Abstract
Aim. The objective of this study was to characterize coordinated molecular changes in the structure and composition of the walls of venous segments of arteriovenous (AV) fistulas evoked by overflow. Methods. Venous tissue samples were collected from 6 hemodialysis patients with AV fistulas exposed to overflow and from the normal cephalic veins of 4 other hemodialysis patients. Total RNA was extracted from the venous tissue samples, and gene expression between the 2 groups was compared using Whole Human Genome DNA microarray 44 K. Microarray data were analyzed by GeneSpring GX software and Ingenuity Pathway Analysis. Results. The cDNA microarray analysis identified 397 upregulated genes and 456 downregulated genes. Gene ontology analysis with GeneSpring GX software revealed that biological developmental processes and glycosaminoglycan binding were the most upregulated. In addition, most upregulation occurred extracellularly. In the pathway analysis, the TGF beta signaling pathway, cytokines and inflammatory response pathway, hypertrophy model, and the myometrial relaxation and contraction pathway were significantly upregulated compared with the control cephalic vein. Conclusion. Combining microarray results and pathway information available via the Internet provided biological insight into the structure and composition of the venous wall of overflow AV fistulas.
1. Introduction
Arteriovenous (AV) fistulas are very useful for determining optimal blood flow for dialysis, but AV fistulas exposed to overflow are thought to increase cardiac output and cause high-output cardiac failure [1, 2].
Measurement of blood flow via an internal shunt was first developed by Krivitski et al., and the monitoring of blood flow via a shunt has since become widespread [3]. We use this technique to monitor the blood flow of AV fistulas at our hospital and correct overflow AV fistulas with surgery.
It is thought that the outflow vein of overflow AV fistulas bears a heavy load: as the vein is exposed to increased arterial flow, the wall dilates, triggering a vascular remodeling process. However, the molecular mechanisms by which the outflow vein is remodeled into a mature fistula remain unclear. By investigating venous remodeling in overflow AV fistulas, candidate genes important to the remodeling process can be discovered and their functional significance investigated. Thus, the identification of relevant genes involved in this process should provide insight into AV fistula maturation.
In this study, we performed a cDNA microarray analysis and compared segments of the venous walls of overflow AV fistulas from 6 hemodialysis patients with the normal cephalic veins of 4 other hemodialysis patients to determine whether there was any difference in their gene expression patterns.
2. Study Population
From June 2009 to September 2010, 548 patients underwent hemodialysis at the Oyokyo Kidney Research Institute in Hirosaki, Japan. During that period, 10 patients underwent surgical ligation to correct an overflow AV fistula. When the operation was performed, we retained a sample of the wall of the overflow AV fistula (Figure 1). The AV fistula specimens were resected from the wall of the vein close to the AV fistula anastomosis. The study was approved by the Bioethics Committee of Oyokyo Kidney Research Institute, and all patients provided their informed consent to the procedure prior to it being performed.
Figure 1.
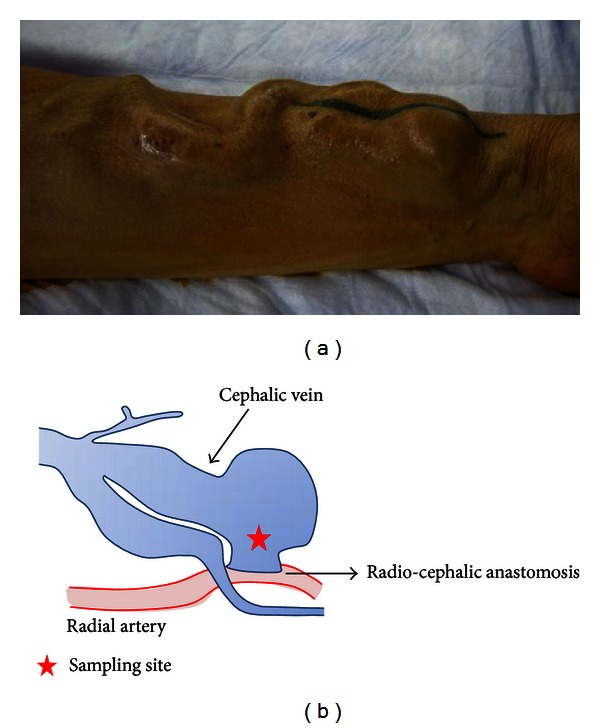
(a) Photograph of overflow AV fistula. (b) Schematic of overflow AV fistula.
3. Inclusion Criteria
The inclusion criteria were as follows: (1) blood access flow greater than 2.0 L/min measured by the color Doppler ultrasound (2) an AV fistula in the lower arm with a distal radio-cephalic anastomosis. In total, 6 patients had overflow AV fistulas that met these criteria. The backgrounds of these patients are detailed in Table 1. We also obtained tissue samples from the lower arm distal cephalic veins of 4 new hemodialysis patients and used these as a control.
Table 1.
Patient characteristics.
| Over flow AVF | Age | Gender | Cause of CRF | Patency period of AV fistula (months) | Blood flow (mL/min) |
|---|---|---|---|---|---|
| 1 | 48 | M | CGN | 56 | 3790 |
| 2 | 83 | F | CGN | 93 | 2760 |
| 3 | 57 | M | CGN | 19 | 3280 |
| 4 | 46 | M | CGN | 22 | 2710 |
| 5 | 75 | M | CGN | 104 | 3520 |
| 6 | 57 | F | IgA | 88 | 2340 |
|
| |||||
| Control | |||||
| 1 | 67 | M | CGN | (−) | (−) |
| 2 | 68 | F | CGN | (−) | (−) |
| 3 | 56 | M | CGN | (−) | (−) |
| 4 | 80 | F | CGN | (−) | (−) |
4. Methods
As noted above, venous tissues were resected from a venous segment of an overflow AV fistula from 6 patients and from a normal cephalic vein from 4 other patients. The surgical specimens were immediately placed in test tubes containing RNAlater (see below for details).
Total RNA was extracted from the venous tissue samples, and gene expression between the 2 groups was compared using Whole Human Genome DNA microarray 44 K (Agilent Technologies, Santa Clara, California). The microarray data were analyzed with GeneSpring GX software and Ingenuity Pathway Analysis.
5. RNA Isolation
Surgical specimens were 0.5 cm or smaller in size and were initially stored in RNA later (Ambion, Austin, TX) overnight at 4 ± 3°C then at –80°C until RNA extraction. Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The total RNA was further purified using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) and then extracted. The quantity and quality of the RNA were determined using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA) and an Agilent Bioanalyzer (Agilent Technologies, Palo Alto, CA).
6. cRNA Amplification and Labeling
Total RNA was amplified and labeled with Cyanine 3 (Cy3) as instructed by the manufacturer of the Agilent Low Input Quick Amp Labeling Kit, one-color (Agilent Technologies, Palo Alto, CA). Briefly, 100 ng of total RNA was reverse transcribed to double-strand cDNA using a poly dT-T7 promoter primer. The primer, template RNA, and quality-control transcripts of known concentration and quality were then denatured at 65°C for 10 min and incubated for 2 hours at 40°C with 5X First-Strand Buffer, 0.1 M DTT, 10 mM dNTP mix, and Affinity Script RNase Block Mix. The Affinity Script enzyme was inactivated at 70°C for 15 min. The resulting cDNA products were then used as templates for in vitro transcription to generate fluorescent cRNA. They were mixed with a transcription master mix in the presence of T7 RNA polymerase and Cy3-labeled CTP and incubated at 40°C for 2 hours. Labeled cRNAs were purified using Qiagen's RNeasy Mini spin columns and eluted in 30 μL of nuclease-free water. After amplification and labeling, cRNA quantity and cyanine incorporation were determined using a Nanodrop ND-1000 spectrophotometer and an Agilent Bioanalyzer.
7. Sample Hybridization
For each hybridization, 1.65 μg of Cy3-labeled cRNA was fragmented and hybridized onto an Agilent Human GE 4x44K v2 Microarray (Design ID: 026652) for 17 hours at 65°C. After washing, the microarrays were scanned using an Agilent DNA microarray scanner.
8. Microarray Data Analysis
The intensity values of each scanned feature were quantified using Agilent feature extraction software (version 10.7.3.1), which performs background subtractions. We only used features flagged as having no errors (present flags) and excluded features that were not positive, not significant, not uniform, not above background levels, saturated, or population outliers (marginal and absent flags). Normalization was performed using Agilent GeneSpring GX version 11.0.2. (per chip: normalization to the 75 percentile shift; per gene: normalization to median across all samples). There are 34,127 probes in total on the Agilent Human GE 4x44K v2 Microarray (Design ID: 026652), excluding control probes. The microarray data were submitted to NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/), sample number [GSE39488].
The altered transcripts were quantified using a comparative method. We applied a P value < 0.05 combined with a >2-fold change in normalized intensity to identify genes with significantly different expression patterns.
9. Gene Ontology Analysis and Pathway Analysis
The gene ontology analysis was performed using Agilent Technologies GeneSpring GX software (11.0.2). Pathway analysis was performed with GenMAPP 2.1 (http://www.genmapp.org/).
10. Results
The cDNA microarray analysis revealed that 397 genes were upregulated and 456 were downregulated (Tables 2 and 3).
Table 2.
Genes significantly upregulated in the remodeled vein compared to the control vein (top 30).
| Probe name | P value | FCAbsolute | Gene symbol |
|---|---|---|---|
| A_23_P106194 | 0.045653246 | 38.85 | FOS |
| A_23_P429998 | 0.021514166 | 27.22 | FOSB |
| A_24_P33895 | 0.001204797 | 25.26 | ATF3 |
| A_23_P46936 | 1.36E − 05 | 24.77 | EGR2 |
| A_23_P96158 | 0.002943707 | 24.55 | KRT17 |
| A_23_P34915 | 0.001120758 | 23.39 | ATF3 |
| A_23_P71037 | 0.004629572 | 21.98 | IL6 |
| A_23_P46429 | 7.94E − 04 | 21.04 | CYR61 |
| A_24_P882732 | 0.036205057 | 19.08 | |
| A_23_P97141 | 0.0211732 | 17.66 | RGS1 |
| A_23_P323751 | 9.21E − 05 | 17.26 | FAM83D |
| A_33_P3316273 | 0.00369598 | 15.81 | CCL3 |
| A_23_P216225 | 0.0219925 | 15.72 | EGR3 |
| A_33_P3295203 | 0.001008955 | 15.65 | HAS1 |
| A_23_P131208 | 2.92E − 04 | 14.26 | NR4A2 |
| A_23_P214080 | 0.00224604 | 13.9 | EGR1 |
| A_33_P3214105 | 8.77E − 04 | 13.44 | ATF3 |
| A_33_P3390793 | 0.004063138 | 13.41 | TRIM36 |
| A_33_P3354607 | 0.001234311 | 13.09 | CCL4 |
| A_23_P79518 | 0.006241249 | 12.95 | IL1B |
| A_23_P1331 | 0.001146301 | 11.08 | COL13A1 |
| A_23_P110569 | 7.10E − 04 | 10.2 | TRIM36 |
| A_23_P166408 | 0.003698398 | 10.04 | OSM |
| A_32_P76627 | 1.51E − 04 | 10.02 | |
| A_23_P207564 | 0.00151482 | 9.94 | CCL4 |
| A_33_P3299066 | 0.001036557 | 9.61 | NR4A2 |
| A_33_P3214393 | 0.008276591 | 9.56 | |
| A_33_P3413741 | 0.032112285 | 9.53 | OXTR |
| A_33_P3271594 | 0.001451045 | 9.49 | TRIM54 |
| A_24_P158089 | 0.003357552 | 8.93 | SERPINE1 |
Table 3.
Genes significantly downregulated in the remodeled vein compared to the control vein (top 30).
| Probe name | FCAbsolute | P value | Gene symbol |
|---|---|---|---|
| A_23_P23783 | 18.99 | 0.009408315 | MYOC |
| A_23_P121545 | 14.63 | 6.67E − 04 | GPM6A |
| A_33_P3368193 | 10.96 | 2.33E − 05 | PNLIPRP3 |
| A_32_P92489 | 8.79 | 0.004908097 | PKD1L2 |
| A_24_P40626 | 8.15 | 0.011296479 | GREM2 |
| A_33_P3221408 | 8.14 | 0.004285622 | NTNG1 |
| A_23_P143526 | 7.15 | 0.004383178 | S100B |
| A_23_P136777 | 7.14 | 3.89E − 04 | APOD |
| A_23_P102331 | 7.10 | 0.003490915 | SCN7A |
| A_33_P3421923 | 7.03 | 0.001119926 | CADM3 |
| A_23_P140384 | 7.00 | 0.026459113 | CTSG |
| A_33_P3363799 | 6.94 | 0.002682039 | NCAM1 |
| A_24_P203134 | 6.80 | 0.024163503 | DCAF12L1 |
| A_24_P280684 | 6.69 | 0.03005707 | FBXO40 |
| A_23_P55544 | 6.51 | 0.004269639 | CCBE1 |
| A_23_P73571 | 6.45 | 0.03981546 | MUM1L1 |
| A_23_P212050 | 6.22 | 0.021448081 | BCHE |
| A_33_P3336557 | 6.12 | 1.20E − 04 | |
| A_23_P121676 | 6.07 | 0.014616995 | CXXC4 |
| A_23_P204885 | 6.01 | 0.007333652 | PCDH20 |
| A_23_P64919 | 5.92 | 0.012492463 | RERGL |
| A_23_P422911 | 5.81 | 6.12E − 04 | HS6ST3 |
| A_23_P146233 | 5.79 | 0.01808302 | LPL |
| A_23_P110624 | 5.76 | 0.003615111 | CTNND2 |
| A_23_P45185 | 5.69 | 0.00549277 | FIGF |
| A_23_P110764 | 5.65 | 0.009343005 | MYOT |
| A_23_P114862 | 5.41 | 0.039056532 | ANGPTL7 |
| A_23_P39251 | 5.31 | 9.92E − 04 | PLIN5 |
| A_23_P111402 | 5.28 | 0.008291814 | RSPO3 |
| A_33_P3400763 | 5.26 | 0.038730744 | PLIN4 |
The gene ontology analysis revealed that biological developmental processes and glycosaminoglycan binding were the most upregulated. In addition, most upregulation occurred extracellularly (Tables 4, 5, and 6).
Table 4.
Statistically overrepresented GO terms in the biological process category (P < 0.001).
| Biological process | ||||||
|---|---|---|---|---|---|---|
| GO accession (with AmiGO link) | GO term | Corrected P value | Count in selection | % count in selection | Count in total | % count in total |
| GO:0032502 | Developmental process | 2.100E − 11 | 77 | 29.8 | 3077 | 17.9 |
| GO:0007275 | Multicellular organismal development | 1.340E − 10 | 67 | 26.0 | 2810 | 16.3 |
| GO:0010033 | Response to organic substance | 3.530E − 10 | 40 | 15.5 | 698 | 4.1 |
| GO:0001568 | Blood vessel development | 9.230E − 10 | 21 | 8.1 | 231 | 1.3 |
| GO:0048514 | Blood vessel morphogenesis | 1.250E − 09 | 19 | 7.4 | 198 | 1.1 |
| GO:0001944 | Vasculature development | 1.250E − 09 | 21 | 8.1 | 238 | 1.4 |
| GO:0048545 | Response to steroid hormone stimulus | 1.360E − 09 | 23 | 8.9 | 183 | 1.1 |
| GO:0001525 | Angiogenesis | 2.930E − 09 | 18 | 7.0 | 139 | 0.8 |
| GO:0009653 | Anatomical structure morphogenesis | 4.930E − 09 | 30 | 11.6 | 1125 | 6.5 |
| GO:0016265 | Death | 5.110E − 09 | 39 | 15.1 | 663 | 3.8 |
| GO:0048646 | Anatomical structure formation involved in morphogenesis | 5.950E − 09 | 18 | 7.0 | 306 | 1.8 |
| GO:0008219 | Cell death | 1.280E − 08 | 37 | 14.3 | 658 | 3.8 |
| GO:0042221 | Response to chemical stimulus | 1.280E − 08 | 53 | 20.5 | 1264 | 7.3 |
| GO:0048856 | Anatomical structure development | 1.320E − 08 | 42 | 16.3 | 2437 | 14.1 |
| GO:0006950 | Response to stress | 2.080E − 08 | 51 | 19.8 | 1642 | 9.5 |
| GO:0048731 | System development | 3.600E − 08 | 38 | 14.7 | 2284 | 13.3 |
| GO:0032570 | Response to progesterone stimulus | 7.370E − 08 | 9 | 3.5 | 21 | 0.1 |
| GO:0006915∣GO:0008632 | Apoptosis | 1.340E − 07 | 31 | 12.0 | 541 | 3.1 |
| GO:0012501∣GO:0016244 | Programmed cell death | 1.900E − 07 | 31 | 12.0 | 549 | 3.2 |
| GO:0042981 | Regulation of apoptosis | 2.470E − 07 | 35 | 13.6 | 796 | 4.6 |
| GO:0032501∣GO:0050874 | Multicellular organismal process | 3.190E − 07 | 70 | 27.1 | 4154 | 24.1 |
| GO:0043067∣GO:0043070 | Regulation of programmed cell death | 3.190E − 07 | 35 | 13.6 | 804 | 4.7 |
| GO:0010941 | Regulation of cell death | 3.310E − 07 | 35 | 13.6 | 807 | 4.7 |
| GO:0009887 | Organ morphogenesis | 3.510E − 07 | 26 | 10.1 | 685 | 4.0 |
| GO:0009628 | Response to abiotic stimulus | 5.270E − 07 | 16 | 6.2 | 357 | 2.1 |
| GO:0009725 | Response to hormone stimulus | 5.390E − 07 | 23 | 8.9 | 358 | 2.1 |
| GO:0048519∣GO:0043118 | Negative regulation of biological process | 6.290E − 07 | 39 | 15.1 | 1756 | 10.2 |
| GO:0009719 | Response to endogenous stimulus | 7.450E − 07 | 24 | 9.3 | 391 | 2.3 |
| GO:0009605 | Response to external stimulus | 7.730E − 07 | 32 | 12.4 | 869 | 5.0 |
| GO:0048513 | Organ development | 1.510E − 06 | 34 | 13.2 | 1682 | 9.8 |
| GO:0009607 | Response to biotic stimulus | 2.160E − 06 | 23 | 8.9 | 385 | 2.2 |
| GO:0070482 | Response to oxygen levels | 3.460E − 06 | 14 | 5.4 | 137 | 0.8 |
| GO:0009266 | Response to temperature stimulus | 3.950E − 06 | 9 | 3.5 | 86 | 0.5 |
| GO:0050896∣GO:0051869 | Response to stimulus | 6.040E − 06 | 89 | 34.5 | 3356 | 19.5 |
| GO:0048523∣GO:0051243 | Negative regulation of cellular process | 8.220E − 06 | 38 | 14.7 | 1606 | 9.3 |
| GO:0009408∣GO:0006951 | Response to heat | 8.220E − 06 | 9 | 3.5 | 61 | 0.4 |
| GO:0006928 | Cellular component movement | 1.200E − 05 | 14 | 5.4 | 450 | 2.6 |
| GO:0050793 | Regulation of developmental process | 1.590E − 05 | 8 | 3.1 | 670 | 3.9 |
| GO:0048869 | Cellular developmental process | 1.600E − 05 | 24 | 9.3 | 1641 | 9.5 |
| GO:0022603 | Regulation of anatomical structure morphogenesis | 2.530E − 05 | 5 | 1.9 | 228 | 1.3 |
| GO:0051239 | Regulation of multicellular organismal process | 2.860E − 05 | 7 | 2.7 | 924 | 5.4 |
| GO:0007565 | Female pregnancy | 3.100E − 05 | 13 | 5.0 | 104 | 0.6 |
| GO:0030154 | Cell differentiation | 4.450E − 05 | 24 | 9.3 | 1576 | 9.1 |
| GO:0042127 | Regulation of cell proliferation | 4.830E − 05 | 29 | 11.2 | 773 | 4.5 |
| GO:0001666 | Response to hypoxia | 6.420E − 05 | 14 | 5.4 | 131 | 0.8 |
| GO:0008284 | Positive regulation of cell proliferation | 7.720E − 05 | 16 | 6.2 | 410 | 2.4 |
| GO:0048522∣GO:0051242 | Positive regulation of cellular process | 7.720E − 05 | 34 | 13.2 | 1806 | 10.5 |
| GO:0051789 | Response to protein stimulus | 8.360E − 05 | 11 | 4.3 | 96 | 0.6 |
| GO:0048518∣GO:0043119 | Positive regulation of biological process | 9.260E − 05 | 35 | 13.6 | 1982 | 11.5 |
| GO:0043627 | Response to estrogen stimulus | 1.000E − 04 | 12 | 4.7 | 98 | 0.6 |
| GO:0009991 | Response to extracellular stimulus | 1.000E − 04 | 6 | 2.3 | 204 | 1.2 |
| GO:0042493∣GO:0017035 | Response to drug | 1.770E − 04 | 17 | 6.6 | 213 | 1.2 |
| GO:0043066 | Negative regulation of apoptosis | 1.790E − 04 | 19 | 7.4 | 345 | 2.0 |
| GO:0043069∣GO:0043072 | Negative regulation of programmed cell death | 2.200E − 04 | 19 | 7.4 | 350 | 2.0 |
| GO:0051384 | Response to glucocorticoid stimulus | 2.200E − 04 | 10 | 3.9 | 70 | 0.4 |
| GO:0050789∣GO:0050791 | Regulation of biological process | 2.440E − 04 | 109 | 42.2 | 7200 | 41.8 |
| GO:0060548 | Negative regulation of cell death | 2.570E − 04 | 19 | 7.4 | 354 | 2.1 |
| GO:0051707∣GO:0009613∣GO:0042828 | Response to other organism | 2.770E − 04 | 17 | 6.6 | 300 | 1.7 |
| GO:0040011 | Locomotion | 2.800E − 04 | 16 | 6.2 | 415 | 2.4 |
| GO:0009611∣GO:0002245 | Response to wounding | 2.800E − 04 | 21 | 8.1 | 507 | 2.9 |
| GO:0031960 | Response to corticosteroid stimulus | 3.860E − 04 | 10 | 3.9 | 75 | 0.4 |
| GO:0050794∣GO:0051244 | Regulation of cellular process | 4.070E − 04 | 108 | 41.9 | 6938 | 40.3 |
| GO:0014070 | Response to organic cyclic substance | 4.200E − 04 | 12 | 4.7 | 114 | 0.7 |
| GO:0051128 | Regulation of cellular component organization | 5.900E − 04 | 6 | 2.3 | 466 | 2.7 |
| GO:0065007 | Biological regulation | 7.620E − 04 | 109 | 42.2 | 7592 | 44.1 |
| GO:0051704∣GO:0051706 | Multiorganism process | 7.900E − 04 | 27 | 10.5 | 706 | 4.1 |
| GO:0031099 | Regeneration | 8.770E − 04 | 6 | 2.3 | 65 | 0.4 |
| GO:0007610 | Behavior | 9.680E − 04 | 12 | 4.7 | 449 | 2.6 |
Table 5.
Statistically overrepresented GO terms in the molecular function category (P < 0.01).
| Molecular function | ||||||
|---|---|---|---|---|---|---|
| GO accession (with AmiGO link) | GO term | Corrected P value | Count in selection | % count in selection | Count in total | % count in total |
| GO:0005539 | Glycosaminoglycan binding | 9.960E − 06 | 14 | 5.4 | 149 | 0.9 |
| GO:0005515∣GO:0045308 | Protein binding | 1.550E − 05 | 170 | 65.9 | 8104 | 47.0 |
| GO:0001871 | Pattern binding | 3.120E − 05 | 14 | 5.4 | 164 | 1.0 |
| GO:0030247 | Polysaccharide binding | 3.120E − 05 | 14 | 5.4 | 164 | 1.0 |
| GO:0008201 | Heparin binding | 6.710E − 05 | 13 | 5.0 | 112 | 0.7 |
| GO:0005126 | Cytokine receptor binding | 7.930E − 05 | 4 | 1.6 | 177 | 1.0 |
| GO:0005125 | Cytokine activity | 2.200E − 04 | 12 | 4.7 | 193 | 1.1 |
| GO:0005114 | Type II transforming growth factor beta receptor binding | 1.143E − 03 | 4 | 1.6 | 7 | 0.0 |
| GO:0008083 | Growth factor activity | 2.365E − 03 | 13 | 5.0 | 160 | 0.9 |
| GO:0005102 | Receptor binding | 3.106E − 03 | 24 | 9.3 | 873 | 5.1 |
| GO:0030246 | Carbohydrate binding | 7.049E − 03 | 14 | 5.4 | 354 | 2.1 |
Table 6.
Statistically overrepresented GO terms in the cellular component category (P < 0.01).
| Cellular component | ||||||
|---|---|---|---|---|---|---|
| GO accession (with AmiGO link) | GO term | Corrected P value | Count in selection | % count in selection | Count in total | % count in total |
| GO:0044421 | Extracellular region part | 2.190E − 09 | 49 | 19.0 | 937 | 5.4 |
| GO:0031012 | Extracellular matrix | 7.420E − 07 | 25 | 9.7 | 339 | 2.0 |
| GO:0005576 | Extracellular region | 3.880E − 06 | 69 | 26.7 | 1923 | 11.2 |
| GO:0005615 | Extracellular space | 1.690E − 05 | 32 | 12.4 | 673 | 3.9 |
| GO:0005578 | Proteinaceous extracellular matrix | 1.200E − 04 | 20 | 7.8 | 309 | 1.8 |
| GO:0060205 | Cytoplasmic membrane-bounded vesicle lumen | 4.070E − 04 | 7 | 2.7 | 44 | 0.3 |
| GO:0031983 | Vesicle lumen | 5.660E − 04 | 7 | 2.7 | 46 | 0.3 |
| GO:0031093 | Platelet alpha granule lumen | 2.468E − 03 | 7 | 2.7 | 41 | 0.2 |
The pathway analysis revealed that the TGF beta signaling pathway, cytokines and inflammatory response pathway, hypertrophy model, and the myometrial relaxation and contraction pathway were upregulated (Table 7).
Table 7.
Pathway analysis results.
| Pathway name | LS_vs_control |
|---|---|
| Alpha6 beta4 integrin signaling pathway | 0.793 |
| Androgen receptor signaling pathway | 0.528 |
| Apoptosis mechanisms | 0.124 |
| B-cell receptor signaling pathway | 0.023 |
| G1 to S cell cycle control | 1 |
| Cell cycle | 0.487 |
| Delta-Notch signaling pathway | 0.226 |
| DNA replication | 1 |
| EGFR1 signaling pathway | 0.856 |
| FAS pathway and stress induction of HSP regulation | 1 |
| Focal Adhesion | 0.003 |
| G13 signaling pathway | 0.269 |
| G protein signaling pathways | 0.258 |
| Hedgehog signaling pathway | 1 |
| Apoptosis modulation by HSP70 | 1 |
| Id signaling pathway | 1 |
| IL-1 signaling pathway | 1 |
| IL-2 signaling pathway | 0.327 |
| IL-3 signaling pathway | 0.371 |
| IL-4 signaling pathway | 0.589 |
| IL-5 signaling pathway | 0.576 |
| IL-6 signaling pathway | 1 |
| IL-7 signaling pathway | 0.498 |
| IL-9 signaling pathway | 1 |
| Human insulin signaling | 0.387 |
| Integrin-mediated cell adhesion | 0.363 |
| Kit receptor signaling pathway | 0.051 |
| MAPK cascade | 1 |
| MAPK signaling pathway | 0.011 |
| mRNA processing (Homo sapiens) | 0.014 |
| Notch signaling pathway | 0.191 |
| Ovarian infertility genes | 1 |
| p38 MAPK signaling pathway (BioCarta) | 0.108 |
| Regulation of actin cytoskeleton | 0.834 |
| Eukaryotic transcription initiation | 0.511 |
| Signal transduction of S1P | 0.384 |
| Signaling of hepatocyte growth factor receptor | 1 |
| T cell receptor signaling pathway | 0.243 |
| TGF-beta receptor signaling pathway | 0.095 |
| TGF beta signaling pathway | 0 |
| TNF-alpha/NF-κB signaling pathway | 0.752 |
| Translation factors | 0.368 |
| Wnt signaling pathway | 0.15 |
| Wnt signaling pathway | 0.051 |
| Acetylcholine synthesis | 1 |
| Alanine and aspartate metabolism | — |
| Biogenic amine synthesis | 1 |
| Cholesterol biosynthesis | 0.644 |
| Eicosanoid synthesis | 1 |
| Electron transport chain | 0.013 |
| Fatty acid beta oxidation 1 | 0.403 |
| Fatty acid beta oxidation 2 | 1 |
| Fatty acid beta oxidation 3 | 1 |
| Beta oxidation meta MAPP | 0.264 |
| Fatty acid omega oxidation | 0.687 |
| Fatty acid biosynthesis | 0.426 |
| Glucocorticoid and mineralcorticoid metabolism | 1 |
| Glutathione metabolism | 0.399 |
| Glycogen metabolism | 0.261 |
| Glycolysis and gluconeogenesis | 0.235 |
| Heme biosynthesis | 1 |
| TCA cycle | 0.24 |
| Mitochondrial LC-fatty acid beta-oxidation | 0.635 |
| Nuclear receptors in lipid metabolism and toxicity | 0.389 |
| Nucleotide metabolism | 0.622 |
| Pentose phosphate pathway | 1 |
| Prostaglandin synthesis and regulation | 1 |
| Statin pathway (PharmGKB) | 1 |
| Steroid biosynthesis | 1 |
| Synthesis and degradation of ketone bodies | 1 |
| Triacylglyceride synthesis | 0.419 |
| Tryptophan metabolism | 0.501 |
| Beta oxidation of unsaturated fatty acids | 1 |
| Urea cycle and metabolism of amino groups | — |
| GPCRs, class A rhodopsin-like | 0.317 |
| GPCRs, class B secretin-like | 1 |
| GPCRs, class C metabotropic glutamate, pheromone | 1 |
| GPCRs, other | 1 |
| Matrix metalloproteinases | 0.652 |
| Monoamine GPCRs | 1 |
| Nuclear receptors | 1 |
| Nucleotide GPCRs | 1 |
| Peptide GPCRs | 0.081 |
| Cytoplasmic ribosomal proteins | 0.025 |
| Small ligand GPCRs | 1 |
| ACE inhibitor pathway (Homo sapiens) | 0.254 |
| Adipogenesis human | 0.101 |
| Blood clotting cascade | 0.155 |
| Calcium regulation in the cardiac cell | 0.431 |
| Circadian exercise | 0.754 |
| Complement activation and classical pathway | 0.646 |
| Complement activation and classical pathway | 0.022 |
| Cytokines and inflammatory response (BioCarta) | 0 |
| Hypertrophy model | 0 |
| Inflammatory response pathway | 0.054 |
| Irinotecan pathway (Homo sapiens) | 0.685 |
| Oxidative stress | 0.402 |
| Proteasome degradation | 0.278 |
| Myometrial relaxation and contraction pathways | 0 |
| Striated muscle contraction | 0.427 |
11. Discussion
AV fistulas are very useful for determining the optimal blood flow for hemodialysis since satisfactory blood access flow is necessary for adequate hemodialysis. When stenotic lesions occur within the vascular system and blood flow is insufficient, a percutaneous transluminal angioplasty or some other intervention is performed. However, overflow AV fistulas increase cardiac output and cause high-output cardiac failure [1].
In the 2005 Japanese Society for Dialysis Therapy Guidelines for Vascular Access Construction and Repair for Chronic Hemodialysis, vascular access flow is said to lead to heart failure when the blood access flow is greater than 1.0–1.5 L/min or when the vascular access flow/cardiac output ratio is >20% [1]. If the vascular access flow is clearly responsible for a decline in cardiac function, then it is necessary to intentionally constrict or occlude the vascular access [1]. Surveillance of blood flow in internal shunts by the Doppler echocardiography has become widespread and overflow AV fistulas are now actively treated. Several recent studies have noted the importance of histological changes in AV fistulas [4, 5].
Microarrays of vascular access have been reported in experimental animal models, but there have been no such analyses in humans [6]. In the present study, venous tissue samples were resected from overflow AV fistulas from 6 hemodialysis patients and from the normal cephalic veins of 4 other hemodialysis patients, and their gene expression patterns were compared.
It is interesting to note that zinc finger-containing transcription factors such as egr1, egr2, and egr3 and immediate early genes such as fos and jun, were found to be remarkably upregulated in the present study; egr1, egr 2, and egr 3 have been implicated in the proliferation and differentiation of many cell types [7, 8], and fos and jun have been linked to the regulation of angiogenesis [9]. Moreover, egr-1, c-jun, and c-fos have been linked to the regulation of free radical scavenging enzymes [10–13]. We also observed the upregulation of free radical scavenging enzyme activity in the walls of the overflow AV fistulas, which may reflect chronic reactive oxygen species formation in overflow AV fistulas.
The pathway analysis indicated that the TGF beta signaling pathway and cytokines and inflammatory response pathway were upregulated. This suggests that overflow AV fistulas may be implicated in chronic inflammation in hemodialysis patients.
Malnutrition, inflammation, and atherosclerosis (MIA syndrome) are common in end-stage renal disease (ESRD) patients, and inflammation has been identified as playing a key role in atherosclerotic cardiovascular disease. Proinflammatory cytokines are pivotal to the inflammation that is, associated with malnutrition and atherosclerosis in ESRD [14]. Our findings suggest that overflow AV fistulas may be implicated in MIA syndrome.
12. Conclusion
Combining microarray results and pathway information available via the Internet provided biological insight into molecular changes in the venous walls of overflow AV fistulas. Despite the small sample size, our study findings suggest that overflow AV fistulas may be implicated in chronic inflammation in hemodialysis patients.
References
- 1.Ohira S, Naito H, Amano I, et al. 2005 Japanese Society for Dialysis Therapy guidelines for vascular access construction and repair for chronic hemodialysis. Therapeutic Apheresis and Dialysis. 2006;10(5):449–462. doi: 10.1111/j.1744-9987.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 2.Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrology Dialysis Transplantation. 2008;23(1):282–287. doi: 10.1093/ndt/gfm549. [DOI] [PubMed] [Google Scholar]
- 3.Krivitski NM. Theory and validation of access flow measurement by dilution technique during hemodialysis. Kidney International. 1995;48(1):244–250. doi: 10.1038/ki.1995.290. [DOI] [PubMed] [Google Scholar]
- 4.Lee T, Roy-Chaudhury P. Advances and new frontiers in the pathophysiology of venous neointimal hyperplasia and dialysis access stenosis. Advances in Chronic Kidney Disease. 2009;16(5):329–338. doi: 10.1053/j.ackd.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, et al. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrology Dialysis Transplantation. 2009;24(9):2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abeles D, Kwei S, Stavrakis G, Zhang Y, Wang ET, García-Cardeña G. Gene expression changes evoked in a venous segment exposed to arterial flow. Journal of Vascular Surgery. 2006;44(4):863–870. doi: 10.1016/j.jvs.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Boyle KB, Hadaschik D, Virtue S, et al. The transcription factors Egr1 and Egr2 have opposing influences on adipocyte differentiation. Cell Death and Differentiation. 2009;16(5):782–789. doi: 10.1038/cdd.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumbrink J, Kirsch KH, Johnson JP. EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. Journal of Cellular Biochemistry. 2010;111(1):207–217. doi: 10.1002/jcb.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marconcini L, Marchio S, Morbidelli L, et al. c-fos-Induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(17):9671–9676. doi: 10.1073/pnas.96.17.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schettler V, Völker K, Schulz EG, Wieland E. Impact of lipid apheresis on Egr-1, c-Jun, c-Fos, and Hsp70 gene expression in white blood cells. Therapeutic Apheresis and Dialysis. 2011;15(1):105–112. doi: 10.1111/j.1744-9987.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 11.Maehara K, Oh-Hashi K, Isobe KI. Early growth-responsive-1-dependent manganese superoxide dismutase gene transcription mediated by platelet-derived growth factor. The FASEB Journal. 2001;15(11):2025–2026. doi: 10.1096/fj.00-0909fje. [DOI] [PubMed] [Google Scholar]
- 12.Wenk J, Brenneisen P, Wlaschek M, et al. Stable overexpression of manganese superoxide dismutase in mitochondria identifies hydrogen peroxide as a major oxidant in the AP-1-mediated induction of matrix-degrading metalloprotease-1. The Journal of Biological Chemistry. 1999;274(36):25869–25876. doi: 10.1074/jbc.274.36.25869. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Sharp FR, Honkaniemi J, Mikawa S, Epstein CJ, Chan PH. DNA fragmentation and prolonged expression of c-fos, c-jun, and hsp70 in kainic acid-induced neuronal cell death in transgenic mice overexpressing human CuZn-superoxide dismutase. Journal of Cerebral Blood Flow and Metabolism. 1997;17(3):241–256. doi: 10.1097/00004647-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—the heart of the matter. Nephrology Dialysis Transplantation. 2002;17(supplement 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]


