Abstract
Diabetic neuropathy (DN) is a widespread disabling disorder comprising peripheral nerves' damage. DN develops on a background of hyperglycemia and an entangled metabolic imbalance, mainly oxidative stress. The majority of related pathways like polyol, advanced glycation end products, poly-ADP-ribose polymerase, hexosamine, and protein kinase c all originated from initial oxidative stress. To date, no absolute cure for DN has been defined; although some drugs are conventionally used, much more can be found if all pathophysiological links with oxidative stress would be taken into account. In this paper, although current therapies for DN have been reviewed, we have mainly focused on the links between DN and oxidative stress and therapies on the horizon, such as inhibitors of protein kinase C, aldose reductase, and advanced glycation. With reference to oxidative stress and the related pathways, the following new drugs are under study such as taurine, acetyl-L-carnitine, alpha lipoic acid, protein kinase C inhibitor (ruboxistaurin), aldose reductase inhibitors (fidarestat, epalrestat, ranirestat), advanced glycation end product inhibitors (benfotiamine, aspirin, aminoguanidine), the hexosamine pathway inhibitor (benfotiamine), inhibitor of poly ADP-ribose polymerase (nicotinamide), and angiotensin-converting enzyme inhibitor (trandolapril). The development of modern drugs to treat DN is a real challenge and needs intensive long-term comparative trials.
1. Introduction
A conduction problem arising in peripheral nerves is called peripheral neuropathy. Depending on the cause, the damage may appear in the axons or the myelin sheaths. The involved neurons may be afferent (sensory), efferent (motor), or both. The size of affected axons is an important issue, since sometimes only the small C unmyelinated and the A-delta fibers are affected. If these are damaged, symptoms move forward to pain sensors in the skin and autonomic neurons. Damage to large sensory fibers, which are the A-alpha and A-beta fibers, causes deficits in the proprioception and vibration sensation that results in muscle-stretch reflexes [1].
Diabetic neuropathy (DN), a microvascular complication of diabetes, comprises disorders of peripheral nerve in people with diabetes when other causes are ruled out. Diabetic peripheral neuropathy (DPN) is associated with considerable mortality, morbidity, and diminished quality of life [2]. The prevalence of neuropathy in diabetic patients is about 30%, whereas up to 50% of patients will certainly develop neuropathy during their disease [3]. In fact, against estimated universal prevalence of diabetes of 472 million by 2030, DPN is likely to affect 236 million persons worldwide causing lots of costs [4]. DPN can be broadly divided into generalized polyneuropathies and focal/multifocal varieties [5, 6]. The generalized form can be further classified into typical and atypical in terms of difference in onset, course, associations, clinical manifestations, and pathophysiology. The typical DPN is a chronic, symmetrical length-dependent sensorimotor polyneuropathy (DSPN) and the most common presentation of the peripheral nervous system damage by diabetes [7]. Therefore, considering the widespread of DN, it is vital to investigate details of its pathophysiology and therapeutic strategies. DN develops on a background of hyperglycemia and associated metabolic imbalances mainly oxidative stress. Hyperglycemia-induced overproduction of free radicals has been recognized as the source of further complications. Studies in the recent years have identified major pathways that are linked to DN, such as stimulated polyol, advanced formation of glycation end products, and other cascades of stress responses [8]. Since oxidative stress leads to such a major influence in the development of DN, in this paper we have highlighted the evidence linking DN, oxidative stress, and its consequences.
Despite efforts to make an early diagnosis and to stop the progression of DN, currently very few drugs are available to cure this disease and the others only provide symptomatic relief. Meanwhile, current goal of treatment of DN is to increase the functionality and quality of life and to diminish pain. In the present review, therapies on the horizon based on oxidative stress have been criticized.
2. Methods
Databases of PubMed, Google Scholar, Web of Science, Embase, Scopus, and DARE were searched up to 30 November 2012, for all relevant studies with DN. The search terms were diabetic neuropathy, oxidative stress, mechanisms, and current and new treatments without limiting search elements. All of relevant human (Table 1) and animal (Table 2) studies were included.
Table 1.
Current pharmacotherapy in DN.
| NNT | Study outcome | Treatment duration | Study design | Daily dose (mg) | Trial size | Trial | Drug used | Drug class |
|---|---|---|---|---|---|---|---|---|
| — | Amitriptyline > placebo | 2 × 6 wk | Crossover | Up to 150 mg | 29 | Max [15] | Amitriptyline | Antidepressants: TCAs: |
| 2.1 | Amitriptyline > placebo | 2 × 6 wk | Crossover | ≤150 mg | 29 | Max et al. [16] | Amitriptyline | |
| 2.2 | Amitriptyline = desipramine > placebo | 2 × 6 wk | Crossover | Amitriptyline: 105 mg; desipramine: 111 mg | 38 | Max et al. [17] | Amitriptyline and desipramine | |
| — | Amitriptyline > maprotiline > placebo | 4 wk | Crossover | 75 mg | 37 | Vrethem et al. [18] | Amitriptyline and maprotiline | |
| — | Amitriptyline > placebo | 2 × 6 wk | Crossover | 25–75 mg | 24 | Morello et al. [19] | Amitriptyline | |
| — | Clomipramine > desipramine > placebo | 6 wk | Crossover | Desipramine: 200 mg and clomipramine: 75 mg (in extensive metabolisers). 50 mg of both drugs (in poor metabolisers) | 19 | Sindrup et al. [20] | Desipramine and clomipramine | |
| Desipramine > placebo | 6 wk | Crossover | 201 mg | 20 | Max et al. [21] | Desipramine | ||
| — | Imipramine > placebo | 5 + 5 wk | Crossover | 100 mg | 12 | Kvinesdal et al. [22] | Imipramine | |
| — | Combination > placebo | 8 wk | Crossover | Nortriptyline: 10 mg; fluphenazine: 0.5 mg | 18 | Gomez-Perez et al. [23] | Nortriptyline and fluphenazine | |
|
| ||||||||
| — | Citalopram > placebo | 2 × 3 wk | Crossover | 40 mg | 15 | Sindrup et al. [24] | Citalopram | SSRI: |
|
| ||||||||
| — | Venlafaxine + gabapentin > placebo in patients who do not respond to gabapentin | 2 × 8 wk | Parallel | — | 11 and 42 | Simpson [25] | Venlafaxine and gabapentin | SNRIs |
| 5.2 for venlafaxine and 2.7 for imipramine. |
Venlafaxine > imipramine > placebo | 4 wk | Crossover | Venlafaxine: 225 mg; imipramine: 150 mg | 29 | Sindrup et al. [26] | Venlafaxine versus imipramine | |
|
| ||||||||
| 4.5 | Venlafaxine > placebo | 6 wk | Parallel | 150–225 mg | 244 | Rowbotham et al. [27] |
Venlafaxine | |
| — | Venlafaxine > placebo | 8 wk | Parallel | 75–150 mg | 60 | Kadiroglu et al. [28] | Venlafaxine | |
| 11 (60 mg group); 5 (120 mg group) |
Duloxetine > placebo | 12 wk | Parallel | 60, 120 mg | 348 | Raskin et al. [29] | Duloxetine | |
| 4.3 (60 mg group); 3.8 (120 mg group) |
Duloxetine > placebo | 12 wk | Parallel | 20, 60, 120 mg | 457 | Goldstein et al. [30] | Duloxetine | |
| 6.3 (60 mg group); 3.8 (120 mg group) |
Duloxetine > placebo | 12 wk | Parallel | 60, 120 mg | 334 | Wernicke et al. [31] | Duloxetine | |
| 5.2 and 4.9 (duloxetine 60 mg once daily and 60 mg BID, resp.) |
Duloxetine > placebo | 3 × 12 wk | Parallel | 60 mg | 1024 | Kajdasz et al. [32] | Duloxetine | |
| 3.6 (300 mg group); 3.3 (600 mg group) |
Pregabalin (300, 600 mg) > placebo | 5 wk | Parallel | 75, 300, 600 mg | 338 | Lesser et al. [33] | Pregabalin | |
| 3.9 | Pregabalin > placebo | 8 wk | Parallel | 300 mg | 146 | Rosenstock et al. [34] | Pregabalin | |
| 4.2 (600 mg group) | Pregabalin (600 mg) > placebo | 6 wk | Parallel | 150, 600 mg | 246 | Richter et al. [35] | Pregabalin | |
| 3.6 | Flexible and fixed > placebo | 12 wk | Parallel | Flexible: 150, 300, 450, 600 mg; fixed: 300, 600 mg | 338 | Freynhagen et al. [36] | Pregabalin | |
| 6.3 (600 mg group) | Pregabalin (600 mg) > placebo | 12 wk | Parallel | 150, 300, or 600 mg | 395 | Tölle et al. [37] | Pregabalin | |
| — | Pregabalin > placebo | 13 wk | Parallel | 600 mg | 167 | Arezzo et al. [38] | Pregabalin | |
| 4.04 (600 mg group); 5.99 (300 mg group); 19.06 (150 mg group) | 150, 300, 600 mg TID > placebo; 600 mg BID > placebo |
5 to 13 wk | Parallel | 150, 300, 600 mg administered TID or BID | — | Freeman et al. [39] | Pregabalin | |
| 4 | Gabapentin > placebo | 8 wk | Parallel | Titrated from 900 to 3600 mg | 165 | Backonja et al. [40] | Gabapentin | |
| — | Gabapentin = placebo | 2 × 6 wk | Crossover | 900 mg | 40 | Gorson et al. [41] | Gabapentin | |
| — | Sodium valproate > placebo | 4 wk | Parallel | 600–1200 mg | 52 | Kochar et al. [42] | Sodium valproate | |
| — | Sodium valproate > placebo | 16 wk | Parallel | 500 mg | 39 | Kochar et al. [43] | Sodium valproate | Anticonvulsants: |
| — | Sodium valproate = placebo | 4 wk | Crossover | 1500 mg | 31 | Otto et al. [44] | Sodium valproate | |
| 4 | Lamotrigine > placebo | 6 wk | Parallel | Titrated from 25 to 400 mg | 59 | Eisenberg et al. [45] | Lamotrigine | |
| — | Lamotrigine = placebo | 19 wk | Parallel | 200, 300, 400 mg | 360 | Vinik et al. [46] | Lamotrigine | |
| — | Lamotrigine = amitriptyline | 6 wk | Crossover, | Lamotrigine: 25, 50, 100 mg twice daily; amitriptyline: 10, 25, 50 mg at night time | 53 | Jose et al. [47] | Lamotrigine and amitriptyline | |
| — | Carbamazepine > placebo | 2 wk | Crossover | 200–600 mg | 30 | Rull et al. [48] | Carbamazepine | |
| — | Oxcarbazepine > placebo | 16 wk | Parallel | 300 mg titrated to a maximum dose of 1800 mg | 146 | Dogra et al. 2005 [49] | Oxcarbazepine | |
| 7.9 (1200 groups); 8.3 (1800 groups) |
Oxcarbazepine > placebo (1200, 1800 mg groups) | 16 wk | Parallel | 600, 1200, 1800 mg | 347 | Beydoun et al. [50] | Oxcarbazepine | |
| — | Oxcarbazepine = placebo | 16 wk | Parallel | 1200 mg | 141 | Grosskopf et al. [51] | Oxcarbazepine | |
| — | Lacosamide > placebo | — | Parallel | 400 mg | 94 | Rauck et al. [52] | Lacosamide | |
| — | Lacosamide (400 mg group) > placebo | 18 wk | Parallel | 200, 400, 600 mg | — | Wymer et al. [53] | Lacosamide | |
|
| ||||||||
| 3.1 | Tramadol > placebo | 6 wk | Parallel | 210 mg | 131 | Harati et al. [54] | Tramadol | |
| 4.3 | Tramadol > placebo | 2 × 4 wk | Crossover | 200–400 mg | 45 | Sindrup et al. [55] | Tramadol | |
| — | Tramadol/acetaminophen > placebo | 8 wk | Parallel | Tramadol: 37.5 mg; acetaminophen: 325 mg | 311 | Freeman et al. [56] | Tramadol/ acetaminophen |
|
| — | Oxycodone > placebo | 6 wk | Parallel | 10–100 mg | 159 | Gimbel et al. [57] | Oxycodone | Opioids: |
| 2.6 | Oxycodone > placebo | 4 wk | Crossover | 10–80 mg | 45 | Watson et al. [58] | Oxycodone | |
| — | Oxycodone + gabapentin > placebo + gabapentin | 12 wk | Parallel | Oxycodone: 10–80 mg + gabapentin: 100–3600 mg | 338 | Hanna et al. [59] | Oxycodone | |
| — | Morphine + gabapentin > morphine > gabapentin > placebo | 4 × 4 wk | Crossover | 120, 60 mg morphine + 2400 mg gabapentin, 3600 mg gabapentin | 57 | Gilron et al. [60] | Morphine | |
| — | Capsaicin > vehicle | 8 wk | Parallel | 0.075% capsaicin | 252 | Anonymous et al. [61] | Capsaicin | |
| — | Capsaicin > vehicle | 8 wk | Parallel | 0.075% capsaicin | — | Scheffler et al. [62] | Capsaicin | |
| — | Capsaicin > vehicle | 8 wk | Parallel | 0.075% capsaicin four times a day | 22 | Tandan et al. [63] | Capsaicin | |
| — | Capsaicin > vehicle | 8 wk | Parallel | 0.075% capsaicin four times a day | Anonymous et al. [64] | Capsaicin | Topical medications: | |
| — | Isosorbide > placebo | 2 × 4 wk | Crossover | 30 mg | 22 | Yuen et al. [65] | Isosorbide dinitrate spray | |
| — | Glyceryl > placebo | 2 × 4 wk | Crossover | — | 48 | Agrawal et al. [66] | Glyceryl trinitrate spray | |
| 4.4 | Lidocaine > placebo | 4 wk | Crossover | 5% lidocaine patch | 40 | Meier et al. [67] | Lidocaine patch | |
| — | Lidocaine significantly improved pain and quality of life | 3 wk study with a 5 wk extension | Open label, flexible dosing | 5% lidocaine patch | 56 | Barbano et al. [68] | Lidocaine patch | |
|
| ||||||||
| — | Mexiletine > placebo | 10 wk | Crossover | 10 mg | 16 | Dejgard et al. [69] | Mexiletine |
Anesthetics/ antiarrhythmics: |
| Mexiletine > placebo | 3 wk | Parallel | 675 mg | 216 | Oskarsson et al. [70] | Mexiletine | ||
| — | Mexiletine = placebo | 3 wk | Parallel | 600 mg | 29 | Wright et al. [71] | Mexiletine | |
|
| ||||||||
| 4 | Dextromethorphan > placebo | 2 × 6 wk | Crossover | Mean 381 mg | 14 | Nelson et al. [72] | Dextromethorphan | NMDA antagonists: |
| 3.2 | Dextromethorphan > placebo | 2 × 9 wk | Crossover | 400 mg | 19 | Sang et al. [73] | Dextromethorphan | |
|
| ||||||||
| 3.03 at 12 weeks | Botulinum toxin > placebo | 24 wk | Parallel | Intradermal of subtype A (20–190 units) into the painful area | 29 | Ranoux et al. [74] | Botulinum toxin | Other drugs: |
| — | Botulinum toxin > placebo | 12 × 12 wk | Crossover | 50 units of subtype A in 1.2 mL 0.9% saline given intradermally into each foot, each injection 4 U subtype A | 18 | Yuan et al. [75] | Botulinum toxin | |
| — | Improved pain and nerve fiber regeneration | 2 × 52 wk | Parallel | 500 and 1,000 mg, three times per day | — | Sima et al. [76] | Acetyl-L-carnitine | |
| — | α-lipoic acid = placebo | 28 wk | Parallel | 600 mg | 509 | Ziegler et al. [77] | α-lipoic acid | |
| — | α-lipoic acid ≥ placebo with clinically meaningful degree | 3 wk | Parallel | 600 mg | 1258 | Ziegler et al. [78] | α-lipoic acid | |
NNT: number needed to treat; TCAs: tricyclic antidepressants; SSRI: selective serotonin reuptake inhibitor; SNRIs: serotonin norepinephrine reuptake inhibitors; NMDA: N-methyl-D-aspartate; TID: three times daily; BID: twice daily.
Table 2.
New therapeutic approaches for DN. DRG: dorsal root ganglion neuron.
| Endpoint | Study populations | Compound | Study |
|---|---|---|---|
| Improvement of peripheral nerve function | Diabetic rats | Salvianolic acid A | Yu et al. [79] |
| Improvement of DN | Animal model of T2D | High-fat diet with menhaden oil | Coppey et al. [80] |
| Improvement of DN | Patients with T2D and neuropathy | Tai Chi exercise | Ahn and Song [81] |
| Improvement of DN | T2DM patients | Beraprost sodium | Shin et al. [82] |
| Improvement of DN | STZ-diabetic rats | Anandamide | Schreiber et al. [83] |
| Improvement of peripheral nerve function | Mouse model of DPN | Thymosin β4 | Wang et al. [84] |
| Improvement of chronic pain, including PDN | Rat model of STZ-induced PDN | Gastrodin | Sun et al. [85] |
| Prevention of progression of DN | Patients enrolled in the aldose reductase inhibitor-diabetes complications | Epalrestat | Hotta et al. [86] |
| Improvement of DN | STZ-diabetic rats | Gliclazide with curcumin | Attia et al. [87] |
| Improvement of DN | STZ-diabetic rats | Bone marrow-derived mononuclear cells | Naruse et al. [88] |
| Neuroprotection effect | In vitro model of high glucose-treated DRG neurons in culture | Galanin | Xu et al. [89] |
| Improvement of DN | — | Baicalein | Yorek [90] |
| Improvement of neuropathic pain | Animal models of neuropathic pain | Brazilian armed spider venom toxin Tx3-3 | Dalmolin et al., [91] |
| Neuroprotection effect Improvement of DN |
STZ-diabetic rats | Magnesium-25 carrying porphyrin-fullerene nanoparticles | Hosseini et al. [92, 93] |
| Maintaining health in diabetes | STZ-diabetic rats | Phosphodiesterase inhibitors | Milani et al. [94] |
| Improve transplant outcome and graft function in diabetes | Isolated rat pancreatic islets | IMOD | Larijani et al. [95] |
| Improve islet transplantation in diabetes | Isolated rat pancreatic islets | Cerium and yttrium oxide nanoparticles | Hosseini and Abdollahi [96, 97] |
3. Clinical Features of DN
The most common form of DN is DSPN that accounts for a large proportion of all peripheral nerve manifestations attributed to diabetes, although, some physicians use the terms DSPN and DN interchangeably. Poor control of blood glucose is an important risk factor for the development of DN starting in the toes and gradually progressing proximally. Once it is diagnosed in the lower limbs, it may develop to the upper limbs with sensory loss [4]. For example, a patient with painful sensory neuropathy due to diabetes might first complain of burning or itchy sensations or even pain in the feet that is called paresthesias. The symptoms distribute on a so-called “glove and stocking” manner, as it starts from the longest axons. The unmyelinated nerve endings in the epidermis are degenerated first [2]. Neuropathic pain is the most disabling symptom observed in around one-third of patients with DN and about 20% of all diabetic patients. Painful DN deleteriously influences quality of life, sleep, mood, and the ability to work [4].
4. Pathogenesis of DN: Interaction of Oxidative Stress with Other Physiological Pathways
Although development of DN is multifactorial and the exact pathogenic mechanism is yet to be understood, a number of theories can be described. The current belief is that hyperglycemia, activation of polyol, advanced glycation end products (AGEs), hexosamine, diacylglycerol/protein kinase C (PKC), oxidative stress, nitric oxide, and inflammation all play key roles in DN. Based on evidence, oxidative stress is involved in all the above pathways (see Figure 1). These mechanisms are described one by one in the next sections of this paper, and then a conclusion is made.
Figure 1.
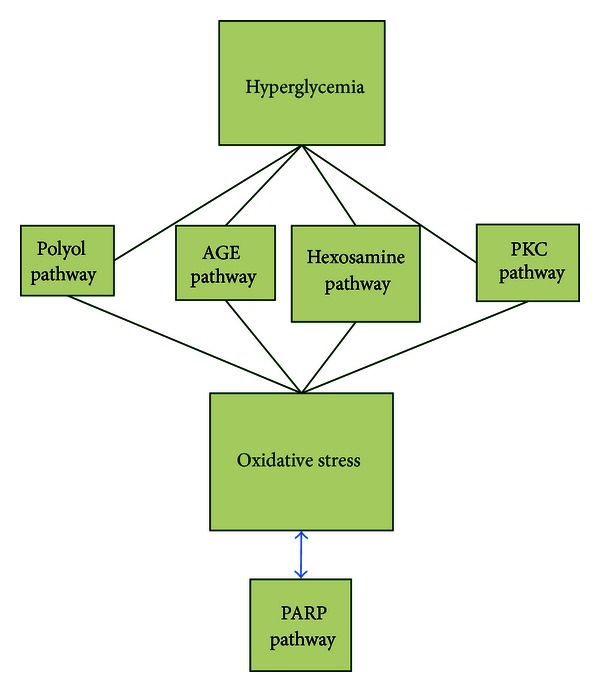
Interaction of oxidative stress with other physiological pathways in DN.
4.1. Hyperglycemia
Excess intracellular glucose is processed by increased flux via one or more glucose metabolism pathways, and thus prolonged hyperglycemia results in progress of chronic complications of diabetes including DN.
4.2. Role of Polyol
Hyperglycemia results in elevated intracellular glucose in nerves, leading to saturation of the normal glycolytic pathway. Extra glucose lightens up the polyol pathway and that produces sorbitol and subsequently fructose by aldose reductase and sorbitol dehydrogenase, respectively. Increased polyol flux causes intracellular hyperosmolarity by an accumulation of impermeable sorbitol and compensatory efflux of other osmolytes such as myoinositol, taurine, and adenosine. In turn, shortage of myoinositol results in exhaustion of phosphatidylinositol and withdraws creation of adenosine triphosphate (ATP). All these processes result in a reduced activity of Na+/K+-ATPase and PKC, impaired axonal transport and structural breakdown of nerves, and finally presents itself as abnormal action potential. Aldose reductase-mediated reduction of glucose to sorbitol is associated with consumption of NADPH, and since NADPH is required for regeneration of reduced glutathione (GSH), this directly contributes to oxidative stress. In addition, formation of fructose from sorbitol promotes glycation, depletes NADPH, and increases AGEs which all result in major redox imbalance (see Figure 2) [9, 10].
Figure 2.
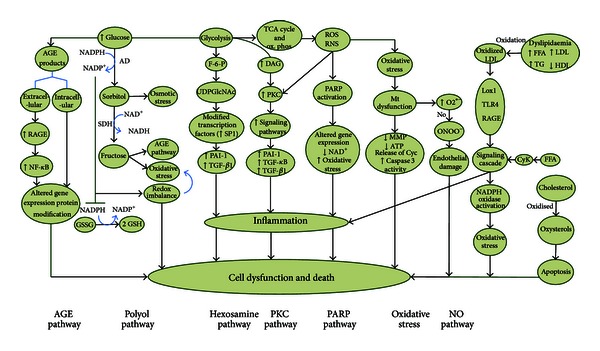
Mechanisms of diabetic neuropathy. The AGE and polyol pathways directly alter the redox capacity of the cell either through depletion of necessary components of glutathione recycling or by direct formation of ROS. The hexosamine, PKC, and PARP pathways indicate damage through expression of inflammation proteins. Dyslipidaemia with high incidence in T2D also linked to DN, and several underlying mechanisms have been identified. AGEs: advanced glycation end products; RAGEs: receptor for advanced glycation end products; NF-κB: nuclear factor kappa B; AD: aldose reductase; SDH: sorbitol dehydrogenase; GSH: glutathione; GSSG: oxidized glutathione; F-6-P: fructose-6 phosphate; UDPGlcNAc: uridine diphosphate-N-acetylglucosamine; PAI-1: plasminogen activator inhibitor-1; TGF-β1: transforming growth factor-β1; DAG: diaceylglycerol; PKC: protein kinase C; ROS: reactive oxygen species; RNS: reactive nitrogen species; PARP: poly ADP-ribose polymerase; Mt: mitochondria; MMPs: mitochondrial membrane potentials; Cyc: cytochrome c; NO: nitric oxide; LDL: low-density lipoprotein; LOX1: oxidised LDL receptor 1; TLR4: toll-like receptor 4; FFA: free fatty acids; TG: triglycerides; HDL: high-density lipoprotein; CyK: cytokine.
4.3. Role of Advanced Glycation End Products (AGEs)
Hyperglycemia accelerates generation of AGEs via attachment of reactive carbohydrate groups to proteins, nucleic acids, or lipids. These groups tend to damage the biological task of proteins, which as a result affects cellular function. Extracellular AGEs also bind to the receptor of AGE (RAGE) and initiate inflammatory flows, activate NADPH oxidases, and generate oxidative stress. Long-term inflammatory responses upregulate RAGE and stimulate nuclear factor kappa B (NFκB). Collectively, the biochemical damage induced by AGEs results in diminished neurotrophic support, impaired nerve blood flow, disrupted neuronal integrity, and impaired repair mechanisms (Figure 2) [3, 10].
4.4. Role of Hexosamine
The hexosamine, an additional factor, is implicated in the pathology of diabetes-induced oxidative stress and its complications. Fructose-6 phosphate, a metabolic intermediate of glycolysis, is shifted from the glycolytic pathway to the hexosamine way where fructose-6 phosphate is converted to uridine diphosphate-N-acetylglucosamine (UDPGlcNAc). Then, the UDPGlcNAc attaches to the serine and threonine residues of transcription factors. Hyperglycemic conditions create additional flux through hexosamine pathway that ultimately result in an increased activation of Sp1, a transcription factor implicated in diabetic complications. Activation of Sp1 leads to overexpression of transforming growth factor-β1 (TGF-β1) and plasminogen activator inhibitor-1 (PAI-1). The PAI-1 is upregulated by both hexosamine and PKC pathways. Collectively, activation of hexosamine pathway is implicated in multiple metabolic derangements in diabetes (see Figure 2) [9].
4.5. Activation of Diacylglycerol Protein Kinase C
Hyperglycemia stimulates formation of diacylglycerol, which then activates PKC. The PKC is an important element in function of nerves and pathogenesis of DN. Activation of PKC initiates an intracellular signaling cascade such as overexpression of PAI-1, NF-κB, and TGF-β. It also increases the production of extracellular matrix and cytokines. Furthermore, it enhances contractility, permeability, and vascular endothelial cell proliferation such as motivation of cytosolic phospholipase A2 and inhibition of Na+/K+ ATPase (Figure 2) [9, 11]. PKC has a unique structural feature that according to redox status of cell facilitates its regulation. An antioxidant can react with catalytic domain to inhibit its activity, while the prooxidants react with regulatory domain to stimulate its activity. On activation, PKC triggers stress genes that phosphorylate transcription factors and thus alter the balance of gene expression resulting in oxidative stress [8].
4.6. Role of Oxidative Stress, Apoptosis, and Poly ADP-Ribose Polymerase
The generation of free radicals is a major factor in development of DN through increased glycolytic process. Oxidative stress and reactive oxygen species (ROS) link the physiological mediators and metabolic initiators implicated in progressive nerve fiber damage, dysfunction, and loss in DN. Simultaneous with generation of free radicals during the glycolytic process, oxidative stress harms mitochondrial DNA, proteins, and membranes [9, 12]. In fact, mitochondrial damage takes place due to surplus formation of ROS or reactive nitrogen species (RNS). Hyperglycemia induces mitochondrial changes such as release of cytochrome C, activation of caspase 3, altered biogenesis and fission, which all lead to a programmed cell death. Reduced mitochondrial action potentials (MMP) with modest ATP are resulted from thrilling entrance of glucose. This process results in surplus transport of oxidant electrons into the mitochondria. Neurotrophic support such as neurotrophin-3 (NT-3) and nerve growth factor (NGF) are also reduced by mitochondrial injury. It should be noted that axons are disposed to hyperglycemic hurt owing to their large content of mitochondria [12]. Oxidative stress in conjunction with hyperglycemia activates poly ADP-ribose polymerase (PARP) which further cleaves nicotinamide adenine dinucleotide (NAD+) to nicotinamide and ADP-ribose residues. This process continues by a link to nuclear proteins and results in changes of gene transcription and expression, NAD+ depletion, oxidative stress, and diversion of glycolytic intermediates to other pathogenic pathways such as PKC and AGEs (Figure 2) [9]. Collectively, the polyol, AGEs, PKC, hexosamine, and PARP, all contribute to neuronal damage together. The AGEs and polyol pathways openly modify the redox capacity of the cell either through weakening of necessary components of glutathione recycling or by direct construction of ROS. The hexosamine, PKC, and PARP pathways are representatives of damage mediated through expression of inflammatory proteins [9].
4.7. Nitric Oxide Deficiency/Impaired Endothelial Function
Vascular factors include impaired nerve perfusion, hypoxia, and nerve energetic defects that are all implicated in the pathogenesis of DN. Nerve blood flow is reduced in DN perhaps mediated via nitric oxide (NO). Overproduction of superoxide anion by the mitochondrial electron transport chain in DN leads to binding of this anion to NO to form the strong oxidant peroxynitrite which is right lethal to endothelial cells. Endothelial cells also produce NO that acts as a vasodilator and antagonizes thrombosis. The NO also defends against inflammation by adjusting (Na+/K+)-ATPase or inhibiting the production of potent vasoconstrictor peptide endothelin (ET)-1 [13, 14]. In addition, hyperhomocysteinemia is associated with impairment of endothelial function, providing a mechanism for its possible involvement in DN. There is a synergistic effect between AGEs and homocysteine in initiation of endothelial damage (Figure 2) [13].
4.8. Inflammation
The nerve tissues in diabetes undergo a proinflammatory process that presents symptoms and develops neuropathy. In addition, inflammatory agents such as C-reactive protein and tumor necrosis factor-α (TNF-α) are present in the blood of both type 1 diabetes (T1D) and type 2 diabetes (T2D) patients. The levels of C-reactive protein and TNF-α correlate with the incidence of neuropathy. Production of the initiating inflammatory mediators such as TNF-α, TGF-β, and NF-κB results from several glucose-induced pathways. Cyclooxygenase-2 (COX-2) is an important enzyme that is upregulated by NF-κB in diabetic peripheral nerves and consecutively generates prostaglandin E2 and ROS that trigger NF-κB. Inducible nitric oxide synthase (iNOS) is an additional inflammatory enzyme which is regulated by NF-κB. Similar to COX-2, iNOS either induces NF-κB or is induced by it. This gives the impression that chronic NF-κB activation is in the center of all the inflammatory elements operating in DN. Subsequent to ischemia reperfusion, an extensive and modest infiltration of macrophages and granulocytes takes place in diabetic peripheral nerves. The cytokines which are induced by NF-κB in Schwann cells, endothelial cells, and neurons lead to absorption of macrophages in the diabetic nerves. Macrophages promote DN via a variety of mechanisms, including making of cytokines, ROS, and proteases, which all result in cellular oxidative damage and myelin breakdown. Excessive macrophage recruitment impairs regeneration of nerves in DN [9, 12].
4.9. Growth Factors
Neurotrophic factors play roles in the development, maintenance, and survival of neuronal tissue. In DN, the Schwann cells are damaged and neurons disintegrate, and the growth factors such as NGF, NT-3 and insulin-like growth factors (IGFs) are affected [9, 11].
5. Differences in the Pathophysiology of T1D and T2D in DN
As noted, hyperglycemia is a fundamental factor in DN. Dyslipidemia and changes in insulin signaling come after hyperglycemia in T2D. Levels of both insulin and C-peptide are reduced in patients with T1D while the neuronal insulin sensitivity is reduced in T2D. Therefore, the circumstances of disease in T1D and T2D are different, and this affects the efficacy of some medications [3].
5.1. Dyslipidemia
Dyslipidemia with high incidence in T2D is linked to DN. Free fatty acids have systemic effects such as release of inflammatory cytokine from adipocytes and macrophages. Plasma lipoproteins, especially low-density lipoproteins (LDLs), can be modified by glycation or oxidation where then binds to extracellular receptors comprising the oxidized LDL receptor 1(LOX1), toll-like receptor 4(TLR4), and RAGEs that activate NADPH oxidase. All these result in oxidative stress. Additionally, cholesterol can be simultaneously oxidized to oxysterols to cause neuronal apoptosis (Figure 2) [3].
5.2. Impaired Insulin Signaling
Insulin has neurotrophic effects and promotes neuronal growth and the survival while it is not involved in uptake of glucose into the neurons. Reduction of this neurotrophic signaling due to insulin resistance (T2D) or insulin deficiency (T1D) contributes to the pathogenesis of DN. In neurons, insulin resistance occurs by inhibition of the PI3K/Akt signalling pathway. Disruption of this pathway leads to mitochondrial dysfunction, oxidative stress, and dysfunction or death of the nerve. Tight glucose control is not as efficacious in patients with T2D, whereas it can reduce neuropathy in patients with T1D. This divergence is most likely related to differences in the underlying mechanisms in terms of hyperglycemia, dyslipidemia, and insulin resistance in T1D and T2D [3].
6. Treatment of DN
6.1. Control of Hyperglycemia
As discussed above, hyperglycemia and/or insulin deficit and their concomitant actions are principally involved in the pathogenesis of DN. Thus, glycemic control gives the impression to be the most effective treatment to delay onset of DN and slowing its progress [14]. By contrast with the results obtained from patients with T1D, glucose control produces less definitive effect in patients with T2D. Therefore, despite the similarities between T1D and T2D, there are dissimilarities in the mechanisms and complications. It seems that glucose control is the only disease-modifying therapy for DN, as uncontrolled diabetes results in a pronounced oxidative stress that can be reversed if patients attain glycemic control. According to assumption that oxidative stress may mediate vascular, microvascular, and specific tissue complications in diabetes, antioxidant therapy remains a vital therapy [3]. Pain management is the other buttress of treatment for DN that can largely improve the quality of patients' life. As shown in Table 1, over the past two decades, enormous efforts have been made by doing randomized placebo-controlled trials to find effective treatments for DN. Based on these studies, several classes of drugs are considered to be effective for the treatment of DN, and also given the pathogenesis of DN, other drugs have been suggested as disease modifying all based on oxidative stress (Figure 3).
Figure 3.
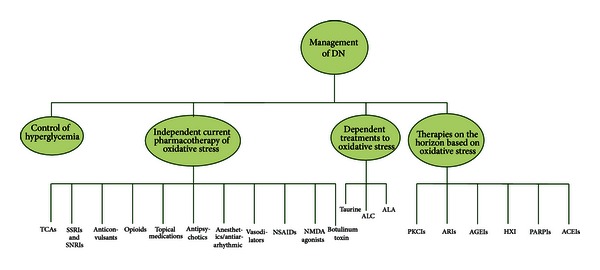
Algorithm for treatment of DN pain. TCAs: tricyclic antidepressants; SSRIs: selective serotonin reuptake inhibitors; SNRIs: serotonin norepinephrine reuptake inhibitors; NSAIDs: nonsteroidal anti-inflammatory drugs; ALC: acetyl-l-carnitine; ALA: α-lipoic acid; PKCIs: protein kinase C inhibitors; ARIs: aldose reductase inhibitors; AGEIs: advanced glycation end product inhibitors; GF: growth factor; PARPIs: poly ADP-ribose polymerase inhibitors; ACEIs: angiotensin converting enzyme inhibitors; HXIs: hexosamine pathway inhibitors.
6.2. Current Pharmacotherapy of DN Independent of Oxidative Stress
6.2.1. Tricyclic Antidepressants (TCAs)
The TCAs are recommended as the first-line therapy to relief pain of DPN for many years, even though they are not specifically endorsed for it. TCAs besides affecting catecholamines, they inhibit sodium and calcium channels, adenosine and N-methyl-D-aspartate (NMDA) receptors on the way to suppress neuronal hyperexcitability. TCAs have many side effects, principally anticholinergic effects [98, 99]. A meta-analysis based on number needed to treat (NNT) for TCAs resulted in the scores of 2 to 3 with a number needed to harm (NNH) of 14.7 [100].
6.2.2. Selective Serotonin Reuptake Inhibitors (SSRIs) andSerotonin Norepinephrine Reuptake Inhibitors (SNRIs)
Because of the relative high rate of adverse effects and several contraindications of TCAs, the SSRIs can be considered for those who do not tolerate TCAs. The SSRIs inhibit presynaptic reuptake of serotonin but not norepinephrine, and unlike TCAs, they lack postsynaptic receptor blocking effects and quinidine-like membrane stabilization. There is limited evidence showing a beneficial role for SSRIs, as they have not been licensed for the treatment of DN pain [14, 99, 101]. Antidepressants with dual selective inhibition of serotonin and norepinephrine (SNRIs) such as duloxetine and venlafaxine are better. SNRIs block the noradrenaline and 5-HT transporters and inhibit monoamine reuptake from the synaptic cleft into the presynaptic terminal which finally result in inhibition of excitatory impulse and pain perception [98, 101–103].
6.2.3. Anticonvulsants
Gabapentin and pregabalin should be used as first-line treatment for DPN pain if there is inadequate response or contraindications to TCAs. Pregabalin and gabapentin bind to the alpha2-delta subunit of the calcium-sensitive channels on the presynaptic neuron and modulate neurotransmitter release [99, 100]. Based on the new guidelines, pregabalin is recommended for the treatment of painful DN [102, 103]. Sodium valproate is probably effective in lessening pain and should be considered for the treatment of painful DN, but its adverse effects are high. For instance, it can worsen glycemic control and weight gain. Lamotrigine, oxcarbazepine and lacosamide should probably not be considered for the treatment of painful DN. Topiramate also lacks adequate support to be used in DN [103].
6.2.4. Opioids
Opioids by interacting with receptors located on neuronal cell membranes prevent neurotransmitter release at the presynaptic nerve terminal and reduce pain [14]. Controlled-release oxycodone, tramadol, and morphine can be exampled [99]. A meta-analysis indicated an NNT of 2.6 for oxycodone, 3.9 for tramadol, and 2.5 for morphine [100] in the treatment of painful DN [103].
6.2.5. Topical Medications
Current topical treatments for DPN pain include capsaicin cream and lidocaine 5% patches. This combination stimulates C fibers to release, and subsequently deplete substance P. Capsaicin application causes degeneration of epidermal nerve fibers as a mechanism of its analgesic effect, and thus caution for its use in insensitive diabetic foot is needed [14]. In a 2009 pooled data analysis, the calculated NNT for capsaicin was 5.7 [53]. Lidocaine blocks voltage-gated channels in damaged nerves [99]. There have been small effective trials with lidocaine. A randomized controlled trial (RCT) in 2003 revealed an NNT of 4.4 for it [67]. Topical clonidine is probably not effective and should not be considered [103].
6.2.6. Antipsychotics
Few atypical antipsychotics have been introduced for painful DN. The newer, less dopamine selective antipsychotics was supported by some animal studies consistent with clinical trials [104]. These compounds may induce negative metabolic effects such as weight gain and insulin resistance [105].
6.2.7. Anesthetics/Antiarrhythmic
These agents are all potent voltage-gated sodium channel antagonists and perhaps are less used in DN [105]. Mexiletine, lidocaine, and tocainide have all shown benefits for painful DN in smaller RCTs. Although tocainide has positive effects, but caution is needed for its cardiotoxicity [105]. Mexiletine has not been found effective [103]. The easiest method to incorporate the utility of the anesthetic/antiarrhythmics in patients with painful DN is the use of 5% lidocaine patches [105].
6.2.8. Vasodilators
Isosorbide dinitrate nasal spray and glyceryl trinitrate transdermal patches have shown positive effects in painful DN [105].
6.2.9. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
In patients with acute painful peripheral neuropathy, simple analgesics such as NSAIDs may provide pain control by modulating the nociceptive and inflammatory pain pathways [14].
6.2.10. NMDA Antagonists
Dextromethorphan, a low affinity NMDA receptor blocker, has been effective in relieving pain and improving quality of life in patients with DN [103].
6.2.11. Botulinum Toxin
This toxin not only works on the acetylcholine channels but also involves other mechanisms. Existing studies lack large number of subjects, and more is required to overcome doubt and debate about efficacy of this toxin [105].
6.3. Treatments Dependent on Oxidative Stress
The strongest sign for the role of oxidative stress in DN is the studies that report positive effects of antioxidants in both animal models and patients. Although, it is impossible to review all the antioxidants that can be effective to prevent or delay the onset of DN, some can be listed such as acetyl-L-carnitine, taurine, β-carotene, free amino acids, vitamin E, curcumin, ascorbic acid, and lipoic acid [8].
6.3.1. Taurine
Taurine is an antioxidant with effects on neuronal calcium signaling. Taurine improves electrophysiological parameters and nerve blood flow and exhibits analgesic properties in patients with DN [14].
6.3.2. Acetyl-L-Carnitine
Acetyl-L-carnitine (ALC), the acetylated ester of the amino acid L-carnitine, as an antioxidant has shown significant reduction in pain of patients with DN [14].
6.3.3. Alpha Lipoic Acid
D-L-α-lipoic acid (ALA) is a potent antioxidant, which has been extensively evaluated in subjects with DN and has shown good effects [14]. But based on new guidelines, there is insufficient evidence to recommend it in treatment of DN [103].
6.4. Nonpharmacologic Agents
Percutaneous electrical nerve stimulation is probably effective in reducing pain and improving sleep in patients who have painful DN. Exercise and acupuncture lack evidence of efficacy in the treatment of painful DN. Low-intensity laser therapy, Reiki therapy, and electromagnetic field treatment (such as magnetized shoe insoles) are probably not effective and should not be considered [103].
6.5. Therapies on the Horizon Based on Oxidative Stress
Since constant tight glucose control is difficult and still a challenge in most cases, additional therapies that target the hyperglycemia-induced complications are now under attention of researchers. To this end, coping against some known pathways that are activated as a consequence of increased oxidative stress and glucose flux is deemed effective to control DN. These pathways and inhibition of them are reviewed below.
6.5.1. Protein Kinase C Inhibitors
It is thought that activation of PKC through hyperglycemia-induced oxidative stress contributes to the generation of free radicals that result in diabetic microvascular complications [14]. Some of the PKC inhibitors such as ruboxistaurin have been shown to exhibit antioxidant effects. Ruboxistaurin has been particularly successful in reducing the progress of DN, and it is pending to get official approval [106].
6.5.2. Aldose Reductase Inhibitors (ARIs)
As stated earlier, hyperglycemia-mediated activation of the polyol pathway can produce oxidative stress that may underlie DN. Aldose reductase is a key enzyme in pathogenesis of DN. ARIs drop the flux of glucose through the polyol or sorbitol pathways resulting in a reduction of intracellular accumulation of sorbitol and fructose [14]. The effects of fidarestat (a novel ARIs) on nerve conduction and the subjective symptoms of DN provided evidence that this treatment can control DN with concomitant reduction in oxidative stress markers [107]. Similarly long-term treatment with epalrestat, an ARI, can effectively delay the progress of DN and improve the symptoms, particularly in subjects with limited microangiopathy and good glycemic control [108]. Treatment with ranirestat (ARIs) also appears to have an effect on motor nerve function in mild to moderate diabetic sensorimotor polyneuropathy (DSP), but the results of this study did not show a statistically significant difference in sensory nerve function relative to placebo [109].
6.5.3. Advanced Glycation End Product Inhibitors (AGEIs)
Accumulation of AGEs and activation of AGE receptors lead to oxidative stress and microvascular damage in DN. Benfotiamine, a derivative of thiamine (vitamin B1), reduces tissue AGEs formation and oxidative stress in subjects with DN with varying doses and duration of treatment [14, 110]. Also aspirin, because of its antioxidant capacity, and aminoguanidine, a free radical scavenger, can inhibit this pathway and reduce oxidative stress in DN [9].
6.5.4. Hexosamine Pathway Inhibitors
As described above, diabetes-induced oxidative stress stimulates hexosamine pathway that implicates in the pathology of DN. Benfotiamine decreases flux through hexosamine pathway resulting in lower oxidative stress. This agent can reduce the pain associated with DN [9].
6.5.5. Inhibition of PARP
As discussed above, PARP activation involves in the pathogenesis of DN, and its inhibition lightens numerous experimental pathologic conditions connected with oxidative stress in DN. Nicotinamide has been shown to act as a PARP inhibitor and an antioxidant in animals that improves complications of early DN [9].
6.5.6. Angiotensin Converting Enzyme Inhibitors
Angiotensin II is a potent vasoconstrictor with proinflammatory properties, which especially in the absence of NO causes thrombosis. It also stimulates intercellular adhesion molecules and vascular adhesion molecules (VCAMs). The role of angiotensin converting enzyme inhibitors in DN is probably through inhibition of angiotensin II. In this regard, trandolapril has shown a small but significant improvement in some complication of DN [14].
7. New Therapeutic Approaches for DN
Despite relative lack of success of interventional agents to reverse or slow established DN, there is still hope to find some good agents. We have summarized a few of new approaches in Table 2 [79–97]. Hopefully, some reviews in the recent years have proven positive effects of natural antioxidants and herbal products such as Satureja species and Urtica dioica in diabetes and its complications [111–116].
8. Conclusion
In the present review, we tried to elaborate the pathogenesis of disease with a focus on oxidative stress and introduced therapies dependent or independent of oxidative stress. Diabetes can injure peripheral nerves in various distributions, and DSPN is the most common presentation in diabetes, which lead to substantial pain, morbidity, and impaired quality of life. Social and health-care costs linked with DN are high. DN develops on a background of hyperglycemia and associated metabolic imbalance. Numerous biochemical mechanisms of neurovascular and nerve damage have been identified in DN, but excessive production of ROS or oxidative stress is thought to be a common etiologic factor.
Treatment of DN always begins with optimizing glycemic control and then control of pain. Regarding role of oxidative stress and consequential factors in pathogenesis of DN, observing positive results with inhibitors of key pathways at the preclinical level is not surprising, but final decision will be made on the basis of clinical trials. If oxidative stress is assumed only as an ancillary player in DN, then antioxidants should be supplemented with conventional treatments. Development of new drugs to treat DN still remains a challenge that needs intensive long-term comparative trials.
Acknowledgment
This paper is the outcome of an in-house financially nonsupported study prepared for the special issue of Oxidative Medicine and Cellular Longevity. Author thanks assistance of INSF.
References
- 1.Peripheral Neuropathy. http://courses.washington.edu/conj/neuron/peripheralNeuropathy.htm.
- 2.Bril V. Treatments for DN. Journal of the Peripheral Nervous System. 2012;2:22–27. doi: 10.1111/j.1529-8027.2012.00391.x. [DOI] [PubMed] [Google Scholar]
- 3.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. The Lancet Neurology. 2012;11(6):521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes—Metabolism Research and Reviews. 2012;1:8–14. doi: 10.1002/dmrr.2239. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. 1997;46(2):S54–S57. doi: 10.2337/diab.46.2.s54. [DOI] [PubMed] [Google Scholar]
- 6.Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 7.Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negi G, Kumar A, Joshi RP, Ruby PK, Sharma SS. Oxidative stress and diabetic neuropathy: current status of antioxidants. Institute of Integrative Omics and Applied Biotechnology. 2011;2(6):71–78. [Google Scholar]
- 9.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacology & Therapeutics. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmood D, Singh BK, Akhtar M. Diabetic neuropathy: therapies on the horizon. Journal of Pharmacy and Pharmacology. 2009;61(9):1137–1145. doi: 10.1211/jpp/61.09.0002. [DOI] [PubMed] [Google Scholar]
- 11.Rajbhandari SM, Piya MK. A brief review on the pathogenesis of human diabetic neuropathy: observations and postulations. International Journal of Diabetes and Metabolism. 2005;13(3):135–140. [Google Scholar]
- 12.Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? Journal of Diabetes Investigation. 2011;2(1):18–32. doi: 10.1111/j.2040-1124.2010.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Head KA. Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Alternative Medicine Review. 2006;11(4):294–329. [PubMed] [Google Scholar]
- 14.Shakher J, Stevens MJ. Update on the management of diabetic polyneuropathies. Diabetes, Metabolic Syndrome and Obesity. 2011;4:289–305. doi: 10.2147/DMSO.S11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Max MB. Endogenous monoamine analgesic systems: amitriptyline in painful diabetic neuropathy. Anesthesia Progress. 1987;34(4):123–127. [PMC free article] [PubMed] [Google Scholar]
- 16.Max MB, Culnane M, Schafer SC. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37(4):589–596. doi: 10.1212/wnl.37.4.589. [DOI] [PubMed] [Google Scholar]
- 17.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. The New England Journal of Medicine. 1992;326(19):1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 18.Vrethem M, Boivie J, Arnqvist H, Holmgren H, Lindström T, Thorell LH. A comparison of amitriptyline and maprotiline in the treatment of painful polyneuropathy in diabetics and nondiabetics. Clinical Journal of Pain. 1997;13(4):313–323. doi: 10.1097/00002508-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Archives of Internal Medicine. 1999;159(16):1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 20.Sindrup SH, Gram LF, Skjold T, Grodum E, Brøsen K, Beck-Nielsen H. Clomipramine vs desipramine vs placebo in the treatment of diabetic neuropathy symptoms. A double-blind cross-over study. British Journal of Clinical Pharmacology. 1990;30(5):683–691. doi: 10.1111/j.1365-2125.1990.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45(1):3–9. doi: 10.1016/0304-3959(91)90157-S. [DOI] [PubMed] [Google Scholar]
- 22.Kvinesdal B, Molin J, Frøland A, Gram LF. Imipramine treatment of painful diabetic neuropathy. Journal of the American Medical Association. 1984;251(13):1727–1730. [PubMed] [Google Scholar]
- 23.Gomez-Perez FJ, Rull JA, Dies H, Rodriquez-Rivera JG, Gonzalez-Barranco J, Lozano-Castañeda O. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy. A double-blind cross-over study. Pain. 1985;23(4):395–400. doi: 10.1016/0304-3959(85)90010-7. [DOI] [PubMed] [Google Scholar]
- 24.Sindrup SH, Bjerre U, Dejgaard A, Brøsen K, Aaes-Jørgensen T, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clinical Pharmacology and Therapeutics. 1992;52(5):547–552. doi: 10.1038/clpt.1992.183. [DOI] [PubMed] [Google Scholar]
- 25.Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. Journal of Clinical Neuromuscular Disease. 2001;3(2):53–62. doi: 10.1097/00131402-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284–1289. doi: 10.1212/01.wnl.0000058749.49264.bd. [DOI] [PubMed] [Google Scholar]
- 27.Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697–706. doi: 10.1016/j.pain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Kadiroglu AK, Sit D, Kayabasi H, Kemal Tuzcu A, Tasdemir N, Yilmaz ME. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. Journal of Diabetes and its Complications. 2008;22(4):241–245. doi: 10.1016/j.jdiacomp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Medicine. 2005;6(5):346–356. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DJ, Lu Y, Detke MJ, Lee TC, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1-2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 31.Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411–1420. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 32.Kajdasz DK, Iyengar S, Desaiah D, et al. Duloxetine for the management of diabetic peripheral neuropathic pain: evidence-based findings from post hoc analysis of three multicenter, randomized, double-blind, placebo-controlled, parallel-group studies. Clinical Therapeutics. 2007;29(11):2536–2546. doi: 10.1016/j.clinthera.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock J, Tuchman M, Lamoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. Journal of Pain. 2005;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115(3):254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 37.Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP., Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. European Journal of Pain. 2008;12(2):203–213. doi: 10.1016/j.ejpain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Arezzo JC, Rosenstock J, LaMoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurology. 2008;8, article 33 doi: 10.1186/1471-2377-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman R, Durso-DeCruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31(7):1448–1454. doi: 10.2337/dc07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus. A randomized controlled trial. Journal of the American Medical Association. 1998;280(21):1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 41.Gorson KC, Schott C, Herman R, Ropper AH, Rand WM. Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. Journal of Neurology Neurosurgery and Psychiatry. 1999;66(2):251–252. doi: 10.1136/jnnp.66.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochar DK, Jain N, Agarwal RP, Srivastava T, Agarwal P, Gupta S. Sodium valproate in the management of painful neuropathy in type 2 diabetes—a randomized placebo controlled study. Acta Neurologica Scandinavica. 2002;106(5):248–252. doi: 10.1034/j.1600-0404.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- 43.Kochar DK, Rawat N, Agrawal RP, et al. Sodium valproate for painful diabetic neuropathy: a randomized double-blind placebo-controlled study. QJM. 2004;97(1):33–38. doi: 10.1093/qjmed/hch007. [DOI] [PubMed] [Google Scholar]
- 44.Otto M, Bach FW, Jensen TS, Sindrup SH. Valproic acid has no effect on pain in polyneuropathy: a randomized, controlled trial. Neurology. 2004;62(2):285–288. doi: 10.1212/wnl.62.2.285. [DOI] [PubMed] [Google Scholar]
- 45.Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology. 2001;57(3):505–509. doi: 10.1212/wnl.57.3.505. [DOI] [PubMed] [Google Scholar]
- 46.Vinik AI, Tuchman M, Safirstein B, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128(1-2):169–179. doi: 10.1016/j.pain.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 47.Jose VM, Bhansali A, Hota D, Pandhi P. Randomized double-blind study comparing the efficacy and safety of lamotrigine and amitriptyline in painful diabetic neuropathy. Diabetic Medicine. 2007;24(4):377–383. doi: 10.1111/j.1464-5491.2007.02093.x. [DOI] [PubMed] [Google Scholar]
- 48.Rull JA, Quibrera R, González-Millán H, Castañeda OL. Symptomatic treatment of peripheral diabetic neuropathy with carbamazepine (Tegretol): double blind crossover trial. Diabetologia. 1969;5(4):215–218. doi: 10.1007/BF01212087. [DOI] [PubMed] [Google Scholar]
- 49.Dogra S, Beydoun S, Mazzola J, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: a randomized, placebo-controlled study. European Journal of Pain. 2005;9(5):543–554. doi: 10.1016/j.ejpain.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Beydoun A, Shaibani A, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: results of a dose-ranging study. Acta Neurologica Scandinavica. 2006;113(6):395–404. doi: 10.1111/j.1600-0404.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 51.Grosskopf J, Mazzola J, Wan Y, Hopwood M. A randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurologica Scandinavica. 2006;114(3):177–180. doi: 10.1111/j.1600-0404.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 52.Rauck RL, Shaibani A, Biton V, Simpson J, Koch B. Lacosamide in painful diabetic peripheral neuropathy: a phase 2 double-blind placebo-controlled study. Clinical Journal of Pain. 2007;23(2):150–158. doi: 10.1097/01.ajp.0000210957.39621.b2. [DOI] [PubMed] [Google Scholar]
- 53.Wymer JP, Simpson J, Sen D, Bongardt S. Efficacy and safety of lacosamide in diabetic neuropathic pain: an 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clinical Journal of Pain. 2009;25(5):376–385. doi: 10.1097/AJP.0b013e318196d2b6. [DOI] [PubMed] [Google Scholar]
- 54.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50(6):1842–1846. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 55.Sindrup SH, Andersen G, Madsen C, Smith T, Brøsen K, Jensen TS. Tramadol relieves pain and allodynia in polyneuropathy: a randomised, double-blind, controlled trial. Pain. 1999;83(1):85–90. doi: 10.1016/s0304-3959(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 56.Freeman R, Raskin P, Hewitt DJ, et al. Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Current Medical Research and Opinion. 2007;23(1):147–161. doi: 10.1185/030079906X162674. [DOI] [PubMed] [Google Scholar]
- 57.Gimbel JS, Richards P, Portenoy RK. Controlled-release oxycodone for pain in diabetic neuropathy: a randomized controlled trial. Neurology. 2003;60(6):927–934. doi: 10.1212/01.wnl.0000057720.36503.2c. [DOI] [PubMed] [Google Scholar]
- 58.Watson CPN, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105(1-2):71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 59.Hanna M, O’Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. European Journal of Pain. 2008;12(6):804–813. doi: 10.1016/j.ejpain.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. The New England Journal of Medicine. 2005;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 61.Treatment of painful diabetic neuropathy with topical capsaicin: a multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Archives of Internal Medicine. 1991;151(11):2225–2229. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- 62.Scheffler NM, Sheitel PL, Lipton MN. Treatment of painful diabetic neuropathy with capsaicin 0.075% Journal of the American Podiatric Medical Association. 1991;81(6):288–293. doi: 10.7547/87507315-81-6-288. [DOI] [PubMed] [Google Scholar]
- 63.Tandan R, Lewis GA, Krusinski PB, Badger GB, Fries TJ. Topical capsaicin in painful diabetic neuropathy. Diabetes Care. 1992;15(1):8–14. doi: 10.2337/diacare.15.1.8. [DOI] [PubMed] [Google Scholar]
- 64.Effect of treatment with capsaicin on daily activities of patients with painful diabetic neuropathy. Capsaicin Study Group. Diabetes Care. 1992;15(2):159–165. doi: 10.2337/diacare.15.2.159. [DOI] [PubMed] [Google Scholar]
- 65.Yuen KCJ, Baker NR, Rayman G. Treatment of chronic painful diabetic neuropathy with isosorbide dinitrate spray: a double-blind placebo-controlled cross-over study. Diabetes Care. 2002;25(10):1699–1703. doi: 10.2337/diacare.25.10.1699. [DOI] [PubMed] [Google Scholar]
- 66.Agrawal RP, Choudhary R, Sharma P, et al. Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Research and Clinical Practice. 2007;77(2):161–167. doi: 10.1016/j.diabres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Meier T, Wasner G, Faust M, et al. Efficacy of lidocaine patch 5% in the treatment of focal peripheral neuropathic pain syndromes: a randomized, double-blind, placebo-controlled study. Pain. 2003;106(1-2):151–158. doi: 10.1016/s0304-3959(03)00317-8. [DOI] [PubMed] [Google Scholar]
- 68.Barbano RL, Herrmann DN, Hart-Gouleau S, Pennella-Vaughan J, Lodewick PA, Dworkin RH. Effectiveness, tolerability, and impact on quality of life of the 5% lidocaine patch in diabetic polyneuropathy. Archives of Neurology. 2004;61(6):914–918. doi: 10.1001/archneur.61.6.914. [DOI] [PubMed] [Google Scholar]
- 69.Dejgard A, Kastrup J, Petersen P. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet. 1988;1(8575-8576):9–11. doi: 10.1016/s0140-6736(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 70.Oskarsson P, Ljunggren JG, Lins PE. Efficacy and safety of mexiletine in the treatment of painful diabetic neuropathy. Diabetes Care. 1997;20(10):1594–1597. doi: 10.2337/diacare.20.10.1594. [DOI] [PubMed] [Google Scholar]
- 71.Wright JM, Oki JC, Graves L. Mexiletine in the symptomatic treatment of diabetic peripheral neuropathy. Annals of Pharmacotherapy. 1997;31(1):29–34. doi: 10.1177/106002809703100103. [DOI] [PubMed] [Google Scholar]
- 72.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48(5):1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 73.Sang CN, Booher S, Gilron I, Parada S, Max MB. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: efficacy and dose-response trials. Anesthesiology. 2002;96(5):1053–1061. doi: 10.1097/00000542-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Annals of Neurology. 2008;64(3):274–283. doi: 10.1002/ana.21427. [DOI] [PubMed] [Google Scholar]
- 75.Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473–1478. doi: 10.1212/01.wnl.0000345968.05959.cf. [DOI] [PubMed] [Google Scholar]
- 76.Sima AAF, Calvani M, Mehra M, Amato A. Acetyl-L-carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo-controlled trials. Diabetes Care. 2005;28(1):89–94. doi: 10.2337/diacare.28.1.89. [DOI] [PubMed] [Google Scholar]
- 77.Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999;22(8):1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- 78.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: a meta-analysis. Diabetic Medicine. 2004;21(2):114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 79.Yu X, Zhang L, Yang X, et al. Salvianolic acid A protects the peripheral nerve function in diabetic rats through regulation of the AMPK-PGC1α-Sirt3 axis. Molecules. 2012;17(9):11216–11228. doi: 10.3390/molecules170911216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coppey LJ, Holmes A, Davidson EP, Yorek MA. Partial replacement with menhaden oil improves peripheral neuropathy in high-fat-fed low-dose streptozotocin type 2 diabetic rat. Journal of Nutrition and Metabolism. 2012;2012:8 pages. doi: 10.1155/2012/950517.950517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahn S, Song R. Effects of Tai Chi Exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. Journal of Alternative and Complementary Medicine. 2012;18(12):1172–1178. doi: 10.1089/acm.2011.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin S, Kim KJ, Chang HJ, et al. The effect of oral prostaglandin analogue on painful diabetic neuropathy: a double-blind, randomized, controlled trial. Diabetes, Obesity and Metabolism. 2012;15(2):185–188. doi: 10.1111/dom.12010. [DOI] [PubMed] [Google Scholar]
- 83.Schreiber AK, Neufeld M, Jesus CH, Cunha JM. Peripheral antinociceptive effect of anandamide and drugs that affect the endocannabinoid system on the formalin test in normal and streptozotocin-diabetic rats. Neuropharmacology. 2012;63(8):1286–1297. doi: 10.1016/j.neuropharm.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Chopp M, Szalad A, et al. Thymosin β4 promotes the recovery of peripheral neuropathy in type II diabetic mice. Neurobiology of Disease. 2012;48(3):546–555. doi: 10.1016/j.nbd.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun W, Miao B, Wang XC, et al. Gastrodin inhibits allodynia and hyperalgesia in painful DN rats by decreasing excitability of nociceptive primary sensory neurons. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039647.e39647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hotta N, Kawamori R, Fukuda M, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabetic Medicine. 2012;29(12):1529–1533. doi: 10.1111/j.1464-5491.2012.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Attia HN, Al-Rasheed NM, Al-Rasheed NM, Maklad YA, Ahmed AA, Kenawy SA. Protective effects of combined therapy of gliclazide with curcumin in experimental DN in rats. Behavioural Pharmacology. 2012;23(2):153–161. doi: 10.1097/FBP.0b013e3283512c00. [DOI] [PubMed] [Google Scholar]
- 88.Naruse K, Sato J, Funakubo M, et al. Transplantation of bone marrow-derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in DN. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027458.e27458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu X, Jiang Liu H, Zhang W, Xu X, Li Z. The effects of galanin on dorsal root ganglion neurons with high glucose treatment in vitro. Brain Research Bulletin. 2012;87(1):85–93. doi: 10.1016/j.brainresbull.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 90.Yorek MA. Treatment of DN with baicalein: intervention at multiple sites. Experimental Neurology. 2011;232(2):105–109. doi: 10.1016/j.expneurol.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Dalmolin GD, Silva CR, Rigo FK, et al. Antinociceptive effect of Brazilian armed spider venom toxin Tx3-3 in animal models of neuropathic pain. Pain. 2011;152(10):2224–2232. doi: 10.1016/j.pain.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 92.Hosseini A, Sharifzadeh M, Rezayat SM, et al. Benefit of magnesium-25 carrying porphyrin-fullerene nanoparticles in experimental diabetic neuropathy. International Journal of Nanomedicine. 2010;5(1):517–523. doi: 10.2147/ijn.s11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hosseini A, Abdollahi M, Hassanzadeh G, et al. Protective effect of magnesium-25 carrying porphyrin-fullerene nanoparticles on degeneration of dorsal root ganglion neurons and motor function in experimental DN. Basic & Clinical Pharmacology & Toxicology. 2011;109(5):381–386. doi: 10.1111/j.1742-7843.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 94.Milani E, Nikfar S, Khorasani R, Zamani MJ, Abdollahi M. Reduction of diabetes-induced oxidative stress by phosphodiesterase inhibitors in rats. Comparative Biochemistry and Physiology Part C. 2005;140(2):251–255. doi: 10.1016/j.cca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Larijani B, Salimi M, Pourkhalili N, et al. Positive response of isolated rat pancreatic islets to IMOD; hopes for better transplant outcome and graft function. Asian Journal of Animal and Veterinary Advances. 2011;6(10):1019–1025. [Google Scholar]
- 96.Hosseini A, Abdollahi M. It is time to formulate an antioxidant mixture for management of diabetes and its complications: notice for phannaceutical industries. International Journal of Pharmacology. 2012;8(1):60–61. [Google Scholar]
- 97.Hosseini A, Abdollahi M. Through a mechanism-based approach, nanoparticles of cerium and yttrium may improve the outcome of pancreatic islet isolation. Journal of Medical Hypotheses and Ideas. 2012;6:4–6. [Google Scholar]
- 98.Jensen TS, Backonja MM, Hernández Jiménez S, Tesfaye S, Valensi P, Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diabetes and Vascular Disease Research. 2006;3(2):108–119. doi: 10.3132/dvdr.2006.013. [DOI] [PubMed] [Google Scholar]
- 99.Lindsay TJ, Rodgers BC, Savath V, Hettinger K. Treating diabetic peripheral neuropathic pain. American Family Physician. 2010;82(2):151–158. [PubMed] [Google Scholar]
- 100.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes care. 2009;32:S414–419. doi: 10.2337/dc09-S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sultan A, Gaskell H, Derry S, Andrew RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology. 2008;8, article 29 doi: 10.1186/1471-2377-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Devitt M. AAN, AANEM, and AAPMR publish guideline for treatment of painful diabetic neuropath. American Family Physician. 2012;86(5):469–470. [Google Scholar]
- 104.Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD004844.pub2.CD004844 [DOI] [PubMed] [Google Scholar]
- 105.Lindsay TJ, vitrikas K, Temporal M, Herndon CM. Diabetic neuropathic pain: real world treatment options. Clinical Medicine Insights: Therapeutics. 2012;4:169–183. [Google Scholar]
- 106.Vinik AI, Bril V, Kempler P, et al. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C β-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clinical Therapeutics. 2005;27(8):1164–1180. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Hotta N, Toyota T, Matsuoka K, et al. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24(10):1776–1782. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]
- 108.Hotta N, Akanuma Y, Kawamori R, et al. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care. 2006;29(7):1538–1544. doi: 10.2337/dc05-2370. [DOI] [PubMed] [Google Scholar]
- 109.Bril V, Hirose T, Tomioka S, Buchanan R. Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care. 2009;32(7):1256–1260. doi: 10.2337/dc08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy—a three-week randomized, controlled pilot study (BEDIP study) International Journal of Clinical Pharmacology and Therapeutics. 2005;43(2):71–77. doi: 10.5414/cpp43071. [DOI] [PubMed] [Google Scholar]
- 111.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomedicine and Pharmacotherapy. 2005;59(7):365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Monfared SSMS, Larijani B, Abdollahi M. Islet transplantation and antioxidant management: a comprehensive review. World Journal of Gastroenterology. 2009;15(10):1153–1161. doi: 10.3748/wjg.15.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasani-Ranjbar S, Larijani B, Abdollah M. A systematic review of iranian medicinal plants useful in diabetes mellitus. Archives of Medical Science. 2008;4(3):285–292. [Google Scholar]
- 114.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. Journal of Pharmacy & Pharmaceutical Sciences. 2012;15(3):447–466. doi: 10.18433/j32w29. [DOI] [PubMed] [Google Scholar]
- 115.Momtaz S, Abdollahi M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. International Journal of Pharmacology. 2010;6(4):454–461. [Google Scholar]
- 116.Mehri A, Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of efficacy and safety of urtica dioica in the treatment of diabetes. International Journal of Pharmacology. 2011;7(2):161–170. [Google Scholar]


