. Deletional bias and the evolution of bacterial genomes. Trends Genet. 2001;17:589–596. doi: 10.1016/s0168-9525(01)02447-7. [DOI] [PubMed] [Google Scholar]
- Moisander PH, Beinart RA, Hewson I, White AE, Johnson KS, Carlson CA, Montoya JP, Zehr JP. Unicellular cyanobacterial distributions broaden the oceanic N2 fixation domain. Science. 2010;327:1512–1514. doi: 10.1126/science.1185468. [DOI] [PubMed] [Google Scholar]
- Montoya JP, Carpenter EJ, Capone DG. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol Oceanogr. 2002;47:1617–1628. [Google Scholar]
- Montoya JP, Holl CM, Zehr JP, Hansen A, Villareal TA, Capone DG. High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature. 2004;430:1027–1032. doi: 10.1038/nature02824. [DOI] [PubMed] [Google Scholar]
- Moore CM, Mills MM, Achterberg EP, et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat Geosci. 2009;2:867–871. [Google Scholar]
- Mulholland MR. The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosciences. 2007;4:37–51. [Google Scholar]
- Mulholland MR, Capone DG. Stoichiometry of nitrogen and carbon utilization in cultured populations of Trichodesmium IMS101: implications for growth. Limnol Oceanogr. 2001;46:436–443. [Google Scholar]
- Mulholland MR, Ohki K, Capone DG. Nutrient controls on nitrogen uptake and metabolism by natural populations and cultures of Trichodesmium (Cyanobacteria) J Phycol. 2001;37:1001–1009. [Google Scholar]
- Mulholland MR, Bronk DA, Capone DG. Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat Microb Ecol. 2004;37:85–94. [Google Scholar]
- Negri AP, Bunter O, Jones B, Llewellyn L. Effects of the bloom-forming alga Trichodesmium erythraeum on the pearl oyster Pinctada maxima. Aquaculture. 2004;232:91–102. [Google Scholar]
- Ohki K. Intercellular localization of nitrogenase in a non-heterocystous cyanobacterium (cyanophyte), Trichodesmium sp NIBB1067. J Oceanogr. 2008;64:211–216. [Google Scholar]
- Ohki K, Zehr JP, Falkowski PG, Fujita Y. Regulation of nitrogen-fixation by different nitrogen-sources in the marine nonheterocystous cyanobacterium Trichodesmium Sp Nibb1067. Arch Microbiol. 1991;156:335–337. [Google Scholar]
- Oneil JM, Roman MR. Ingestion of the Cyanobacterium Trichodesmium Spp by Pelagic Harpacticoid Copepods Macrosetella, Miracia and Oculostella. Hydrobiologia. 1994;293:235–240. [Google Scholar]
- Orchard E, Webb E, Dyhrman S. Characterization of phosphorus-regulated genes in Trichodesmium spp. Biol Bull. 2003;205:230–231. doi: 10.2307/1543268. [DOI] [PubMed] [Google Scholar]
- Orchard ED, Webb EA, Dyhrman ST. Molecular analysis of the phosphorus starvation response in Trichodesmium spp. Environ Microbiol. 2009;11:2400–2411. doi: 10.1111/j.1462-2920.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- Orchard ED, Benitez-Nelson CR, Pellechia PJ, Lomas MW, Dyhrman ST. Polyphosphate in Trichodesmium from the low-phosphorus Sargasso Sea. Limnol Oceanogr. 2010;55:2161–2169. [Google Scholar]
- Orcutt KM, Rasmussen U, Webb EA, Waterbury JB, Gundersen K, Bergman B. Characterization of Trichodesmium spp. by genetic techniques. Appl Environ Microbiol. 2002;68:2236–2245. doi: 10.1128/AEM.68.5.2236-2245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt KM, Ren SS, Gundersen K. Detecting proteins in highly autofluorescent cells using quantum dot antibody conjugates. Sensors. 2009;9:7540–7549. doi: 10.3390/s90907540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW. Microscale physiological and ecological studies of aquatic cyanobacteria: macroscale implications. Microsc Res Tech. 1996;33:47–72. doi: 10.1002/(SICI)1097-0029(199601)33:1<47::AID-JEMT6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Bebaut BM. Direct measurement of O2-depleted microzones in marine oscillatoria: relation to N2 fixation. Science. 1988;22:442–445. doi: 10.1126/science.241.4864.442. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Priscu JC, Brawner DL. Immunochemical localization of nitrogenase in marine Trichodesmium aggregates: relationship to N(2) fixation potential. Appl Environ Microbiol. 1989;55:2965–2975. doi: 10.1128/aem.55.11.2965-2975.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenik B, Ren Q, Dupont CL, et al. Genome sequence of Synechococcus CC9311: insights into adaptation to a coastal environment. P Natl Acad Sci USA. 2006;103:13555–13559. doi: 10.1073/pnas.0602963103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partensky F, Hess WR, Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev. 1999;63:106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug H, Musat N, Adam B, Moraru CL, Lavik G, Vagner T, Bergman B, Kuypers MM. Carbon and nitrogen fluxes associated with the cyanobacterium Aphanizomenon sp. in the Baltic Sea. ISME J. 2010;4:1215–1223. doi: 10.1038/ismej.2010.53. [DOI] [PubMed] [Google Scholar]
- Popa R, Weber PK, Pett-Ridge J, Finzi JA, Fallon SJ, Hutcheon ID, Nealson KH, Capone DG. Carbon and nitrogen fixation and metabolite exchange in and between individual cells of Anabaena oscillarioides. ISME J. 2007;1:354–360. doi: 10.1038/ismej.2007.44. [DOI] [PubMed] [Google Scholar]
- Post AF, Rihtman B, Wang Q. Decoupling of ammonium regulation and ntcA transcription in the diazotrophic marine cyanobacterium Trichodesmium sp. IMS101. ISME J. 2012;6:629–637. doi: 10.1038/ismej.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufert-Bebout L, Paerl HW, Lassen C. Growth, nitrogen fixation, and spectral attenuation in cultivated Trichodesmium species. Appl Environ Microbiol. 1993;59:1367–1375. doi: 10.1128/aem.59.5.1367-1375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JBE, Biswas H, Schulz KG, LaRoche J, Riebesell U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochem Cycles. 2007;21:GB2028. 6 PP., doi: 10.1029/2006GB002898. [Google Scholar]
- Rijkenberg MJ, Langlois RJ, Mills MM, Patey MD, Hill PG, Nielsdottir MC, Compton TJ, LaRoche J, Achterberg EP. Environmental forcing of nitrogen fixation in the eastern tropical and sub-tropical North Atlantic Ocean. PLoS ONE. 2011;6:e28989. doi: 10.1371/journal.pone.0028989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe KL, Barbeau K, Mann EL, Haygood MG. Acquisition of iron by Trichodesmium and associated bacteria in culture. Environ Microbiol. 2011;14:1681–1695. doi: 10.1111/j.1462-2920.2011.02653.x. [DOI] [PubMed] [Google Scholar]
- Roman MR. Ingestion of blue-green-alga Trichodesmium by harpacticoid copepod, macrosetella-gracilis. Limnol Oceanogr. 1978;23:1245–1248. [Google Scholar]
- Romans KM, Carpenter EJ, Bergman B. Buoyancy regulation in the colonial diazotrophic cyanobacterium Trichodesmium Tenue – ultrastructure and storage of carbohydrate, polyphosphate, and nitrogen. J Phycol. 1994;30:935–942. [Google Scholar]
- Rosetti V, Bagheri HC. Advantages of the division of labour for the long-term population dynamics of cyanobacteria at different latitudes. Proc Biol Sci. 2012;279:3457–3466. doi: 10.1098/rspb.2012.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin M, Berman-Frank I, Shaked Y. Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat Geosci. 2011;4:529–534. [Google Scholar]
- Rust MJ, Golden SS, O'Shea EK. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science. 2011;331:220–223. doi: 10.1126/science.1197243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandh G, El-Shehawy R, Diez B, Bergman B. Temporal separation of cell division and diazotrophy in the marine diazotrophic cyanobacterium Trichodesmium erythraeum IMS101. FEMS Microbiol Lett. 2009;295:281–288. doi: 10.1111/j.1574-6968.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- Sandh G, Ran L, Xu L, Sundqvist G, Bulone V, Bergman B. Comparative proteomic profiles of the marine cyanobacterium Trichodesmium erythraeum IMS101 under different nitrogen regimes. Proteomics. 2011;11:406–419. doi: 10.1002/pmic.201000382. [DOI] [PubMed] [Google Scholar]
- Sandh G, Xu L, Bergman B. Diazocyte development in the marine diazotrophic cyanobacterium Trichodesmium. Microbiology. 2012;158:345–352. doi: 10.1099/mic.0.051268-0. [DOI] [PubMed] [Google Scholar]
- Sandmann G, Peleato ML, Fillat MF, Lazaro MC, Gomez-Moreno C. Consequences of the iron-dependent formation of ferredoxin and flavodoxin on photosynthesis and nitrogen fixation on Anabaena strains. Photosynth Res. 1990;26:119–125. doi: 10.1007/BF00047083. [DOI] [PubMed] [Google Scholar]
- Sanudo-Wilhelmy SA, Kustka AB, Gobler CJ, Hutchins DA, Yang M, Lwiza K, Burns J, Capone DG, Raven JA, Carpenter EJ. Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature. 2001;411:66–69. doi: 10.1038/35075041. [DOI] [PubMed] [Google Scholar]
- Scanlan DJ, Ostrowski M, Mazard S, Dufresne A, Garczarek L, Hess WR, Post AF, Hagemann M, Paulsen I, Partensky F. Ecological genomics of marine Picocyanobacteria. Microbiol Mol Biol Rev. 2009;73:249–299. doi: 10.1128/MMBR.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmeister BE, Antonelli A, Bagheri HC. The origin of multicellularity in cyanobacteria. BMC Evol Biol. 2011;11:45. doi: 10.1186/1471-2148-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock TB, Huncik K, Beauchesne KR, Villareal TA, Moeller PD. Identification of trichotoxin, a novel chlorinated compound associated with the bloom forming Cyanobacterium, Trichodesmium thiebautii. Environ Sci Technol. 2011;45:7503–7509. doi: 10.1021/es201034r. [DOI] [PubMed] [Google Scholar]
- Shi T, Sun Y, Falkowski PG. Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS101. Environ Microbiol. 2007;9:2945–2956. doi: 10.1111/j.1462-2920.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tyson GW, DeLong EF. Metatranscriptomics reveals unique microbial small RNAs in the ocean's water column. Nature. 2009;459:266–269. doi: 10.1038/nature08055. [DOI] [PubMed] [Google Scholar]
- Shi T, Ilikchyan I, Rabouille S, Zehr JP. Genome-wide analysis of diel gene expression in the unicellular N(2)-fixing cyanobacterium Crocosphaera watsonii WH 8501. ISME J. 2010;4:621–632. doi: 10.1038/ismej.2009.148. [DOI] [PubMed] [Google Scholar]
- Sohm JA, Mahaffey C, Capone DG. Assessment of relative phosphorus limitation of Trichodesmium spp. in the North Pacific, North Atlantic, and the north coast of Australia. Limnol Oceanogr. 2008;53:2495–2502. [Google Scholar]
- Sohm JA, Webb EA, Capone DG. Emerging patterns of marine nitrogen fixation. Nat Rev Microbiol. 2011a;9:499–508. doi: 10.1038/nrmicro2594. [DOI] [PubMed] [Google Scholar]
- Sohm JA, Subramaniam A, Gunderson T, Carpenter EJ, Capone DG. Nitrogen fixation by Trichodesmium spp. and unicellular diazotrophs in the North Pacific Subtropical Gyre. J Geophys Res Biogeo. 2011b;116:G03002. doi: 10.1029/2010JG001513. [Google Scholar]
- Staal M, Meysman FJ, Stal LJ. Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans. Nature. 2003;425:504–507. doi: 10.1038/nature01999. [DOI] [PubMed] [Google Scholar]
- Staal M, Rabouille S, Stal LJ. On the role of oxygen for nitrogen fixation in the marine cyanobacterium Trichodesmium sp. Environ Microbiol. 2007;9:727–736. doi: 10.1111/j.1462-2920.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- Stal LJ. Is the distribution of nitrogen-fixing cyanobacteria in the oceans related to temperature? Environ Microbiol. 2009;11:1632–1645. doi: 10.1111/j.1758-2229.2009.00016.x. [DOI] [PubMed] [Google Scholar]
- Stihl A, Sommer U, Post AF. Alkaline phosphatase activities among populations of the colony-forming diazotrophic cyanobacterium Trichodesmium spp. (cyanobacteria) in the Red Sea. J Phycol. 2001;37:310–317. [Google Scholar]
- Stockel J, Welsh EA, Liberton M, Kunnvakkam R, Aurora R, Pakrasi HB. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. P Natl Acad Sci USA. 2008;105:6156–6161. doi: 10.1073/pnas.0711068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam A, Brown CW, Hood RR, Carpenter EJ, Capone DG. Detecting Trichodesmium blooms in SeaWiFS imagery. Deep Sea Res. 2002;49(Pt II):107–121. [Google Scholar]
- Swingley WD, Chen M, Cheung PC, et al. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. P Natl Acad Sci USA. 2008;105:2005–2010. doi: 10.1073/pnas.0709772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treangen TJ, Rocha EP. Horizontal transfer, not duplication, drives the expansion of protein families in prokaryotes. PLoS Genet. 2011;7:e1001284. doi: 10.1371/journal.pgen.1001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk KA, Rees AP, Zehr JP, Pereira N, Swift P, Shelley R, Lohan M, Woodward EM, Gilbert J. Nitrogen fixation and nitrogenase (nifH) expression in tropical waters of the eastern North Atlantic. ISME J. 2011;5:1201–1212. doi: 10.1038/ismej.2010.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell T, Maranon E, Poulton AJ, Bowie AR, Harbour DS, Woodward EMS. Large-scale latitudinal distribution of Trichodesmium spp. in the Atlantic Ocean. J Plankton Res. 2003;25:405–416. [Google Scholar]
- Van Mooy BA, Fredricks HF, Pedler BE, et al. Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature. 2009;458:69–72. doi: 10.1038/nature07659. [DOI] [PubMed] [Google Scholar]
- Van Mooy BA, Hmelo LR, Sofen LE, Campagna SR, May AL, Dyhrman ST, Heithoff A, Webb EA, Momper L, Mincer TJ. Quorum sensing control of phosphorus acquisition in Trichodesmium consortia. ISME J. 2012;6:422–429. doi: 10.1038/ismej.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verne J. Vingt mille lieues sous les mers. France: Pierre-Jules Hetzel; 1869. [Google Scholar]
- Verne J. Twenty Thousand Leagues under The Sea. London, England: Sampson Low & Co; 1872. [Google Scholar]
- Villareal TA, Carpenter EJ. Buoyancy regulation and the potential for vertical migration in the oceanic cyanobacterium Trichodesmium. Microb Ecol. 2003;45:1–10. doi: 10.1007/s00248-002-1012-5. [DOI] [PubMed] [Google Scholar]
- Webb EA, Moffett JW, Waterbury JB. Iron stress in open-ocean cyanobacteria (Synechococcus Trichodesmium, and Crocosphaera spp.): identification of the IdiA protein. Appl Environ Microbiol. 2001;67:5444–5452. doi: 10.1128/AEM.67.12.5444-5452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry TK, Siegel DA. Spatial and temporal distribution of Trichodesmium blooms in the world's oceans. Global Biogeochem Cycles. 2006;20:GB4016. doi: 10.1029/2005GB002673. [Google Scholar]
- White AE, Spitz YH, Karl DM, Letelier RM. Flexible elemental stoichiometry in Trichodesmium spp. and its ecological implications. Limnol Oceanogr. 2006a;51:1777–1790. [Google Scholar]
- White AE, Spitz YH, Letelier RM. Modeling carbohydrate ballasting by Trichodesmium spp. Mar Ecol Prog Ser. 2006b;323:35–45. [Google Scholar]
- White AE, Karl DM, Bjorkman KM, Beversdorf LJ, Letelier RM. Production of organic matter by Trichodesmium IMS101 as a function of phosphorus source. Limnol Oceanogr. 2010;55:1755–1767. [Google Scholar]
- Whittaker S, Bidle KD, Kustka AB, Falkowski PG. Quantification of nitrogenase in Trichodesmium IMS 101: implications for iron limitation of nitrogen fixation in the ocean. Environ Microbiol Rep. 2011;3:54–58. doi: 10.1111/j.1758-2229.2010.00187.x. [DOI] [PubMed] [Google Scholar]
- Wilcox M, Mitchison GJ, Smith RJ. Mutants of Anabaena cylindrica altered in heterocyst spacing. Arch Microbiol. 1975;103:219–223. doi: 10.1007/BF00436353. [DOI] [PubMed] [Google Scholar]
- Wille N. Die Schizophyceen der plankton-expedition. In: Hensen V, editor. Ergebnisse der Plankton-Expedition der Humbol-Stiftung. Germany: Lipsius and Tischer, Kiel and Leipzig; 1904. p. 88. [Google Scholar]
- Yooseph S, Nealson KH, Rusch DB, et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature. 2010;468:60–66. doi: 10.1038/nature09530. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Kudela RM. Nitrogen cycle of the open ocean: from genes to ecosystems. Ann Rev Mar Sci. 2011;3:197–225. doi: 10.1146/annurev-marine-120709-142819. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Limberger RJ, Ohki K, Fujita Y. Antiserum to nitrogenase generated from an amplified DNA fragment from natural populations of Trichodesmium spp. Appl Environ Microbiol. 1990;56:3527–3531. doi: 10.1128/aem.56.11.3527-3531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JP, Wyman M, Miller V, Duguay L, Capone DG. Modification of the Fe protein of nitrogenase in natural populations of Trichodesmium thiebautii. Appl Environ Microbiol. 1993;59:669–676. doi: 10.1128/aem.59.3.669-676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JP, Harris D, Dominic B, Salerno J. Structural analysis of the Trichodesmium nitrogenase iron protein: implications for aerobic nitrogen fixation activity. FEMS Microbiol Lett. 1997;153:303–309. doi: 10.1111/j.1574-6968.1997.tb12589.x. [DOI] [PubMed] [Google Scholar]
- Zehr JP, Waterbury JB, Turner PJ, Montoya JP, Omoregie E, Steward GF, Hansen A, Karl DM. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean. Nature. 2001;412:635–638. doi: 10.1038/35088063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


