Abstract
The present study builds on previous work showing that infusion of adeno-associated virus type 9 (AAV9) into the cisterna magna (CM) of nonhuman primates resulted in widespread transduction throughout cortex and spinal cord. Transduction efficiency was severely limited, however, by the presence of circulating anti-AAV antibodies. Accordingly, we compared AAV9 to a related serotype, AAV7, which has a high capsid homology. CM infusion of either AAV7 or AAV9 directed high level of cell transduction with similar patterns of distribution throughout brain cortex and along the spinal cord. Dorsal root ganglia and corticospinal tracts were also transduced. Both astrocytes and neurons were transduced. Interestingly, little transduction was observed in peripheral organs. Our results indicate that intrathecal delivery of either AAV7 or AAV9 directs a robust and widespread cellular transduction in the central nervous system and other peripheral neural structures.
Samaranch and colleagues compare the transduction profiles of AAV9-GFP and AAV7-GFP in nonhuman primates. They show that intrathecal delivery of either vector leads to equally high levels of cell transduction with similar patterns of distribution throughout the brain cortex and spinal cord, including the dorsal root ganglia and corticospinal tracts. Importantly, using this approach, they observe virtually no transduction of peripheral organs.
Introduction
Recent findings with adeno-associated virus type 9 (AAV9; Foust et al., 2008; Gray et al., 2011; Samaranch et al., 2012; Federici et al., 2012) have aroused considerable enthusiasm for its clinical potential. The primary reason for this interest is that AAV9 shows a remarkable ability to breach the blood–brain barrier after intravenous injection (Foust et al., 2008; Foust and Kaspar, 2009; Gray et al., 2011; Samaranch et al., 2012), and it also seems to be relatively efficient in transducing various brain tissues (Gray et al., 2011), although concerns remain about its sensitivity to circulating anti-AAV antibodies (Gray et al., 2011; Samaranch et al., 2012) and its ability to trigger cell-mediated immune responses in the brain if directing expression of a nonself protein (Ciesielska et al., 2013). Recently, we reported that injection of AAV9 into the cisterna magna (CM) of nonhuman primates (NHPs) directs more extensive transduction of large structures like the cortex with much less vector than that achieved by intravenous injection (Samaranch et al., 2012). We describe here that AAV9 and its close homolog, AAV7 (82% capsid identity; Daya and Berns, 2008), behave similarly in transducing brain cortex, and we report the ability of both vectors to transduce spinal cord structures, which is no less impressive. Both vectors evinced a pronounced ability to transduce motor neurons and dorsal root ganglia (DRG). These findings suggest that these vectors may find application in the treatment of spinal diseases and neuropathic pain.
Material and Methods
Animals
Four adult NHPs (Macaca fascicularis) were included in this study (Table 1). These animals received a single injection of either AAV7 (n=2) or AAV9 (n=2) vector encoding a self-complementary DNA sequence of green fluorescent protein (GFP) under the control of a chicken β-actin (CBA) and cytomegalovirus (CMV) promoter, respectively. On the day of surgery, a stock solution of vector (∼2.0×1013 vector genomes [vg]/mL) was combined 1:1 with vehicle (saline, 5% sorbitol and 0.001% pluronic F-68) and 2 mL of vector was infused into the CM. Viral particles were manufactured by the Research Vector Core at Children's Hospital of Philadelphia as previously described (Matsushita et al., 1998; Wright et al., 2003). Briefly, vectors were produced in packaging cells by standard helper free transfection method (triple plasmid transfection). GFP gene plasmid was designed encoding the transgene under control of the CBA or CMV promoter. Recombinant viral particles were purified by double-CsCl ultracentrifugation and phosphate-buffered saline (PBS) dialysis. Particles were quantified by real-time PCR and vector titers were expressed as viral genomes per milliliter.
Table 1.
Experimental Summary
| |
AAV7 |
AAV9 |
||
|---|---|---|---|---|
| NHP 1 | NHP 2 | NHP 3 | NHP 4 | |
| Sex | Female | Male | Male | Male |
| Age | 23 y, 7 m | 5 y, 5 m | 4 y, 3 m | 5 y, 4 m |
| Weight (kg) | 3.9 | 3.7 | 2.3 | 3.0 |
| Dose (×1013 vg) | 2.0 | 2.0 | 1.8 | 1.8 |
| Antibody titera | 1:100 | <1:50 | <1:50 | <1:50 |
Neutralizing antibody titer against AAV capsid.
NHP, nonhuman primate; vg, vector genomes; AAV7, adeno-associated vector serotype 7; AAV9, adeno-associated vector serotype 9; y, year; m, month.
All animals were tested for the presence of anti-AAV antibodies (Table 1) as previously described (Bevan et al., 2011), and all animals had antibody titers of less than 1:100. No adverse clinical signs were observed throughout the study. All procedures were carried out in accordance with the UCSF Institutional Animal Care and Use Committee (San Francisco, CA) and Institutional Animal Care and Use Committee at Valley Biosystems Inc. (Sacramento, CA).
Vector delivery
All monkeys were infused with vector in the CM as described previously (Samaranch et al., 2012). Briefly, after induction of deep anesthesia, the animal's head was placed in a stereotactic frame and the body was flexed in a prone position. A 3-mL syringe, mounted onto the micromanipulator, was manually guided into the CM. Once the needle was inside the CM, the correct location was verified by aspiration of a small volume of cerebrospinal fluid (CSF) and 2 mL of vector was infused at 0.5 mL/min with a pump (3500 Medfusion, Strategic Applications Inc., Lake Villa, IL).
Tissue processing
Necropsies were performed 3 weeks after vector administration. NHPs were transcardially perfused with PBS followed by 4% paraformaldehyde (PFA)/PBS, postfixed by immersion in 4% PFA/PBS overnight, and then transferred to 30% (w/v) sucrose. Brain, spinal cord, and DRG were collected and processed for histological analysis. A cryostat (HM 500M; Thermo Scientific, Waltman, MA) was used for cutting serial 40-μm sections of whole brain, 30-μm sections of spinal cord, 15-μm sections of DRG, and 6-μm sections of peripheral organs. All sections were stored in cryoprotectant solution until further use.
Immunohistochemistry
Immunoperoxidase staining
A polyclonal antibody against GFP (rabbit anti-GFP, 1:1000, G10362 Molecular Probes, Life Technologies™, Foster City, CA) was used to detect the expression of the transgene by polymer-enhanced immunohistochemistry. Briefly, sections were washed in PBS, endogenous peroxidase activity was blocked in 1% H2O2/30% alcohol/PBS, and then sections were rinsed in PBST (PBS/1% Tween 20). Sections were then incubated in Background Sniper® blocking solution (Biocare Medical) followed by a 24-hr incubation at 4°C with primary antibody against GFP in Da Vinci® Green diluent (Biocare Medical). The next day, sections were washed in PBST and incubated in Rabbit Mach 2 HRP-polymer (Biocare Medical) at room temperature for 1 hr. All sections were chromogenically developed with 3,3′-diaminobenzidine (DAB; DAB Peroxidase Substrate Kit, SK-4100, Vector Laboratories) according to the manufacturer's suggestions.
A monoclonal antibody against glial fibrillary acidic protein (GFAP; mouse anti-GFAP, 1:100,000, MAB360 Millipore), a polyclonal antibody against ionized calcium binding adaptor molecule 1 (Iba1; rabbit anti-Iba1, 1:1000, CP290C Biocare Medical) and a monoclonal one against major histocompatibility complex class II (MHC II, mouse anti-MHCII; 1:300, M3887-30 US Biological) were used separately to detect immune responses.
Double immunofluorescent staining
The phenotype of GFP-positive cells was examined by double immunofluorescence staining. Brain sections were washed with PBST, blocked for 1 hr in 20% normal horse serum (Jackson Immuno Research), and then incubated overnight at 4°C with a mixture of anti-GFP (rabbit, 1:200, G10362 Molecular Probe) and a specific antibody for neurons (anti-NeuN, mouse monoclonal, 1:100, MAB377 Millipore), fibrous astrocytes (mouse anti-GFAP, 1:500, MAB360 Dako), or protoplasmic astrocytes (rabbit anti-S100, 1:300, CP021 Biocare Medical). Anti–choline acetyltransferase antibody (goat anti-ChAT, 1:500, AB144P Millipore) was used to detect motor neurons in spinal cord sections. Additionally, to determine the type of sensory DRG cell bodies transduced, DRG sections were costained for GFP and either β-III tubulin (mouse monoclonal, clone TUJ1, 1:100, MAB1637 Millipore) or NF-200 (mouse monoclonal, clone RT-97, 1:100, CBL212 Millipore) to identify myelinated fibers and neurons. After incubation with primary antibodies, sections were washed in PBST, incubated with a cocktail of the corresponding secondary antibodies in PBST for 2 hr at room temperature, washed in PBS and wet-mounted on frosted slides. To avoid autofluorescence due to lipofuscin, all sections were incubated in 0.3% Sudan Black B solution for 5 min and then washed in PBS followed by distilled water.
Results
Comparable cellular tropism of AAV serotypes 7 and 9 in the CNS
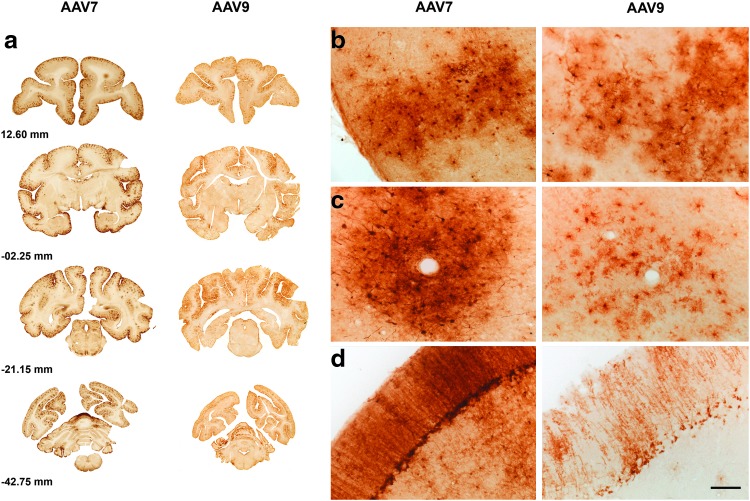
We have recently shown that infusion of AAV9-GFP into the CM of NHPs leads to a widespread transduction of the entire brain (Samaranch et al., 2012). In the present study, we investigated whether AAV7, which is highly homologous to AAV9, had a similar distribution and tropism to that shown by AAV9. Our results indicated that both serotypes transduced cortical cells throughout the NHP brain from prefrontal to occipital cortex and also in the cerebellum (Fig. 1a). Examination of representative sections from different brain regions revealed strong cortical GFP expression (Fig. 1b). GFP-positive cells were found only sparsely in deep brain nuclei such as striatum (data not shown). GFP-positive cells in AAV7-treated animals were found clustered around blood vessels throughout the brain, a very similar pattern to what we observed in the AAV9-infused animals in this study (Fig. 1c) and to our previous data (Samaranch et al., 2012). After either AAV9 or AAV7 administration, transduction of cells in the cerebellum was prominent (Fig. 1d). Although AAV7-GFP resulted in an apparently stronger transduction signal compared to AAV9, distribution and spread of the GFP expression was very similar with both viral vectors (Fig. 1).
FIG. 1.
Transgene expression after AAV7-GFP or AAV9-GFP vector administration into cisterna magna. Representative brain sections from prefrontal to occipital cortex of AAV7- or AAV9-treated animals showed a widespread distribution of GFP transgene using chromogenic immunohistochemistry (a). Immunostained sections against GFP showed transduction of cells with either neuron-like or glia-like morphology (b). Strong level of expression was found proximal to blood vessel lumen (c) and in cerebellar cells (d). Scale bar: 100 μm (b, c, d). GFP, green fluorescent protein.
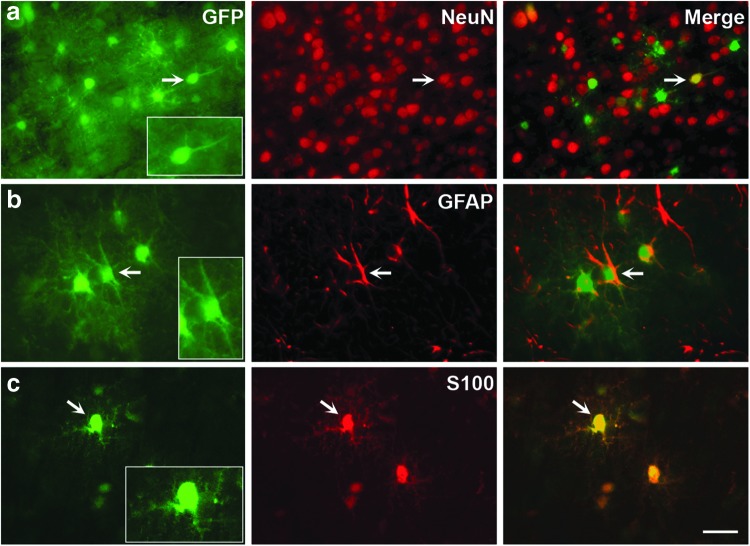
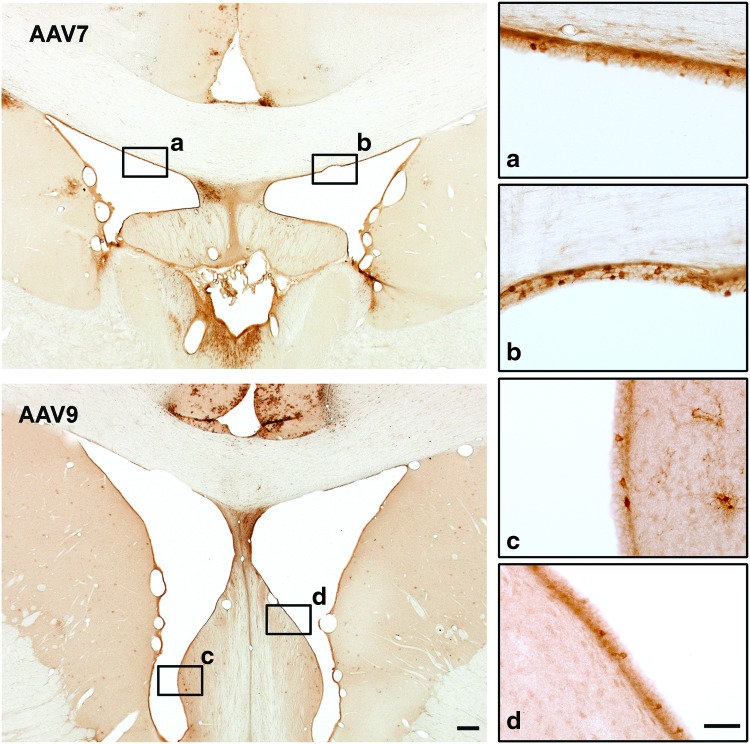
Phenotypic analysis of transduced cells in AAV7 animals showed that this vector transduced neurons (GFP+/NeuN+; Fig. 2a) and astrocytes, both fibrous (GFP+/GFAP+; Fig. 2b) and protoplasmic (GFP+/S100+; Fig. 2c), also very similarly to AAV9 transduction (Samaranch et al., 2012; Snyder et al., 2011). Interestingly, AAV delivery into the CM resulted in the transduction of ependymal cells lining the lateral ventricles (Fig. 3).
FIG. 2.
Cellular phenotypic analysis of AAV7-GFP transduced cells. Brain sections were costained against GFP and neuronal (NeuN) or astrocytic (GFAP, S100) markers. Both neurons (a) as well as fibrous (b) and protoplasmic astrocytes (c) were transduced. Arrows depict examples of GFP-positive cells expressing the different markers. Scale bar: 50 μm.
FIG. 3.
Transduction of ependymal cells lining the ventricles. Chromogenic GFP staining revealed strong transduction of ependymal cells in the lateral ventricles after both AAV7 and AAV9 injection. Scale bar: 500 μm; 50 μm (a–d). Color images available online at www.liebertpub.com/hum
Concerns about peripheral toxicity arise when viral vectors or other molecules are being delivered to the CNS. In our previous work, AAV9 delivery to CM resulted in sparse GFP expression in the spleen and liver (Samaranch et al., 2012). Similarly NHPs that received CM infusion of AAV7 presented with weak transduction in these structures, confirming that CSF delivery results in low vector exposure of peripheral organs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum).
Spinal cord transduction after AAV7 or AAV9 delivery
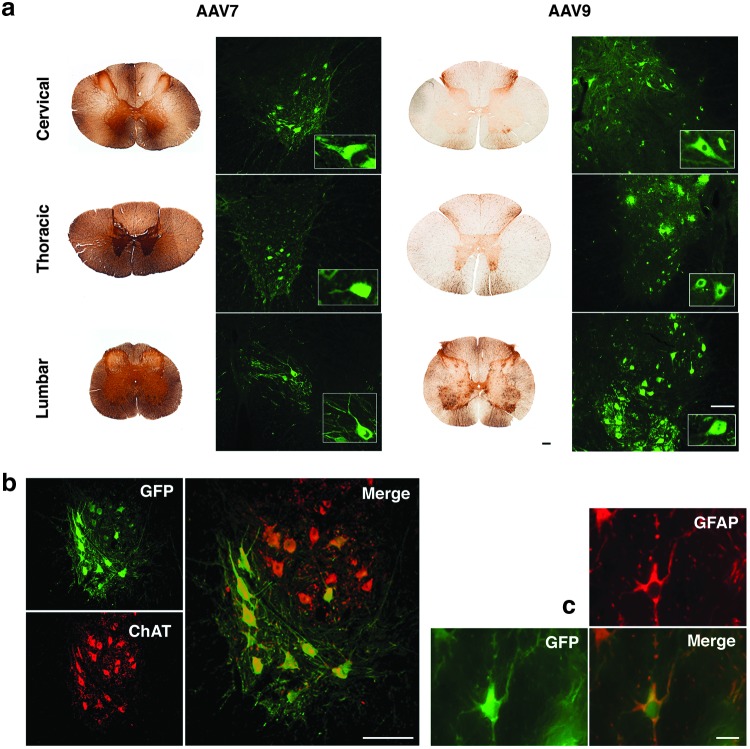
Although there were some intrasegmental differences in the number of GFP-positive cells after exposure to AAV9 or AAV7, DAB and immunofluorescent GFP staining revealed the presence of GFP-positive cells at all spinal cord levels (i.e., cervical, thoracic, and lumbar; Fig. 4a). Double labeling of spinal cord sections confirmed that most of the cells transduced in the ventral horns were motor neurons (GFP+/ChAT+; Fig. 4b), but some astrocytes were also transduced (GFP+/GFAP+; Fig. 4c). Interestingly, the majority of GFP-positive motor neurons and fibers in the spinal cord were found in the lumbar region (Fig. 5a). In addition, we observed transduction of astrocytes (GFP+/GFAP+) limited to the ventral funiculi in thoracic and lumbar, but not cervical, regions (Fig. 5b). It is noteworthy that this phenotypic transduction of astrocytes was not found in monkeys with low GFP expression in the brain. Altogether, our data show that the infusion of either AAV7 or AAV9 leads to a comprehensive and strong transduction of motor neuron pools throughout the spinal cord.
FIG. 4.
Robust transduction of spinal cord after AAV7-GFP or AAV9-GFP administration. GFP-positive cells were found at cervical, thoracic and lumbar spinal regions after both chromogenic (left AAV7 and AAV9 columns) and fluorescent staining (right AAV7 and AAV9 columns) (a). Cellular phenotype analysis revealed that both motoneurons (GFP+/ChAT+) (b) and astrocytes (GFP+/GFAP+) (c) were transduced after AAV7 or AAV9 delivery. Scale bar: (a) 500 μm (chromogenic images) or 200 μm (fluorescence images); (b) 200 μm; (c) 50 μm. ChAT, choline acetyltransferase.
FIG. 5.

Corticostriatal fibers and spinal nerve transduction after AAV serotypes 7 and 9 infusion. High transduction levels were found in corticospinal tracts (CSt, GFP+) mainly at lumbar levels (a). In addition, levels with high transduction also showed astrocytes transduced (GFP+) (b). Peripheral spinal nerves at all levels were also transduced (GFP+) (c). Scale bar: 500 μm (a, b); 100 μm (c).
Peripheral nervous system transduction after AAV7 or AAV9 delivery
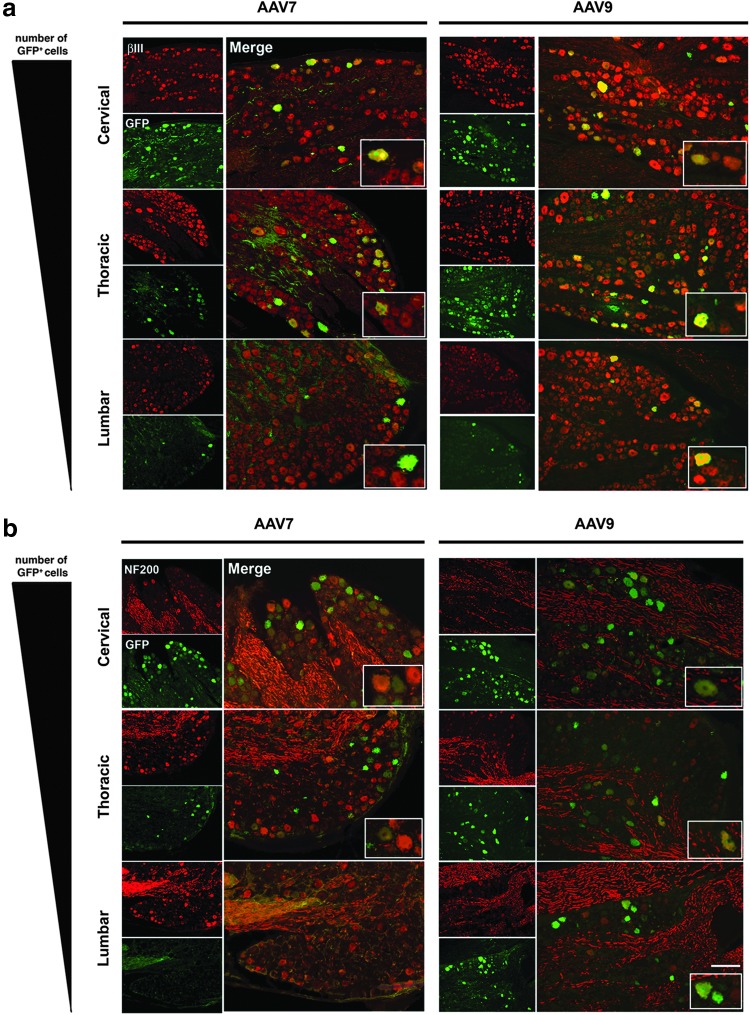
On the basis of the broad transduction achieved throughout the CNS, we investigated whether these AAV serotypes were able to transduce components of the peripheral nervous system bathed by the CSF, such as spinal nerves and/or DRG. After vector administration we observed a robust GFP expression in fibers of the dorsal and ventral roots (Fig. 5c). DRG neurons were transduced within all segments of the spinal cord (GFP+/β-III+), with the majority of GFP+ somas found in DRG of the cervical region, proximal to the site of injection (Fig. 6a). Transduction of myelinated type-A DRG neurons (GFP+/NF-200+) was observed at cervical and thoracic levels, but NF-200–positive myelinated neurons in lumbar region were only found in AAV9 monkeys (Fig. 6b). In addition, some satellite glial cells surrounding DRG neurons were also GFP positive (Fig. 7).
FIG. 6.
Peripheral nervous system transduction. Representative sections of dorsal root ganglia at three levels (cervical, thoracic, lumbar) were stained to characterize transduced neuronal subtypes. GFP-positive cells were colocalized with sensory neuron (GFP+/green; β-III tubulin+/red) (a) and myelinated neuron (GFP+/green; NF-200+/red) (b) markers. Scale bar: 200 μm (a, b).
FIG. 7.
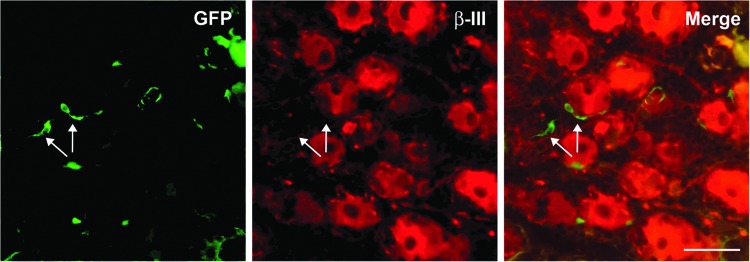
Glia-like cells transduced in dorsal root ganglia. Double fluorescent immunohistochemistry revealed some GFP-positive satellite glia cells proximal to β-III tubulin–positive DRG neurons. Scale bar: 50 μm.
Discussion
In this study, we compared transduction of spinal cord and cortex by AAV9-GFP and AAV7-GFP. Previously we showed that infusion of AAV9 into the CM of NHPs was about 10-fold more efficient in transducing the brain than was vascular delivery, with the added advantage of avoiding transduction of liver (Samaranch et al., 2012). AAV7, highly homologous to AAV9, has not been studied in this infusion modality. We were interested, therefore, in evaluating how the two vectors compared in efficiency and tropism. In view of the homology between the two serotypes in amino acid sequence of their capsid protein (Daya and Berns, 2008), it is unsurprising that the tropism and distribution of the two vectors matched closely. Both serotypes, when infused into CM transduced brain cortex remarkably well with transduced astrocytes and neurons abundantly present throughout, from prefrontal to occipital regions. Bergmann glial cells in the cerebellum and some spear neurons in the brainstem nuclei were also transduced. This cortical and cerebellar transduction pattern observed with both AAV9 and AAV7 vectors matches with a vector flow from CSF into the brain and cerebellum matters through the perivascular space (Virchow–Robin space). A recent study in mouse brain demonstrates how intrathecally delivered tracers get transported into the brain interstitium following para-arterial influx of subarachnoid CSF and paravenous efflux of interstitial fluid back into the CSF (Iliff et al., 2012). Indeed, in our previous work of AAV2 parenchymal delivery in rat brains, we reported that viral particles spread moving through brain perivascular space (Hadaczek et al., 2006). Based on the results of the present study, AAV9 and AAV7 virion particles appear to use the same pathway into the brain when injected into the CM. In sum, we conclude that AAV7 is functionally indistinguishable from AAV9 in our assays.
An important aspect of this study was the striking transduction of spinal cord neurons by both vectors. Motor neurons throughout the spinal cord were transduced, with perhaps a modest tendency toward greater transduction in lumbar segments. However, the small number of animals in this study precludes any rigorous quantitative analysis. This avidity of both AAV7 and AAV9 serotype for motor neurons after intrathecal infusion clearly has strong implications for the treatment of amyotrophic lateral sclerosis, spinomuscular atrophy, and other motor neuron diseases (Veldink et al., 2004). Specificity for these cells might be further improved with motor neuron–specific promoters.
Another remarkable observation was the ability of AAV7 and AAV9 to transduce DRG. Intrathecal AAV8 shows a similar propensity in rats (Storek et al., 2008; Jacques et al., 2012). That NHP DRGs are accessible by AAV7 and AAV9 suggests utility for these serotypes in transducing DRG. Given the central role DRG play in transmission of pain signals and maintenance of pathological pain, both AAV2 (Milligan et al., 2005) and AAV8 (Storek et al., 2008; Jacques et al., 2012) have been used to therapeutic effect in rat models of neuropathic pain. Clearly, this area is ripe for a more intensive survey of other AAV serotypes in terms of efficiency and specificity.
A potential advantage of intrathecal delivery of AAV7 and AAV9 is reduced exposure of peripheral organs to vector. This has two salient practical implications. First, it may reduce the potential for peripheral transgene toxicity. Second, and perhaps more importantly, it reduces the amount of vector required for effective CNS transduction (Gray et al., 2011; Samaranch et al., 2012). However, as we have previously observed, there is only modest protection from circulating antibodies against AAV by resorting to intrathecal administration rather than intravenous (Samaranch et al., 2012). Nevertheless, there seems to be some protection as long as neutralizing antibody titers are not too high, as in the present study. Human infants tend to have low titers (Calcedo et al., 2009, 2011), and this augurs well for the use of intrathecal AAV in the treatment of familial spinal cord diseases.
Supplementary Material
Acknowledgments
This study was supported by a grant to K.S.B. from National Institutes of Health, National Institute of Neurological Disorders and Stroke (R01NS073940-01). We also thank Dr. Brian Kaspar and Adam Bevan for helping us with the neutralizing antibody determinations.
Author Disclosure Statement
No competing financial interests exist.
References
- Bevan A.K. Duque S. Foust K.D., et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R. Vandenberghe L.H. Gao G., et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R. Morizono H. Wang L., et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielska A. Hadaczek P. Mittermeyer G., et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol. Ther. 2013;21:158–166. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S. Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici T. Taub J.S. Baum G.R. Gray S.J. Grieger J.C. Matthews K.A., et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- Foust K.D. Kaspar B.K. Over the barrier and through the blood: to CNS delivery we go. Cell Cycle. 2009;8:4017–4018. doi: 10.4161/cc.8.24.10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D. Nurre E. Montgomery C.L., et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2008;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.J. Matagne V. Bachaboina L., et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P. Yamashita Y. Mirek H., et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff J.J. Wang M. Liao Y., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques S.J. Ahmed Z. Forbes A., et al. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol. Cell. Neurosci. 2012;49:464–474. doi: 10.1016/j.mcn.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Matsushita T. Elliger S. Elliger C., et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Milligan E.D. Sloane E.M. Langer S.J., et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol. Pain. 2005;1:9. doi: 10.1186/1744-8069-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch L. Salegio E.A. San Sebastian W., et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum. Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder B.R. Gray S.J. Quach E.T. Huang J.W. Leung C.H. Samulski R.J., et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum. Gene Ther. 2011;22:1129–1135. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Storek B. Reinhardt M. Wang C., et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldink J.H. Van den Berg L.H. Wokke J.H.J. The future of motor neuron disease: the challenge is in the genes. J. Neurol. 2004;251:491–500. doi: 10.1007/s00415-004-0322-6. [DOI] [PubMed] [Google Scholar]
- Wright J.F. Qu G. Tang C. Sommer J.M. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr. Opin. Drug Discov. Devel. 2003;6:174–178. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.