Abstract
Cholangiocarcinoma (CC) is curable only in early stages by complete surgical resection. Thus, in advanced disease stages in which a complete removal of the tumor mass is no longer possible and palliative chemotherapy achieves only modest success, therapeutics employing new methods of action are desperately needed. Oncolytic viruses employed in clinical studies have been shown to spread preferentially in cancer cells. Beyond that, virotherapeutic cell killing can be enhanced by virus-based expression of suicide genes. We engineered a measles vaccine virus (MeV) vector expressing super cytosine deaminase (SCD), a fusion protein of yeast cytosine deaminase and uracil phosphoribosyltransferase, which converts the prodrug 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) and subsequently to 5-fluorouridine-monophosphate. This novel vector was evaluated using three different human-derived CC cell lines. In vitro, all CC cell lines were found to be permissive to MeV infection. Partial blocking of MeV-mediated oncolysis could be overcome by employment of the SCD transgene together with administration of 5-FC. In vivo, intratumoral application of SCD-armed MeV together with a systemic 5-FC treatment showed a significant reduction in tumor size in a TFK-1 xenograft mouse model when compared with virus-only treatment. In a second animal experiment employing a HuCCT1 xenograft tumor model, an enhanced SCD-armed MeV vector, in which the SCD transgene was expressed from a different genomic position, led not only to reduced tumor volumes, but also to a significant survival benefit. On the basis of these encouraging preclinical data on employment of SCD-armed MeV for the virotherapeutic treatment of chemotherapy-resistant CC, a clinical virotherapy trial is set up currently.
Lange and colleagues engineer a measles vaccine virus (MeV) vector expressing an enzyme that converts 5-fluorocytosine (5-FC) prodrug into 5-fluorouracil-monophosphate. Partial blocking of MeV-mediated oncolysis could be overcome by vector and 5–FC co-administration in cell lines. In vivo, intratumoral vector injection along with systemic 5–FC treatment resulted in significant tumor reduction in a xenograft mouse model.
Introduction
Malignant tumors of the biliary tract comprise cholangiocellular and gallbladder carcinomas and are the second most common primary hepatic tumors, accounting for approximately 20% of the deaths from hepatobiliary malignancies, which cause 13% of the total cancer mortality worldwide (Patel, 2001; Jemal et al., 2011). Epidemiologic studies suggest an increasing incidence in Western countries during the last decades (Blechacz and Gores, 2008; Skipworth et al., 2011).
The only curative option for patients with gallbladder or bile duct cancer is surgical resection. Unfortunately, most cholangiocarcinomas (CC) remain clinically silent until having reached an advanced and then unresectable stage. Although the rate of resectability has been reported to be as high as 65%, curative resection or margin-free resection rates are less than 50% (Blechacz and Gores, 2008; Akamatsu et al., 2011). Accordingly, the overall 5-year survival rate amounts to only 5%–10% (Mosconi et al., 2009). Therefore, novel approaches in both adjuvant and palliative settings of malignant biliary tract tumors are urgently required.
Oncolytic viruses are under investigation as a new treatment modality against several different tumor types. Viruses from different families have been tested preclinically and clinically, including adenovirus, herpes simplex virus, vaccinia virus, reovirus, Newcastle disease virus, and measles vaccine virus (MeV) (Liu and Kirn, 2008; Lech and Russell, 2010). MeV has been shown to be an oncolytic vector infecting a broad range of tumor entities, and it has a long-standing safety record through administration to patients in huge vaccination programs (Griffin et al., 2008). MeV is currently under clinical investigation as a new treatment modality against ovarian cancer (Galanis et al., 2010), multiple myeloma, and glioblastoma multiforme [reviewed by Msaouel et al. (2009) and Lech and Russell (2010)], and, notably, in these trials no dose-limiting toxicity has been reached so far (Russell et al., 2012).
Oncolytic viruses have been armed with suicide genes that convert nontoxic prodrugs into toxic substances, leading to a localized chemotherapy at sites of viral gene expression and thus to minimized side effects compared with conventional chemotherapies [overview in Cattaneo et al. (2008)]. For oncolytic MeV, arming with Escherichia coli purine nucleoside phosphorylase that toxifies fludarabine or 6-methylpurine-2′-deoxyriboside (MeP-dR) has shown preclinical effectivity in lymphoma (Ungerechts et al., 2007a, 2010), pancreatic cancer (Bossow et al., 2011), and an immunocompetent model of murine colon carcinoma (Ungerechts et al., 2007b). However, fludarabine can cause cytotoxicity when administered systemically (Sioka and Kyritsis, 2009). Recently, an armed MeV for chemovirotherapy of head and neck squamous cell carcinoma has been published (Zaoui et al., 2012).
To date, there have been only a very limited number of publications testing virotherapy against CC. These investigations employed either adenovirus (Pederson et al., 1997; Stackhouse et al., 2000; Zhu et al., 2006; Kojima et al., 2009) or herpes simplex virus (Jarnagin et al., 2006) enhanced by cytosine deaminase/5-fluorocytosine (5-FC) (Pederson et al., 1997; Stackhouse et al., 2000), uracil phosphoribosyltransferase/5-fluorouracil (5-FU) (Kojima et al., 2009), and/or radiotherapy (Pederson et al., 1997; Stackhouse et al., 2000; Jarnagin et al., 2006). No studies have involved MeV so far.
Thus, we sought to develop a new MeV-based suicide gene therapy for the virotherapeutic treatment of CC. We constructed a virus with 100% genetic identity to the measles vaccine Schwarz strain (Schwarz, 1962) and armed it by introducing the gene for a prodrug convertase with enhanced effectivity, super cytosine deaminase (SCD) (Graepler et al., 2005). SCD, a fusion of yeast cytosine deaminase and uracil phosphoribosyltransferase, converts the clinically approved antimycotic 5-FC into the clinically approved chemotherapeutic 5-FU and further into 5-fluorouridine-monophosphate, which leads to inhibition of DNA and protein synthesis (Longley et al., 2003). This suicide gene–prodrug combination has been tested in the context of an oncolytic vaccinia virus in a clinical trial on hepatocellular carcinoma that was completed recently (Clinical Trial NCT00978107; sponsor: Transgene SA, France).
In our study, we first tested this novel armed MeV in vitro in different human CC cell lines. MeV was able to replicate in all cell lines tested, although to different extents. Of note, in one CC cell line that was only partially permissive to virus-mediated lysis, addition of 5-FC was found to improve the extent of tumor cell death significantly. In vivo, SCD-armed MeV vectors proved to be effective in xenograft mouse models of human CC, leading to a significant reduction of tumor volume and prolonged survival.
Materials and Methods
Generation of recombinant MeV
We constructed a MeV-Schwarz cDNA by RT-PCR from an original measles vaccine batch (Mérieux, Sanofi-Pasteur, Leimen, Germany). Details on the primers, the cloning, and the rescue strategy can be obtained from the authors upon request. In brief, the viral vectors were constructed as follows:
Viral cDNA was inserted into a plasmid containing regulatory sequences (promoter, terminator) recognized by T7 RNA polymerase. In this cDNA, an empty additional transcription unit (ATU) was integrated into genome position three by inserting a double-stranded synthetic DNA sequence (synthesized from the 5′-phosphorylated oligonucleotides CTAGTCCTCCATCATTGTTATAAAAAACTTAGGAACCAGGTCCATACACCGTACGCTCGAGGCGCGCGACGT and CTAGACGTCGCGCGCCTCGAGCGTACGGTGTATGGACCTGGTTCCTAAGTTTTTTATAACAATGATGGAGGA) into the SpeI restriction site at genome position 3374. Two transgenic open reading frames (SCD or DsRed) were inserted via restriction sites compatible to the unique PauI cloning site within the ATU. The resulting recombinant MeVs were named MeV P-SCD and MeV P-DsRed and were rescued by transfection of BSR-T7 cells [a kind gift from K.-K. Conzelmann, Ludwig-Maximilians-University Munich, Germany (Buchholz et al., 1999)] with 7.5 μg viral cDNA and a mixture of plasmids encoding the viral proteins N, P, and L under the control of the T7-promoter (250, 150, and 150 ng, respectively) using FuGene HD (Roche, Basel, Switzerland).
Viral cDNA was inserted into a plasmid containing regulatory sequences (promoter, terminator) derived from cytomegalovirus (CMV) and recognized by RNA polymerase II. In this cDNA, an empty ATU was integrated into genome position one. This ATU was synthesized by fusion PCR using primer pairs (i) GAGCGGATAACAATTTCACACAGG and TATAACAATGATGGATGGCGCGCCTCGAGATATCCCTAATCCTGCTCTT and (ii) CGCGCCATCCATCATTGTTATAAAAAACTTAGGATTCAAGATCCTATT and CCTATTAGTGCCCCTGTTAGTTT. The SCD ORF was integrated via restriction sites compatible to the unique AscI cloning site within the ATU. The virus was rescued by transfection of Vero cells with 5 μg viral cDNA and a mixture of plasmids encoding the viral proteins N, P, and L under the control of the CMV-promoter (500, 100, and 500 ng, respectively) using FuGene HD and was named MeV ld-SCD.
Cell culture
Human CC cell lines RBE (Enjoji et al., 1997) and HuCCT1 (Miyagiwa et al., 1989) cells were purchased from Riken Cell Bank (Riken BRC, Tsukuba, Japan). TFK-1 cells (Saijyo et al., 1995) were a kind gift from Dr. Nischalke (Rheinische Friedrich-Wilhelms-University Bonn, Germany). All CC cells were obtained in 2008. Vero African green monkey kidney cells were obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany) in 2005. All cells were passaged for fewer than 3 months after resuscitation. Riken Cell Bank and the German Collection of Microorganisms and Cell Cultures regularly employ the short tandem repeat polymorphism analysis test for human cell lines. All CC cell lines were grown in RPMI-1640 (Biochrom, Berlin, Germany), Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM; Biochrom). All cell lines were cultured in medium supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Pasching, Austria) at 37°C in a humidified atmosphere containing 5% CO2.
Production and titration of MeV
Vero cells (1×107/plate) were seeded in 15 cm plates. The next day, cells were washed with phosphate-buffered saline (PBS; PAA Laboratories) and infected at a multiplicity of infection (MOI) of 0.03 in Opti-MEM (Invitrogen, Carlsbad, CA). After 3 hr, the medium was replaced with DMEM supplemented with 10% FCS. About 54 hr later, when most of the cells showed cytopathic effects, the medium was removed, cells were scraped into 1 ml Opti-MEM, and lysed by one freeze–thaw cycle. After centrifugation (1,900×g, 15 min, 4°C), the supernatant was stored at −80°C.
Viral titers were determined by endpoint dilution assay and calculated by the method of Kärber and Spearman on Vero cells (Kärber, 1931).
Quantification of CD46 expression by flow cytometry
Cells were washed with PBS and detached with Accutase (PAA Laboratories). Cells were then washed with PBS and incubated on ice for 30 min with a PE-labeled anti-human CD46 antibody or a PE-conjugated IgG1 isotype control (eBioscience, San Diego, CA) diluted 1:20 in PBS containing 10% FCS. After washing with PBS to remove free antibody, cells were resuspended in PBS containing 6.6% FCS and 1.3% paraformaldehyde. Flow cytometry analysis was performed on a FACS Calibur (Becton Dickinson, Franklin Lakes, NJ) and analyzed using Cell Quest (Becton Dickinson). Mean fluorescence index (Anderson et al., 2004) is the ratio of the mean FL-3 height of antiCD46-stained cells and the respective isotype control.
Replication of MeV
About 2×105 CC cells in six-well plates were washed with PBS and infected with MeV P-DsRed in 1 ml Opti-MEM at MOI of 0.1 for 3 hr. After infection, the medium was replaced with RPMI-1640 containing 5% FCS. Directly after adsorption and every 24 hr, supernatants were collected and cells were scraped into 1 ml Opti-MEM. Virus was released by one freeze–thaw cycle. Viral titers were determined as described above.
In vitro cell viability experiments
CC cells in 24-well plates (7.5×104/well) were washed with PBS and infected with MeV in 250 μl Opti-MEM for 3 hr. The inoculum was replaced with RPMI-1640 containing 10% FCS and varying concentrations of 5-FC (Roche, Mannheim, Germany) and the cells were incubated for 96 hr. To determine sensitivity toward the activated prodrug, cells were incubated with varying concentrations of 5-FU (Medac, Wedel, Germany). Cell viability was evaluated by phase contrast microscopy (IX50; Olympus, Tokyo, Japan; analySIS, Soft Imaging System, Münster, Germany), sulforhodamine B (SRB), and lactate dehydrogenase (LDH) assay.
SRB assay
Protein mass was evaluated by SRB assay as described before (Skehan et al., 1990). In brief, cells were washed with cold PBS twice, fixed in 10% trichloroacetic acid at 4°C for 30 min, and stained with 0.04% SRB dye (Sigma-Aldrich, Taufkirchen, Germany) in 1% acetic acid. The stained cell mass was solubilized in 10 mM Tris base (pH 10.5). Optical density was measured at 550 nm and related to untreated cells.
LDH assay
Cell lysis was quantified with the LDH-P mono kit (Biocon, Voehl/Marienhagen, Germany) according to the manufacturer's recommendations. To determine the relative LDH release, LDH concentration was measured in the supernatant as well as in the cells that were lysed with 0.1% Triton X-100 (Carl Roth, Karlsruhe, Germany) in PBS. Values represent the ratio of LDH activity in the supernatant and the total LDH activity in each well.
Immunoblotting
CC cells (2×106/plate) were seeded in 10 cm plates. Cells were infected as described above. About 54 hr after infection, cells were washed with PBS and harvested in lysis buffer (50 μM Tris, 150 μM NaCl, 1% NP40). Lysates were subjected to three freeze–thaw cycles and centrifuged (7,000×g, 4°C, 10 min), and supernatants were stored at −80°C. Samples were prepared with Roti Load Buffer (Carl Roth), separated on 10% SDS-polyacrylamide gels with PAGE buffer, and transferred onto polyvinylidene difluoride membranes (Hybond-P; Amersham Biosciences, Piscataway, NJ). After blocking, membranes were incubated with anti-vinculin (mouse antibody; Sigma-Aldrich; 1:6,000), anti-measles N Protein (rabbit antibody; Abcam, Cambridge, United Kingdom; 1:6,000), or anti-SCD (rat antibody, a kind gift from Transgene, Illkirch-Graffenstaden, France; 1:2,000) antibodies overnight. Membranes were washed in TBS-T and incubated with secondary antibodies (peroxidase-conjugated anti-mouse, anti-rabbit or anti-rat, goat antibody [Invitrogen], 1:30,000 in Roti-Block). After washing, proteins were detected with ECL (Millipore, Billerica, MA) on Hyperfilm-ECL (Amersham Biosciences).
In vivo experiments
All animal experiments were conducted according to the German law for the care and use of laboratory animals and were approved by the local authorities. TFK-1 cells (1×107 cells each in 100 μl PBS) were injected subcutaneously into the right flank of 4–6-week-old female CanN.Cg-Foxn1nu/Crl mice (Charles River Laboratories, Sulzfeld, Germany). In a second experiment, HuCCT1 cells were implanted subcutaneously in Hsd:Atymic Nude-Foxn1nu (Harlan Laboratories, Venray, The Netherlands) mice. Tumors were measured with a caliper three times per week and tumor volume was calculated from the ellipsoid volume formula (length×width×width×π/6) (Tomayko and Reynolds, 1989). When tumors reached a volume of about 100 mm3, mice were randomized into four treatment groups: control, control+5-FC, MeV, and MeV+5-FC. Mice received intratumoral injections of MeV (either 1×106 pfu/dose of MeV P-SCD for TFK-1 or 2×106 pfu/dose of MeV ld-SCD for HuCCT1 in 100 μl Opti-MEM) or Opti-MEM alone once daily on days 0–4. Mice randomized to the 5-FC groups received daily intraperitoneal injections of 5-FC (500 mg/kg body weight/dose in PBS) on days 5–11. Animals were sacrificed when tumor volumes reached 2,000 mm3, weight loss over 20% of body weight occurred, or ulcerating tumors were observed.
Statistical analysis
For comparison between groups, either the unpaired Student's t-test or two-way analysis of variance (ANOVA) was used. The Kaplan–Meier method was used to calculate survival curves. Statistical analysis was done with GraphPad Prism 4.03 (GraphPad Software, San Diego, CA) and JMP 9.0.1 (SAS Institute, Cary, NC). Values of p less than 0.05 were considered statistically significant.
Results
Generation and characterization of an armed MeV
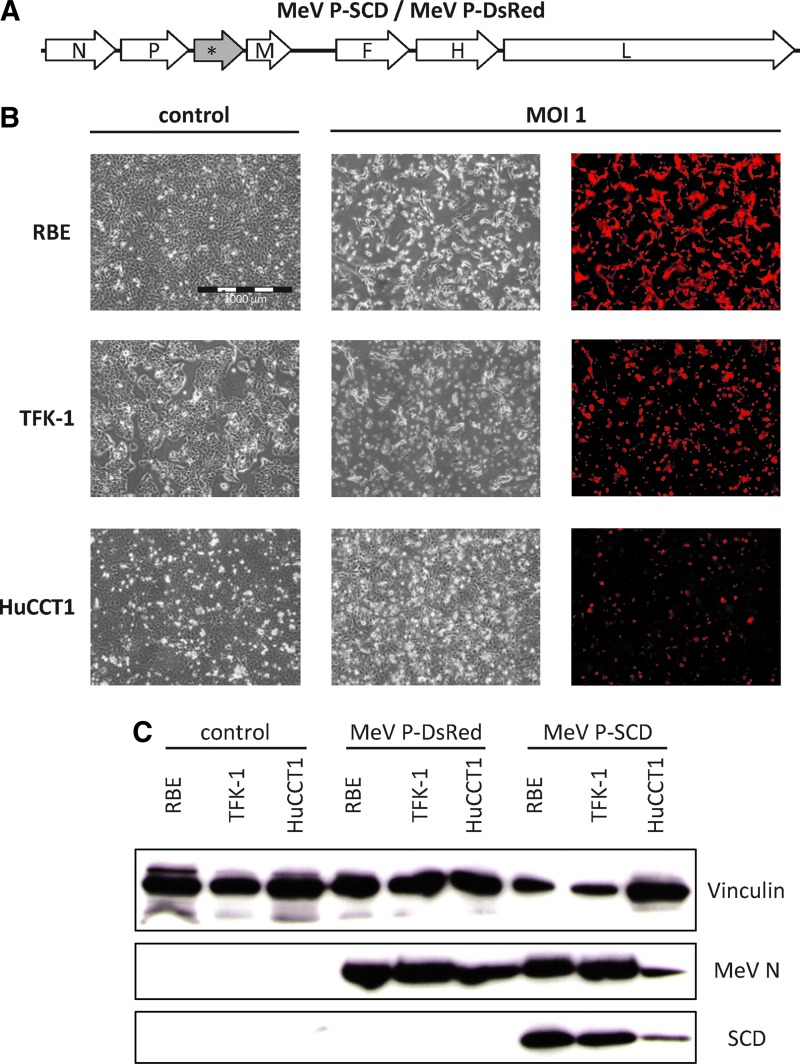
To enhance the oncolytic properties of MeV, we first generated a suicide gene-armed MeV expressing the SCD (Graepler et al., 2005) from genome position 3 (Fig. 1A). For this purpose, the virus backbone was produced by RT-PCR from a batch of the Schwarz vaccine and demonstrated to exhibit 100% identity with this clinically well-established measles vaccine strain. We introduced an ATU into the viral genome and then either the SCD open reading frame or that coding for the reporter gene DsRed (Fig. 1A), resulting in vectors MeV P-SCD or MeV P-DsRed.
FIG. 1.
Generation and characterization of armed measles vaccine virus (MeV). (A) Open reading frames encoding super cytosine deaminase (SCD; yeast cytosine deaminase fused to uracil phosphoribosyltransferase) or DsRed were inserted into an empty additional transcription unit (*) at genome position 3 of the Schwarz measles vaccine strain. (B) Cells were infected with MeV-DsRed at a multiplicity of infection (MOI) of 1 and pictures were taken at 2 days postinfection. Mock-infected cells (left panels) and infected cells are shown. The scale bar (1,000 μm) in the top-left panel applies to all panels. (C) Cells were mock-infected or infected with either MeV P-DsRed or MeV P-SCD at an MOI of 0.1 and harvested 54 hr later. Immunoblotting was performed employing antibodies against human vinculin (top panel), MeV N protein (middle panel), or SCD (bottom panel).
To test for the susceptibility toward MeV, human CC cell lines RBE, TFK-1, and HuCCT1 were infected with MeV P-DsRed and analyzed by phase-contrast and fluorescence microscopy. All human CC cell lines could be infected with MeV P-DsRed successfully (Fig. 1B). Syncytia formation, a fusion of neighboring cells that is the typical MeV-mediated cytopathic effect, was observed in all three cell lines, although to different extents. The cytopathic effect of MeV was found to be most profound in TFK-1 cells and was slightly weaker in RBE cells. In contrast, infection of HuCCT1 cells showed only a low-grade infection with limited formation of syncytia (Fig. 1B).
Expression of virus-encoded proteins was confirmed by western blotting in all three cell lines (Fig. 1C). However, HuCCT1 cells showed a weaker expression of virus-encoded proteins (viral N-protein [MeV N] and transgenic SCD), which was in line with the weaker expression of DsRed and the less pronounced cytopathic effect.
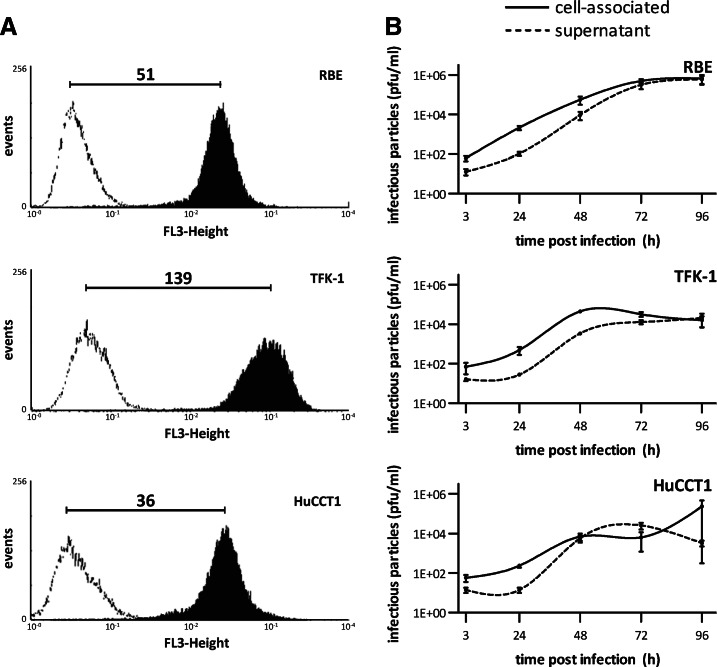
Human CC cell lines express the MeV receptor CD46 and support MeV replication
Overexpression of the MeV receptor CD46 has been shown to be a prerequisite for the typical cytopathic effect of MeV and thus seems to play an important role in MeV-mediated oncolysis. The minimum mean fluorescence index needed for substantial MeV-mediated oncolysis has been shown to be between 25 and 50 (Anderson et al., 2004). Thus, we determined CD46 expression on three human CC cell lines (Fig. 2A). The mean fluorescence index of TFK-1 cells was found to be 139±29 (mean±SD), which was highest among the three cell lines; the indices of RBE and HuCCT1 cells were 51±14 and 36±5, respectively. Thus, all human CC cell lines under investigation were found to express CD46 to an extent sufficient to support MeV-mediated oncolysis.
FIG. 2.
CD46 receptor expression and replication of MeV P-DsRed in human cholangiocarcinoma (CC) cell lines. (A) Cells were stained with a PE-labeled anti-CD46 antibody (black histogram) or an isotype control (white histogram) and analyzed by flow cytometry. Numbers represent the ratios of the mean fluorescence indexes of the black histogram:white histogram of three independent experiments. Values represent mean of three independent experiments. (B) Cells were infected with MeV P-DsRed at MOI 0.1. Cells and supernatant were harvested daily over 4 days and viral titers were determined on Vero cells. Values represent mean of three independent experiments±SEM.
To quantify replication of oncolytic MeV, viral growth curves were performed (Fig. 2B). MeV P-DsRed replicated efficiently in all three cell lines. In TFK-1 cells, the amount of cell-associated MeV reached a plateau as early as 48 hr after infection (compared with 72 hr for RBE cells), which was caused by viral oncolysis already causing profound cell death at this time point. Replication in HuCCT1 cells appeared to be much slower than in RBE and TFK-1 cells, an observation being consistent with the weaker transgene expression observed by immunoblotting and microscopy (compare Fig. 1).
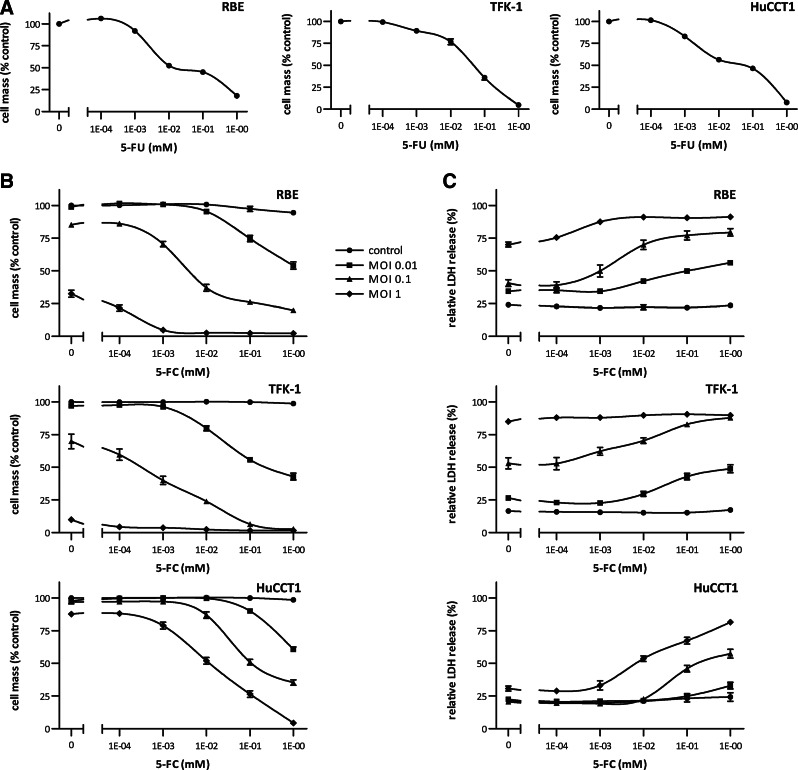
Suicide gene therapy leads to enhanced cell killing and can be employed to overcome partial resistance of virotherapy
Sensitivity toward the activated form of the prodrug is a prerequisite for efficient suicide gene therapy. Thus, we first investigated the effect of 5-FU on our selected human CC cell lines. Concentrations of 0.01–0.1 mM 5-FU were found to reduce the tumor cell mass in all three CC cell lines to 50% of the control with an almost complete cell killing at 1 mM (Fig. 3A).
FIG. 3.
Cytotoxic effects of 5-FU and virotherapeutic treatment with MeV P-SCD+prodrug 5-FC in human CC cell lines. (A) Cytotoxic effects of 5-FU on CC cell lines RBE, TFK-1, and HuCCT1. Cells were treated with various concentrations of 5-FU for 4 days. Then, remnant cell masses were measured in a sulforhodamine B (SRB) cytotoxicity assay. Values were normalized to the untreated control and represent mean±SEM from three independent experiments performed in quadruplicates. (B) Cells were infected with MeV P-SCD at MOIs 0.01, 0.1, and 1 and incubated with varying concentrations of 5-FC for 4 days. Remnant cell masses were measured in an SRB cytotoxicity assay. Values were normalized to the untreated control and represent mean±SEM from three independent experiments performed in quadruplicates. (C) Cytotoxicity was measured in a lactate dehydrogenase (LDH) release assay. Values represent the mean ratio between LDH in the supernatant and the total LDH in each well±SEM from three independent experiments performed in quadruplicates.
To assess both virus-mediated oncolysis alone and cytotoxicity induced by the combination therapy, the effects of a range of MOIs and concentrations of 5-FC were tested via SRB and LDH assays (Fig. 3). As others have observed differences in cell survival depending on the time of prodrug addition in MeV-mediated suicide gene therapy (Ungerechts et al., 2007a), we also compared prodrug addition at 3, 24, 48, or 72 hr post infection (our unpublished results). As we found that early administration of 5-FC (i.e., at 3 hpi) yields much better results in terms of oncolytic efficiency than any later administration (i.e., at 24, 48, or 72 hpi), prodrug was added ab initio in all further experiments.
MeV P-SCD proved to be highly cytotoxic in TFK-1 cells. At an MOI of 1, even without the addition of 5-FC, 80%–90% of the cells were killed (Fig. 3B). A similar effect was observed with RBE cells, where about 70% of the cells were killed at an MOI of 1. In both cell lines, the addition of the prodrug 5-FC led to a significantly reduced tumor cell survival. For example, cell death after infection at MOI 0.1 was significantly enhanced by addition of 1 mM 5-FC (1) in RBE cells from 85.3%±1% remaining cell mass (mean±SEM) to 20.6%±1% and (2) in TFK-1 cells from 69.9%±5% to 2.7%±1% remaining cell mass (p<0.001 for both comparisons) (Fig. 3B). These observations were confirmed in LDH cytotoxicity assays, which correlated closely with the rates of cell mass reduction measured in the SRB assay (Fig. 3C). However, in HuCCT1 cells, where viral replication was found to be less effective than in the other two cell lines, even an MOI of 1 did not lead to a significant tumor cell killing. Importantly, by addition of 5-FC, this partial blocking of MeV-mediated oncolysis was overcome and cell survival was found to be significantly reduced (p<0.001): from 87.7%±1% (MeV P-SCD at an MOI of 1) to 4.4%±1% remaining cell mass (MeV P-SCD at an MOI of 1 plus addition of 1 mM 5-FC). This increase in cytotoxicity was confirmed in the LDH assay.
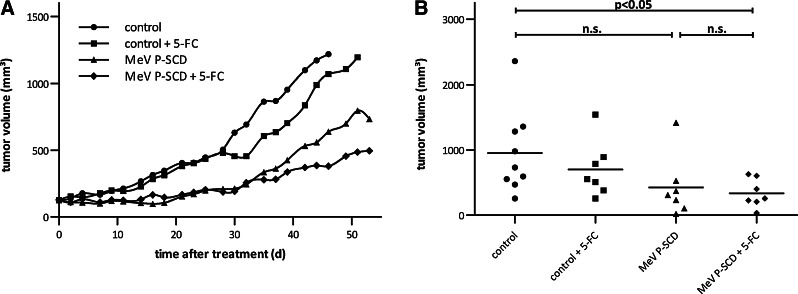
Intratumoral virotherapy of human CC xenografts exhibits a potent antitumor effect and extends survival
Next, the in vivo efficacy of MeV P-SCD was tested in a mouse xenograft model of human CC. For this purpose, TFK-1 cells were implanted into the right flank of nude mice. When tumors reached a volume of about 100 mm3, they were intratumorally injected for 5 consecutive days with MeV P-SCD, followed by 7 days of systemic/intraperitoneal treatment with 5-FC. Treatment with virus and prodrug significantly inhibited tumor growth (p<0.05) compared with the untreated control (Fig. 4A). However, treatment with MeV P-SCD only did not significantly influence tumor growth compared with the control group. About 39 days after initiation of treatment (Fig. 4B), mean tumor volumes of mice treated with the combination therapy were found to be smaller (336 mm3) than mean tumor volumes of mice treated with MeV P-SCD only (424 mm3). Treatment with vehicle control and 5-FC did not influence tumor volume compared with vehicle control only. Tumors from all treatment groups (including the control group) developed central tumor necrosis starting at approximately day 80 after tumor implantation: in most cases, this led to the development of necrotic cysts (data not shown), interfering with tumor volume measurements. Therefore, long-term tumor volume measurements were not feasible and thus survival of mice could not be evaluated in this specific xenograft model.
FIG. 4.
Antitumor effect of MeV P-SCD+prodrug 5-FC in a TFK-1 human CC xenograft mouse model. TFK-1 CC cells (1×107 in 100 μl phosphate-buffered saline [PBS]) were injected subcutaneously into the right flank of female nude mice. When tumors reached a volume of about 100 mm3, mice were randomized into four treatment groups: control (9 mice), control+prodrug 5-FC (7 mice), MeV P-SCD only (7 mice), and MeV P-SCD+prodrug 5-FC (7 mice). Mice were treated daily with intratumoral injections of MeV P-SCD (106 pfu per dose) or vehicle control (days 0–4), followed by seven injections of 5-FC (500 mg/kg body weight) intraperitoneally (days 5–11). (A) Mean tumor volume for each group. (B) Individual tumor volumes at day 39 (n.s., not significant).
As a consequence, we conducted a second in vivo experiment, now employing the HuCCT1 cell line for which we have found a partial blocking of MeV oncolysis in vitro. HuCCT1 tumor-bearing mice were treated with the same schedule as described above. To further enhance the anti-tumor effect, a high-level suicide gene-expressing MeV vector was used for this experiment: MeV ld-SCD, in which the SCD transgene is expressed from genome position 1 instead of position 3 (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/hum). This was based on the transcription gradient of measles virus with mRNA abundance decreasing with increasing distance of the respective gene from the MeV promoter (Cattaneo et al., 1987). This virus was demonstrated to exhibit enhanced oncolytic efficacy in comparison to MeV P-SCD in vitro, both in the virus-only setting as well as in combination with the prodrug (Supplementary Fig. S1B).
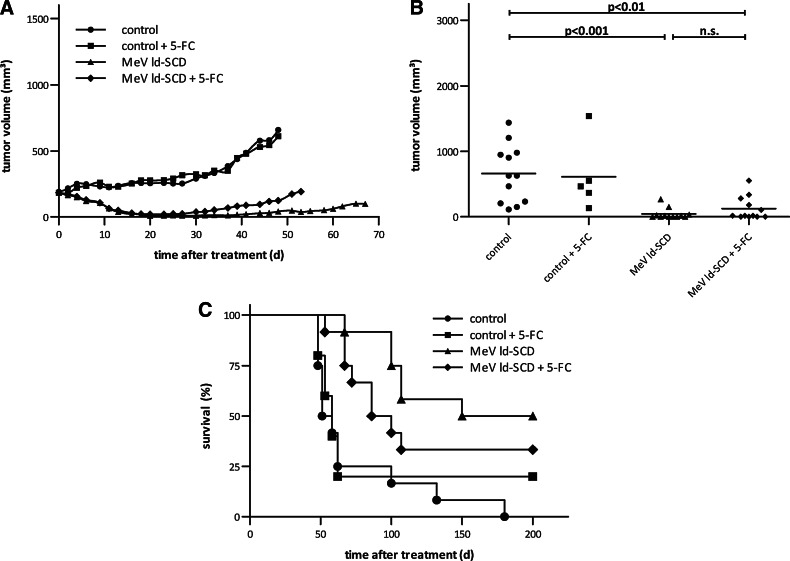
Tumors from both virus-treated groups were found to regress in the course of oncolytic treatment, but slightly reinitiated their growth from day 30 on (Fig. 5A). Mean tumor volumes after 48 days (Fig. 5B) were significantly larger in the control group (658 μl) than in groups treated with MeV ld-SCD, both with and without addition of 5-FC (124 μl, p<0.01, and 42 μl, p<0.001, respectively). Tumor volume was slightly larger in the MeV ld-SCD+5-FC group compared to MeV ld-SCD only; however, this difference was not statistically significant (p>0.05). Treatment with MeV ld-SCD±5-FC (median survival time: 175 days [MeV ld-SCD only] and 93 days [MeV ld-SCD+5-FC]) significantly prolonged survival (Fig. 5C) compared with the control group (55 days; p<0.01 for all comparisons). No significant differences were observed between treatment with MeV ld-SCD and MeV ld-SCD+5-FC (Fig. 5C). Therapy with vehicle control and 5-FC did not influence tumor volume or survival compared with vehicle control only. Taken together, these in vivo data encourage the further clinical development of SCD-armed MeV for the virotherapeutic treatment of chemotherapy-resistant CC.
FIG. 5.
Antitumor effect of MeV ld-SCD+prodrug 5-FC in a HuCCT1 human CC xenograft mouse model. HuCCT1 CC cells (1×107 in 100 μl PBS) were injected subcutaneously into the right flank of female nude mice. When tumors reached a volume of about 100 mm3, mice were randomized into four treatment groups: control (12 mice), control+prodrug 5-FC (5 mice), MeV ld-SCD only (12 mice), and MeV ld-SCD+prodrug 5-FC (12 mice). Mice were treated daily with intratumoral injections of MeV ld-SCD (2×106 pfu per dose) or vehicle control (days 0–4), followed by seven intraperitoneal injections of 5-FC (500 mg/kg body weight; days 5–11). (A) Mean tumor volume for each group. (B) Individual tumor volumes at day 48 (n.s., not significant). (C) Kaplan–Meier survival analysis for the individual treatment groups.
Discussion
Diagnosis of CC usually takes place late in the course of the disease, which either makes surgery not feasible or results in an incomplete resection of the tumor mass. Therefore, novel therapies against advanced stage CC are urgently needed. A multitude of new biological compounds are currently under development, involving, for example, targeted small molecules and immunotherapeutics, but so far, no significant improvements in treating this fatal tumor disease have been achieved (Skipworth et al., 2011).
In this study, we demonstrated that three human CC cell lines overexpress the CD46 receptor, which is the entry gateway for MeV and needed for efficient MeV-mediated oncolysis. We constructed a MeV armed with SCD, a fusion of two yeast-derived genes that convert 5-FC to 5-FU and facilitates subsequent conversion to 5-FUMP. This armed MeV vector (MeV P-SCD) was able to efficiently replicate in three different human CC cell lines, leading to expression of virus-encoded proteins and to profound cell death in two of three cell lines under investigation, even without any utilization of the MeV-encoded suicide gene function. Additional treatment with 5-FC significantly enhanced the tumoricidal effect. Importantly, in HuCCT1 cells that exhibit a partial blocking of MeV-mediated lysis alone, the additional treatment with 5-FC resulted in a strong cytotoxic effect. We also evaluated the potential benefit of using a MeV vector with the SCD gene inserted into genome position 1 instead of position 3.
In vivo, we showed that the tumor volume of mice bearing TFK-1 tumors was significantly reduced compared with mock controls when treated with MeV P-SCD in combination with 5-FC. Survival in this model was not evaluated because of development of necrosis in the tumors. Survival of HuCCT1-tumor bearing mice was significantly prolonged compared with mock controls when treated with MeV ld-SCD alone or in combination with 5-FC. However, in this tumor model, we did not observe superior effects of the combination therapy compared with treatment with virus alone. This missing enhancement of tumor cell killing when making use of our suicide gene armament might have been due to the following several reasons.
SCD-mediated conversion of 5-FC into 5-FU and toxic metabolites might have been insufficient. However, when employing reverse-phase HPLC for a direct quantification of 5-FC conversion into newly produced 5-FU, an almost complete conversion within 24 hr was measured in vitro, even when input concentrations of up to 1 mM 5-FC were used (our unpublished results). Thus, we conclude that our 5-FC prodrug convertase SCD [which is identical with the FCU1 suicide gene used in (Erbs et al., 2000)] is strong enough to produce sufficiently high enough levels of 5-FU in tumor cells, at least under our in vitro conditions. Interestingly, in the context of a vaccinia virus (MVA)-based expression of SCD/FCU1, a maximum concentration of 5.5 ng 5-FU/mg of tumor tissue has been observed 3–8 days post infection in xenograft human colon carcinoma tumors at 1 hr after daily single oral dosing of 100 mg 5-FC/kg body weight (Erbs et al., 2008). This tumor-based concentration of 5-FU was calculated as being 20 times higher as compared with the 5-FU concentration being achieved when applying 5-FU systemically under the conditions of standard chemotherapy regimes (Erbs et al., 2008). Thus, there is strong evidence that SCD/FCU1 constitutes a highly effective system for the intratumoral conversion of 5-FC into 5-FU under both in vitro and in vivo conditions.
There also might be a basal inability of 5-FU to kill tumor cells of human CC origin. However, when we directly incubated our selected human CC cell lines with 5-FU, concentrations as low as 0.1–0.01 mM 5-FU (Fig. 3A) were found to reduce the CC tumor cell mass in all three cell lines to 50% of the control. Furthermore, an almost complete cell killing was achieved at a concentration of 1 mM 5-FU (Fig. 3A). These results were found to be quite comparable with the results that we achieve in human hepatoma cell lines (Hep3B, HepG2, and PLC/PRF/5), being also of primary liver origin (our unpublished results). Thus, we conclude that 5-FU is functionally active in human CCs.
-
Timing of 5-FC prodrug application might have been suboptimal under our current regime, starting with the 5-FC application “only” when 5 days of consecutive vector application had been fully accomplished. This procedure was inferred from the postulate that early prodrug administration potentially leads to an early abrogation of virus replication (Wildner et al., 1999; Seo et al., 2005; Ungerechts et al., 2007a, 2010). Notably, this is in discrepancy to our recent results of testing HuCCT1 tumor cells under our in vitro conditions where we found that an early administration of 5-FC (i.e., already at 3 hpi) yields much better results in terms of oncolytic efficiency than any later administration (i.e., at 24–72 hpi) (our unpublished results). This finding is supported by the results of a recent systematic dissection of which specific timing of prodrug addition would be optimal in a human xenograft setting when making use of a vector-encoded suicide gene function by systemic application of a respective prodrug: when using a replication-conditional HSV-1-based virotherapeutic vector expressing yeast cytosine deaminase, it was observed that earlier 5-FC administration led to a greater tumor cell killing than any later 5-FC administration both in vitro and in vivo (Yamada et al., 2012).
The optimal time for prodrug administration might be when virus replication is at its peak. However, pinpointing down the exact time point for peak virus replication in each and every tumor patient would require vector-encoded tracking tools [e.g., soluble marker proteins such as carcinoembryonic antigen (Peng et al., 2002) or membrane channel proteins like sodium iodide symporter (Dingli et al., 2004)], which would enable the repetitive monitoring of virus replication and gene expression in a noninvasive manner. So far, the tools for arming and tracking of viruses have not been combined.
Another aspect is that long-term MeV replication in tumors has been observed before [our own unpublished data, and Peng et al. (2002)]. In these animals, repeated administration of the prodrug might be beneficial, whereas in other animals exhibiting continuous tumor growth with no signs of ongoing viral replication, additional cycles of virus plus optimized administration of the respective prodrug might be required to cause late-stage tumor regressions. Thus, by generation of novel virotherapeutics which combine tools for arming and tracking, individual decisions for repeated administrations of a respective prodrug or a respective oncolytic virus together with a respective prodrug would be feasible, leading to an individualized treatment schedule possibly achieving optimized results. It is of interest that repeated cycling has already been applied successfully for MeV in the treatment of xenograft lymphomas before (Ungerechts et al., 2010).
-
Also, dosing of 5-FC might have been suboptimal under our current regime applying a 5-FC standard dose of 500 mg/kg body weight, which is quite often used in mouse xenograft tumor models (You et al., 2009; Deng et al., 2011). However, in a recent preclinical study (Erbs et al., 2008) animal groups treated with adenovirus-based vectors (Ad-FCU1) exhibited statistically significant differences in tumor sizes between the group treated with Ad-FCU1 plus 5-FC and the other groups only when 5-FC was given at 1,000 mg/kg body weight per day. Explicitly, at 500 and 200 mg/kg body weight per day, no signs of oncolytic effectiveness were observed.
Therefore, one other explanation for our failure to find an in vivo enhancement of the well-proven in vitro basic oncolytic effect of our prototypic MeV-based virotherapeutic MeV ld-SCD in the HuCCT1 xenograft mouse model when using the SCD suicide gene function by systemic application of the prodrug 5-FC could be an underdosing of the 5-FC prodrug: 1,000 mg/kg body weight per day potentially could do much better than 500 mg/kg body weight per day if this does not go along with a 5-FC based systemic toxicity, which in prior work already has been described for this dosing (Sia et al., 2012).
To our knowledge, this is the first study investigating the oncolytic effect of MeV in CC. As a result, we could demonstrate that CC represents a viable target for measles vaccine virotherapy. Furthermore, our findings suggest that even partial resistance to MeV-mediated oncolysis can be overcome by using an improved, suicide gene-armed vector combined with the corresponding prodrug. We translated the results obtained in vitro into a tumor xenograft animal experiment and showed that treatment with the SCD-armed MeV vectors leads to a significant decrease in tumor size and a survival benefit compared with the control groups; however, addition of the prodrug 5-FC did not achieve further benefits under the chosen conditions of timing of 5-FC application and 5-FC dosing (500 mg/kg body weight).
In the context of future clinical studies on CC and also other primary hepatic tumors such as hepatocellular carcinoma, it is of great importance that we recently could show that direct intrahepatic injections of MeV ld-SCD in conjunction with a systemic 5-FC prodrug administration are safe in both transgenic mice and rhesus macaques (Völker et al., 2013). Moreover, clinical studies being performed at the Mayo Clinic (Rochester, MN) have shown that intravenous, intraperitoneal, and intracerebral injections of MeV-based virotherapeutics are well tolerated (Galanis et al., 2010). On basis of our encouraging preclinical safety and efficiency data on employment of SCD-armed MeV for the virotherapeutic treatment of chemotherapy-resistant CC, a clinical virotherapy trial is set up currently.
Supplementary Material
Acknowledgments
We are grateful to I. Smirnow and A. Schenk for their excellent technical support and to Dr. S. Berchtold and C. Yurttas for providing additional data during the revision process.
This work was supported in part by grants from the Bundesministerium für Bildung und Forschung (German Federal Ministry of Education and Research, Grant 01GU0503, to U.M.L.) and by the Deutsche Forschungsgemeinschaft (German Research Foundation, SFB 773-Project C3, to U.M.L. and SFB 773-Integrated Graduate School, to S.L.).
Author Disclosure Statement
No competing financial interests exist.
References
- Akamatsu N. Sugawara Y. Hashimoto D. Surgical strategy for bile duct cancer: advances and current limitations. World J. Clin. Oncol. 2011;2:94–107. doi: 10.5306/wjco.v2.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.D. Nakamura T. Russell S.J. Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer. Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Blechacz B.R.A. Gores G.J. Cholangiocarcinoma. Clin. Liver Dis. 2008;12:131–150. doi: 10.1016/j.cld.2007.11.003. ix. [DOI] [PubMed] [Google Scholar]
- Bossow S. Grossardt C. Temme A., et al. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther. 2011;18:598–608. doi: 10.1038/cgt.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz U.J. Finke S. Conzelmann K.K. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 1999;73:251–259. doi: 10.1128/jvi.73.1.251-259.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Rebmann G. Schmid A., et al. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Miest T. Shashkova E.V. Barry M.A. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L.-Y. Wang J.-P. Gui Z.-F. Shen L.-Z. Antitumor activity of mutant bacterial cytosine deaminase gene for colon cancer. World J. Gastroenterol. 2011;17:2958–2964. doi: 10.3748/wjg.v17.i24.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D. Peng K.W. Harvey M.E., et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Enjoji M. Sakai H. Nawata , et al. Sarcomatous and adenocarcinoma cell lines from the same nodule of cholangiocarcinoma. In Vitro Cell Dev. Biol. Anim. 1997;33:681–683. doi: 10.1007/s11626-997-0125-z. [DOI] [PubMed] [Google Scholar]
- Erbs P. Regulier E. Kintz J., et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- Erbs P. Findeli A. Kintz J., et al. Modified vaccinia virus Ankara as a vector for suicide gene therapy. Cancer Gene Ther. 2008;15:18–28. doi: 10.1038/sj.cgt.7701098. [DOI] [PubMed] [Google Scholar]
- Galanis E. Hartmann L.C. Cliby W.A., et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graepler F. Lemken M.-L. Wybranietz W.A., et al. Bifunctional chimeric SuperCD suicide gene—YCD: YUPRT fusion is highly effective in a rat hepatoma model. World J. Gastroenterol. 2005;11:6910–6919. doi: 10.3748/wjg.v11.i44.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.E. Pan C.-H. Moss W.J. Measles vaccines. Front. Biosci. 2008;13:1352–1370. doi: 10.2741/2767. [DOI] [PubMed] [Google Scholar]
- Jarnagin W.R. Zager J.S. Hezel M., et al. Treatment of cholangiocarcinoma with oncolytic herpes simplex virus combined with external beam radiation therapy. Cancer Gene Ther. 2006;13:326–334. doi: 10.1038/sj.cgt.7700890. [DOI] [PubMed] [Google Scholar]
- Jemal A. Bray F. Center M.M., et al. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kärber G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931;162:480–483. [Google Scholar]
- Kojima Y. Honda K. Hamada H. Kobayashi N. Oncolytic gene therapy combined with double suicide genes for human bile duct cancer in nude mouse models. J. Surg. Res. 2009;157:e63–e70. doi: 10.1016/j.jss.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Lech P.J. Russell S.J. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev. Vaccines. 2010;9:1275–1302. doi: 10.1586/erv.10.124. [DOI] [PubMed] [Google Scholar]
- Liu T.-C. Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- Longley D.B. Harkin D.P. Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- Miyagiwa M. Ichida T. Tokiwa T., et al. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev. Biol. 1989;25:503–510. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- Mosconi S. Beretta G.D. Labianca R., et al. Cholangiocarcinoma. Crit. Rev. Oncol. Hematol. 2009;69:259–270. doi: 10.1016/j.critrevonc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Msaouel P. Dispenzieri A. Galanis E. Clinical testing of engineered oncolytic measles virus strains in the treatment of cancer: an overview. Curr. Opin. Mol. Ther. 2009;11:43–53. [PMC free article] [PubMed] [Google Scholar]
- Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- Pederson L.C. Buchsbaum D.J. Vickers S.M., et al. Molecular chemotherapy combined with radiation therapy enhances killing of cholangiocarcinoma cells in vitro and in vivo. Cancer Res. 1997;57:4325–4332. [PubMed] [Google Scholar]
- Peng K.-W. Facteau S. Wegman T., et al. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Russell S.J. Peng K.-W. Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijyo S. Kudo T. Suzuki M., et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J. Exp. Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- Schwarz A.J. Preliminary tests of a highly attenuated measles vaccine. Am. J. Dis. Child. 1962;103:386–389. doi: 10.1001/archpedi.1962.02080020398042. [DOI] [PubMed] [Google Scholar]
- Seo E. Abei M. Wakayama M., et al. Effective gene therapy of biliary tract cancers by a conditionally replicative adenovirus expressing uracil phosphoribosyltransferase: significance of timing of 5-fluorouracil administration. Cancer Res. 2005;65:546–552. [PubMed] [Google Scholar]
- Sia K.C. Huynh H. Chinnasamy N., et al. Suicidal gene therapy in the effective control of primary human hepatocellular carcinoma as monitored by noninvasive bioimaging. Gene Ther. 2012;19:532–542. doi: 10.1038/gt.2011.131. [DOI] [PubMed] [Google Scholar]
- Sioka C. Kyritsis A.P. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother. Pharmacol. 2009;63:761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- Skehan P. Storeng R. Scudiero D., et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Skipworth J.R.A. Olde Damink S.W.M. Imber C., et al. Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol. Ther. 2011;34:1063–1078. doi: 10.1111/j.1365-2036.2011.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackhouse M.A. Pederson L.C. Grizzle W.E., et al. Fractionated radiation therapy in combination with adenoviral delivery of the cytosine deaminase gene and 5-fluorocytosine enhances cytotoxic and antitumor effects in human colorectal and cholangiocarcinoma models. Gene Ther. 2000;7:1019–1026. doi: 10.1038/sj.gt.3301196. [DOI] [PubMed] [Google Scholar]
- Tomayko M.M. Reynolds C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- Ungerechts G. Frenzke M.E. Yaiw K.-C., et al. Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Ther. 2010;17:1506–1516. doi: 10.1038/gt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerechts G. Springfeld C. Frenzke M.E., et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007a;67:10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- Ungerechts G. Springfeld C. Frenzke M.E., et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol. Ther. 2007b;15:1991–1997. doi: 10.1038/sj.mt.6300291. [DOI] [PubMed] [Google Scholar]
- Völker I. Bach P. Coulibaly C., et al. Intrahepatic application of suicide gene-armed measles virotherapeutics: a safety study in transgenic mice and rhesus macaques. Hum. Gene Ther. Clin. Dev. 2013;24:11–22. doi: 10.1089/humc.2012.242. [DOI] [PubMed] [Google Scholar]
- Wildner O. Morris J.C. Vahanian N.N., et al. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999;6:57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- Yamada S. Kuroda T. Fuchs B.C., et al. Oncolytic herpes simplex virus expressing yeast cytosine deaminase: relationship between viral replication, transgene expression, prodrug bioactivation. Cancer Gene Ther. 2012;19:160–170. doi: 10.1038/cgt.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M.-H. Kim W.-J. Shim W., et al. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J. Gastroenterol. Hepatol. 2009;24:1393–1400. doi: 10.1111/j.1440-1746.2009.05862.x. [DOI] [PubMed] [Google Scholar]
- Zaoui K. Bossow S. Grossardt C., et al. Chemovirotherapy for head and neck squamous cell carcinoma with EGFR-targeted and CD/UPRT-armed oncolytic measles virus. Cancer Gene Ther. 2012;19:181–191. doi: 10.1038/cgt.2011.75. [DOI] [PubMed] [Google Scholar]
- Zhu Z.B. Chen Y. Makhija S.K., et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int. J. Oncol. 2006;29:1319–1329. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.