Abstract
Peridinin is known as the main light-harvesting pigment in photosynthesis in the sea and exhibits exceptionally high energy transfer efficiencies to chlorophyll a. This energy transfer efficiency is thought to be related to the intricate structure of peridinin, which possesses allene and ylidenbutenolide functions in the polyene backbone. There are, however, no studies on the relationship between the structural features of peridinin and its super ability for energy transfer. We then focused on the subjects of why peridinin possesses a unique allene group and how the allene function plays a role in the exceptionally high energy transfer. Toward elucidation of the exact role of the allene function, we now describe the syntheses of three relatively unstable allene-modified derivatives of peridinin along with the results of the Stark spectroscopy of peridinin and the synthesized peridinin derivatives.
Introduction
Peridinin (1) was first isolated in 1890 from planktonic algae dinoflagellates, which are causally linked to red tides,1 and displays an atypical C37 carbon skeleton for carotenoids possessing an allene and a ylidenbutenolide function in conjugation with a π-electron conjugated system.2 This highly functionalized C37-norcarotenoid is a very attractive target molecule for synthetic organic chemists because of both its characteristic structure and its tremendous ability for highly effective energy transfer in photosynthesis. The first total synthesis of enantiomerically pure peridinin was achieved by Ito et al. in 1990.3 We reported the second total synthesis of peridinin in 2002, featuring high stereochemical control of the six asymmetric carbons and the geometry of the seven double bonds in the molecule.4 The third total synthesis was described by Brückner’s group in 2006, employing a novel strategy using (+)-diethyl tartrate and (−)-actinol,5 and the fourth one was achieved by de Lera’s group in 2007, featuring three-component coupling using a dihalogenated C8 linchpin unit.6
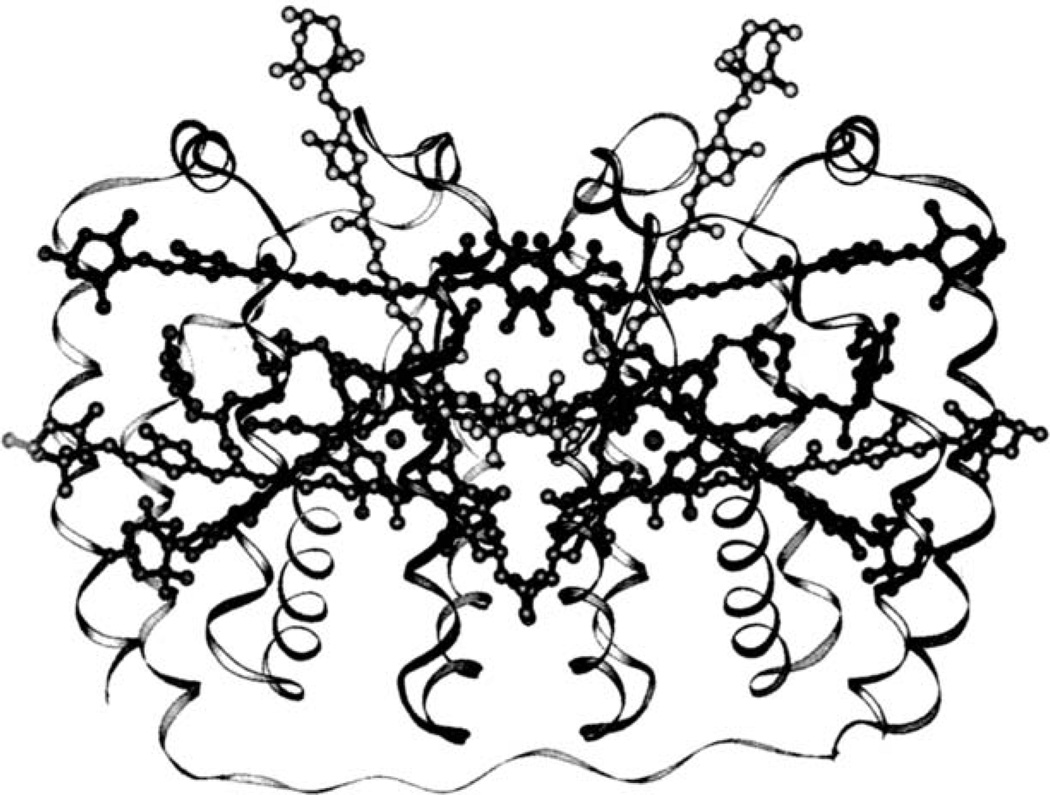
This carotenoid has been known as the main light-harvesting pigment in photosynthesis in the sea and forms the peridinin-chlorophyll a (Chl a)-protein (PCP) complex found in dinoflagellates. The crystal structure of the main form of the PCP trimer from Amphidinium carterae was determined by X-ray crystallography as shown in Fig. 1.7 Each of the polypeptides binds eight peridinin molecules and two Chl a molecules, and the allene function of peridinin exists in the center of the PCP. In this complex, a so-called antenna pigment, peridinin exhibits exceptionally high (>95%) energy transfer efficiencies to Chl a.8 In addition, the presence of an intramolecular charge transfer (ICT) excited state of peridinin has been proposed based on an anomalous strong solvent dependence of its singlet excited state lifetime.9 This particular excited state is thought to be related to the intricate structure of peridinin, which has allene and ylidenbutenolide functions in the polyene backbone. In particular, the ylidenbutenolide function provides the molecule asymmetry and produces an evident dipole moment in the molecule.10 Recently, it was proposed that the presence of the ICT excited state promotes dipolar interactions with Chl a in the PCP complex and facilitates energy transfer via a dipole mechanism.11 Furthermore, the Stark spectroscopy of peridinin was studied to try to determine the change in electrostatic properties produced on excitation within the absorption band.10 However, the precise nature of the ICT excited state and its role in light-harvesting is not yet entirely clear, and there are no studies on the relation between the structural features of peridinin and its super ability for the energy transfer in the PCP complex. In particular, no studies on the function of the allene group have been reported so far, probably because the synthesis of various kinds of desired peridinin derivatives has not been easy. We have therefore focused on the subjects of why peridinin possesses a unique allene group and how the allene function plays a role in the exceptionally high energy transfer and began with the synthesis of allene-modified derivatives of peridinin.
Fig. 1.
Crystal structure of PCP complex.
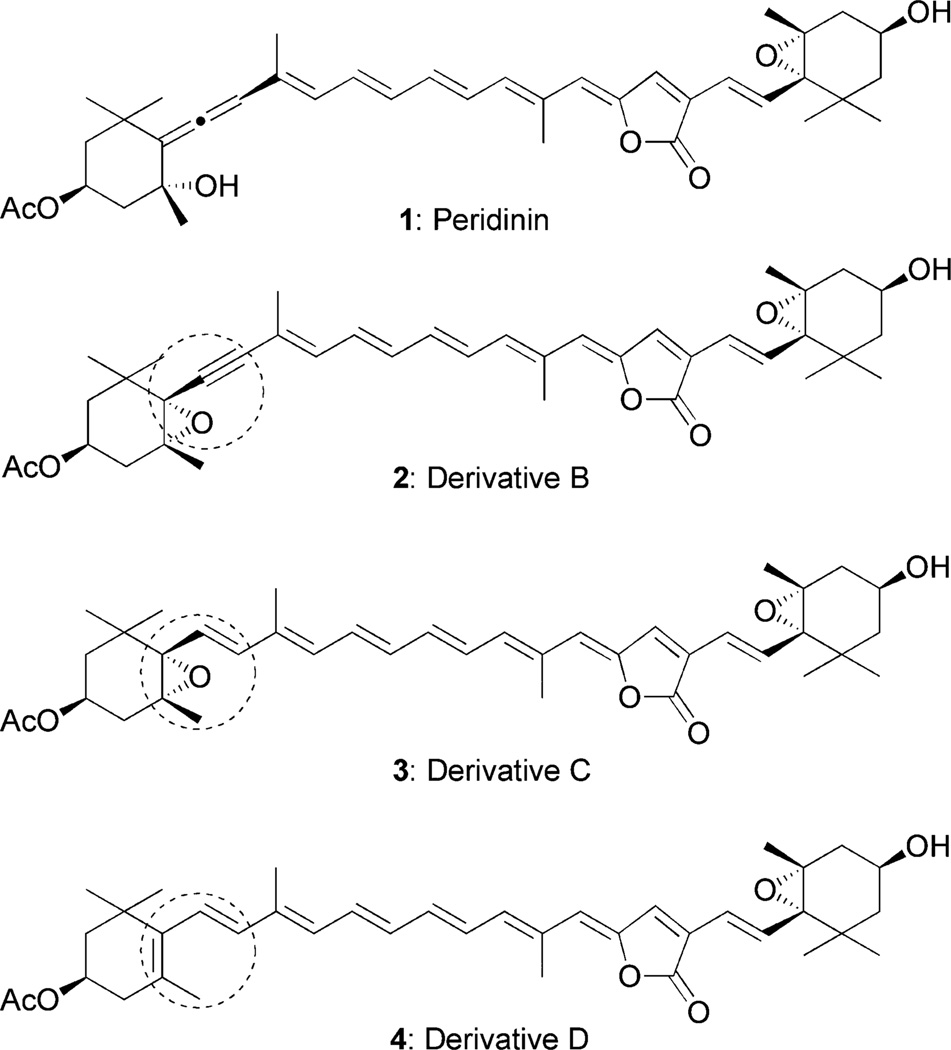
In order to understand the exact roles of the allene group, we designed the peridinin derivatives B, C, and D. Derivative B (2) possesses an epoxy-acetylene, derivative C (3) has an epoxy-olefin, and derivative D (4) has a conjugating olefin group instead of the hydroxy-allene group (Fig. 2). These derivatives would provide useful information on the roles of the allene group by comparing the Stark spectroscopy and solvent dependence data of their singlet excited state lifetimes with those of peridinin. We now describe the results of the synthetic studies on these peridinin derivatives and their Stark spectroscopy results compared with those of peridinin.
Fig. 2.
Structures of peridinin derivatives.
Results
Retrosynthetic analysis
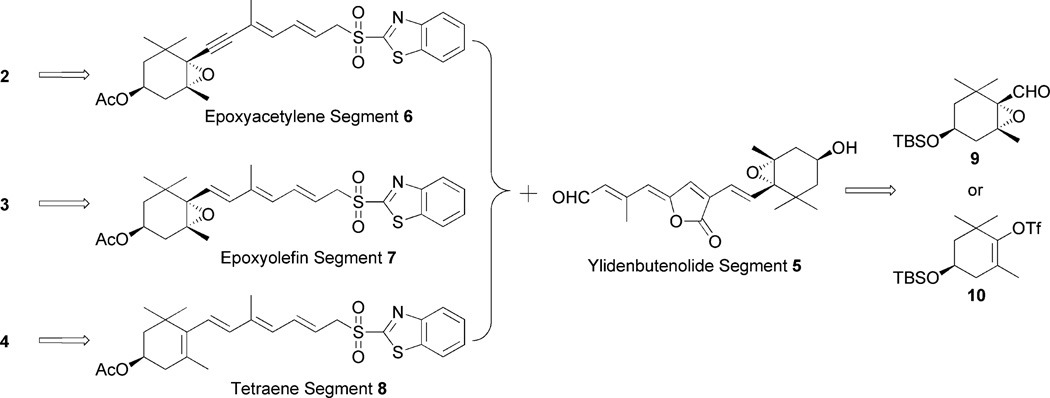
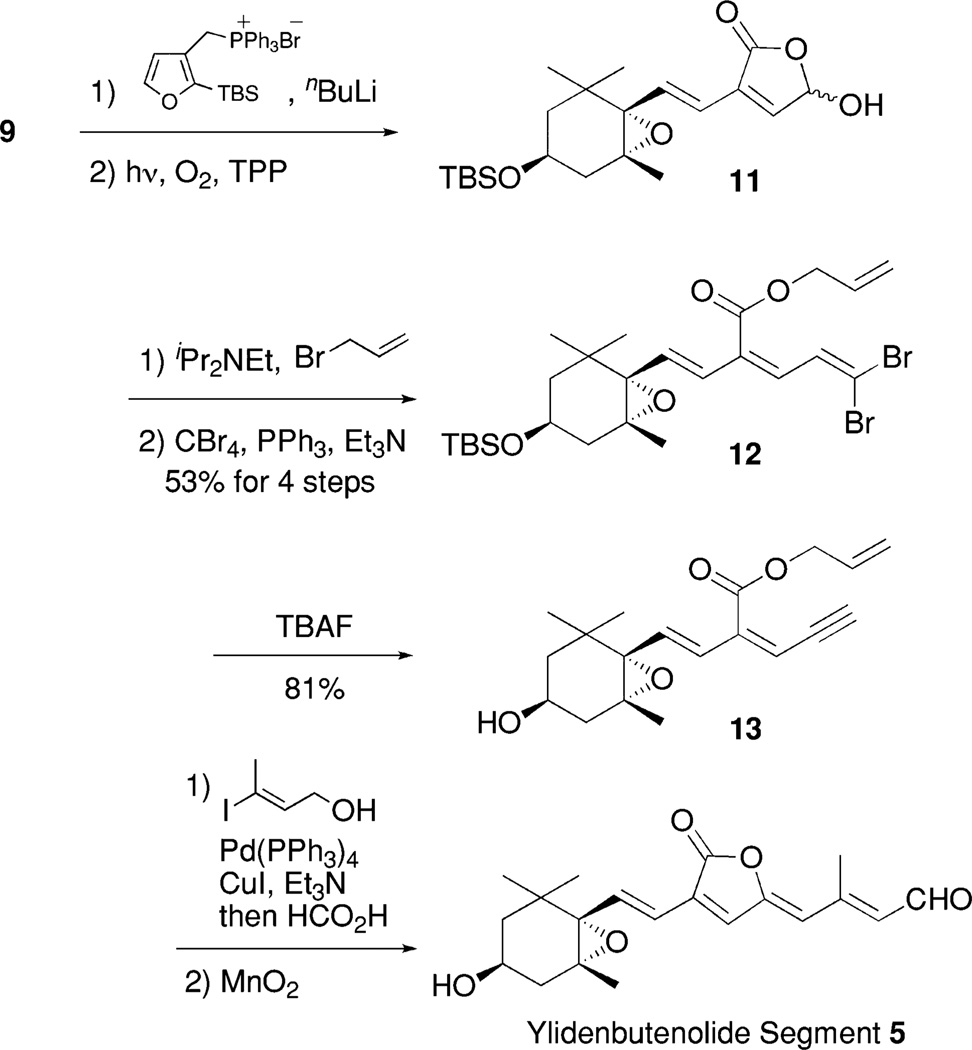
According to the stereocontrolled synthesis of peridinin, which we previously established,4 we planned to synthesize three peridinin derivatives B, C, and D by coupling between the common ylidenbutenolide half-segment 54 and the corresponding allene-modified half-segments 6, 7, and 8 using the modified Julia olefination reaction, respectively (Fig. 3). Both half-segments would be synthesized from the optically homogenous epoxyaldehyde derivative 912 and (−)-vinyltriflate 10,3b,c both of which had been prepared from (−)-actinol. The outline of the synthesis of ylidenbutenolide segment 5 is described in Scheme 1 as reported previously.4 This synthesis includes a Pd-catalyzed three-step one-pot ylidenbutenolide formation from allylester 13, which was synthesized from 9 in a stereocontrolled manner.
Fig. 3.
Retrosynthetic analysis.
Scheme 1.
Synthesis of ylidenbutenolide segment.
Synthesis of peridinin derivative B (2)
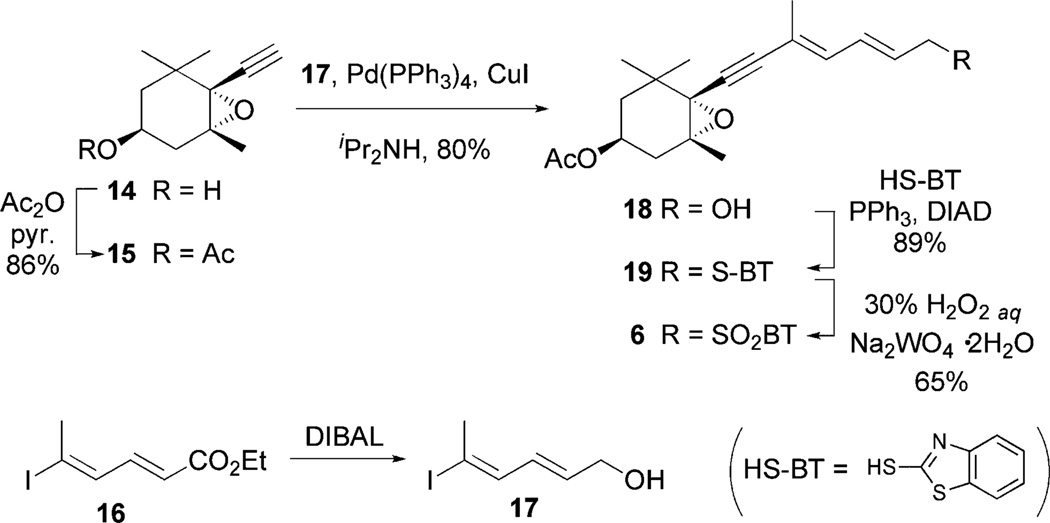
First, the synthesis of derivative B (2) is described. The stereocontrolled synthesis of the epoxyacetylene half-segment 6 is shown in Scheme 2. Acetoxy derivative 15 was prepared starting from (−)-epoxyaldehyde 9 through alcohol 1413 in 53% overall yield. Meanwhile, vinyl iodide 17, which is a component of 6, was prepared from known ester 164 by DIBAL reduction. The resulting vinyl iodide 17 was not purified because of its instability. Sonogashira cross-coupling14 between 15 and 17 in the presence of catalytic amounts of Pd(PPh3)4 and CuI in diisopropylamine produced the desired alcohol 18 in 80% yield, which was transformed into the acetylene segment 6 using the Mitsunobu reaction with 2-mercaptobenzothiazole, followed by oxidation of the resulting sulfide with aqueous 30%H2O2 and Na2WO4·2H2O.15 The all-trans structure depicted for 6 was confirmed by 1H NMR spectroscopic analysis.
Scheme 2.
Synthesis of epoxyacetylene segment 6.
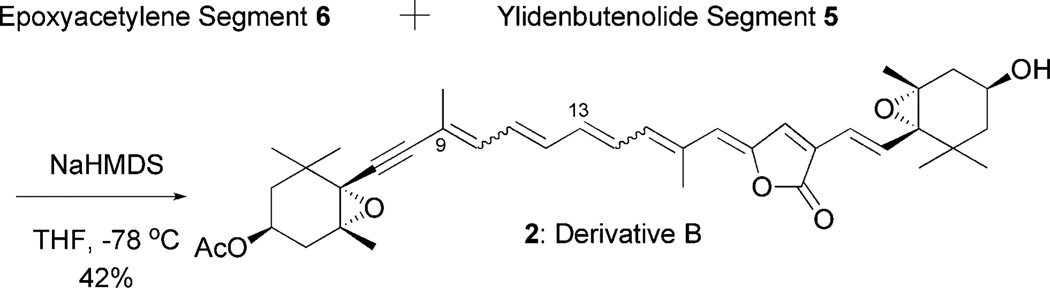
With epoxyacetylene segment 6 and ylidenbutenolide segment 5 in hand, the crucial modified Julia olefination16 was explored as the final key step in the synthesis of derivative B (2). The reaction of an anion derived from 6 with 5 at −78 °C smoothly proceeded within 5 min in the dark to produce the peridinin derivatives in 42% yield as a mixture of stereoisomers (Scheme 3).
Scheme 3.
Synthesis of peridinin derivative B (2).
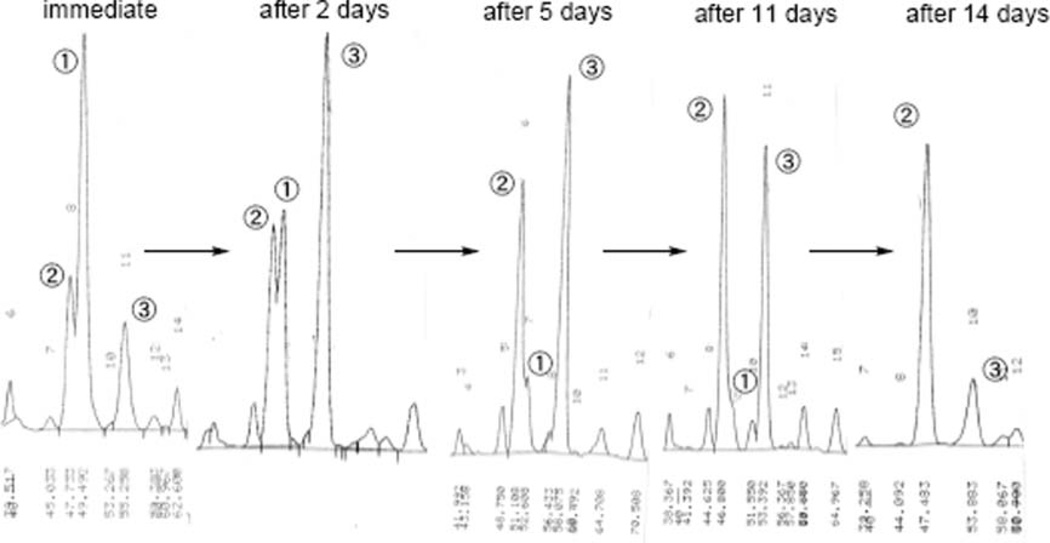
Based on the previous experiments with our carotenoid syntheses4,17 and the reports of Brückner’s and de Lera’s groups that the modified Julia olefination of polyene compounds generally produced the Z-isomer at the connected double bond,18 we tried to isomerize the connected double bond monitoring by HPLC as shown in Fig. 4. The resulting mixture was allowed to stand in benzene at room temperature under fluorescent light in an argon atmosphere. The isomerization under fluorescent light was faster than that in the dark.
Fig. 4.
Isomerization of peridinin derivative B (2).
After 2 days, we observed that the initially generated major peak (peak 1 in the immediate situation) changed to another major peak (peak 3). After 11 days, while peak 3 gradually decreased, peak 2 increased. After 14 days, peak 2 became the major peak in an equilibrium state. We isolated all peaks by both mobile-phase and reverse-phase HPLC, and elucidated their structures by NMR (400 and 750 MHz). Thus, we clarified that peak 1 was (9E,13Z)-isomer 2, peak 2 was (9Z,13E)-isomer 2, and peak 3 was (9E,13E)-all-trans derivatve B (2). All-trans derivative B did not isomerize to the 9Z-isomer at −20 °C but gradually isomerized at room temperature in the dark. Obviously, all-trans isomer B was unstable at room temperature and easily isomerized to the 9Z-isomer, which was the most stable isomer19 and showed a much shorter maximum absorption spectrum (438.0 nm) than that of peridinin (454.0 nm).
Synthesis of peridinin derivative C (3)
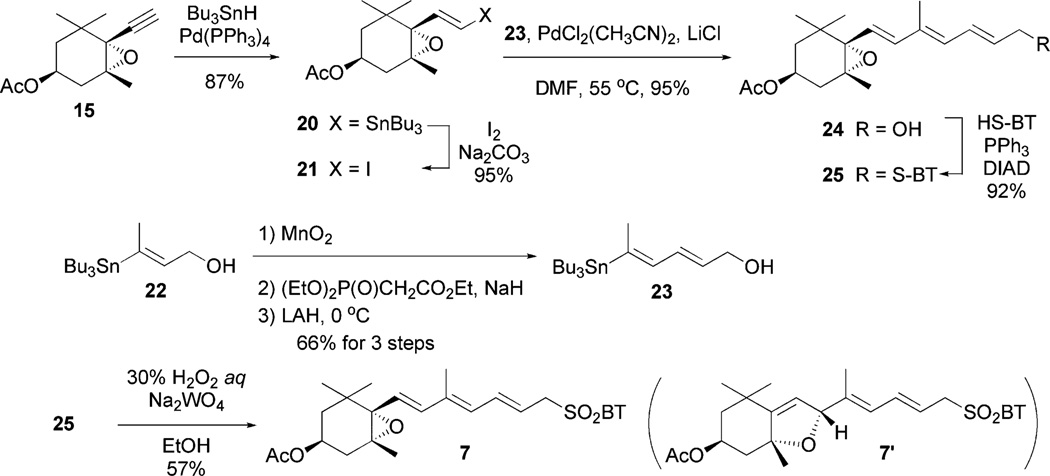
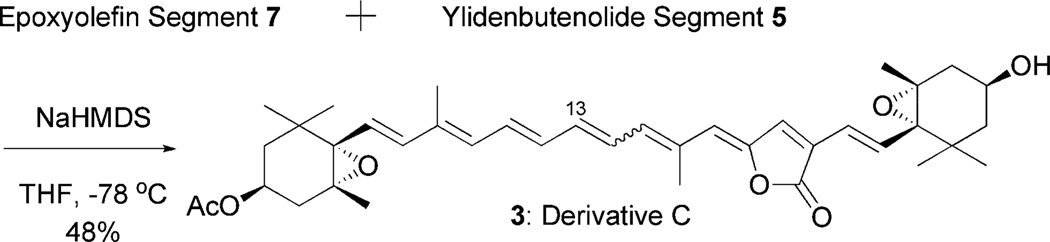
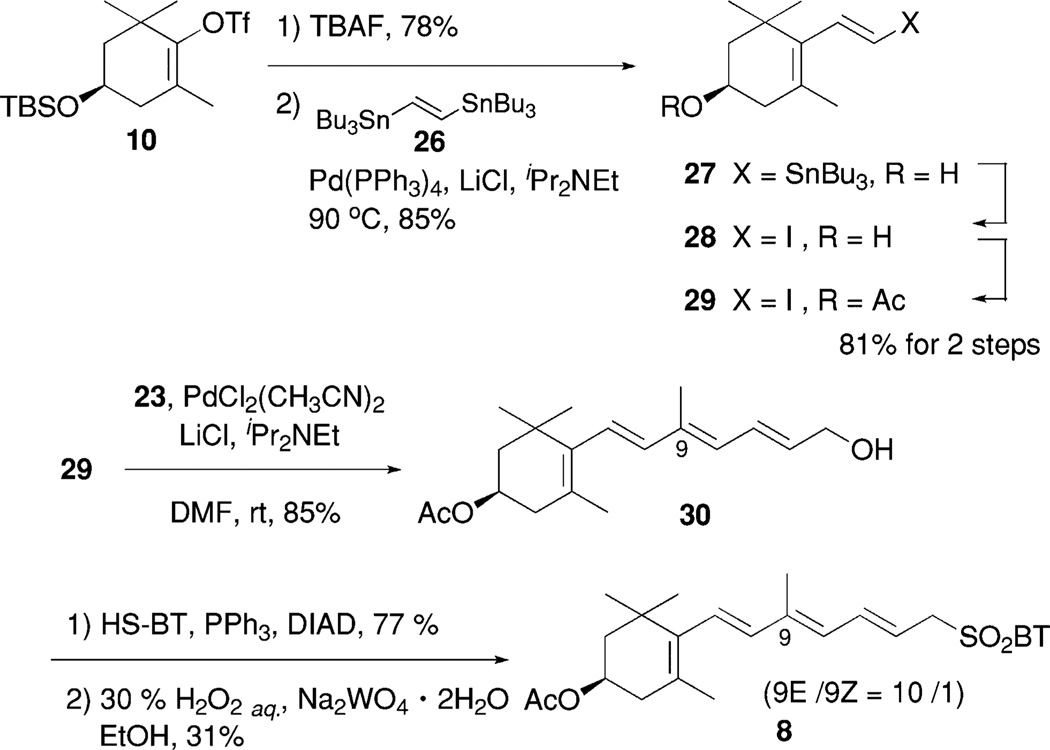
Derivative C (3) would be synthesized by the modified Julia olefination of the epoxyolefin segment 7 with the ylidenbutenolide segment 5 (Schemes 4 and 5). The segment 7 would be obtained by a coupling between vinyl iodide 21 and vinylstannane 23. The Pd-catalyzed hydrostannylation of acetate 15 produced 20 in 87% yield, which was rather better than that of the corresponding alcohol derivative 14 (68%).6 The obtained stannane 20 was transformed into the corresponding vinyl iodide 21 in excellent yield. Meanwhile, vinyl stannane 23 was prepared by MnO2 oxidation of 2220 followed by the Horner-Emmons reaction and then LAH reduction. The Stille cross-coupling reaction21 of acetyl iodide 21 with vinyl stannane 23 in the presence of PdCl2(CH3CN)2 and LiCl gave the desired alcohol 24 in 95% yield as a single isomer. The Stille cross-coupling of the reversed combination, namely acetyl stannane 20 and vinyl iodide 17, did not produce a desirable result. The alcohol 24 was transformed into sulfide 25 under Mitsunobu reaction conditions in excellent yield. Oxidation of 25 using the same reagents as for the preparation of 6 (30%H2O2 and Na2WO4) gave the desired 7. However, the use of 30% H2O2 and (NH4)6Mo7O24 gave a mixture of 7 and 7′ in low yield, and the ratio was not reproducible (1–4 : 1). It is noteworthy that sulfone 7 was easily isomerized to 7′ by trace amounts of hydrochloric acid in CDCl3.
Scheme 4.
Synthesis of epoxyolefin segment 7.
Scheme 5.
Synthesis of peridinin derivative C (3).
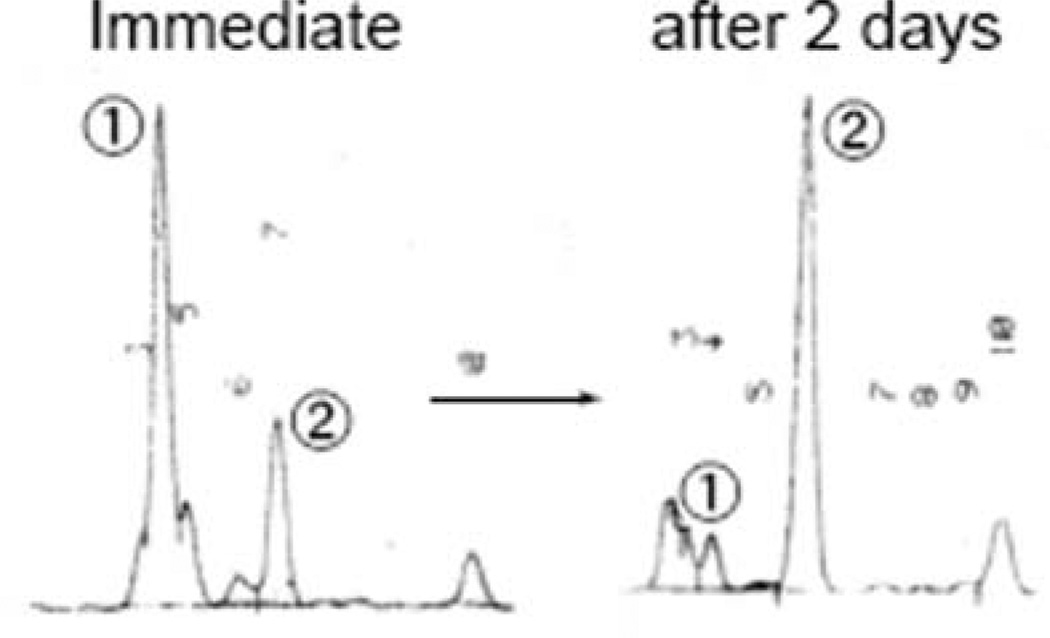
The desired 7 was reacted with the ylidenbutenolide segment 5 under the same modified Julia olefination reaction condition as in the case of derivative B to produce a mixture composed of two major components in mobile phase HPLC (acetone/hexane = 1/10). Each of them proved to be structural isomers by mass spectroscopy and showed very similar absorption spectra instead of a peak at around 310 nm absorption, which was usually assigned to the Z-configuration.22 In order to isomerize at the connected double bond and to obtain the desired all-trans isomer 3, the mixture was allowed to stand for 2 days under the same conditions as in the case of derivative B (2). In the HPLC of the isomerized mixture, we observed that the initially produced major component (peak 1) clearly changed to another one (peak 2), and peak 2 finally comprised more than 79% of the mixture (Fig. 5).We then isolated both peaks 1 and 2 and elucidated their structures by NMR (400 and 750 MHz). The structure of peak 2 was assigned to the all-trans derivative C (3) by rigorous analysis of coupling constants and NOE experiments (750 MHz), and peak 1 was also the 13Z-isomer. The electronic spectrum of derivative C (3) showed a maximum absorption (450.0 nm) similar to that of peridinin (454.0 nm).
Fig. 5.
Isomerization of peridinin derivative C (3).
Synthesis of peridinin derivative D (4)
Next was the synthesis of derivative D (4), which has no epoxide and possesses nine conjugating double bonds compared with the eight conjugating feature of peridinin. Stille coupling of the corresponding alcohol derived from vinyl triflate 10 with bisstannane 2623 in the presence of catalytic amounts of Pd(PPh3)4 and LiCl proceeded smoothly, and the desired coupling product 27 was obtained in 85% yield (Scheme 6). The obtained stannane 27 was transformed into the corresponding vinyl iodide followed by acetylation to produce 29 in 81% yield over two steps. The second Stille cross-coupling of 29 with vinyl stannane 23, which was used in the synthesis of 3, afforded tetraene alcohol 30 as a single isomer. In this coupling, the reaction proceeded smoothly at room temperature, and the absence of iPr2NEt gave a mixture with the 9Z-isomer of 30 in a ratio of eight to one by NMR. The amount of 9Z-isomer seemed to increase at higher reaction temperature, for instance, 9E/9Z = 3/1 at 50 °C. The desired sulfone 8 was obtained from 30 by the Mitsunobu reaction with 2-mercaptobenzothiazole, followed by oxidation of the resulting sulfide with aqueous 30%H2O2 andNa2WO4·2H2O,15 as a mixture of 9E/9Z = 10/1 in 31% yield. The use of 30% H2O2 and (NH4)6Mo7O24, and mCPBA gave a complex mixture. Oxidation of allylic sulfide to the corresponding sulfone in conjugated polyenes was still problematic.
Scheme 6.
Synthesis of tetraene segment 8.
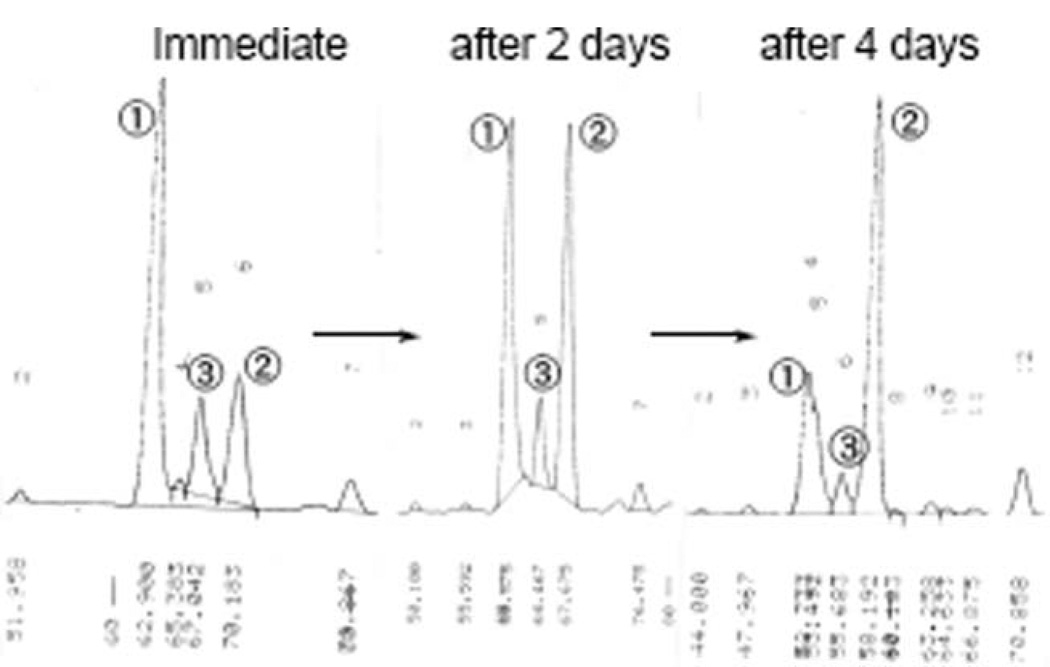
As shown in Scheme 7, the modified Julia olefination between sulfone 8 and aldehyde 5 proceeded successfully, and the reaction was over within 5 min in the dark to produce the coupling products as a mixture of stereoisomers in almost 47% yield, in which peak 1 was estimated to comprise 60% by HPLC analysis (Fig. 6). Isomerization was again attempted under the same conditions. After 4 days, a large amount of peak 1, which was a major component of the mixture immediately after the reaction, changed to peak 2 (peak 2 : peak 1 > 3 : 1 based on HPLC analysis). The peak 3, presumed to be a 9Z-isomer, nearly disappeared after 4 days. The compound of peak 2 was isolated by mobile and reverse phase HPLC and its structure was elucidated by NMR (400 and 750 MHz) to be the desired all-trans isomer 4. The obtained derivative D (4) showed the maximum absorption at 459.0 nm, which was similar to that of peridinin. The synthesized 4 was, however, rather unstable and was gradually decomposed within one week at room temperature under argon atmosphere. In contrast, peridinin could be stored without notable decomposition after ten days under similar circumstances.
Scheme 7.
Synthesis of peridinin derivative D (4)..
Fig. 6.
Isomerization of peridinin derivative D (4).
Results of Stark measurement
The maximum absorptions of the electronic spectra of peridinin (1) and the synthesized derivatives (2–4) in hexane are summarized in Table 1. Evidently, derivative D showed the longest λmax. Next, the Stark spectra of peridinin (1) and the derivatives B–D (2–4) were obtained. Stark spectra can determine the change in electrostatic properties and estimate the change in the static dipole moment (|Δµ|) between the ground state and the excited state. The large dipole moment would allow for strong dipolar interaction between peridinin and Chl a in PCP, and would contribute to high energy transfer.10 The Stark spectra of peridinin and derivatives B, C and D were recorded in methyl methacrylate polymer at 77 K and the results are listed in Table 1.24 The |Δµ| values were corresponding to the CT absorption band. As a result, peridinin showed the largest |Δµ| value among all of them. Namely, peridinin yielded a |Δµ| value of 5.42 (×10−29 C·m), derivative B (2) showed 2.47, derivative C (3) showed 4.22, and derivative D (4) showed 4.25. The |Δµ| value of peridinin is in agreement with that of Grondelle’s group.10 The difference in the |Δµ| values is evidently attributable to the difference in the functional groups. Although peridinin possesses fewer conjugating double bonds and shows a shorter λmax than derivative D, the |Δµ| value of peridinin is the largest among the four compounds. Thus, we have understood that the unique allene group contributes to production of the large dipole moment in the molecule. These results strongly suggest that the allene group of peridinin is essential for formation of the effective ICT state, which would allow the quantitative energy transfer to Chl a in the PCP complex. The exact role of the allene function for the large |Δµ| value is under investigation from a spectroscopic point of view.24
Table 1.
Results of UV and Stark spectra of peridinin and its derivatives
| λmax (nm) | |Δµ| (×10−29 C·m) | |
|---|---|---|
| Peridinin | 454.0 | 5.42 |
| Derivative B (2) | 438.0 | 2.47 |
| Derivative C (3) | 450.0 | 4.22 |
| Derivative D (4) | 459.0 | 4.25 |
Conclusion
In summary, we synthesized three kinds of relatively unstable allene-modified derivatives of peridinin, derivatives B, C, and D, which respectively possessed an epoxy-acetylene, an epoxyolefin, and a conjugating olefin groups instead of the allene group. In addition, the Stark spectra of peridinin and these derivatives were obtained, and showed that the |Δµ| value of peridinin was the largest among the four compounds. These results apparently show that the allene group of peridinin effectively contributes to production of the large dipole moment in the molecule, which would result in the high energy transfer efficiencies to Chl a in the PCP complex. Ultrafast time-resolved optical absorption spectroscopic experiments in addition to Stark spectroscopy of the synthesized compounds are currently in progress to further understand the exact role of the allene group.
Experimental
General synthetic procedures
All commercially available reagents were used without further purification. All solvents were used after distillation. Tetrahydrofuran (THF), diethyl ether, benzene, toluene, and dimethoxyethane (DME) were refluxed over and distilled from sodium-benzophenone ketyl. Dichloromethane was refluxed over and distilled from P2O5. Dimethylformamide (DMF) was distilled from CaH2 under reduced pressure. Triethylamine, diisopropylamine, and diisopropylethylamine were refluxed over and distilled from KOH. Preparative separation was performed by column chromatography on silica gel. 1H NMR and 13C NMR spectra were recorded on 400 MHz and 750 MHz spectrometers, and chemical shifts were represented as δ values relative to the internal standard TMS. IR spectra were recorded on a FT-IR Spectrometer. High-resolution mass spectra (HRMS) were measured on a ESI-TOF MS.
(1S,2R,4S)-4-Acetoxy-1,2-epoxy-1-ethynyl-2,6,6-trimethylcyclohexanol (15)
To a solution of acetylene 14 (215 mg, 1.21 mmol) in pyridine (5 mL) was added acetic anhydride (0.18 mL, 1.94 mmol) at room temperature and the reaction mixture was stirred for 18 h at the same temperature. A saturated aqueous CuSO4 solution was added, and then the resulting mixture was extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (5% ethyl acetate in hexane) afforded acetate 15 (209 mg, 77%): [α]24.0D −20.3 (c 1.18, CHCl3); IR (neat, cm−1) 3281, 2966, 2930, 2872, 1738, 1460, 1367, 1242, 1157, 1105, 1043; 1H NMR (CDCl3, 400 MHz) δ 4.85 (m, 1H), 2.42 (s, 1H), 2.37 (dd, J = 15.1, 5.7 Hz, 1H), 2.01 (s, 3H), 1.79 (dd, J = 15.1, 6.4 Hz, 1H), 1.60 (dd, J = 13.8, 3.4 Hz, 1H), 1.52 (s, 3H), 1.38 (dd, J = 13.5, 8.24 Hz, 1H), 1.27 (s, 3H), 1.16 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.1, 80.2, 74.2, 66.9, 64.9, 63.1, 39.7, 35.6, 33.5, 28.4, 25.9, 21.7, 21.3; ESI-HRMS m/z calcd for C13H18O3Na (M + Na)+ 245.1154, found 245.1164.
(2E,4E)-7-[(1′S,2′R,4′S)-4′-Acetoxy-1′,2′-epoxy-2′,6′,6′-trimethylcyclohexa-1′-yl]5-methylhepta-2,4-diene-6-yn-1-ol (18)
To a solution of ester 16 (1.11 g, 1.89 mmol) in dichloromethane (18.9 mL) was added dropwise diisobutylaluminium hydride (1.0 M in toluene, 4.56 mL, 4.56 mmol) at −78 °C. After the reaction mixture was stirred for 5 min at the same temperature, aqueous potassium sodium (+)-tartrate tetrahydrate solution was added and then resulting mixture was extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. to afford crude vinyl iodide 17, which was used to the next reaction without further purification.
To a solution of crude vinyl iodide 17 and acetylene 15 (420 mg, 1.89 mmol) in diisopropylamine (9.45 mL) was added tetrakis(triphenylphosphine)palladium (262 mg, 0.23 mmol) and CuI (40 mg, 0.21 mmol). After being stirred for 1.5 h at room temperature, the reaction mixture was poured into a saturated aqueous NH4Cl solution, and then the resulting mixture was extracted with diethyl ether. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (from 30% to 50% ethyl acetate in hexane) afforded the conjugated acetylene derivative 18 (484 mg, 81%): [α]24.0D +15.1 (c 1.07, CHCl3); IR (neat, cm−1) 3458, 2965, 2926, 1738, 1448, 1370, 1244, 1043; 1H NMR (CDCl3, 400 MHz) δ 6.50 (dd, J = 14.8, 11.4Hz, 1H), 6.39 (d, J = 11.2Hz, 1H), 5.90 (dt, J = 14.9, 5.5 Hz, 1H), 4.87 (m, 1H), 4.23 (d, J = 5.5 Hz, 2H), 2.38 (dd, J = 14.9, 5.7 Hz, 1H), 2.01 (s, 3H), 1.91 (s, 3H), 1.80 (dd, J = 14.8, 6.4 Hz, 1H), 1.62 (dd, J = 13.8, 3.5 Hz, 1H), 1.51 (s, 3H), 1.39 (dd, J = 13.5, 8.3Hz, 1H), 1.27 (s, 3H), 1.16 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 170.3, 135.4, 134.2, 125.9, 118.0, 89.3, 85.5, 67.1, 65.6, 63.9, 62.9, 39.8, 35.7, 34.0, 28.6, 26.1, 21.9, 21.3, 17.4; ESI-HRMSm/z calcd for C19H26O4Na (M + Na)+ 341.1729, found 341.1740.
2-(((2′E,4′E)-7′-((1″S,2″R,4″S)-4″-Acetoxy-1″,2″-epoxy-2″, 6″,6″-trimethylcyclohexylidene-1″-yl)-5′-methylhepta-2,4-diene-6-yn)sulfanyl)benzothiazole (19)
To a solution of 18 (100 mg, 0.31 mmol), 2-mercaptobenzothiazole (68 mg, 0.41 mmol) and triphenylphosphine (107 mg, 0.41 mmol) in THF (4 mL) was added dropwise diisopropyl azodicarboxylate (0.09 mL, 0.44 mmol) at 0 °C. The reaction mixture was stirred for 1 h at room temperature and all solvents were removed in vacuo. To the residue was added diethyl ether and the precipitate was removed by filtration through a pad of Celite to give the crude products as a solution, which was concentrated in vacuo. Purification by short silica gel column chromatography (from 5% to 10% ethyl acetate in hexane) afforded the thioether 19 (127 mg, 87%): [α]26.0D +8.5 (c 0.97, CHCl3); IR (neat, cm−1) 2967, 2926, 1736, 1460, 1427, 1367, 1242, 1042; 1H NMR (CDCl3, 400 MHz) δ 7.87 (m, 1H), 7.75 (m, 1H), 7.42 (m, 1H), 7.30 (m, 1H), 6.60 (dd, J = 14.9, 11.2 Hz, 1H), 6.36 (d, J = 11.5 Hz, 1H), 5.93 (m, 1H), 4.87 (m, 1H), 4.08 (d, J = 7.6 Hz, 2H), 2.37 (ddd, J = 14.9, 5.7, 0.9 Hz, 1H), 2.00 (s, 3H), 1.89 (s, 3H), 1.79 (dd, J = 15.1, 6.6 Hz, 1H), 1.61 (m, 1H), 1.48 (s, 3H), 1.38 (dd, J = 13.7, 8.2 Hz, 1H), 1.24 (s, 3H), 1.14 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.3, 165.8, 153.2, 135.4, 135.0, 129.8, 128.9, 126.1, 124.3, 121.5, 120.9, 118.9, 89.2, 86.1, 67.1, 65.6, 63.9, 39.9, 35.9, 34.1, 28.7, 26.2, 21.9, 21.4, 17.5, −0.02; ESI-HRMS m/z calcd for C26H29NO3S2Na (M + Na)+ 490.1487, found 490.1467.
2-(((2′E,4′E)-7′-((1″S,2″R,4″S)-4″-Acetoxy-1″,2″-epoxy-2″, 6″,6″-trimethylcyclohexylidene-1″-yl)-5′-methylhepta-2,4-diene-6-yn)sulfonyl)benzothiazole (6)
To a solution of the thioether 19 (133 mg, 0.28 mmol) in ethanol (3 mL) was added dropwise a solution of ammonium heptamolybdate tetrahydrate (527 mg, 0.43 mmol) in hydrogen peroxide (30 wt.% in water, 1.4 mL) at 0 °C. After being stirred for 30 min at room temperature, the reaction mixture was poured into water and then extracted with diethyl ether. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography afforded the sulfone 6 (from 10% to 20% ethyl acetate in hexane) (79 mg, 58%): [α]24.0D +9.6 (c 0.29, CHCl3); IR (neat, cm−1) 2967, 2928, 1736, 1472, 1368, 1333, 1244, 1150, 1028; 1H NMR (CDCl3 , 400 MHz) δ 8.23 (m, 1H), 8.02 (m, 1H), 7.64 (m, 2H), 6.46 (dd, J = 14.9, 11.5 Hz, 1H), 6.31 (d,J = 11.4 Hz, 1H), 5.69 (m, 1H), 4.87 (m, 1H), 4.31 (d, J = 7.8 Hz, 2H), 2.37 (dd, J = 15.1, 5.7 Hz, 1H), 2.01 (s, 3H), 1.78 (dd, J = 15.1, 6.4 Hz, 1H), 1.72 (s, 3H), 1.60 (m, 1H), 1.47 (s, 3H), 1.37 (dd, J = 13.7, 8.4 Hz, 1H),1.23 (s, 3H), 1.13 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 170.3, 165.4, 152.6, 135.7, 134.0, 128.1, 127.7, 125.4, 122.4, 121.5, 117.7, 88.7, 87.3, 67.1, 65.7, 63.8, 58.7, 39.9, 35.8, 34.1, 28.6, 26.2, 21.9, 21.4, 17.5, −0.02; ESI-HRMSm/z calcd for C26H29NO5S2Na (M + Na)+ 522.1385, found 522.1381.
Peridinin derivative B (2)
To a solution of acetylene segment 6 (22 mg, 0.044 mmol) and ylidenbutenolide segment 5 (15 mg, 0.044 mmol) in THF (0.87 mL) was added dropwise sodium bis(trimethylsilyl)amide (1.0 M in THF, 0.12 mL, 0.12 mmol) at −78 °C in the dark. After being stirred for 5 min at the same temperature, the reaction mixture was poured into water and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography (from 30% to 50% ethyl acetate in hexane) in the dark afforded peridinin derivative 2 (5 mg, 18%) as a mixture of the isomers as a red film. A solution of a mixture of all-trans-peridinin derivative 2 and its cis-isomer in benzene was left at room temperature under the fluorescence light. After 11 days, partial separation by preparative HPLC [column: Develosil CN-UG (0.6 × 25 cm); mobile phase: acetone/n-hexane = 1/10; flow rate: 2.0 mL/min; UVdetect: 438 nm; retention time: (all-trans isomer) 51 min, (9Z, 13E-isomer) 58 min] in the dark, and HPLC [column: YMC Carotenoid C30 (10 × 250 mm); reverse phase: acetonitrile/methanol/water = 50/48/2; flow rate: 2.0 mL/min; UVdetect: 438 nm; retention time: (all-trans isomer) 22 min] in the dark, was afforded the desired optically active peridinin derivative 2 as a red film: IR (neat, cm−1) 3455, 2924, 2853, 2367, 1701, 1655, 1561, 1460, 1419, 1379, 1259, 1121, 1041; 1H NMR (C6D6, 750 MHz) δ 7.57 (d, J = 15.5 Hz, 1H), 6.61 (d, J = 11.7 Hz, 1H), 6.56 (d, J = 15.5 Hz, 1H), 6.42 (dd, J = 13.9, 12.3 Hz, 1H), 6.38 (dd, J = 14.3, 12.1 Hz, 1H), 6.30 (d, J = 11.8 Hz, 1H), 6.26 (dd, J = 14.2, 11.5 Hz, 1H), 6.15 (s, 1H), 6.13 (dd, J = 14.2, 11.7 Hz, 1H), 5.18 (s, 1H), 5.07 (m, 1H), 3.75 (m, 1H), 2.25 (dd, J = 14.8, 3.5 Hz, 1H), 2.20 (ddd, J = 14.5, 4.2, 1.0 Hz, 1H), 2.11 (s, 3H), 1.79 (s, 3H), 1.68 (s, 3H), 1.62 (m, 1H), 1.46 (s, 3H), 1.41 (m, 2H), 1.35 (m, 1H), 1.34 (s, 3H), 1.31 (s, 3H), 1.13 (s, 3H), 1.09 (s, 3H), 1.08 (s, 3H), 1.06 (m, 1H); 13C NMR (C6 D6, 188 MHz) δ 169.3, 168.3, 147.7, 137.3,136.8, 136.4, 136.3, 135.1, 135.0, 134.9, 130.9, 130.0, 125.4, 122.3, 119.5, 118.2, 90.1, 89.1, 70.5, 67.4, 67.2, 65.6, 64.1, 63.9, 47.3, 41.1, 40.4, 36.2, 35.3, 34.4, 29.5, 29.0, 26.6, 25.3, 22.1, 20.9, 19.9, 17.7, 15.6; ESI-HRMS m/z calcd for C39H48O7Na (M + Na)+ 651.3298, found 651.3276.
trans-2-[(1′S,2′R,4′S)-4′-Acetoxy-1′,2′-epoxy-2′,6′,6′-trimethylcyclohexyl]-1-(tributylstannyl)ethylene (20)
To a solution of acetylene 15 (649 mg, 2.92 mmol), tetrakis(triphenylphosphine) palladium (169 mg, 0.15 mmol) in THF (29 mL) was added dropwise tributyltin hydride (1.55 mL, 5.84 mmol) at −78 °C. After being stirred for 15 min at room temperature and the reaction mixture was filtered through a pad of silica gel to give the crude products as a solution, which was concentrated in vacuo. Purification by silica gel column chromatography afforded 20 (1.31 g, 87%): [α]23.0D −49.44 (c 0.89, CHCl3); IR (neat, cm−1) 3466, 2959, 2926, 2872, 2854, 1739, 1462, 1419, 1377, 1365, 1242, 1184, 1155, 1118, 1097, 1070, 1030; 1H NMR (CDCl3, 400 MHz) δ 6.23 (d, J = 19.0 Hz, 1H), 6.16 (d, J = 19.2 Hz, 1H), 4.92 (m, 1H), 2.38 (dd, J = 14.8, 5.7 Hz, 1H), 2.01 (s, 3H), 1.75 (dd, J = 14.8, 6.8 Hz, 1H), 1.64 (dd, J = 13.2, 3.4 Hz, 1H), 1.49 (m, 6H), 1.35 (m, 1H), 1.30 (m, 6H), 1.19 (s, 3H), 1.16 (s, 3H), 0.97 (s, 3H), 0.88 (m, 15H); 13C NMR (CDCl3, 100 MHz) δ 170.4, 142.1, 132.4, 72.2, 67.9, 64.7, 41.5, 36.8, 34.3, 29.2, 28.6, 27.3, 25.5, 21.5, 20.3, 13.8, 9.6; ESI-HRMS m/z calcd for C25H46O3SnNa (M + Na)+ 537.2371, found 537.2363.
trans-2-[(1S,2R,4S)-4-Acetoxy-1,2-epoxy-2,6,6-trimethylcyclohexyl]-1-iodoethylene (21)
To a solution of iodine (445 mg, 1.75 mmol) and Na2CO3 (372 mg, 3.51 mmol) in dichloromethane (7 mL) was added dropwise a solution of 20 (450 mg, 0.88 mmol) in dichloromethane (2 mL) at 0 °C. After stirring for 5 min at 0 °C, the mixture was poured into a saturated aqueous Na2S2O3 solution and then extracted with chloroform. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography afforded iodide 21 (273 mg, 89%): [α]23.0D −68.0 (c 1.01, CHCl3); IR (neat, cm−1) 2965, 2932, 1736, 1603, 1468, 1365, 1242, 1032; 1H NMR (CDCl3, 400 MHz) δ 6.77 (d, J = 14.2 Hz, 1H), 6.28 (d, J = 14.2 Hz, 1H), 4.89 (m, 1H), 2.37 (dd, J = 14.9, 5.5 Hz, 1H), 2.01 (s, 3H), 1.76 (dd, J = 15.1, 6.8 Hz, 1H), 1.63 (dd, J = 13.5, 3.4 Hz, 1H), 1.35 (dd, J = 13.5, 8.9 Hz, 1H), 1.22 (s, 3H), 1.15 (s, 3H), 0.99 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.3, 141.3, 79.8, 72.5, 67.3, 65.1, 41.3, 36.6, 34.3, 28.4, 25.4, 21.5, 20.2; ESI-HRMS m/z calcd for C13H19IO3Na (M + Na)+ 373.0277, found 373.0277.
Ethyl (2E,4E)-5-(tributylstannyl)hexa-2,4-dienate (22a)
A mixture of 22 (1.0 g, 2.77 mmol) and manganese dioxide (16.6 g) in THF (17 mL) was stirred at room temperature for 6 h. The precipitate was filtered through a pad of Celite, and the filtrate was concentrated in vacuo to afford crude aldehyde, which was used in the next reaction without further purification.
To a solution of triethyl phosphonoacetate (0.72 mL, 3.6 mmol) in THF (13 mL) was added sodium hydride (133 mg, 3.32 mmol) at 0 °C and the mixture was stirred for 10 min. To this mixture was added a solution of the crude aldehyde in THF (3 mL) at 0 °C. After being stirred for 5 min at room temperature, the reaction mixture was poured into water and then extracted with ethyl acetate. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography afforded ethyl ester 22a (936 mg, 79% for 2 steps): IR (neat, cm−1) 2961, 2928, 2870, 2852, 1716, 1620, 1462, 1419, 1367, 1340, 1304, 1265, 1234, 1180, 1132, 1095, 1076, 1043; 1H NMR (CDCl3, 400 MHz) δ 7.67 (dd, J = 15.3, 11.2 Hz, 1H), 6.34 (d, J = 11.3 Hz, 1H), 5.79 (d, J = 15.1 Hz, 1H), 4.21 (q, J = 7.1 Hz, 2H), 2.13 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 167.8, 157.9, 137.6, 136.5, 119.9, 60.1, 29.0, 27.3, 20.6, 14.3, 13.6, 9.2; ESI-HRMS m/z calcd for C20H38O2SnNa (M + Na)+ 453.1795, found 453.1777.
(2E,4E)-5-(Tributylstannyl)hexa-2,4-dien-1-ol (23)
To a suspension of lithium aluminium hydride (36 mg, 0.95 mmol) in THF (6 mL) was added dropwise a solution of 22a (338 mg, 0.79 mmol) in THF (2 mL) at 0 °C. After being stirred for 10 min at the same temperature, Rochelle salt was carefully added. The reaction mixture was stirred for 30 min at room temperature and then extracted with ethyl acetate. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography afforded 23 (223 mg, 73%): IR (neat, cm−1) 3327, 2957, 2920, 2852, 1460, 1417, 1375, 1340, 1292, 1089, 1005; 1H NMR (CDCl3, 400 MHz) δ 6.64 (dd, J = 15.1, 10.5 Hz, 1H), 6.19 (d, J = 10.5 Hz, 1H), 5.78 (dt, J = 14.8, 5.9 Hz, 1H), 4.22 (t, J = 5.8 Hz, 2H), 2.00 (s, 3H), 1.49 (m, 6H), 1.30 (m, 6H), 0.89 (m, 15H); 13C NMR (CDCl3, 100 MHz) δ 145.2, 138.0, 130.9, 126.3, 63.7, 29.2, 27.5, 19.9, 13.8, 9.2.
(2E,4E,6E)-7-[(1′S,2′R,4′S)-4′-Acetoxy-1′,2′-epoxy-2′,6′,6′-trimethylcyclohexa-1′-yl]-5-methylhepta-2,4,6-trien-1-ol (24)
To a solution of iodide 21 (560 mg, 1.6 mmol) and (2E,4E)-5-(tributylstannyl)hexa-2,4-dien-1-ol 23 (915 mg, 2.40 mmol) in DMF (8 mL) was added bis(acetonitrile)dichloropalladium(II) (21 mg, 0.05 mmol) and lithium chloride (136 mg, 3.20 mmol). After being stirred for 10 min at 55 °C, the reaction mixture was poured into water and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography afforded coupling product 24 (482 mg, 94%) as a yellow oil: [α]23.0D −41.6 (c 1.02, CHCl3); IR (neat, cm−1) 3443, 2964, 2928, 1736, 1450, 1365, 1244, 1030; 1H NMR (CDCl3, 400 MHz) δ 6.60 (dd, J = 14.2, 10.3 Hz, 1H), 6.27 (d, J = 15.8 Hz, 1H), 6.10 (d, J = 11.2 Hz, 1H), 5.88 (d, J = 15.8 Hz, 1H), 5.86 (m, 1H), 4.93 (m, 1H), 4.24 (m, 2H), 2.40 (dd, J = 15.1, 5.8 Hz, 1H), 2.01 (s, 3H), 1.88 (s, 3H), 1.77 (dd, J = 14.8, 6.8 Hz, 1H), 1.66 (dd, J = 13.2, 3.4 Hz, 1H), 1.34 (m, 1H), 1.18 (s, 3H), 1.15 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.3, 137.4, 134.5, 132.8, 130.4, 127.5, 123.7, 70.3, 67.7, 65.5, 63.5, 41.4, 36.8, 34.7, 28.6, 25.5, 21.4, 20.2, 12.8; ESI-HRMS m/z calcd for C19H28O4Na (M + Na)+ 343.1885, found 343.1883.
2-(((2′E,4′E,6′E)-7′-((1″S,2″R,4″S)-4″-Acetoxy-1″,2″-epoxy-2″,6″,6″-trimethylcyclohexylidene-1″-yl)-5′-methylhepta-2,4,6-triene)sulfanyl)benzothiazole (25)
To a solution of 24 (330 mg, 1.03 mmol), 2-mercaptobenzothiazole (241 mg, 1.44 mmol) and triphenylphosphine (378 mg, 1.44 mmol) in THF (10 mL) was added dropwise diisopropyl azodicarboxylate (0.32 mL, 1.65 mmol) at 0 °C. The reaction mixture was stirred for 10 min at room temperature and the all solvents were removed in vacuo. To a residue was added diethyl ether and the precipitate was removed by filtration through a pad of Celite to give the crude products as a solution, which was concentrated in vacuo. Purification by short silica gel column chromatography afforded the thioether 25 (444 mg, 92%): [α]23.0D −25.6 (c 1.08, CHCl3); IR (neat, cm−1) 2964, 2926, 1734, 1460, 1427, 1365, 1242, 1028; 1H NMR (CDCl3, 400 MHz) δ 7.87 (m, 1H), 7.75 (m, 1H), 7.40 (m, 1H), 7.28 (m, 1H), 6.71 (dd, J = 14.7, 11.3 Hz, 1H), 6.24 (d, J = 15.5 Hz, 1H), 6.07 (d, J = 11.4 Hz, 1H), 5.88 (d, J = 15.5 Hz, 1H), 5.86 (m, 1H), 4.93 (m, 1H), 4.11 (d, J = 7.5 Hz, 2H), 2.38 (dd, J = 15.8, 5.7 Hz, 1H), 2.01 (s, 3H), 1.87 (s, 3H), 1.76 (dd, J = 14.8, 6.8 Hz, 1H), 1.65 (dd, J = 13.2, 3.4 Hz, 1H), 1.33 (m, 1H), 1.16 (s, 3H), 1.14 (s, 3H), 0.96 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.3, 166.1, 153.2, 137.2, 135.3, 134.9, 130.9, 129.9, 127.4, 125.9, 124.2, 124.1, 121.5, 120.9, 70.3, 67.6, 65.5, 41.4, 36.7, 36.1, 34.7, 28.5, 25.5, 21.4, 20.2, 12.8; ESI-HRMS m/z calcd for C26H31NO3S2Na (M + Na)+ 492.1643, found 492.1640.
2-(((2′E,4′E,6′E)-7′-((1″S,2″R,4″S)-4″-Acetoxy-1″,2″-epoxy-2″,6″,6″-trimethylcyclohexylidene-1″-yl)-5′-methylhepta-2,4,6-triene)sulfonyl)benzothiazole (7)
To a solution of the thioether 25 (30 mg, 0.064 mmol) in ethanol (0.64 mL) was added dropwise a solution of sodium tungstate(VI) dihydrate (42 mg, 0.128 mmol) in hydrogen peroxide (30 wt.% in water, 0.51 mL) at 0 °C. After being stirred for 50 min at room temperature, the reaction mixture was poured into water and then extracted with diethyl ether. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography afforded the sulfone 7 (18 mg, 56%) as a yellow solid: [α]24.0D −22.5 (c 0.79, CHCl3); IR (neat, cm−1) 3471, 2930, 2865, 1736, 1637, 1473, 1381, 1334, 1240, 1147, 1116, 976, 763; 1H NMR (CDCl3, 400 MHz) δ 8.22 (d, J = 7.8 Hz, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.65 (m, 2H), 6.59 (dd, J = 14.7, 11.0 Hz, 1H), 6.20 (d, J = 15.6, 1H), 6.02 (d, J = 15.6 Hz, 1H), 5.90 (d, J = 15.6 Hz, 1H), 5.64 (dt, J = 15.1, 7.8 Hz, 1H), 4.91 (m, 1H), 4.31 (d, J = 7.8 Hz, 1H), 2.36 (dd, J = 15.1, 5.7, 1H), 2.00 (s, 3H), 1.78 (dd, J = 15.2, 6.5 Hz, 1H), 1.71 (s, 3H), 1.63 (m, 1H), 1.37 (dd, J = 13.7, 8.5 Hz, 1H), 1.13 (s, 3H), 1.12 (s, 3H), 0.95 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.7, 165.9, 153.0, 137.5, 137.2, 129.5, 128.3, 128.0, 125.8, 125.7, 122.7, 116.5, 70.5, 67.9, 65.9, 59.3, 41.7, 37.1, 35.0, 28.9, 25.8, 21.7, 20.5, 13.1; ESI-HRMS m/z calcd for 26H31NO5S2Na (M + Na)+ 524.1541, found 524.1524.
Peridinin derivative C (3)
To a solution of olefin segment 7 (22 mg, 0.044 mmol) and ylidenbutenolide segment 5 (15 mg, 0.044 mmol) in THF (0.65 mL) was added dropwise sodium bis(trimethylsilyl)amide (1.0 M in THF, 0.13 mL, 0.13 mmol) at −78 °C in the dark. After being stirred for 5 min at the same temperature, the reaction mixture was poured into water and then extracted with diethyl ether. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography (from 30% to 50% ethyl acetate in hexane) in the dark afforded peridinin derivative 3 (11 mg, 40%) as a mixture of the isomers as a red film. A solution of a mixture of all-trans-peridinin derivative 3 and its cis-isomer in benzene was left at room temperature under the fluorescence light. After 2 days, partial separation by preparative HPLC [column: Develosil CN-UG (0.6 × 25 cm); mobile phase: acetone/n-hexane = 1/10; flow rate: 2.0 mL/min; UV detect: 450 nm; retention time: (all-trans-isomer) 49 min, (15-cis-isomer) 43 min] in the dark, and HPLC [column: YMC Carotenoid C30 (10 × 250 mm); reverse phase: acetonitrile/methanol/water = 87/10/3; flow rate: 2.0 mL/min.; UVdetect: 450 nm; retention time: (all-trans-isomer) 30 min, (15-cis-isomer) 24 min] in the dark, afforded the desired optically active peridinin derivative 3 as a red film: IR (neat, cm−1) 3327, 2924, 1741, 1712, 1462, 1377, 1259, 1153, 1028; 1H NMR (C6 D6, 750 MHz) δ 7.57 (d, J = 15.5 Hz, 1H), 6.68 (d, J = 15.4 Hz, 1H), 6.62 (dd, J = 14.0, 12.3 Hz, 1H), 6.56 (d, J = 15.5 Hz, 1H), 6.45 (dd, J = 14.1, 11.9 Hz, 1H), 6.38 (dd, J = 14.3, 11.2 Hz, 1H), 6.33 (d, J = 11.7 Hz, 1H), 6.26 (dd, J = 14.2, 11.3 Hz, 1H), 6.17 (d, J = 11.7 Hz, 1H), 6.15 (s, 1H), 5.92 (d, J = 15.5 Hz, 1H), 5.20 (s, 1H), 5.19 (m, 1H), 3.86 (m, 1H), 2.35 (dd, J = 14.7, 5.3 Hz, 1H), 2.19 (ddd, J = 14.7, 5.1, 1.1 Hz, 1H), 2.13 (s, 3H), 1.79 (s, 3H), 1.72 (s, 3H), 1.71 (m, 1H), 1.62 (dd, J = 14.8, 7.2 Hz, 1H), 1.42 (m, 2H), 1.35 (m, 1H), 1.13 (s, 3H), 1.12 (s, 3H), 1.09 (s, 3H), 1.09 (s, 3H), 1.08 (s, 3H), 1.05 (s, 3H), 1.05 (m, 1H); 13C NMR (C6D6, 188 MHz) δ 169.3, 168.3, 147.5, 138.1, 137.7, 137.1, 136.4, 135.8, 134.7, 134.6, 134.4, 132.5, 131.4, 129.9, 125.1, 125.0, 122.4, 118.5, 70.5, 70.3, 67.7, 67.5, 65.8, 63.9, 47.3, 42.2, 41.2, 37.3, 35.3, 35.1, 29.5, 28.8, 25.7, 25.3, 21.0, 19.9, 15.6, 12.9; ESI-HRMS m/z calcd for C39H48O7Na (M + Na)+ 651.3298, found 651.3276.
(4S) - 4 -Hydroxy - 2, 6, 6 - trimethylcyclohex- 1 - enyltrifluoromethanesulfonate (10a)
To a solution of 10 (200 mg, 0.50 mmol) in THF (2.47 mL) was added tetra-n-butylammonium fluoride (1.0 M in THF, 1.49 mL, 1.49 mmol) at room temperature. After being stirred for 45 min at the same temperature, the reaction mixture was poured into a saturated aqueous NH4Cl solution and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (from 10% to 50% ethyl acetate in hexane) afforded alcohol 10a (112 mg, 78%) as a colorless oil: [α]23.0D −25.4 (c 0.99, CHCl3); IR (neat, cm−1) 3359, 2932, 2361, 1686, 1404, 1210, 1067, 913; 1H NMR (CDCl3, 400 MHz) δ 4.11 (m, 1H), 2.50 (ddd, J = 17.0, 5.5, 1.9 Hz, 1H), 2.18 (ddd, J = 16.5, 9.2, 0.9 Hz, 1H), 1.86 (ddd, J = 12.4, 3.7, 0.3 Hz, 1H), 1.77 (s, 3H), 1.68 (dd, J = 11.9, 11.9 Hz, 1H), 1.22 (s, 3H), 1.17 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 149.3, 123.9, 64.1, 49.0, 41.3, 37.1, 27.7, 27.0, 17.9; FAB-HRMS m/z calcd for C39H51O7Na (M + H)+ 288.0716, found 289.0759.
trans-2-[(4S)-4-Hydroxy-2,6,6-trimethylcyclohexene]-1-(tributylstannyl) ethylene (27)
To a solution of alcohol 10a (388 mg, 1.17 mmol) and bisstannane 26 (855 mg, 1.41 mmol) in DMF (5.86 mL) was added diisopropylethylamine (0.61 mL, 3.52 mmol), tetrakis(triphenylphosphine)palladium (67 mg, 0.059 mmol), and lithium chloride (99 mg, 2.34 mmol) After being stirred for 1 h at 90 °C, the reaction mixture was poured into water and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (from 0% to 30% ethyl acetate 3% triethylamine in hexane) afforded 27 (451 mg, 85%) as a colorless oil: [α]23.0D −72.0 (c 0.84, CHCl3); IR (neat, cm−1) 3360, 2924, 2855, 2361, 1579, 1464, 1174, 1045, 691; 1H NMR (CDCl3, 400 MHz) δ 6.30 (d, J = 19.6Hz, 1H), 5.90 (d, J = 19.2 Hz, 1H), 3.98 (m, 1H), 2.35 (dd, J = 16.5, 5.5 Hz, 1H), 2.00 (dd, J = 16.1, 9.5 Hz, 1H), 1.75 (ddd, J = 19.0, 3.6, 2.3 Hz, 1H), 1.70 (s, 3H), 16.0–1.47 (m, 6H), 1.45 (dd, J = 12.3 Hz, 1H), 1.06 (s, 3H), 1.04 (s, 3H), 0.90 (m, 15H); 13C NMR (CDCl3, 100 MHz) δ 145.4, 141.5, 133.9, 124.9, 65.5, 48.6, 42.5, 36.9, 30.4, 29.6, 28.8, 27.6, 21.7, 14.1, 9.9.
trans-2-[(4S)-4-Hydroxy-2,6,6-trimethylcyclohexene]-1-iodoethylene (28)
To a solution of iodide (728 mg, 2.87 mmol), Na2CO3 (608 mg, 5.74 mmol) in dichloromethane (11.4 mL) was added dropwise a solution of stannane 27 (654 mg, 1.44 mmol) in dichloromethane (3 mL) at 0 °C. After stirred for 5 min at 0 °C, the mixture was poured into a saturated aqueous Na2S2O3 solution and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (from 5%to 30% ethyl acetate in hexane) afforded iodide 28 (379 mg, 94%) as a colorless oil: [α]23.0D −115.0 (c 0.64, CHCl3); IR (neat, cm−1) 3301, 2955, 1590, 1466, 1364, 1166, 1047, 945, 781; 1H NMR (CDCl3 , 400 MHz) δ 6.89 (d, J = 14.6 Hz, 1H), 5.96 (d, J = 14.6 Hz, 1H), 3.95 (m, 1H), 2.33 (dd, J = 17.0, 5.5 Hz, 1H), 1.94 (ddd, J = 17.0, 9.6, 1.4 Hz, 1H), 1.73 (ddd, J = 12.4, 3.7, 1.4 Hz, 1H), 1.66 (s, 3H), 1.42 (dd, J = 11.9, 11.9 Hz, 1H), 1.02 (s, 6H); 13C NMR (CDCl3 , 100 MHz) δ 143.5, 139.0, 128.2, 79.5, 65.1, 48.2, 42.4, 36.8, 30.2, 28.7, 21.7; FAB-HRMS m/z calcd for C11H17IO7 (M + H)+ 293.0397, found 293.0423.
trans-2-[(4S)-4-Acetoxy-2,6,6-trimethylcyclohexene]-1-iodoethylene (29)
To a solution of iodide 28 (379 mg, 1.30 mmol) in pyridine (5.19 mL) was added acetic anhydride (0.24 mL, 2.59 mmol) at room temperature, and the reaction mixture was stirred for 16 h at the same temperature. A saturated aqueous CuSO4 solution was added and then the resulting mixture was extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (30% ethyl acetate in hexane) afforded acetate 29 (374 mg, 86%) as a colorless oil: [α]25.0D −99.8 (c 1.10, CHCl3); IR (neat, cm−1) 2963, 2867, 1740, 1588, 1466, 1242, 1117, 1035, 968; NMR (CDCl3, 400 MHz) δ 6.91 (d, J = 14.6 Hz, 1H), 6.00 (d, J = 15.1 Hz, 1H), 5.02 (m, 1H), 2.39 (dd, J = 16.9, 5.4 Hz, 1H), 2.04 (s, 3H), 2.01 (m, 1H), 1.76 (ddd, J = 11.9, 3.2, 1.8 Hz, 1H), 1.67 (s, 3H), 1.55 (dd, J = 11.5, 11.5 Hz, 1H), 1.07 (s, 3H), 1.04 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 171.1, 143.3, 139.2, 127.8, 79.8, 68.3, 43.9, 38.4, 36.4, 30.0, 28.5, 21.8, 21.6; FAB-HRMS m/z calcd for C39H51O7 (M + H)+ 335.0502, found 335.0541.
(2E,4E,6E)-7-[(4′S)-4′-Acetoxy-2′,6′,6′-trimethylcyclohexene]-5-methylhepta-2,4,6-trien-1-ol (30)
To a solution of acetate 29 (194 mg, 0.58 mmol) and (2E,4E)-5-(tributylstannyl)hexa- 2,4-dien-1-ol 23 (247 mg, 0.64 mmol) in DMF (2.9 mL) was added diisopropylethylamine (0.30 mL, 1.74 mmol), bis(acetonitrile)dichloropalladium(II) (7 mg, 0.03 mmol) and lithium chloride (49 mg, 1.16 mmol). After being stirred for 50 min at room temperature, the reaction mixture was poured into water and then extracted with ethyl acetate. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by silica gel column chromatography (from 10% to 30% ethyl acetate in hexane) afforded 30 (151 mg, 85%) as a yellow oil: [α]23.0D −91.0 (c 1.33, CHCl3); IR (neat, cm−1) 3414, 2961, 2924, 1736, 1366, 1244, 1030; 1H NMR (CDCl3 , 400 MHz) δ 6.63 (dd, J = 15.1, 11.2 Hz, 1H), 6.13–5.96 (m, 3H), 5.88 (td, J = 12.1, 5.9 Hz, 1H), 5.06 (m, 1H), 4.24 (m, 1H), 2.44 (dd, J = 17.0, 5.72 Hz, 1H), 2.06 (m, 1H), 2.05 (s, 3H), 1.92 (s, 3H), 1.77 (ddd, J = 12.2, 3.4, 1.8 Hz, 1H), 1.71 (s, 3H), 1.56 (m, 1H), 1.10 (s, 3H), 1.06 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.8, 138.3, 137.7, 135.9, 132.0, 129.3, 127.9, 125.8, 125.6, 68.4, 63.7, 43.9, 38.4, 36.6, 29.9, 28.4, 21.5, 21.4, 12.6; ESI-HRMS m/z calcd for C19H28O3Na (M + Na)+ 327.1936, found 327.1940.
2-(((2′E,4′E,6′E)-7′-((4″S)-4″-Acetoxy-1″,2″-epoxy-2″,6″,6″-trimethylcyclohexene) -5′ -methylhepta -2,4,6- triene)sulfanyl)benzothiazole (30a)
To a solution of alcohol 30 (110 mg, 0.29 mmol), 2-mercaptobenzothiazole (68 mg, 0.41 mmol) and triphenylphosphine (107 mg, 0.41 mmol) in THF (3 mL) was added dropwise diisopropyl azodicarboxylate (0.09 mL, 0.47 mmol) at 0 °C. The reaction mixture was stirred for 10 min at room temperature and the solvents were removed in vacuo. To the residue was added diethyl ether and the precipitate was removed by filtration through a pad of Celite to give the crude products as a solution, which was concentrated in vacuo. Purification by short silica gel column chromatography (from 10% to 30% ethyl acetate in hexane) afforded thioether 30a (111 mg, 84%): [α]23.0D −64.1 (c 0.93, CHCl3); IR (neat, cm−1) 2963, 2926, 1734, 1460, 1427, 1363, 1244, 1030; 1H NMR (CDCl3 , 400 MHz) δ 7.87 (m, 1H), 7.75 (m, 1H), 7.41 (m, 1H), 7.27 (m, 1H), 6.74 (dd, J = 14.6, 11.2 Hz, 1H), 6.09–6.04 (m, 2H), 6.02 (d, J = 10.0 Hz, 1H), 5.88 (m, 1H), 5.04 (m, 1H), 4.11 (d, J = 7.6 Hz, 2H), 2.43 (dd, J = 17.0, 5.5 Hz, 1H), 2.08 (dd, J = 17.0, 9.4 Hz, 1H), 2.04 (s, 3H), 1.91 (s, 3H), 1.73 (m, 1H), 1.69 (s, 3H), 1.56 (m, 1H), 1.08 (s, 3H), 1.05 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 170.7, 166.2, 153.2, 138.2, 137.6, 136.3, 135.3, 131.2, 128.9, 126.7, 126.1, 125.9, 125.6, 124.2, 121.5, 120.9, 68.2, 43.9, 38.3, 36.6, 36.2, 29.9, 28.4, 21.4, 21.4, 12.56; ESI-HRMS m/z calcd for C26H31NO2S2Na (M + Na)+ 476.1694, found 476.1696.
2-(((2′E,4′E,6′E)-7′-((4″S)-4″-Acetoxy-2″,6″,6″-trimethylcyclohexene)-5′-methylhepta-2,4,6-triene)sulfonyl)benzothiazole (8)
To a solution of the thioether 30a (205 mg, 0.45 mmol) in ethanol (9.0 mL) was added dropwise a solution of sodium tungstate(VI) dihydrate (164 mg, 0.50 mmol) in hydrogen peroxide (30 wt.% in water, 5,42 mL) at 0 °C. After being stirred for 50 min at room temperature, the reaction mixture was poured into water and then extracted with diethyl ether. The organic layers were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography (from 10% to 30% ethyl acetate in hexane) afforded the sulfone 8 (68 mg, 31%): [α]23.0D −43.5 (c 1.50, CHCl3); IR (neat, cm−1) 2963, 1728, 1630, 1471, 1364, 1330, 1244, 1148, 1026, 970; 1H NMR (CDCl3 , 400 MHz) δ 8.24 (m, 1H), 7.99 (m, 1H), 7.63 (m, 2H), 6.60 (dd, J = 14.9, 11.3Hz, 1H), 6.10 (d, J = 16.2Hz, 1H), 5.99 (d, J = 16.3Hz, 1H), 5.98 (d, J = 11.4 Hz, 1H), 5.62 (m, 1H), 5.04 (m, 1H), 4.33 (d, J = 7.4 Hz, 2H), 2.37 (m, 1H), 2.09 (m, 1H), 2.04 (s, 3H), 1.80–1.65 (m, 1H), 1.74 (s, 3H), 1.60–1.50 (m, 1H), 1.43 (s, 3H), 0.97 (s, 3H), 0.88 (s, 3H); 13C NMR (CDCl3 , 100 MHz) δ 171.5, 166.3, 153.3, 139.2, 138.5, 137.8, 137.6, 133.9, 129.5, 128.6, 128.3, 128.2, 127.6, 126.1, 123.0, 116.1, 69.2, 59.8, 44.7, 38.7, 37.8, 29.5, 28.9, 22.12, 20.8, 13.2; ESI-HRMS m/z calcd for C26H31NO4S2Na (M + Na)+ 508.1592, found 508.1547.
Peridinin derivative D (4)
To a solution of sulfone 8 (22 mg, 0.045 mmol) and aldehyde 5 (16 mg, 0.045 mmol) in THF (0.68 mL) was added dropwise sodium bis(trimethylsilyl)amide (1.0 M in THF, 0.14 mL, 0.14 mmol) at −78 °C in the dark. After being stirred for 5 min at the same temperature, the reaction mixture was poured into water and then extracted with diethyl ether. The organic layers were combined, washed with brine, dried over MgSO4, filtered and concentrated in vacuo. Purification by short silica gel column chromatography (from 30% to 50% ethyl acetate in hexane) in the dark afforded peridinin derivative 4 (13 mg, 47%) as a mixture of the isomers as a red film. A solution of a mixture of all-trans-peridinin derivative 4 and its isomer in benzene was left at room temperature under fluorescent light. After 4 days, partial separation by preparative HPLC[column: Develosil CN-UG (0.6 × 25 cm); mobile phase: acetone/n-hexane = 1/10; flow rate: 2 mL/min; UVdetect: 459 nm; retention time: (all-trans-isomer) 68 min, (15-cis-isomer) 61 min] in the dark, and HPLC [column: YMC Carotenoid C30 (10 × 250 mm); reverse phase: acetonitrile/methanol/water = 87/10/3; flow rate: 2.0 mL/min; UVdetect: 459 nm; retention time: (all-trans-isomer) 34 min] in the dark afforded the desired optically active peridinin derivative 4 as a red film: IR (neat, cm−1) 3449, 2924, 2853, 2363, 1751, 1655, 1509, 1364, 1242, 1124, 1034; 1H NMR (C6D6, 750 MHz) δ 7.57 (d, J = 15.5 Hz, 1H), 6.68 (dd, J = 13.7, 12.3 Hz, 1H), 6.57 (d, J = 15.5 Hz, 1H), 6.49 (dd, J = 14.1, 12.0 Hz, 1H), 6.42 (dd, J = 14.1, 12.0 Hz, 1H), 6.36 (d, J = 11.4 Hz, 1H), 6.34 (dd, J = 14.1, 11.0Hz, 1H), 6.27 (d, J = 15.8Hz, 1H), 6.26 (d, J = 11.4Hz, 1H), 6.19 (d, J = 16.1 Hz, 1H), 6.17 (s, 1H), 5.29 (m, 1H), 5.22 (s, 1H), 3.76 (m, 1H), 2.46 (dd, J = 17.1, 5.9 Hz, 1H), 2.20 (ddd, J = 14.2, 4.8, 1.0 Hz, 1H), 2.15 (s, 3H), 2.13 (m, 1H), 1.87 (m, 1H), 1.84 (s, 3H), 1.68 (s, 3H), 1.64 (dd, J = 11.9, 11.9 Hz, 1H), 1.42 (m, 2H), 1.13 (s, 3H), 1.13 (s, 3H), 1.09 (s, 3H), 1.08 (s, 3H), 1.07 (s, 3H), 1.06 (m, 1.16); 13C NMR (C6D6, 188 MHz) δ 169.8, 168.3, 147.4, 139.0, 138.1, 137.7, 137.2, 136.3, 134.6, 134.3, 134.1, 131.6, 129.6, 126.7, 126.4, 125.1, 122.4, 118.5, 70.4, 68.1, 67.4, 63.8, 47.3, 44.4, 41.2, 38.8, 36.8, 35.3, 30.1, 29.5, 28.6, 25.3, 21.5, 21.0, 19.9, 15.6, 12.7; ESI-HRMS m/z calcd for C39H50O6Na (M + Na)+ 637.3505, found 637.3517.
Supplementary Material
Acknowledgements
We thank Dr Thomas Netscher of DSM Nutritional Products, Ltd., for the donation of (−)-actinol, and Mr Keigo Yoshida and co-workers of the Institute for Life Science Research at Asahi Chemical Industry Co., Ltd., for their high-resolution mass spectra measurements. This work was supported by a Grant-in- Aid for Science Research on Priority Areas 16073222 from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by a Maching Fund Subsidy for a Private University. HH thanks Nissan Science Foundation and HFSP for financial support. This work was also supported in the lab of HAF by a grant from the National Institute of Health (GM-30353) and by the University of Connecticut Research Foundation.
Footnotes
Electronic supplementary information (ESI) available: Further experimental, characterisation and spectroscopic details. See DOI: 10.1039/b907456b
Contributor Information
Takashi Iwashita, Email: iwashita@sunbor.or.jp.
Harry A. Frank, Email: harry.frank@uconn.edu.
Hideki Hashimoto, Email: hassy@sci.osaka-cu.ac.jp.
Shigeo Katsumura, Email: katsumura@kwansei.ac.jp.
Notes and references
- 1.Schutt F. Ber. Deut. Bot. Ges. 1890;8:9. [Google Scholar]
- 2.Stain H, Svec WA, Aitzetmuller K, Grandolfo MC, Katz JJ, Kjosen H, Norgard S, Liaaen-Jensen S, Haxo FH, Wegfahrt P, Rapoport HJ. Am. Chem. Soc. 1971;93:1823. [Google Scholar]; Strain HH, Svec WA, Webfahrt P, Rapoport H, Haxo FT, Nogard S, Kjosen H, Liaaen-Jensen S. Acta Chem. Scand. Ser. B. 1976;30:109. [Google Scholar]; Johansen JE, Borch G, Liaaen-Jensen JE. Phytochemistry. 1980;19:441. [Google Scholar]
- 3.Ito M, Hirata Y, Shibata A, Tsukida K. J. Chem. Soc., Perkin Trans. 1. 1990:197. [Google Scholar]; Yamano Y, Ito M. J. Chem. Soc., Perkin Trans. 1. 1993:1599. [Google Scholar]; Ito M, Yamano Y, Sumiya S, Wada A. Pure Appl. Chem. 1994;66:939. [Google Scholar]; Yamano Y, Tode C, Ito M. J. Chem. Soc., Perkin Trans. 1. 1995:1895. [Google Scholar]
- 4.Furuichi N, Hara H, Osaki T, Mori H, Katsumura S. Angew. Chem. Int. Ed. 2002;41:1023. doi: 10.1002/1521-3773(20020315)41:6<1023::aid-anie1023>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; Furuichi N, Hara H, Osaki T, Nakano MM, Mori H, Katsumura S. J. Org. Chem. 2004;69:7949. doi: 10.1021/jo048852v. [DOI] [PubMed] [Google Scholar]
- 5.Olpp T, Brückner R. Angew. Chem. Int. Ed. 2006;45:4023. doi: 10.1002/anie.200600502. [DOI] [PubMed] [Google Scholar]
- 6.Vaz B, Dominguez M, Alvarez R, de Lera AR. Chem. Eur. J. 2007;13:1273. doi: 10.1002/chem.200600959. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann E, Wrench PM, Sharples FP, Hiller RG, Welte W, Diederichs K. Science. 1996;272:1788. doi: 10.1126/science.272.5269.1788. [DOI] [PubMed] [Google Scholar]
- 8.Song PS, Koba P, Prezelin BB, Haxo FT. Biochemistry. 1976;15:4422. doi: 10.1021/bi00665a012. [DOI] [PubMed] [Google Scholar]; Mimuro M, Nishimura Y, Takaichi S, Yamano Y, Ito M, Nagaoka S, Yamazaki I, Katoh T, Nagashima U. Chem Phys. Lett. 1993;213:576. [Google Scholar]; Bautista JA, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR, Frank HA. J. Phys. Chem. A. 1999;103:2267. [Google Scholar]
- 9.Akimoto S, Takaichi S, Ogata T, Nishimura Y, Yamazaki I, Mimuro M. Chem. Phys. Lett. 1996;260:147. [Google Scholar]; Bautista JA, Connors RE, Raju BB, Hiller RG, Sharples FP, Gosztola D, Wasielewski MR, Frank HA. J. Phys. Chem. B. 1999;103:8751. [Google Scholar]; Chatterjee N, Niedzwiedzki DM, Kajikawa T, Hasegawa S, Katsumura S, Frank HA. Chem. Phys. Lett. 2008;463:219. doi: 10.1016/j.cplett.2008.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Premvardhan L, Papagiannakis E, Hiller RG, Grondelle RV. J. Phys. Chem. B. 2005;109:15589. doi: 10.1021/jp052027g. [DOI] [PubMed] [Google Scholar]
- 11.Zigmantas D, Hiller RG, Sundstroem V, Polivka T. Proc. Natl. Acad. Sci. USA. 2002;99:16760. doi: 10.1073/pnas.262537599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuba M, Furuichi N, Katsumura S. Chem. Lett. 2002:1248. [Google Scholar]
- 13.Baumeler A, Brade W, Haag A, Eugster CH. Helv. Chim. Acta. 1990;73:700. [Google Scholar]
- 14.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467. [Google Scholar]; Takahashi K, Kuroyama Y, Sonogashira K, Hagihara N. Synthesis. 1980:627. ; for a review: [Google Scholar]; Sonogashira K. Comprehensive Organic Synthesis. Vol. 3. Pergamon Press; 1991. p. 521. [Google Scholar]
- 15.Schulz HS, Freyermuth SR, Buc SR. J. Org. Chem. 1963;28:1140. [Google Scholar]
- 16.Baudin JB, Hareau G, Julia SA, Ruel O. Tetrahedron Lett. 1991;32:1175. [Google Scholar]; Baudin JB, Hareau G, Julia SA, Ruel O. Bull. Soc. Chim. Fr. 1993;130:336. [Google Scholar]; Baudin JB, Hareau G, Julia SA, Lorne R, Ruel O. Bull. Soc. Chim. Fr. 1993;130:856. [Google Scholar]; Blakemore PR, Cole WPJ, Kocienski A, Morley J. Synlett. 1998:26. Recent review: [Google Scholar]; Blakemore PR. J. Chem. Soc., Perkin Trans. 1. 2002:2563. [Google Scholar]
- 17.Kuki M, Koyama Y, Nagae H. J. Phys. Chem. 1991;95:7171. [Google Scholar]; Murakami Y, Nakano M, Shimofusa T, Furuichi N, Katsumura S. Org. Biomol. Chem. 2005;3:1372. doi: 10.1039/b500316d. [DOI] [PubMed] [Google Scholar]
- 18.Brückner R, Song A. Synlett. 2005;2:289. [Google Scholar]; Vaz B, Alvarez R, Souto JA, de Lera AR. Synlett. 2005;2:294. [Google Scholar]
- 19.A similar phenomenon of E- and Z-isomerization was reported in the synthesis of pyrrhoxanthin: Vaz B, Dominguez M, Alvarez R, de Lera AR. J. Org. Chem. 2006;71:5914. doi: 10.1021/jo060490z..
- 20.Lipshutz BH, Kozlowski JA, Wilhelm RS. J. Org. Chem. 1984;49:3943. [Google Scholar]; Chen SH, Horvath RF, Joglar J, Fisher MJ, Danishefsky SJ. J. Org. Chem. 1991;56:5834. [Google Scholar]; Kunishima M, Hioki K, Kono K, Kato A, Tani S. J. Org. Chem. 1997;62:7542. [Google Scholar]; Betzer JF, Delaloge F, Muller B, Pancrazi A, Prunet J. J. Org. Chem. 1997;62:7768. [Google Scholar]
- 21.Stille JK. Pure Appl. Chem. 1985;57:1771. [Google Scholar]; Stille JK. Angew. Chem. Int. Ed. Engl. 1986;25:508. [Google Scholar]; Farina V. chapter 3.4. In: Abel EW, Stone FGA, Wilkinson G, editors. Comprehensive Organometallic Chemistry II. Vol. 12. Oxford: Elsevier; 1995. p. 161. [Google Scholar]
- 22.Koyama Y, Takii T, Saiki K, Tsuda K. Photobiochem. Photobiophys. 1983;5:139. [Google Scholar]; Hu Y, Hashimoto H, Moine G, Hengartner U, Koyama Y. J. Chem. Soc., Perkin Trans. 2. 1997:2699. [Google Scholar]
- 23.Corey EJ, Wollenberg RH. J. Org. Chem. 1975;40:3788. [Google Scholar]; Renldo AF, Labadie JW, Stille JK. Org. Syn. 1989;67:86. [Google Scholar]
- 24.The detailed results and analysis of the Stark spectra will be reported in a paper in the field of physics. For the experimental procedure, see: Yanagi K, Kobayashi T, Hashimoto H. Phys. Rev. B. 2003;67:115122. Yanagi K, Shimizu M, Hashimoto H. J. Phys. Chem. B. 2005;109:992. doi: 10.1021/jp046929d. Nakagawa K, Suzuki S, Fujii R, Gardiner AT, Cogdell RJ, Nango M, Hashimoto H. J. Phys. Chem. B. 2008;112:9467. doi: 10.1021/jp801773j..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.