Abstract
Cisplatin causes infertility due to ovarian toxicity. The toxicity mechanism is unknown, but evidence suggests oxidative stress. In this study, the effect of mirtazapine on cisplatin-induced infertility and oxidative stress in rats was investigated. 64 female rats were divided into 4 groups of 16. Except for the controls that received physiologic saline only, all were administered with cisplatin (5 mg/kg i.p.) and mirtazapine (15 mg/kg p.o.) or mirtazapine (30 mg/kg p.o.) for 10 days. After this period, six rats from each group were randomly selected, and malondialdehyde (MDA), myeloperoxidase (MPO), nitric oxide (NO), total gluthatione (tGSH), gluthatione peroxidase (GPx), superoxide dismutase (SOD), and 8-hydroxy-2 deoxyguanine (8-OH Gua) levels were measured in their ovarian tissues. Reproductive functions of the remaining rats were examined for 6 months. The MDA, MPO, NO groups and 8-OH Gua levels were higher in the cisplatin-treated groups than the controls, which was not observed in the mirtazapine and cisplatin groups. GSH, GPx, and SOD levels were reduced by cisplatin, which was prevented by mirtazapine. Cisplatin caused infertility by 70%. The infertility rates were, respectively, 40% and 10% for the 15 and 30 mg/kg mirtazapine administered groups. In conclusion, oxidative stress induced by cisplatin in the rat ovary tissue causes infertility in the female rats. Mirtazapine reverses this in a dose-dependent manner.
1. Introduction
Cisplatin, a platinum derivative, is a chemotherapeutic agent used for the treatment of solid tumors [1]. Cisplatin is asserted as an intermediately gonadotoxic agent [2]. Moreover, it has been demonstrated that cisplatin-associated infertility is caused by the toxic effect on the primordial follicles. Since the primordial follicles are not able to regenerate, the damage caused by the exposure to toxic agents may lead to ovarian insufficiency and infertility [3]. The severe adverse effects occurring during cancer chemotherapy restrict the appropriate use of anticancer drugs [4]. The anti-cancer drugs, particularly those used in early childhood and in reproductive period, may cause several complications such as ovarian insufficiency and infertility [5]. Therefore, in recent years, trials have been initiated on several methods to prevent infertility in patients given chemotherapy [6]. The mechanism of action of cisplatin toxicity on ovaries has not been explained thoroughly. However, it is thought that increased production of free oxygen radicals and decreased production of antioxidants have an impact on the occurrence of cisplatin toxicity [7, 8]. It has been claimed that organ damage related to free radicals occurs as a consequence of disrupted antioxidant defense mechanisms. Moreover, it was reported that the toxicity caused by cisplatin in the tissues was closely related to the increased lipid peroxidation [9, 10]. This literature knowledge suggests that antioxidant treatment might be helpful to prevent cisplatin-related ovarian toxicity and therefore infertility due to this toxicity. Mirtazapine that we tested in this trial is an antidepressant drug used in the treatment of major depression. In recent years, researchers have begun to conduct studies about the antioxidant activity of mirtazapine along with its antidepressant effect. It was reported that it could be used as a cell protector because of its inhibitor effects particularly on the antioxidant parameters [11, 12]. In literature research, we found no information about preventing infertility with mirtazapine in rats with oxidative ovarian damage due to cisplatin administration. Thus, the purpose of this study was to demonstrate whether mirtazapine would be efficacious for preventing infertility occurred in rats with oxidative ovarian damage due to cisplatin administration, and to define the association of oxidative stress in ovarian tissues with infertility.
2. Materials and Method
2.1. Animals
For the experiment, a total of 96 albino Wistar female rats weighing between 165-170 g were used. The rats were provided by the Medical Experimental Practice and Research Center of Ataturk University. The animals were kept in room temperature (22°C) in groups and were fed ad libitum. Ataturk University Local Ethical Committee of Experimental Animals approved that all the steps of this study were compliant with ethical rules.
2.2. Chemical Substances
The thiopental sodium used in the experiment was provided by Ibrahim Etem Ulagay (Istanbul, Turkey); cisplatin and mirtazapine were provided by Organon Pharmaceuticals (New Jersey, USA).
The rats used in this study were divided into four groups—cisplatin group (Cis), 15 mg/kg of mirtazapine + cisplatin group (Mirt-15), 30 mg/kg of mirtazapine + cisplatin group (Mirt-30), and control group (C). The rats in Cis group (n = 16) were injected with cisplatin at a dosage of 5 mg/kg intraperitoneally (i.p.). The rats in Mirt-15 group (n = 16) and those in Mirt-30 group (n = 16) were given mirtazapine orally at dosages of 15 mg/kg and 30 mg/kg, respectively. Subsequently, one hour later, cisplatin was administered intraperitoneally 5 mg/kg. The rats in group C (n = 16) were given distilled water orally in equal volumes. This procedure was applied throughout 10 days. At the end of the period, six rats from each group were randomly selected and sacrificed using high-dose anesthetic (50 mg/kg of thiopental i.p.). Their ovaries were removed, and the levels of malondialdehyde (MDA), myeloperoxidase (MPO), nitric oxide (NO), total gluthatione (tGSH), gluthatione peroxidase (GPx), superoxide dismutase (SOD), and 8-hydroxy-2 deoxyguanine (8-OH Gua), were measured.
Other animals were housed in an appropriate environment for reproduction. Reproductive functions of the remaining rats (n = 10 of each group) were examined for 6 months. Rats not getting pregnant and not giving birth during this period were accepted as infertile.
2.3. Biochemical Analysis of Ovarian Tissue
Whole ovarian tissue was weighed and homogenized on ice with 2-mL relevant buffer. The buffers were 0.5% hexadecyltrimethyl ammonium bromide (pH 6), potassiumphosphate buffer formyeloperoxidase analysis, 1.15% potassium chloride solution for malondialdehyde analysis, and pH 7.5 phosphate buffer for the other analyses. Then, they were centrifuged at 4°C, 10,000 rpm for 15 min. The supernatant was used for analysis.
2.4. MDA Analysis
The concentrations of ovarian mucosal lipid peroxidation were determined by estimating MDA using the thiobarbituric acid test [13]. 0.5 mL homogenate was added to a solution containing 0.2 mL of 80 g/L sodium lauryl sulfate, 1.5 mL of 200 g/L acetic acid, 1.5 mL of 8 g/L of 2-thiobarbiturate, and 0.3 mL of distilled water. The mixture was incubated at 98°C for 1 h. Upon cooling, 5 mL of n-butanol : pyridine (15 : l) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4000 rpm. The absorbance of the supernatant was measured at 532 nm. The standard curve was obtained by using 1,1,3,3-tetramethoxypropane.
2.5. MPO Analysis
The activity of MPO in the total homogenate was measured according to the method of Wei and Frenkel with some modifications [14]. The supernatant was used to determine MPO activity using 1,3 mL 4-aminoantipyrine-2% phenol (25 mM) solution. 25 mM 4-aminoantipyrine-2% phenol solution and 0.0005% 1.5 mL H2O2 were added and equilibrated for 3-4 min. After establishing the basal rate, a 0.2 mL sample suspension was added and quickly mixed. Increases in absorbance at 510 nm for 4 min at 0.1 min intervals were recorded.
2.6. NO Analysis
Nitric oxide levels were measured by the Griess reaction [15, 16]. Nitric oxide measurement is difficult because of its brief half life. Therefore, nitrate and nitrite levels, which are stable end products of nitric oxide metabolism, were used. 100 μL Griess reagent and 100 μL of metaphosphoric acid were added to the supernatant, and a deep purple azo compound occurred. The Griess reagent consists of 0.5 g sulfanilamide, 12.5 g phosphoric acid, and 0.05 g N-(1-napthyl)-ethylenediamine in 500 mL distilled water. Absorbance of the deep purple azo compound was measured at 540 nm wave length by photometric measurement. This azo chromophore accurately determines nitrite concentration as a marker of NO.
2.7. tGSH Analysis
The amount of GSH in the total homogenate was measured according to the method of Sedlak and Lindsay with some modifications [17]. 1500 μL of measurement buffer (200 mM Tris-HCl buffer containing 0.2 mM EDTA at pH 7.5), 500 μL of supernatant, 100 μL of 5,5-dithiobis (2-nitrobenzoic acid) (10 mM), and 7900 μL of methanol were added to a tube and vortexed and incubated for 30 min in 37°C. The absorbance was measured at 412 nm using a spectrophotometer. The standard curve was obtained by using reduced glutathione.
2.8. GPx Analysis
GPx activity was determined according to the method of Lawrence and Burk [18]. The absorbance at 340 nm was recorded for 5 minutes.
2.9. SOD Analysis
SOD activity was determined according to the method of Sun et al. [19]. 2450 µL of measurement mixture (0.3 mM xanthine, 0.6 mM EDTA, 150 μM nitroblue tetrazolium (NBT), 0.4 M Na2CO3, and 1 g/L bovine serum albumin), 500 μL supernatant, and 50 μL xanthine oxidase (167 U/L) was vortexed. Then it was incubated for 10 min. At the end of the reaction, formazan occurs. Absorbance of the purple-colored formazan was measured at 560 nm.
2.10. Measurement of 8-OH Gua by High-Performance Liquid Chromatography (HPLC)
At first, the DNA was isolated from ovarian tissue for the measurement of 8-OH Gua, using the modified method of Shigenaga et al. [20]. Approximately, 50 mg of DNA was hydrolyzed with 0.5 mL of formic acid (60%, v/v) for 45 min at 150°C [21]. Formic acid was removed by freeze-drying. Before analysis by HPLC, DNA samples were redissolved in the eluent (final volume, 200 μL).
The amount of 8-OH Gua and guanine (Gua) was measured by using an HPLC system equipped with an electrochemical detector (HP Agilent 1100 module series, E.C.D. HP 1049 A), as described previously [21, 22]. The amount of 8-OH Gua and Gua was analyzed on a 250 mm × 4.6 mm Supelco LC-18-S reverse-phase column. The mobile phase was 50 mM potassium phosphate, pH 5.5, with acetonitrile (97-volume acetonitrile and 3-volume potassium phosphate), and the flow rate was 1.0 mL/min. The detector potential was set at −0.80 V for measuring the oxidized base. Gua and 8-OH Gua (25 pmol) were used as standards. The 8-OH Gua levels were expressed as the number of 8-OH Gua molecules/105 Gua molecules [23].
2.11. Statistical Analysis
All data were subjected to one-way analysis of variance (ANOVA) using statistical package for the social sciences (SPSS) version 15.0 software. The differences among groups were analyzed using the least significant difference (LSD) option and a P value < 0.05 was considered to be statistically significant. The results are presented as the mean ± standard error of the mean (SEM).
3. Results
All oxidant and antioxidant parameters, measured in the ovarian tissue of the rat groups, are shown in Table 1. The amounts of MDA found in the ovarian tissue of the Cis group rats were higher than the C group (P < 0.001). The MDA levels of the Mirt-15 and the Mirt-30 groups were found to be less than the Cis group (resp., P < 0.05, P < 0.01) (Figure 1). MPO, NO, and 8-OH Gua levels in the ovarian tissues of the rats in the Cis group were found to be the highest (P < 0.001 compared with the C group). MPO, NO, and 8-OH Gua levels of the Mirt-15 and the Mirt-30 groups were found to be lower than the Cis group (P < 0.001) (Figures 2, 3, and 4). The Cis group had the lowest amount of tGSH, one of the nonenzymatic antioxidant parameters, in the groups (P < 0.001 compared with the C group). The amount of tGSH in the Mirt-15 and the Mirt-30 groups was found to be higher than the Cis group (resp., P < 0.05, P < 0.001) (Figure 5). GRx activities, which is an enzymatic antioxidant parameter, measured in the Cis group were lower than in the C group (P < 0.001). GRx activity of the Mirt-30 group was higher than in the Cis group (P < 0.001) (Figure 6). The activities of SOD, another enzymatic antioxidant parameter, were measured lower than those in the C group (P < 0.001). The activities of SOD in the Mirt-15 and the Mirt-30 groups were measured higher than in the Cis group (P < 0.001) (Figure 7).
Table 1.
Oxidative stress and DNA damage parameters in the study groups.
| Groups | MDA | MPO | tGSH | GRx | SOD | NO | 8-OH Gua |
|---|---|---|---|---|---|---|---|
| Cis | 18.33 ± 3.04 | 23.17 ± 1.49 | 6.50 ± 0.76 | 9.83 ± 1.14 | 11.50 ± 0.76 | 20.67 ± 1.99 | 2.60 ± 0.16 |
| Mirt-15 | 10.83 ± 1.62 | 12.17 ± 1.2 | 10.83 ± 0.95 | 15.00 ± 2.08 | 19.33 ± 0.88 | 11.33 ± 1.16 | 1.53 ± 0.14 |
| Mirt-30 | 7.67 ± 0.88 | 8.00 ± 0.97 | 16.50 ± 1.06 | 22.50 ± 1.38 | 29.33 ± 1.54 | 7.00 ± 0.97 | 1.21 ± 0.11 |
| C | 5.67 ± 0.88 | 5.83 ± 0.60 | 20.50 ± 0.76 | 26.50 ± 1.18 | 35.17 ± 1.18 | 3.50 ± 0.67 | 1.02 ± 0.07 |
Figure 1.
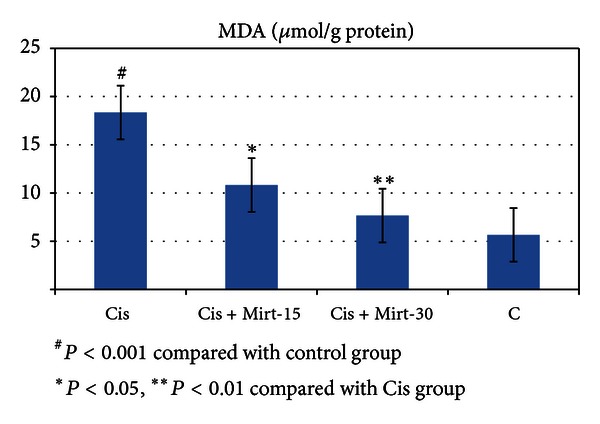
MDA levels of study groups.
Figure 2.
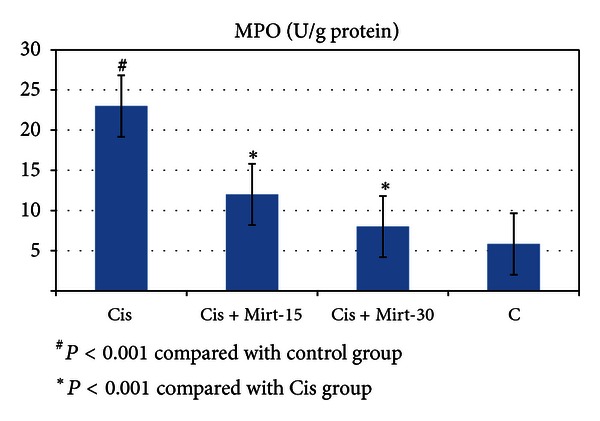
MPO activity of study groups.
Figure 3.
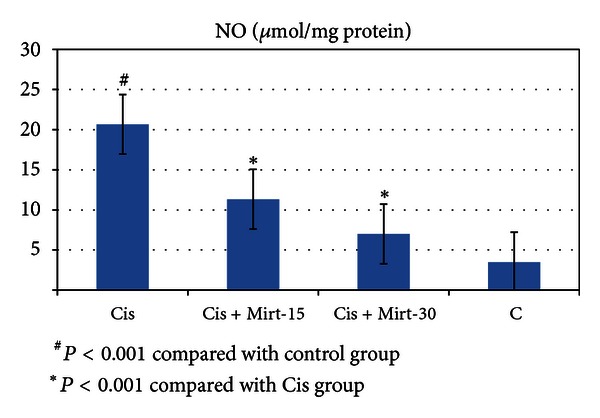
NO levels of study groups.
Figure 4.
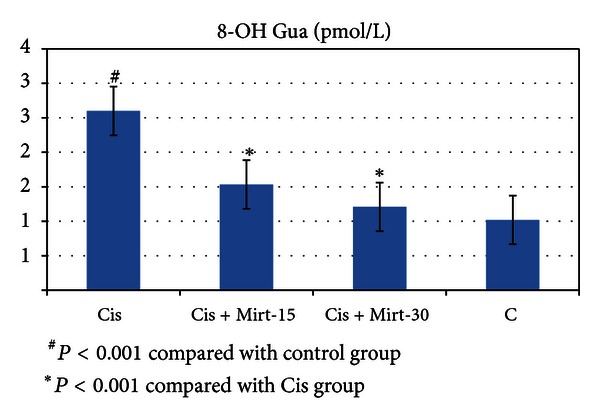
8-OH Gua levels of study groups.
Figure 5.
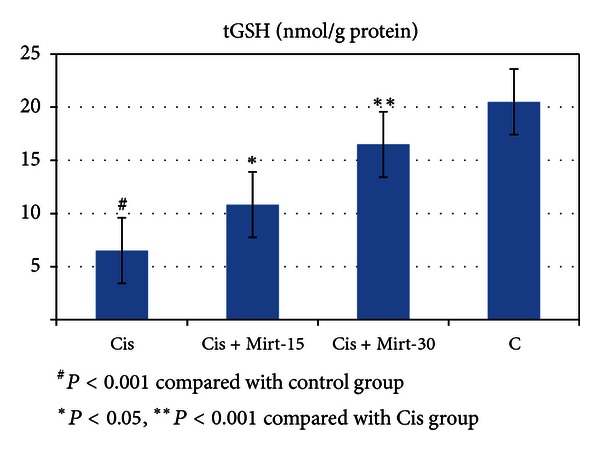
tGSH levels of study groups.
Figure 6.
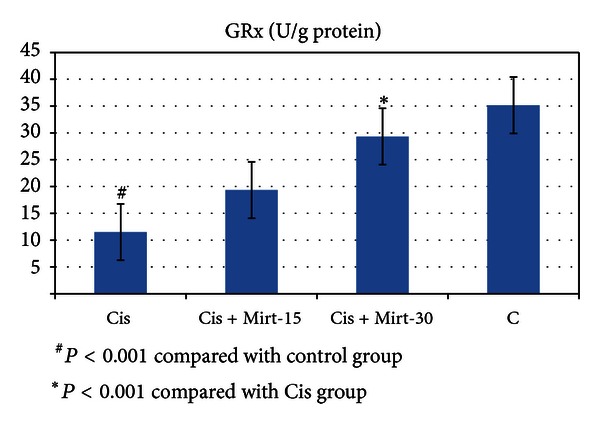
MPO activity of study groups.
Figure 7.
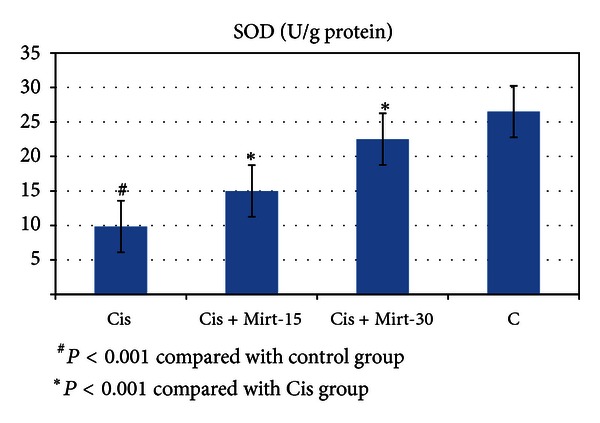
SOD activity of study groups.
As shown in Table 2, Cisplatin caused infertility by 70% in the Cis group. The infertility rates decreased by 40% and 10% for the Mirt-15 and Mirt-30 groups, respectively.
Table 2.
Reproduction and infertility rate in the study groups.
| Groups | Animals taken for reproduction | Animals giving birth | Infertile animals | ||
|---|---|---|---|---|---|
| n | n | % | n | % | |
| Cis | 10 | 3 | 30 | 7 | 70 |
| Mirt-15 | 10 | 6 | 60 | 4 | 60 |
| Mirt-30 | 10 | 9 | 90 | 1 | 10 |
| C | 10 | 10 | 100 | 0 | 0 |
4. Discussion
In this study, it was researched whether mirtazapine was efficacious to prevent infertility occurring in rats with cisplatin-induced oxidative ovarian damage. The data obtained in the study demonstrated that cisplatin caused significant oxidative stress in the ovarian tissues of rats. Moreover, in the Cis group, where oxidative stress was significant, the rate of infertility was significantly higher in comparison with the other groups. Cisplatin is used in the treatment of many solid tumors, mainly testicular and ovarian tumors. However, it was reported that cisplatin caused severe adverse effects such as nephrotoxicity, neurotoxicity, gastric toxicity, and infertility [24]. It was reported that chemotherapeutic medicines leading to either temporary or permanent infertility severely affected the ovaries and hormonal balance [25]. The deterioration of the oxidants/antioxidants balance in the tissues in favour of the oxidants is considered as oxidative stress. As the results of our study demonstrated, in ovarian tissues of animals administered cisplatin, there was an increase in the levels of MDA and MPO, which are oxidant parameters, while the levels of antioxidants such as tGSH, GPx, and SOD were decreased. MDA is the end-product of lipid peroxidation. Lipid peroxidation is known to be the most harmful effect of free radicals in the cell [26]. It was reported that cisplatin caused oxidative damage in the ovarian tissue increasing MDA concentration and decreasing GSH concentration [27]. Furthermore, in oxidative ovarian damage occurring due to ischemia reperfusion, both MDA and MPO levels were found to be significantly increased in comparison with the healthy ovarian tissue [28]. In our study, for ovarian tissues of the Cis group rats in which MDA was found to be increased, MPO activity was also increased. MPO, which is known to play important roles in tissue damage, is reduced to hypochlorous acid in the presence of hydrogen peroxide and chloride anions. The hypochlorous acid is a powerful oxidant and causes damage to vascular endothelium [29]. There was no previous study about that effect of mirtazapine over MPO activity in ovarian tissue. However, in the kidney tissue of rats given cisplatin, a significant increase in MPO activity was noticed [30]. This knowledge in the literature supports the results of our study. In the ovaries of the rats given cisplatin, GSH and GPx levels were decreased in comparison with the C group. GSH is a nonenzymatic endogenous antioxidant parameter, but GPx is an enzymatic endogenous antioxidant. Under physiological conditions, the oxidant/antioxidant balance is maintained with predominance of antioxidants. The disruption of this equilibrium causes tissue damage named oxidative stress. Therefore, oxidant/antioxidant balance is used to assess if tissue damage emerges [31]. GSH, an endogenous antioxidant, protects the cells against oxidative damage, keeping the –SH groups of proteins reduced and preventing them from reacting with free radicals [32]. GPx reduces oxidized glutathione (GSSG) by transferring one electron from NADPH to the disulfide bonds of GSSG [33]. SOD, another protective enzyme against free oxygen radicals, catalyzes the transformation of superoxide molecule into hydrogen peroxide and molecular oxygen [34]. Low SOD activity found in ovarian tissues of rats given cisplatin indicates that the oxidative stress has occurred. In ovarian tissues of rats given cisplatin, the concentration of 8-OH Gua was found to be significantly higher in comparison with Mirt-15, Mirt-30, and C groups. The 8-OH Gua is an important marker reflecting DNA oxidation and is formed by breaking off the hydrogen from the nucleic acids with the action of toxic oxygen radicals such as hydroxyl radical in injured tissues [35]. Cisplatin creates inchain and interchain cross-linking by interacting with DNA. This cross-link formation inhibits the transcription and replication of DNA. If cisplatin-induced DNA is not repaired, cell death occurs [36]. There are studies demonstrating that 8-OH Gua concentrations significantly increase in damaged ovarian tissue caused by oxidative stress in comparison with healthy tissues [37]. In our study, mirtazapine, which we tested to prevent cisplatin-induced oxidative ovarian damage, dose dependently decreased the concentrations of oxidant parameters and raised the concentrations of antioxidant parameters. Previous studies also reported that mirtazapine prevented cisplatin-induced oxidative damage in the kidney tissue [30].
Furthermore, mirtazapine significantly prevented cisplatin-associated infertility. In several studies conducted, it was found that the incidence of depression was high among cancer patients because of the adverse effects of chemotherapy [38]. The severity of depression and anxiety was found to be higher among infertile women in comparison with HIV-positive women and women with cancer and cardiac disorders. In some studies, it was found that the infertility was associated with anxiety disorder [39–41]. Recent studies demonstrated antioxidant and antiulcer activities of mirtazapine along with its antidepressant and sedative properties [42]. In conclusion, cisplatin leads to oxidative stress in the ovarian tissue of rats. Cisplatin also causes infertility in female rats. Mirtazapine prevented dose-dependent cisplatin-induced oxidative stress in the ovarian tissue and the infertility. It is hypothesized that mirtazapine prevents the infertility through its antioxidant, sedative, and antidepressant activities. The results of our study demonstrate that mirtazapine can be useful for preventing cisplatin-induced infertility.
References
- 1.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney International. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 2.Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstetrics & Gynecology. 2003;101(2):251–257. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 3.Yucebilgin MS, Terek MC, Ozsaran A, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2004;44(1):6–9. doi: 10.1111/j.1479-828X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. Ca-A Cancer Journal for Clinicians. 2003;53(1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Sommezer M, Oktay K. Fertility reservation in female patients. Human Reproduction Update. 2004;10(3):251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 6.Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. Journal of Clinical Oncology. 2005;23(19):4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 7.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Experimental and Toxicologic Pathology. 2009;61(3):223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Weijl NI, Wipkink-Bakker A, Lentjes EGWM, Berger HM, Cleton FJ, Osanto S. Cisplatin combination chemotherapy induces a fall in plasma antioxidants of cancer patients. Annals of Oncology. 1998;9(12):1331–1337. doi: 10.1023/a:1008407014084. [DOI] [PubMed] [Google Scholar]
- 9.Greggi Antunes LM, D’arc J, Bianchi MDLP. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacological Research. 2000;41(4):405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 10.Wozniak K, Czechowska A, Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitor STI571. Chemico-Biological Interactions. 2004;147(3):309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Berger MM, Shenkin A, Revelly JP, et al. Copper, selenium, zinc, and thiamine balances during continuous venovenous hemodiafiltration in critically ill patients. The American Journal of Clinical Nutrition. 2004;80(2):410–416. doi: 10.1093/ajcn/80.2.410. [DOI] [PubMed] [Google Scholar]
- 12.Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol and Alcoholism. 2009;44(2):148–154. doi: 10.1093/alcalc/agn118. [DOI] [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Frenkel K. In vivo formation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Research. 1991;51(16):4443–4449. [PubMed] [Google Scholar]
- 15.Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clinical Chemistry. 1995;41(6):904–907. [PubMed] [Google Scholar]
- 16.Moshage H, Kok B, Huizenga JR, Jansen PLM. Nitrite and nitrate determinations in plasma: a critical evaluation. Clinical Chemistry. 1995;41(6):892–896. [PubMed] [Google Scholar]
- 17.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry. 1968;25(C):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium deficient rat liver. Biochemical and Biophysical Research Communications. 1976;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 20.Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2’-deoxyguanosine and 8- oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods in Enzymology. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- 21.Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochemical Journal. 1996;318(1):21–23. doi: 10.1042/bj3180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radical Research Communications. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- 23.Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8-hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Research. 1996;56(11):2546–2549. [PubMed] [Google Scholar]
- 24.Pogach LM, Lee Y, Gould S, Giglio W, Meyenhofer M, Huang HFS. Characterization of cis-platinum-induced Sertoli cell dysfunction in rodents. Toxicology and Applied Pharmacology. 1989;98(2):350–361. doi: 10.1016/0041-008x(89)90239-1. [DOI] [PubMed] [Google Scholar]
- 25.DeVita VT, Jr., Hellman S, Rosenberg SA. Cancer: Principals and Practice of Oncology. 7th edition. Philadelphia, Pa, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 26.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research. 1998;39(8):1529–1542. [PubMed] [Google Scholar]
- 27.Borekci B, Yapca OE, Turan MI, Gul MA, Isaoglu U. Preventıon of ovarian oxidative stress—related infertility associated with cisplatin in rats with thiamine pyrophosphate. Latin American Journal of Pharmacy. In press. [Google Scholar]
- 28.Isaoglu U, Yilmaz M, Sener E, et al. The impaired balances of oxidant/antioxidant and COX-1/COX-2 in ovarian ischemia-reperfusion injury and prevention by nimesulide. Latin American Journal of Pharmacy. 2012;31(10):1481–1488. [Google Scholar]
- 29.Korthuis RJ, Granger DN. Reactive oxygen metabolites, neutrophils, and the pathogenesis of ischemic-tissue/reperfusion. Clinical Cardiology. 1993;16(4):I19–I26. doi: 10.1002/clc.4960161307. [DOI] [PubMed] [Google Scholar]
- 30.Sener MT, Sener E, Tok A, et al. Biochemical and histologic study of lethal cisplatin nephrotoxicity prevention by mirtazapine. Pharmacological Reports. 2012;64(594):594–602. doi: 10.1016/s1734-1140(12)70855-1. [DOI] [PubMed] [Google Scholar]
- 31.Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Tissue damage and oxidant/antioxidant balance. Euroasian Journal of Medicine. 2013;(45):47–49. doi: 10.5152/eajm.2013.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1-2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 33.Young IS, Woodside JV. Antioxidants in health and disease. Journal of Clinical Pathology. 2001;54(3):176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology and Medicine. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 35.Grollman AP, Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends in Genetics. 1993;9(7):246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- 36.Jordan P, Carmo-Fonseca M. Molecular mechanisms involved in cisplatin cytotoxicity. Cellular and Molecular Life Sciences. 2000;57(8-9):1229–1235. doi: 10.1007/PL00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingec M, Isaoglu U, Yilmaz M, et al. Prevention of ischemia-reperfusion injury in rat ovarian tissue with the on-off method. Journal of Physiology and Pharmacology. 2011;62(5):575–582. [PubMed] [Google Scholar]
- 38.Farrell C, Heaven C, Beaver K, Maguire P. Identifying the concerns of women undergoing chemotherapy. Patient Education and Counseling. 2005;56(1):72–77. doi: 10.1016/j.pec.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Anderson KM, Sharpe M, Rattray A, Irvine DS. Distress and concerns in couples referred to a specialist infertility clinic. Journal of Psychosomatic Research. 2003;54(4):353–355. doi: 10.1016/s0022-3999(02)00398-7. [DOI] [PubMed] [Google Scholar]
- 40.Kainz K. The role of the psychologist in the evaluation and treatment of infertility. Women’s Health Issues. 2001;11(6):481–485. doi: 10.1016/s1049-3867(01)00129-3. [DOI] [PubMed] [Google Scholar]
- 41.King RB. Subfecundity and anxiety in a nationally representative sample. Social Science & Medicine. 2003;56(4):739–751. doi: 10.1016/s0277-9536(02)00069-2. [DOI] [PubMed] [Google Scholar]
- 42.Bilici M, Ozturk C, Dursun H, et al. Protective effect of mirtazapine on indomethacin-induced ulcer in rats and its relationship with oxidant and antioxidant parameters. Digestive Diseases and Sciences. 2009;54(9):1868–1875. doi: 10.1007/s10620-008-0560-z. [DOI] [PubMed] [Google Scholar]


