Abstract
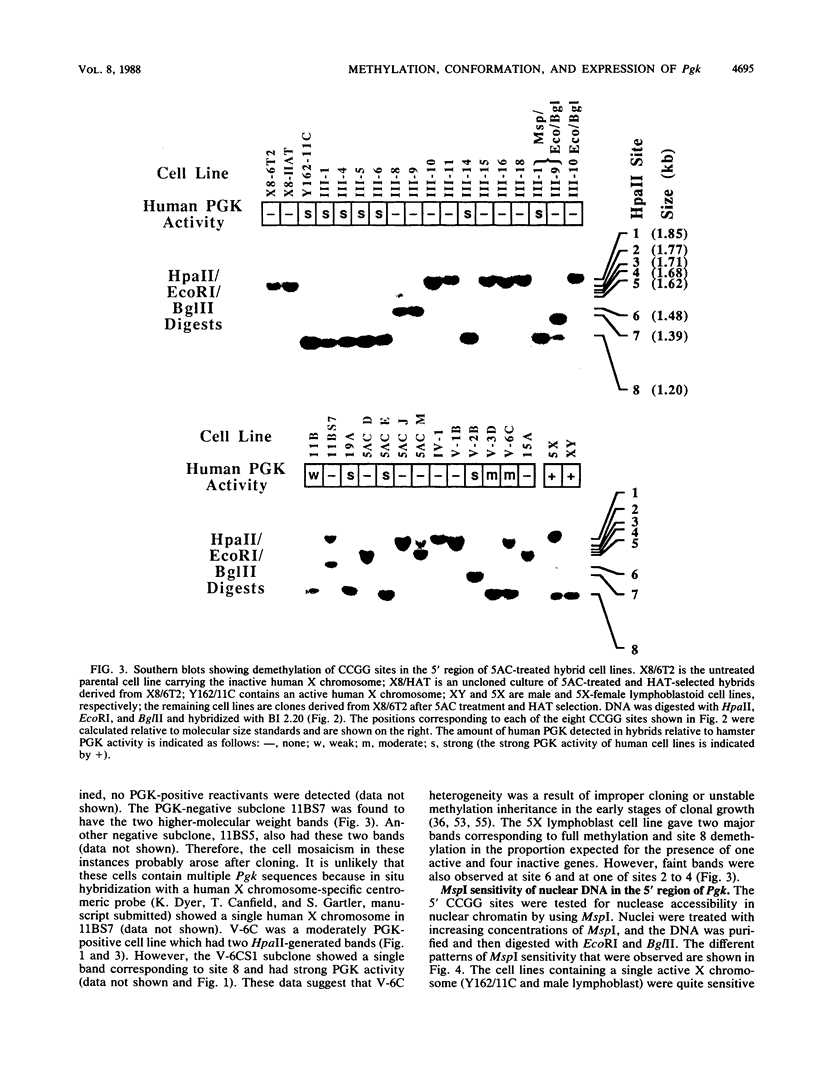
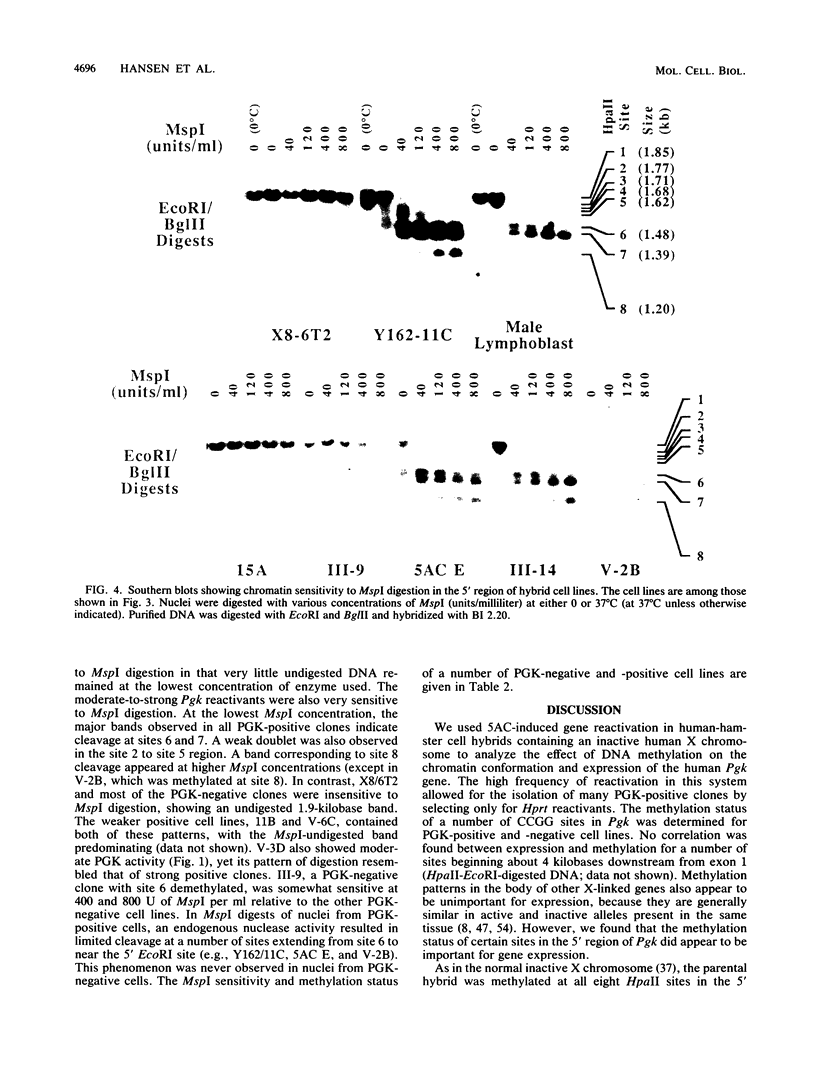
X8/6T2, a hamster-human hybrid cell line which contains an inactive human X chromosome, was treated with 5-azacytidine and selected for derepression of hypoxanthine-guanine phosphoribosyltransferase. Clones were examined for coreactivation of the phosphoglycerate kinase gene (Pgk). Of 68 of these hybrids, approximately 20% expressed measurable human phosphoglycerate kinase (PGK) activity. A 600-base-pair region of the Pgk 5' CpG cluster was examined for the methylation status of eight CCGG sites (site 1 being 5'-most) in a number of PGK-negative and PGK-positive cell lines. The inactive X chromosome is normally methylated at all eight sites, and this was also true for the majority of X8/6T2 cells. However, several PGK-negative hybrids were demethylated in the site 3 to site 6 region. PGK activity correlated with demethylation at both sites 6 and 7. The data for PGK-positive and -negative hybrids indicate that demethylation at or near site 7 was necessary for reactivation of Pgk. Chromatin sensitivity to MspI digestion in the nuclei of male lymphoblastoid cells and several PGK-positive and PGK-negative hybrids was examined. PGK-positive cell lines were hypersensitive to digestion, while PGK-negative hybrids were resistant. Cleavage at sites 6 and 7 was observed in all PGK-positive cell lines at each MspI concentration examined. Sites 7 and 8 were less accessible to digestion than site 6. Cleavage in the site 2 to site 5 region was observable at the lowest MspI concentration. In most PGK-positive hybrids, a nonspecific endogenous nuclease detected the presence of a hypersensitive region spanning at least 450 base pairs, bounded at the 3' end near HpaII site 6. Nuclease hypersensitivity appears to be related to promoter activity, because sites 7 and 8 are in transcribed regions of the gene. These data indicate that specific sites within the CpG cluster have a dominant controlling influence over the Pgk promoter conformation and the transcriptional activation of Pgk.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Busslinger M., deBoer E., Wright S., Grosveld F. G., Flavell R. A. The sequence GGCmCGG is resistant to MspI cleavage. Nucleic Acids Res. 1983 Jun 11;11(11):3559–3569. doi: 10.1093/nar/11.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Cooper D. N. Eukaryotic DNA methylation. Hum Genet. 1983;64(4):315–333. doi: 10.1007/BF00292363. [DOI] [PubMed] [Google Scholar]
- Cullen C. R., Hubberman P., Kaslow D. C., Migeon B. R. Comparison of factor IX methylation on human active and inactive X chromosomes: implications for X inactivation and transcription of tissue-specific genes. EMBO J. 1986 Sep;5(9):2223–2229. doi: 10.1002/j.1460-2075.1986.tb04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Dracopoli N. C., Rettig W. J., Albino A. P., Esposito D., Archidiacono N., Rocchi M., Siniscalco M., Old L. J. Genes controlling gp25/30 cell-surface molecules map to chromosomes X and Y and escape X-inactivation. Am J Hum Genet. 1985 Jan;37(1):199–207. [PMC free article] [PubMed] [Google Scholar]
- Ellis N., Keitges E., Gartler S. M., Rocchi M. High-frequency reactivation of X-linked genes in Chinese hamster X human hybrid cells. Somat Cell Mol Genet. 1987 May;13(3):191–204. doi: 10.1007/BF01535202. [DOI] [PubMed] [Google Scholar]
- Gardiner-Garden M., Frommer M. CpG islands in vertebrate genomes. J Mol Biol. 1987 Jul 20;196(2):261–282. doi: 10.1016/0022-2836(87)90689-9. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Andina R., Gant N. Ontogeny of X-chromosome inactivation in the female germ line. Exp Cell Res. 1975 Mar 15;91(2):454–457. doi: 10.1016/0014-4827(75)90127-5. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gidoni D., Dynan W. S., Tjian R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. 1984 Nov 29-Dec 5Nature. 312(5993):409–413. doi: 10.1038/312409a0. [DOI] [PubMed] [Google Scholar]
- Hors-Cayla M. C., Heuertz S., Frezal J. Coreactivation of four inactive X genes in a hamster x human hybrid and persistence of late replication of reactivated X chromosome. Somatic Cell Genet. 1983 Nov;9(6):645–657. doi: 10.1007/BF01539470. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Cedar H. Effect of CpG methylation on Msp I. Nucleic Acids Res. 1983 Jun 11;11(11):3571–3580. doi: 10.1093/nar/11.11.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M. X chromosome reactivation in oocytes of Mus caroli. Proc Natl Acad Sci U S A. 1981 May;78(5):3093–3097. doi: 10.1073/pnas.78.5.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lester S. C., Korn N. J., DeMars R. Derepression of genes on the human inactive X chromosome: evidence for differences in locus-specific rates of derepression and rates of transfer of active and inactive genes after DNA-mediated transformation. Somatic Cell Genet. 1982 Mar;8(2):265–284. doi: 10.1007/BF01538681. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Melton D. W., Caskey C. T., Martin G. R. Methylation of the mouse hprt gene differs on the active and inactive X chromosomes. Mol Cell Biol. 1986 Mar;6(3):914–924. doi: 10.1128/mcb.6.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Schmidt M., Axelman J., Cullen C. R. Complete reactivation of X chromosomes from human chorionic villi with a switch to early DNA replication. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2182–2186. doi: 10.1073/pnas.83.7.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Monk M. Methylation and the X chromosome. Bioessays. 1986 May;4(5):204–208. doi: 10.1002/bies.950040505. [DOI] [PubMed] [Google Scholar]
- Murray E. J., Grosveld F. Site specific demethylation in the promoter of human gamma-globin gene does not alleviate methylation mediated suppression. EMBO J. 1987 Aug;6(8):2329–2335. doi: 10.1002/j.1460-2075.1987.tb02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlofsky A., Chasin L. A. A domain of methylation change at the albumin locus in rat hepatoma cell variants. Mol Cell Biol. 1985 Jan;5(1):214–225. doi: 10.1128/mcb.5.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. L., Caldwell M., Fialkow P. J. Studies of skin tumorigenesis in PGK mosaic mice: many promoter-independent papillomas and carcinomas do not develop from pre-existing promoter-dependent papillomas. Int J Cancer. 1987 Feb 15;39(2):261–265. doi: 10.1002/ijc.2910390223. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Singer-Sam J., Keith D. H. Methylation of the PGK promoter region and an enhancer way-station model for X-chromosome inactivation. Prog Clin Biol Res. 1985;198:211–222. [PubMed] [Google Scholar]
- Riley D. E., Canfield T. K., Gartler S. M. Chromatin structure of active and inactive human X chromosomes. Nucleic Acids Res. 1984 Feb 24;12(4):1829–1845. doi: 10.1093/nar/12.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. E., Goldman M. A., Gartler S. M. Chromatin structure of active and inactive human X-linked phosphoglycerate kinase gene. Somat Cell Mol Genet. 1986 Jan;12(1):73–80. doi: 10.1007/BF01560729. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M., Chasin L. A. Isolation of mammalian cell mutants deficient in glucose-6-phosphate dehydrogenase activity: linkage to hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1975 Feb;72(2):493–497. doi: 10.1073/pnas.72.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluz H. P., Jiricny J., Jost J. P. Genomic sequencing reveals a positive correlation between the kinetics of strand-specific DNA demethylation of the overlapping estradiol/glucocorticoid-receptor binding sites and the rate of avian vitellogenin mRNA synthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7167–7171. doi: 10.1073/pnas.83.19.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Inokuchi K., Nienhuis A. W. Site-specific demethylation and normal chromatin structure of the human dihydrofolate reductase gene promoter after transfection into CHO cells. Mol Cell Biol. 1987 Aug;7(8):2830–2837. doi: 10.1128/mcb.7.8.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Goldstein S. Interclonal variation in methylation patterns for expressed and non-expressed genes. Nucleic Acids Res. 1982 Jul 24;10(14):4293–4304. doi: 10.1093/nar/10.14.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Hennighausen L., Battey J., Leder P. Chromatin structure and protein binding in the putative regulatory region of the c-myc gene in Burkitt lymphoma. Cell. 1984 Jun;37(2):381–391. doi: 10.1016/0092-8674(84)90368-4. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Stein R., Sciaky-Gallili N., Razin A., Cedar H. Pattern of methylation of two genes coding for housekeeping functions. Proc Natl Acad Sci U S A. 1983 May;80(9):2422–2426. doi: 10.1073/pnas.80.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D., D'Urso M., Martini G., Persico M., Tufano V., Battistuzzi G., Luzzatto L. Specific methylation pattern at the 3' end of the human housekeeping gene for glucose 6-phosphate dehydrogenase. EMBO J. 1984 Sep;3(9):1987–1995. doi: 10.1002/j.1460-2075.1984.tb02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo D., Martini G., Migeon B. R., Dono R. Expression of the G6PD locus on the human X chromosome is associated with demethylation of three CpG islands within 100 kb of DNA. EMBO J. 1988 Feb;7(2):401–406. doi: 10.1002/j.1460-2075.1988.tb02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Dintzis S., Toniolo D., Persico G., Lunnen K. D., Axelman J., Migeon B. R. Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3' CpG clusters: implications for X chromosome dosage compensation. Nucleic Acids Res. 1984 Dec 21;12(24):9333–9348. doi: 10.1093/nar/12.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Studies of X chromosome DNA methylation in normal human cells. Nature. 1982 Feb 25;295(5851):667–671. doi: 10.1038/295667a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Mohandas T., Shapiro L. J. Stability of DNA methylation of the human hypoxanthine phosphoribosyltransferase gene. Somat Cell Mol Genet. 1986 Mar;12(2):153–161. doi: 10.1007/BF01560662. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisraeli J., Adelstein R. S., Melloul D., Nudel U., Yaffe D., Cedar H. Muscle-specific activation of a methylated chimeric actin gene. Cell. 1986 Aug 1;46(3):409–416. doi: 10.1016/0092-8674(86)90661-6. [DOI] [PubMed] [Google Scholar]