Abstract
Objective
Adherence to antiretroviral therapies (ART) is the strongest predictor of viral suppression among individuals infected with HIV, however, limited data exists to understand the patterns of adherence that confer the greatest benefit across different ART regimens.
Design
Longitudinal data pooled from 16 studies conducted between 1997 and 2009 across the United States.
Methods
Adherence was measured using Medication Event Monitoring System. Percentage of time with sufficient drug concentrations (covered time) and the length of the longest treatment interruption during the 28 days prior to plasma HIV-RNA measurements were calculated. Logistic regression with generalized estimating equations was used to estimate medication-specific adherence estimates on detectable HIV-RNA (>400 copies/ml).
Results
One thousand and eighty-eight participants with 3795 HIV-RNA measures were studied. Both lower covered time and greater longest interruption showed dose– response relationships with the odds of detectable HIV-RNA; however, estimates did not vary by medication regimen. Compared with 93–100% coverage, periods of 0–25% covered time had a three-fold increased risk of detectable HIV-RNA [odds ratio (OR)=3.22, 95% confidence interval (CI): 2.48–4.19]. Similarly, compared to longest interruptions of 0–48 h, longest interruptions of 21–28 days had a nearly four-fold increased risk of detectable HIV-RNA (OR=3.65, 95% CI: 2.77, 4.81).
Conclusion
We found that adherence was consistently strongly associated with treatment response across ART regimens. Of the patterns of adherence, longer interruptions may have greater impact than covered time. Future research should investigate additional methods for examining adherence patterns, understanding the determinants of consecutive missed doses and the evaluation of interventions designed to address interruptions in treatment.
Keywords: antiretroviral therapy, medication adherence, protease inhibitors, reverse transcriptase inhibitors, viral load
Introduction
Adherence to antiretroviral therapies (ART) is the strongest predictor of HIV-RNA suppression among individuals infected with HIV [1–3]. Average adherence, measured as the percentage of prescribed doses taken, has dominated adherence research. However, since the advent of ART, adherence measurement has evolved making more precise measures of medication-taking possible. Medication-taking patterns may be highly variable with differential impact on outcomes [4]. As a result, there has been increased interest in exploring the impact of multiple dimensions of ART adherence [5, 6].
The use of Medication Event Monitoring System (MEMS) enables the examination of patterns of ART use. Recent research using MEMS has demonstrated that nonstructured treatment interruptions are associated with resistance and other adverse clinical outcomes [7–9]. Interruptions in nonnucleoside reverse-transcriptase inhibitor (NNRTI)-based regimens have also been shown to be associated with an increased risk of viral rebound among those with low-to-moderate overall adherence [10]. However, existing studies using MEMS to examine ART adherence are small, without comparisons of adherence patterns across multiple ART regimens. If different patterns of adherence have differential impact on outcomes, more aggressive intervention may be warranted for patterns conferring higher risk.
The goal of the current study, therefore, was to examine the impact of patterns of adherence on HIV-RNA using a large pooled MEMS data set from the Multisite Adherence Collaboration in HIV 14 study. Additionally, we sought to determine the differential impact of covered time and interruptions in treatment by different ART regimen types on HIV-RNA.
Methods
Multisite adherence collaboration in HIV 14 study
The MACH14 study has been described elsewhere [11]. Briefly, data was pooled from 16 studies conducted between 1997 and 2009 at 14 institutions in 12 states across the United States. To be eligible for inclusion, studies were required to have a longitudinal study design, collected adherence data using MEMS and collected HIV-RNA and clinical outcomes. The overall study population included data from 2860 individuals followed over a mean of 18 months.
Study population
MEMS data were available from 2498 participants with a total of 478 242 individual MEMS openings in the MACH14 study. We restricted the sample to individuals with dosing schedules of one, two, or three daily doses, sufficient follow-up to establish patterns (≥4 weeks), at least one HIV-RNA measured during the MEMS monitoring period and without protocol-driven interruptions in MEMS monitoring.
By design, some MACH14 studies monitored multiple drugs using multiple MEMS at the same time per participant. In some cases, there was only a brief period of overlapping MEMS from the same individual (≤5 days), and these were included assuming that they represented transitions between caps. In cases of more than 5 days overlap, we selected one MEMS to include based on the monitored medicine using the following prioritization: NNRTI, boosted protease inhibitor, protease inhibitor, other. If there were multiple medicines of the same type monitored simultaneously from one individual, we selected the cap with the greatest number of openings.
After the above restrictions, the sample included 1088 individuals with 3795 HIV-RNA measures, with an average of 3.5 HIV-RNA measures per person (SD: 3.2, range: 1–30).
Statistical analysis
We restricted MEMS data to the 28 days prior to each HIV-RNA measurement. The outcome of interest was a dichotomous variable indicating detectable plasma HIVRNA (>400 copies/ml). We chose this cutoff due to the varying sensitivity of HIV-RNA assays during the years data was collected.
Standard descriptive statistics were used to describe sociodemographics and characteristics associated with the 28-day periods preceding HIV-RNA measurements. A categorical variable classified the medication regimens as: NNRTI, boosted protease inhibitor, protease inhibitor, or other regimen type (included two nucleoside reverse-transcriptase inhibitors with no other reported drug, fusion inhibitors, or unspecified/unknown regimens).
To measure adherence, we used an approach that took advantage of the MEMS data by capturing both dose frequency and dose timing. Our goal was to estimate the fraction of time during the 28 days that drug levels were in therapeutic range. Beginning with elapsed time between MEMS openings, we considered any lapse that exceeded 3 h from the prescribed time as ‘noncovered’ time. Noncovered time was calculated according to the dosing schedule. For once, twice and thrice daily dosing schedules, noncovered time was a sum of the time over the previous 28 days when lapses between openings exceeded 27, 15 and 11 h between openings, respectively. For each schedule, credit was given for the last dose taken (i.e., subtracted from the total noncovered time) as 24, 12 and 8 h, respectively, in order to roughly correspond to therapeutic range of drug levels. We calculated the covered time by expressing the total amount of noncovered time/hours as a percentage of all time within the previous 28 days and subtracted it from 100%. The longest interruption was the longest amount of consecutive noncovered time over the 28 days.
We created categorical variables with five categories of comparable groups for both covered time and longest interruption. The categories for covered time were 0–25%, 26–50%, 51–75%, 76–92% and 93–100% (reference). The categories for the longest interruption were 0–48 h (reference), 2–7 days, 7–14 days, 14–21 days and 21 days or longer. We created a covariate to account for the noncovered time not accounted for by the longest interruption. We created a variable indicating deciles of the percentage of noncovered time that was accountable for by the longest interruption in order to determine the impact of consecutive missed doses at various levels of coverage.
For both adherence measures (covered time and longest interruption), graphical analysis was conducted by plotting observed adherence by the proportion with detectable HIV-RNA by medication regimen, using the loess function to smooth the data.
We considered two approaches to handle the clustering of the data: logistic regression models with generalized estimating equations (GEEs) including a fixed effect for site and random effects models accounting for repeated measures from individuals nested within study sites. Upon examining the intraclass correlations (ICC) from marginal models for both approaches to determine which better represented the data structure, we conducted all regression analysis using GEE models with clustering by individual, robust standard errors and a fixed effect for site, as the ICC within site was low (ICC=0.090) compared with within individuals (ICC=0.758), and the random effects model was unstable and imprecise.
We fitted a series models to determine the effects of covered time and the longest interruption in the prior 28 days on detectable HIV-RNA. Models with the categorical variables described above were estimated separately for covered time and longest interruption including interaction terms to obtain medication-type specific parameter estimates. In the longest interruption models, we adjusted for the remaining noncovered time. Other potential confounders (age, sex, race, educational attainment) were examined independently and excluded if not associated with detectable HIVRNA (P>0.10). We compared the predicted probabilities from these models with detectable HIV-RNA using area under the curve (AUC) analysis. Finally, we examined the deciles of the percentage of noncovered time that was accounted for by the longest interruption, both graphically and in models predicting detectable HIV RNA.
Additional sensitivity analyses included the following: examination of the final models adjusted for treatment experience at baseline; restricting the final model to those who were ART-naive at baseline; examining the change in main effects after adjusting for calendar year; and examination of the final models excluding those with less than 5% and more than 95% covered time because these groups have limited variability in adherence patterns. All analyses were conducted with Stata version 11.0 (College Station, Texas, USA).
Results
Table 1 displays the demographic characteristics of the 1088 participants in the sample and the time-varying characteristics from the 28-days prior to HIV RNA measures. Thirty-one percent of the sample was women with a mean age of 40 years. Approximately, one-third of the participants were treatment-naive at baseline. Half of the patients (51%) were taking protease inhibitors, 14% NNRTIs, 8% boosted-protease inhibitors, and 27% other regimens. Thirty-eight percent of HIV-RNA measures were detectable (>400 copies/ml). The mean average covered time was 56%. The mean longest interruption was 7.1 days. The average covered time was lower (P<0.0001) and the longest interruption was longer (P<0.0001) in the 28-day periods prior to detectable HIV-RNA.
Table 1.
Time fixed demographic and time-varying characteristics of 1088 participants from the MACH14 study.
| Time-fixed | % | Mean (SD) | Median (IQR) |
|---|---|---|---|
| Women | 31 | ||
| Age, in years | 40.7 (8) | 40.1 (35–46) | |
| Race/ethnicity | |||
| African–American/Black | 44 | ||
| White | 30 | ||
| Hispanic/Latino | 19 | ||
| Other | 7 | ||
| Educational attainment | |||
| Some high-school | 23 | ||
| HS graduate/some college | 63 | ||
| College graduate or higher | 14 | ||
| Treatment-naive at baseline | 28 | ||
| Number of HIV RNA measures per person | 3.5 (3) | 3 (1–5) | |
| Time-varying | % | Mean (SD) | Median (IQR) |
| Medication regimen type | |||
| NNRTI | 14 | ||
| Boosted-PI | 8 | ||
| PI | 51 | ||
| Other | 27 | ||
| HIV-RNA (copies/ml) | 18 846 | 400 (400–1486) | |
| Detectable HIV RNA (>400 copies/mL) | 38 | ||
| Covered time (%) | 56 (35) | 64 (20–89) | |
| Detectable HIV RNA | 43 (33)a | 39 (8–79) | |
| Undetectable HIV RNA | 63 (33) | 74 (40–92) | |
| Length of longest interruption, in days | 7.1 (9) | 2.5 (0.7–10.0) | |
| Detectable HIV RNA | 10.1 (8)b | 6.3 (1.4–18.5) | |
| Undetectable HIV RNA | 5.3 (10) | 1.5 (0.6–6.1) | |
NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Difference between detectable and undetectable (t= 17.3, P<0.0001).
Difference between detectable and undetectable (t= 16.5, P<0.0001).
Percentage covered time in previous 28 days
Table 2 presents the distribution of percentage-covered time by medication regimen. Of the five categories, within each regimen the highest proportion of patients was in the 0–25% adherence range. Patients on NNRTI regimens had the largest proportion in the most adherent group (28%), compared with 16, 19 and 18 percent in boosted-protease inhibitor, protease inhibitor and others, respectively.
Table 2.
Distribution of adherence (coverage and longest interruption in prior 28 days) and undetectable HIV RNA (<400 copies/ml) in 3795 28-day periods, by medication regimen.
| NNRTI (n = 547) | Boosted-PI (n = 315) | PI (n = 1919) | Other (n = 1014) | Total (n = 3795) | |
|---|---|---|---|---|---|
| Covered time | |||||
| 93–100% | 153 (28) | 49 (16) | 359 (19) | 182 (18) | 743 (20) |
| 76–92% | 83 (15) | 68 (22) | 458 (24) | 223 (22) | 832 (22) |
| 51–75% | 87 (16) | 69 (22) | 361 (19) | 183 (18) | 700 (18) |
| 26–50% | 68 (12) | 42 (13) | 268 (14) | 134 (13) | 512 (13) |
| 0–25% | 156 (29) | 87 (28) | 473 (25) | 295 (29) | 1008 (27) |
| Length of longest interruption | |||||
| <48 h | 236 (43) | 127 (40) | 960 (50) | 436 (43) | 1759 (46) |
| >2 ≤ 7 days | 104 (19) | 79 (25) | 452 (24) | 242 (24) | 877 (23) |
| >7 ≤ 14 days | 56 (10) | 45 (14) | 189 (10) | 120 (12) | 410 (11) |
| >14 ≤ 21 days | 40 (7) | 20 (6) | 84 (4) | 65 (6) | 209 (6) |
| >21 ≤ 28 days | 111 (20) | 44 (14) | 234 (12) | 151 (15) | 540 (14) |
| Treatment-naive at baseline | 124 (23) | 27 (9) | 832 (45) | 415 (41) | 1398 (38) |
| Undetectable HIV RNA | |||||
| All | 310 (57) | 161 (51) | 1273 (66) | 627 (62) | 2371 (62) |
| Treatment-naive | 77 (62) | 19 (70) | 585 (70) | 289 (70) | 970 (69) |
| Treatment-experienced | 233 (55) | 142 (49) | 667 (64) | 329 (56) | 1371 (59) |
NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor. All values represent N (%).
Table 3 presents the results of the covered time by medication regimen interaction model. The odds ratios (ORs) represent the odds of having a detectable HIV-RNA comparing each average adherence group to 93– 100% adherence, within medication type. There was an increased risk of detectable HIV-RNA at 26–50% of covered time in the NNRTI [OR=2.43, 95% confidence interval (CI): 1.31–4.53] and protease inhibitor regimens (OR=1.74, 95% CI: 1.18–2.57), but not in the boosted protease inhibitor (OR=1.04, 95% CI: 0.46–2.34) or other regimens (OR=1.57, 95% CI: 0.86–2.87). Within all medication types, there were statistically significant differences in the odds of detectable HIV-RNA comparing the lowest (0–25%) to the highest covered group, with the odds of detectable HIV-RNA ranging from three to four times higher among the lowest group across all regimens. The interaction terms were not statistically significant (all terms P>0.05).
Table 3.
Odds ratios and 95% confidence intervals of detectable HIV RNA, percentage covered time and longest interruption in treatment, by medication regimen type.
| Modela | NNRTI (n = 547) | Boosted-PI (n = 315) | PI (n = 1919) | Other (n = 1014) | Total (n = 3795) |
|---|---|---|---|---|---|
| Covered time | |||||
| 93–100% | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 76–92% | 1.01 (0.60, 1.64) | 0.83 (0.46, 1.52) | 1.00 (0.73, 1.36) | 1.21 (0.76, 1.92) | 1.03 (0.83, 1.28) |
| 51–75% | 1.11 (0.61, 2.00) | 0.63 (0.31, 1.26) | 1.22 (0.87, 1.73) | 1.13 (0.63, 2.00) | 1.11 (0.86, 1.42) |
| 26–50% | 2.43 (1.31, 4.53) | 1.04 (0.46, 2.34) | 1.74 (1.18, 2.57) | 1.57 (0.86, 2.87) | 1.68 (1.28, 2.22) |
| 0–25% | 4.08 (2.09, 8.22) | 2.77 (1.27, 6.00) | 3.06 (2.07, 4.52) | 3.36 (2.03, 5.54) | 3.22 (2.48, 4.19) |
| Length of longest interruptionb | |||||
| <48 h | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| >2 ≤ 7 days | 1.12 (0.61, 1.52) | 1.24 (0.73, 2.09) | 1.35 (1.06, 1.73) | 0.89 (0.59, 1.35) | 1.15 (0.96, 1.40) |
| >7 ≤ 14 days | 1.91 (1.10, 3.33) | 2.46 (1.36, 4.45) | 2.16 (1.50, 3.11) | 1.92 (1.13, 3.24) | 2.06 (1.58, 2.68) |
| >14 ≤ 21 days | 3.37 (1.75, 6.50) | 4.38 (1.57, 12.2) | 2.65 (1.72, 4.09) | 2.29 (1.29, 4.08) | 2.74 (2.04, 3.67) |
| >21 ≤ 28 days | 4.38 (2.17, 8.81) | 3.43 (1.49, 7.91) | 3.21 (2.20, 4.68) | 4.21 (2.60, 6.85) | 3.65 (2.77, 4.81) |
NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor. All interaction terms were P > 0.05.
All models were estimated using generalized estimating equations with robust standard errors and a fixed effect for study site.
Longest interruption models were also adjusted for remaining noncovered time from the prior 28 days.
Longest interruption in previous 28 days
Table 2 also presents the distributions of the longest interruption within medication regimens. Between 40–50% of the longest interruptions across all regimens fell into the 0–48 h category. Only 9% of patients on boosted protease inhibitor regimens were treatment naive at baseline. Additionally, the proportion undetectable was higher among those who were treatment naive at baseline, compared to those who were treatment experienced across all medication types.
Table 3 also presents the odds ratios of detectable HIV-RNA comparing varying lengths of interruption by medication type. Within each medication there was a dose–response relationship observed with the odds of a detectable HIV-RNA rising incrementally with each increasing longest interruption, compared to interruptions that were 0–48 h. Although the boosted protease inhibitor group may not appear to follow this pattern, the lack of precision in the estimates warrant caution. The odds of a detectable HIV-RNA were 35% higher in the protease inhibitor group for interruptions between 2–7 days (OR=1.35, 95% CI: 1.06–1.73) compared with interruptions of 0–48 h, but not statistically significant in the NNRTI, boosted protease inhibitor or other regimens. For all medications comparing to 0–48 h, the odds of a detectable HIV-RNA were more than two-folds higher for longest interruptions that were between 7–14 days (ORs ranged from 1.91 to 2.46). Comparing to interruptions of 0–48 h, the odds of a detectable HIV-RNA were between two to four times higher for interruptions of 14–21 days (ORs ranged from 2.29 to 4.38) and from three to four times higher for interruptions that 21 days or longer (ORs ranged from 3.21 to 4.38) across all medication types. The interaction terms were not statistically significant (all terms P>0.05).
None of the potential demographic confounders (age, sex, race, educational attainment) were statistically significant when included with the main covariates of interest (P>0.10 for all) and were not included in the final models.
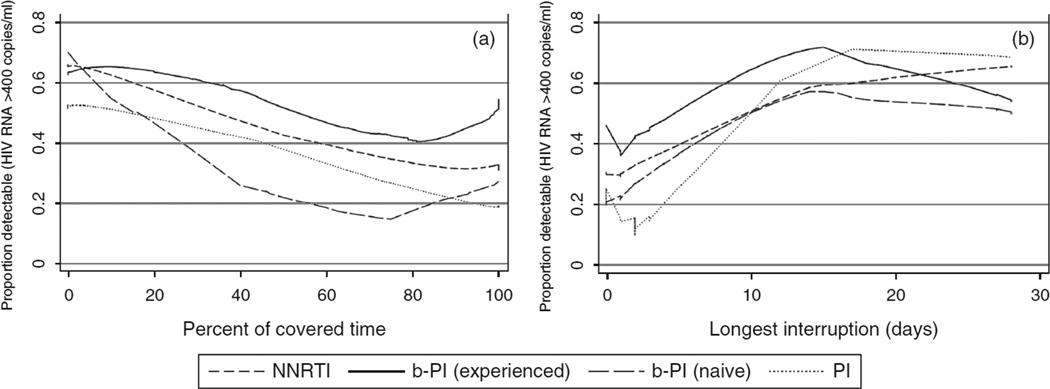
Figure 1 depicts percentage covered time (panel A) and longest interruption (panel B) in the previous 28 days by the proportion detectable within each medication regimen. The boosted protease inhibitor group was further stratified by previous treatment experience. Boosted protease inhibitors with prior treatment showed the highest overall proportion detectable HIV-RNA, followed by NNRTIs, protease inhibitors, and boosted protease inhibitors without treatment experience. There was a sharper increase in the proportion detectable from 0 to 14 days in the longest interruption compared with covered time, followed by a flattening and overlapping pattern. Although both measures were only fair in predicting detectable HIV-RNA, the longest interruption had a slightly higher AUC (0.664 vs. 0.651, P<0.01).
Fig. 1.
Proportion detectable by percentage covered time. Proportion detectable (HIV RNA >400 copies/ml) by (a) percentage covered time and (b) longest interruption in previous 28 days, by medication regimen. b-PI, boosted protease inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor.
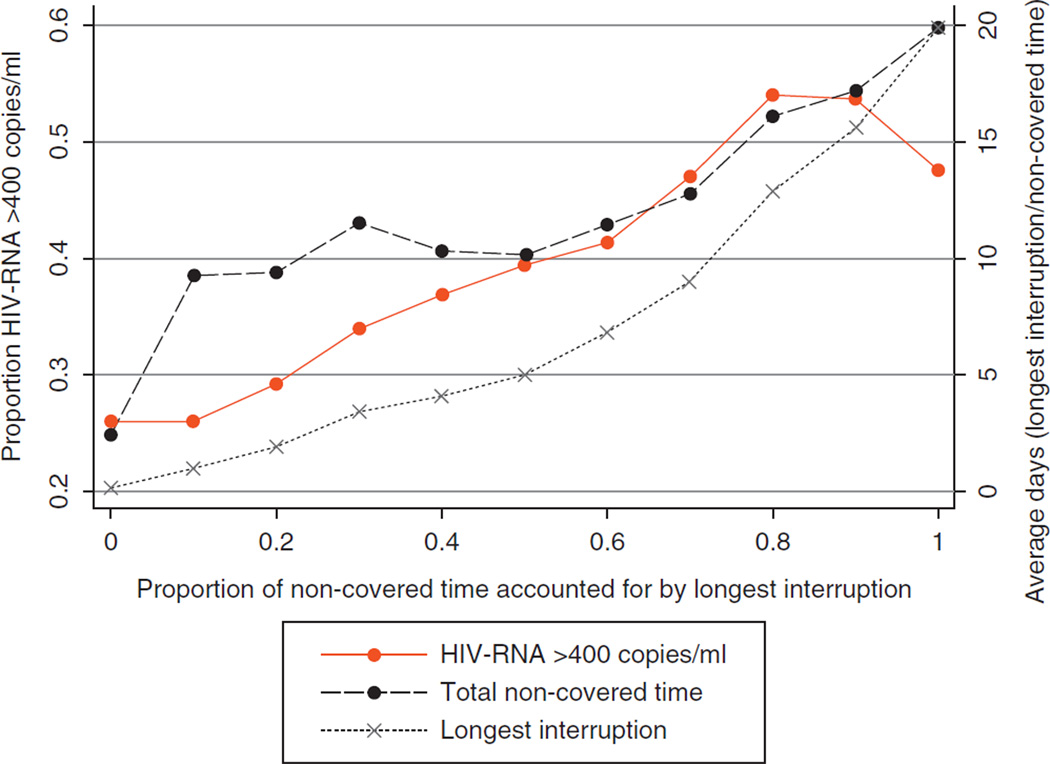
Figure 2 depicts deciles of noncovered time accounted for by the longest interruption and the variations in patterns observed. With increasing deciles of noncovered time, the proportion detectable, the average longest interruption and the average noncovered time increase. With increasing deciles of noncovered time accounted for by the longest interruption, there was a 9% increase in the odds of a detectable HIV RNA (OR=1.09, 95% CI: 1.07–1.12). After adjusting for the total amount of covered time in the 28 days, there was a 5% increase in the odds of a detectable HIV-RNA (OR=1.05, 95% CI: 1.02–1.07) with each decile.
Fig. 2.
Proportion detectable (HIV RNA >400 copies/ml), average noncovered time (in days) and average longest interruption (in days) by deciles of noncovered time accounted for by the longest interruption.
Sensitivity analyses
Table 4 details the results of the two final models (categories of covered time and the longest interruption) excluding those with very high (>95%) and very low (<5%) covered time. The ORs estimated in these models demonstrate stronger associations than observed in the final models overall. In addition, the models also demonstrated increased risk for detectable HIV-RNA at 76–92% coverage (OR=1.47, 95% CI: 1.08–2.04) and for interruptions between 2–7 days (OR=1.21, 95% CI: 1.00–1.46) compared to the highest adherence groups.
Table 4.
Odds ratios and 95% confidence intervals of detectable HIV RNA by categories of covered time and the longest interruption, excluding less than 5% and more than 95% covered time.
| Excluding less than 5% and more than 95% covered time |
OR (95% CI) |
|---|---|
| Covered time | |
| 93–100% | 1.00 |
| 76–92% | 1.47 (1.08, 2.04) |
| 51–75% | 1.56 (1.61, 3.50) |
| 26–50% | 2.38 (1.61, 3.50) |
| 0–25% | 4.23 (2.82, 6.34) |
| Length of longest interruption | |
| <48 h | 1.00 |
| >2 ≤ 7 days | 1.21 (1.00, 1.46) |
| >7 ≤ 14 days | 2.12 (1.62, 2.78) |
| >14 ≤ 21 days | 2.62 (1.85, 3.72) |
| >21 ≤ 28 days | 3.75 (2.46, 5.72) |
CI, confidence interval; OR, odds ratio.
Additional sensitivity analyses (data not shown) adjusting for treatment experience at baseline or calendar year demonstrated no impact on the associations observed (e.g., <5% change in magnitude across associations compared to final model). When restricting the final model to treatment-naive individuals, the associations between detectable HIV-RNA and longest interruption were in the same direction, but greater in magnitude than those from the full sample.
Discussion
There are two main findings from this research. First, patterns of adherence have different impact on the risk of detectable HIV-RNA. More specifically, missing days of medication consecutively may have a greater impact on being detectable than missing the same amount of time in a nonconsecutive manner. As the percentage of noncovered time that was accounted for by the longest interruption increased, there were incremental increases in the odds of detectable HIV-RNA, after controlling for the amount of noncovered time. Second, although there were different results in terms of the strength of the associations by medication regimen in models examining covered time and interruptions, the interactions were not statistically significant. Still, there were some important differences including a greater impact of covered time inNNRTI and protease inhibitor regimens of shorter interruptions in the protease inhibitor regimen. NNRTI and boosted protease inhibitors have longer half-lives than nonboosted protease inhibitors, which may partially explain the relationships between detectable HIV-RNA with fewer missed consecutive days.
It is important to discuss any potential misinterpretations of the results. We are examining whether or not HIVRNA was detectable without taking previous viral suppression or baseline HIV-RNA into account, therefore, we are neither examining virologic rebound, nor virologic success. Moreover, the ORs examine the odds of detectable HIV-RNA comparing to the most adherent group within each medication class. The observed data in Fig. 1 show the proportion detectable by covered time and longest interruption by medication type. Note that both graphs show that the boosted protease inhibitor regimens have the highest levels of detectable HIV-RNA, followed by NNRTI and protease inhibitor, even though the ORs were the weakest for the boosted protease inhibitor group for moderate covered time. One potential explanation for this finding is that the boosted protease inhibitor group are primarily salvage regimens that were commonly prescribed between the years 2000 and 2008 when the data were collected. Although our data limits us in determining the exact regimens patients were taking beyond the monitored drug, only 9% of the boosted protease inhibitor patients were treatment naive at baseline, suggesting most were likely to be salvage regimens.
Although it was initially thought that extremely high adherence to ART (>95%) was necessary to achieve viral suppression [3], it is now understood that more moderate levels of adherence can achieve suppression when using medications with longer half-lives such as NNRTIs and boosted protease inhibitors [12]. Similar findings have been described for viral rebound among patients on NNRTI regimens [10]. Although the current analysis suggests that consecutive interruptions may be a more important driver of viral outcomes overall, the sensitivity analysis excluding those at the extremes of covered time showed that when compared to the most adherent category, all categories of both covered time and the longest interruption were associated with detectable HIV RNA. Further, the percentage of noncovered time due to the longest interruption and covered time were both independently associated with lack of suppression, suggesting that a fuller description of clinically relevant patient behavior is accomplished by accounting for both measures.
Although consecutive interruptions seem to be harmful, it is unclear at what length a consecutive interruption may begin to have a negative impact. In this study there was a clear dose–response relationship with each increasing week of interrupted time, however, the increased risk was statistically significant starting at interruptions between 7 and 14 days. For protease inhibitor-based regimens, the increased risk was statistically significant at 2–7 days. However, this does not suggest that shorter interruptions are well tolerated. Our data does not suggest a tolerable lower bound. Evidence from randomized controlled trials of structured and CD4 guided treatment interruptions demonstrated deleterious effects of week-long interruptions on clinical outcomes [13–15], however, small studies of shorter interruptions such as 5 days on/2 days off appeared to be as effective as continuous therapy [16, 17]. Recently, the deleterious impact of interruptions greater than 48 h, with an average of 11 days, on the development of resistance was demonstrated [7], findings that are consistent with the results presented here. Additional research with varied outcomes (i.e., rebound, resistance) is needed to determine whether there are tolerable lower bounds for consecutive interruptions.
There are several implications of this study. Although the correlates of self-reported adherence have been well characterized, self-reported measures do not allow for precision in estimates of dose timing, and, therefore, the determinants and correlates of objectively measured interruptions in medication taking are not well understood. Future research should focus on the individual, interpersonal and structural determinants of consecutive missed doses and the evaluation of interventions designed to address interruptions. Innovative wireless technologies have been developed and evaluated as interventions to improve adherence [18] and these technologies can be implemented with the goal of detecting interruptions before viral rebound occurs. Individual barriers to adherence including side effects, mental health and drug use [19, 20], as well as inadequate social support, lack of physician support and insufficient patient-provider communication around adherence may also contribute to interruptions. Patient–provider communication should focus on the patterns of medication taking and work toward shortening and eliminating interruptions in treatment. Structural barriers may significantly impact interruptions, particularly in settings with unstable funding sources, high treatment costs, supply shortages and pharmacy stock outs [21]. Future research should also focus on developing novel methods for capturing the variation in patterns of medication taking among individuals with chronic conditions.
The results should also be interpreted in light of several limitations. First, we cannot be certain that stopping the use of MEMS signified stopping medication, or that openings meant for certain that the patient took the medication at the time of the opening. The latter is concerning for patients who may remove more than one dose with each opening for later use (’pocket-dosing’). There may have been misclassification by medication regimen, resulting in a reduction of power to detect differences between the regimens. In particular, MEMS among patients classified as ‘others’ were predominantly monitoring an nucleoside reverse transcriptase inhibitor, with no additional information about the regimen, and it is likely that a substantial proportion were misclassified. Finally, baseline HIV-RNA was not available because data were not uniformly collected across studies at treatment initiation.
There are additional limitations in the scope of the current analysis due to the challenges of pooling data from multiple studies. We were not able to examine many covariates including detailed treatment histories or resistance. The estimates presented may, therefore, be subject to bias due to time-varying confounders that may have been affected by prior treatment, or by time-varying confounders that were common antecedents of adherence and HIV-RNA [22]. This is particularly concerning due to the lack of discrimination exhibited by either measure and suggests potential bias due to prior treatment, resistance or lack of compliance with MEMS. The lack of standardization across studies resulted in nonsystematic HIV-RNA measurement timing and method (e.g. protocol-driven, clinical care). Finally, there may have been differences between the populations under study not accounted for in the analysis, introducing additional bias. Compared to previous studies, we have less concern regarding external validity due to the large sample size representing several distinct geographic regions and populations in the United States; however, we acknowledge that these results may not be generalizable to non-US populations.
This study provides support for examining patterns of adherence beyond average adherence. Our results suggest that consecutive interruptions may have a greater impact on HIV-RNA than the same number of sporadically missed doses. The impact of occasional missed doses is more pronounced with NNRTI and shorter half-life unboosted protease inhibitor regimens. That long-term interruptions have been associated with increased risk of HIV as well as non-HIV adverse events, including death, highlight the importance of interruptions in determining long-term outcomes. These findings suggest that a greater focus on treatment interruptions and their causes will be important in maximizing treatment outcomes for individuals living with HIV.
Acknowledgements
The National Institute of Mental Health (R01MH078773) and the Agency for Healthcare Research and Quality (T32HS01965) provided funding for this research. The original grants of individual participating studies are: R01DA11869, R01MH54907, R01NR04749, R01NR04749, R01MH68197, R01DA13826, K23MH01862, R01MH01584, R01AI41413, R01MH61173, NIH/NIAID AI38858, AI069419, K02DA017277, R01DA15215, NIMH P01MH49548, R01MH58986, R01MH61695, CC99-SD003, CC02-SD-003 and R01DA015679.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Nonadherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15:2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 6.Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S103–S106. doi: 10.1097/00126334-200212153-00003. [DOI] [PubMed] [Google Scholar]
- 7.Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 8.Parienti JJ, Ragland K, Lucht F, de la Blanchardiere A, Dargere S, Yazdanpanah Y, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin Infect Dis. 2010;50:1192–1197. doi: 10.1086/651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, Larouze B, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38:1311–1316. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 10.Parienti JJ, Das-Douglas M, Massari V, Guzman D, Deeks SG, Verdon R, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni J, Bangsberg DR, et al. A Closer Look at Depression and Its Relationship to HIV Antiretroviral Adherence. Ann Behav Med. 2011;42:352–360. doi: 10.1007/s12160-011-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 13.Dybul M, Nies-Kraske E, Daucher M, Hertogs K, Hallahan CW, Csako G, et al. Long-cycle structured intermittent versus continuous highly active antiretroviral therapy for the treatment of chronic infection with human immunodeficiency virus: effects on drug toxicity and on immunologic and virologic parameters. J Infect Dis. 2003;188:388–396. doi: 10.1086/376535. [DOI] [PubMed] [Google Scholar]
- 14.Ananworanich J, Nuesch R, Le Braz M, Chetchotisakd P, Vibhagool A, Wicharuk S, et al. Failures of 1 week on, 1 week off antiretroviral therapies in a randomized trial. AIDS. 2003;17:F33–F37. doi: 10.1097/00002030-200310170-00001. [DOI] [PubMed] [Google Scholar]
- 15.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SJ, Kityo C, Hallahan CW, Kabuye G, Atwiine D, Mbamanya F, et al. A randomized, controlled, trial of short cycle intermittent compared to continuous antiretroviral therapy for the treatment of HIV infection in Uganda. PLoS One. 2010;5:e10307. doi: 10.1371/journal.pone.0010307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen CJ, Colson AE, Sheble-Hall AG, McLaughlin KA, Morse GD. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clinical Trials. 2007;8:19–23. doi: 10.1310/hct0801-19. [DOI] [PubMed] [Google Scholar]
- 18.Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14:1340–1346. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011;16:1297–1313. doi: 10.1111/j.1365-3156.2011.02828.x. [DOI] [PubMed] [Google Scholar]
- 20.Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S123–S127. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 21.Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernan MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–586. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]