Abstract
The phosphoprotein plastin was originally identified as an abundant transformation-induced polypeptide of chemically transformed neoplastic human fibroblasts. This abundant protein is normally expressed only in leukocytes, suggesting that it may play a role in hemopoietic cell differentiation. Protein microsequencing of plastin purified from leukemic T lymphocytes by high-resolution two-dimensional gel electrophoresis produced eight internal oligopeptide sequences. An oligodeoxynucleotide probe corresponding to one of the oligopeptides was used to clone cDNAs from transformed human fibroblasts that encoded the seven other oligopeptides predicted for human plastin. Sequencing and characterization of two cloned cDNAs revealed the existence of two distinct, but closely related, isoforms of plastin--l-plastin, which is expressed in leukocytes and transformed fibroblasts, and t-plastin, which is expressed in normal cells of solid tissues and transformed fibroblasts. The leukocyte isoform l-plastin is expressed in a diverse variety of human tumor cell lines, suggesting that it may be involved in the neoplastic process of some solid human tumors.
Full text
PDF








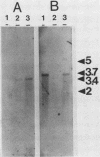
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Cooper C. S., Blair D. G., Oskarsson M. K., Tainsky M. A., Eader L. A., Vande Woude G. F. Characterization of human transforming genes from chemically transformed, teratocarcinoma, and pancreatic carcinoma cell lines. Cancer Res. 1984 Jan;44(1):1–10. [PubMed] [Google Scholar]
- Cooper H. L., Feuerstein N., Noda M., Bassin R. H. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. doi: 10.1128/mcb.5.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Goldman D., Goldin L. R., Rathnagiri P., O'Brien S. J., Egeland J. A., Merril C. R. Twenty-seven protein polymorphisms by two-dimensional electrophoresis of serum, erythrocytes, and fibroblasts in two pedigrees. Am J Hum Genet. 1985 Sep;37(5):898–911. [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Merril C. R. Human lymphocyte polymorphisms detected by quantitative two-dimensional electrophoresis. Am J Hum Genet. 1983 Sep;35(5):827–837. [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Merril C. R., Polinsky R. J., Ebert M. H. Lymphocyte proteins in Huntington's disease: quantitative analysis by use of two-dimensional electrophoresis and computerized densitometry. Clin Chem. 1982 Apr;28(4 Pt 2):1021–1025. [PubMed] [Google Scholar]
- Goldstein D., Djeu J., Latter G., Burbeck S., Leavitt J. Abundant synthesis of the transformation-induced protein of neoplastic human fibroblasts, plastin, in normal lymphocytes. Cancer Res. 1985 Nov;45(11 Pt 2):5643–5647. [PubMed] [Google Scholar]
- Goldstein D., Leavitt J. Expression of neoplasia-related proteins of chemically transformed HuT fibroblasts in human osteosarcoma HOS fibroblasts and modulation of actin expression upon elevation of tumorigenic potential. Cancer Res. 1985 Jul;45(7):3256–3261. [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Hendricks M., Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. doi: 10.1073/pnas.78.9.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I., Hamaguchi H. Evidence for the close linkage between lymphocyte cytosol polypeptide with molecular weight of 64,000 (LCP1) and esterase D. Am J Hum Genet. 1985 Nov;37(6):1106–1111. [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Bushar G., Kakunaga T., Hamada H., Hirakawa T., Goldman D., Merril C. Variations in expression of mutant beta actin accompanying incremental increases in human fibroblast tumorigenicity. Cell. 1982 Feb;28(2):259–268. doi: 10.1016/0092-8674(82)90344-0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Goldman D., Merril C., Kakunaga T. Changes in gene expression accompanying chemically-induced malignant transformation of human fibroblasts. Carcinogenesis. 1982;3(1):61–70. doi: 10.1093/carcin/3.1.61. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Leavitt J., Kakunaga T. Expression of a variant form of actin and additional polypeptide changes following chemical-induced in vitro neoplastic transformation of human fibroblasts. J Biol Chem. 1980 Feb 25;255(4):1650–1661. [PubMed] [Google Scholar]
- Leavitt J., Latter G., Lutomski L., Goldstein D., Burbeck S. Tropomyosin isoform switching in tumorigenic human fibroblasts. Mol Cell Biol. 1986 Jul;6(7):2721–2726. doi: 10.1128/mcb.6.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J., Leavitt A., Attallah A. M. Dissimilar modes of expression of beta- and gamma-actin in normal and leukemic human T lymphocytes. J Biol Chem. 1980 Jun 10;255(11):4984–4987. [PubMed] [Google Scholar]
- Lin C. S., Leavitt J. Cloning and characterization of a cDNA encoding transformation-sensitive tropomyosin isoform 3 from tumorigenic human fibroblasts. Mol Cell Biol. 1988 Jan;8(1):160–168. doi: 10.1128/mcb.8.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Lin J. J., Yamashiro-Matsumura S., Thomas G. P., Topp W. C. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [PubMed] [Google Scholar]
- Matsushima K., Shiroo M., Kung H. F., Copeland T. D. Purification and characterization of a cytosolic 65-kilodalton phosphoprotein in human leukocytes whose phosphorylation is augmented by stimulation with interleukin 1. Biochemistry. 1988 May 17;27(10):3765–3770. doi: 10.1021/bi00410a037. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Paterson H., Reeves B., Brown R., Hall A., Furth M., Bos J., Jones P., Marshall C. Activated N-ras controls the transformed phenotype of HT1080 human fibrosarcoma cells. Cell. 1987 Dec 4;51(5):803–812. doi: 10.1016/0092-8674(87)90103-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Varma M., Aebi U., Fleming J., Leavitt J. A 60-kDa polypeptide in mammalian cells with epitopes related to actin. Exp Cell Res. 1987 Nov;173(1):163–173. doi: 10.1016/0014-4827(87)90342-9. [DOI] [PubMed] [Google Scholar]
- Witt D. P., Brown D. J., Gordon J. A. Transformation-sensitive isoactin in passaged chick embryo fibroblasts transformed by Rous sarcoma virus. J Cell Biol. 1983 Jun;96(6):1766–1771. doi: 10.1083/jcb.96.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]