Abstract
Numerous studies have demonstrated brain region- and subunit-specific abnormalities in the expression of subunits of the AMPA subtype of glutamate receptors in schizophrenia. In addition, abnormalities in the expression of proteins that regulate the forward trafficking of AMPA receptors through the cell have been reported. These findings suggest abnormal trafficking of AMPA receptors as a mechanism underlying dysregulated glutamate neurotransmission in schizophrenia. AMPA receptor subunits (GluR1-4) assemble to form AMPA receptor complexes in the lumen of the endoplasmic reticulum (ER). These subunits undergo the posttranslational modification of N-linked glycosylation in the ER and the Golgi apparatus before the assembled receptors are transported to the plasma membrane. In this study, we measured expression of AMPA receptors and the extent of their N-glycosylation using Western blot analysis in the dorsolateral prefrontal cortex in subjects with schizophrenia (N=35) and a comparison group (N=31). N-glycosylation was assessed using molecular mass shift assays following digestion with endoglycosidase H (Endo H), which removes immature high mannose-containing sugars, and with peptide-N-glycosidase F (PNGase F), which removes all N-linked sugars. Of the four AMPA receptor subunits, only GluR4 was significantly increased in schizophrenia. GluR2 and GluR4 were both sensitive to Endo H and PNGase F treatment. Endo H-mediated deglycosylation of GluR2 resulted in a significantly smaller pool of GluR2 protein to shift in schizophrenia, reflecting less N-linked high mannose and/or hybrid sugars on the GluR2 protein in this illness. This was confirmed by immunoisolation of GluR2 and probing with Concanavalin A, a mannose specific lectin; in subjects with schizophrenia GluR2 was significantly less reactive to Concanavalin A. Altered N-linked glycosylation of the GluR2 subunit in schizophrenia suggests abnormal trafficking of AMPA receptors from the ER to the synaptic membrane in schizophrenia.
Keywords: postmortem brain, psychosis, glutamate receptors, prefrontal cortex
Introduction
The glutamate hypothesis of schizophrenia proposes that there is decreased glutamate neurotransmission in limbic brain structures in this illness. Initial research efforts focused on NMDA receptor hypofunction, given that the administration of noncompetitive NMDA receptor antagonists such as phencyclidine (PCP) and ketamine can evoke the cardinal symptoms of schizophrenia in healthy individuals, and can exacerbate psychotic symptoms in patients with schizophrenia (Javitt and Zukin, 1991; Jentsch and Roth, 1999; Krystal et al., 1994; Tamminga, 1999; Tsai and Coyle, 2002). More recently, the possibility of AMPA receptor dysfunction has been proposed to explain abnormalities in glutamate neurotransmission associated with the pathophysiology of schizophrenia. The initial depolarization of the postsynaptic membrane by activated AMPA receptors is a prerequisite for activation of NMDA receptors at the glutamate synapse, thus abnormalities of the AMPA receptor may result in altered NMDA-mediated transmission.
Although numerous studies conducted in postmortem brain have reported abnormalities in expression of AMPA receptors in schizophrenia, the findings have been variable (Beneyto and Meador-Woodruff, 2004, 2006; Breese et al., 1995; Corti et al., 2011; Deakin et al., 1989; Dracheva et al., 2005; Eastwood et al., 1997b; Eastwood et al., 1995; Kerwin et al., 1990; Kerwin et al., 1988; Nishikawa et al., 1983). Discrepancies in these studies may suggest that other mechanisms crucial for regulation of glutamate receptors may be dysregulated in schizophrenia. In particular, recent data suggest abnormalities in trafficking of AMPA receptors in schizophrenia (Hammond et al., 2010; Hammond et al., 2011). Several proteins that associate with AMPA receptors and regulate their trafficking have been found to be abnormally expressed in cortex in schizophrenia (Beneyto and Meador-Woodruff, 2006; Drummond et al., 2012; Hammond et al., 2010; Mirnics et al., 2000; Toyooka et al., 2002).
AMPA receptor subunits are N-linked glycosylated in the lumen of the ER before being assembled into the AMPA receptor complexes (Kaplan et al., 1987; Silberstein and Gilmore, 1996). The initial N-linked oligomannose glycans are trimmed and replaced by more elaborate glycans in the ER and subsequently in the Golgi apparatus, before the AMPA receptors are trafficked to the plasma membrane. Thus, the extent of N-glycosylation can serve as a marker for the intracellular localization of AMPA receptors and their subunits. In addition, N-glycosylation of AMPA receptor subunits is associated with AMPA receptor regulation, having roles in biosynthesis, degradation, protein folding, trafficking, and stabilization of AMPA receptors at the plasma membrane. It is likely that N-glycosylation of AMPA receptor subunits plays a critical role in efficacy and plasticity of glutamatergic synapses.
We have previously reported data consistent with abnormal forward trafficking of AMPA receptors in postmortem brain in schizophrenia, that we interpreted as reflecting disturbances in AMPA receptor trafficking in proximal intracellular structures (Hammond et al., 2010). In this study, we determined the extent of N-linked glycosylation as an index of proximal trafficking of AMPA receptors, with the hypothesis that there are abnormalities in N-linked glycosylation of AMPA receptor subunits in schizophrenia, consistent with a model of abnormal receptor trafficking.
Methods
Subjects, tissue acquisition and sample preparation
Samples used in the study (Table 1) were from the Mount Sinai Medical Center brain collection (Harvey et al. 1992; Davidson et al. 1995; Powchik et al. 1998). Patients were diagnosed with schizophrenia using DSM-III-R criteria. Each subject had psychotic symptoms before the age of 40, and at least 10 years of hospitalization with a diagnosis of schizophrenia made by two clinicians. Patients were recruited prospectively and underwent extensive antemortem clinical assessment. Consent was obtained from the next of kin for each subject. All subjects were without a history of alcoholism, substance abuse, death by suicide, coma for more than 6 h before death, or any neuropathological evidence of neurodegenerative disorders. Comparison subjects were selected using a formal blinded medical chart review instrument. Assessment instruments included the CERAD battery, the Clinical Dementia Rating Scale, and the Positive and Negative Symptom Scale (Powchik et al., 1998).
Table 1.
Subject demographics.
| Comparison group | Schizophrenia | |
|---|---|---|
| N | 31 | 35 |
| Sex | 12m/19f | 23m/12f |
| Tissue pH | 6.4 ± 0.2 | 6.4 ± 0.3 |
| PMI (hours) | 8.1 ± 6.9 | 12.5 ± 6.6 |
| Age (years) | 78 ± 14 | 74 ± 12 |
Values are expressed as means ± standard deviation. Male (m), female (f), postmortem interval (PMI).
Brains were collected at autopsy and cut in the coronal plane in 10 mm slabs. The dorsolateral prefrontal cortex (DLPFC) was dissected from tissue slabs, snap frozen, pulverized into powder, and stored at −80°C until used. Samples were reconstituted and homogenized in ice-cold buffer (5 mM Tris-HCl (pH 7.4), 0.32 M sucrose, with a protease inhibitor tablet (Complete Mini, Roche Diagnostics, Mannheim, Germany) using a Power Gen 125 homogenizer (Thermo Fisher Scientific, Waltham, MA), and protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific, Waltham, MA).
Western Blot Analysis
Samples were diluted with ultrapure water (Milli-Q A10, Millipore, Bellarica, CA) and mixed with 6× protein sample buffer (0.25 M Tris-HCl, pH 6.8, 5 mM EDTA, 5 mM EGTA, 50 mM dithiothreitol, 6% SDS, 30% glycerol, and bromophenol blue as the tracking dye), heated at 95°C for 10 min and resolved for 1.5 h at 180 V on 4–12% gradient sodium dodecyl sulfate–polyacrylamide (SDS-polyacrylamide) gels (Life Technologies Corporation, Carlsbad, CA). Proteins were transferred from SDS-polyacrylamide gels to either polyvinylidene fluoride (PVDF) (for total protein level experiments) or to nitrocellulose (for immunoisolation and lectin binding experiments) membranes (BioRad, Hercules, CA) using a BioRad semi-dry transblotter. The membranes were blocked in LiCor blocking buffer (LiCor, Lincoln, NE), and then incubated overnight at 4°C in LiCor blocking buffer with 0.1% Tween 20 and with the following primary antibodies: anti-GluR1 rabbit polyclonal (Millipore, Bellarica, CA, # 07–660; 1:1000), anti-GluR2 mouse monoclonal (US Biological, Swampscott, MA, # G3500; 1:6000), anti-GluR3 (Millipore, # MAB5416; 1:1000), anti-GluR4 rabbit polyclonal (Millipore, # 06–308; 1:1,000) or anti-β-tubulin mouse monoclonal (Millipore, # 05–661; 1:100 000). The membranes were washed in phosphate-buffered saline with 0.1% Tween 20 (PBST) and probed with IR-dye labeled secondary anti-mouse or anti-rabbit antibodies (LiCor, Lincoln, NE; 1:10 000) for 1 h at room temperature. The membranes were rinsed three times for 15 min with PBST.
Enzymatic deglycosylation
Samples (50 μg total protein per reaction) were incubated at 37°C for 16 h with two different glycosidases, endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGase F) (QA-Bio, Palm Desert, CA) according to the manufacturer’s protocol. A decrease in the molecular mass of each AMPA receptor subunit protein due to the removal of oligosaccharides was measured as change in migration distance after separation of deglycosylated proteins on 4–12% gradient SDS-polyacrylamide gels followed by Western blot analysis, using the same antisera described above for each of the AMPA receptor subunits (Bauer et al., 2008).
Lectin-binding assays
We assayed the nature of N-glycosylation of each of AMPA receptor subunit using a lectin binding precipitation assay, by measuring the binding of each AMPA receptor subunit to a panel of lectins recognizing different glycans (Table 2). To solubilize plasma membranes, brain homogenates were incubated using end-over-end rotation at 4°C overnight in a buffer containing 1% Triton-X. Samples were centrifuged at 2300 g to remove cellular debris. The resulting supernatants (400 μg total protein) were mixed with 20 μg of each biotin conjugated lectin (Vector Laboratories, Burlingame, CA) and subsequently incubated using rotation at 4°C overnight. Next, samples were incubated with 20 μl of streptavidin coated magnetic beads (Life Technologies Corporation, Carlsbad, CA) for 2 h at room temperature. The beads were next washed three times with 0.5 ml buffer containing 10 mM Tris-Cl pH 7.4, 0.5% Tween 20, 0.1% BSA, protease inhibitors, and beads were isolated from the supernatant with a magnet (Dynal MPC-S, Life Technologies Corporation, Carlsbad, CA). Proteins bound to lectins were eluted from a magnetically isolated beads by incubating them with 2× protein loading buffer for 10 min at 95°C. Samples were resolved using SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and blots probed with antisera to each of the AMPA receptor subunits as described above.
Table 2.
Glycan specificity of lectins.
| Abbreviation | Name | Primary specificity |
|---|---|---|
| LEL | Lycopersicon Esculentum Lectin | GlcNAc (2 or more) |
| HHL | Hippeastrum Hybrid Lectin | Mannose |
| NPL | Narcissus Pseudonarcissus Lectin | Mannose (prefers 2 or more) |
| Con A | Concanavalin A | Glucose, Mannose (2 or more) |
| PNA | Peanut Agglutinin | galactosyl GalNAc (O-linked sugars) |
(GlcNAc) N-Acetylglucosamine; (GalNAc) N-Acetylgalactosamine.
Immunoisolation
The presence of high mannose-containing glycans on the GluR2 subunit was analyzed by immunoisolating the GluR2 subunit from DLPFC homogenates (400 μg) from a subset of eight schizophrenia patients and eight comparison subjects, and probing immunoisolated GluR2 protein with Concanavalin A (ConA).
Samples were incubated using an end-over-end rotation at 4°C overnight with 1% Triton-X to solubilize cellular membranes. To remove insoluble cell debris, samples were centrifuged at 16 000 g. The supernatants were incubated using rotation at 4°C overnight with 2 μg of anti-GluR2 mouse monoclonal antibody, and then for 4 h at the same temperature with 40 μl Protein G conjugated Dynabeads (Life Technologies Corporation, Carlsbad, CA). The beads were washed twice with PBST, and isolated proteins were eluted by adding 30 μl of 2× protein loading buffer and boiling for 10 min. The proteins were separated using SDS–polyacrylamide gels, transferred to nitrocellulose membranes, and probed with biotin conjugated Con A (Vector Laboratories, Burlingame, CA, 1:10 000) for 2 h at room temperature. Blots were washed extensively with Tris-buffered saline containing 0.1% Tween 20, and subsequently incubated with IR-dye 800CW labeled Streptavidin (LiCor, Lincoln, NE; 1:10 000) to visualize biotinylated Con A bound to the GluR2 subunit. The amount of immunoisolated GluR2 protein in each of samples was measured concurrently, by separating half of each immunoisolated sample using SDS–polyacrylamide gels, and blotting with anti-GluR2 goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, sc-7611, 1:1000), and the secondary anti-goat antibody (LiCor, Lincoln, NE; 1:10 000) for 1 h at room temperature.
Data analysis
The near-infrared fluorescence value for each protein band corresponding to AMPA subunit proteins, β-tubulin or biotinylated ConA were collected using a LiCor Odyssey scanner and were expressed as raw integrated intensity with top-bottom median intralane background subtraction using Odyssey V3.0 analytical software (Bauer et al., 2009).
For total AMPA subunit expression level studies, protein expression from each subject was normalized to intralane β-tubulin (Bauer et al., 2009; Funk et al., 2009; Hammond et al., 2010). For analysis of N-glycosylation of AMPA receptor subunits, we quantified three dependent variables: (1) Endo H-sensitivity of GluR2 and GluR4, (2) PNGase F-sensitive molecular mass shifts for GluR2 and GluR4, and (3) amount of ConA-reactive GluR2. Endo H-sensitivity of GluR2 and GluR4 proteins was quantified for each subject as the ratio of intensities of the Endo H-sensitive band to the Endo H-insensitive band (Bands 1 and 2, respectively, in Figure 2B). PNGase F-sensitive molecular mass shifts for GluR2 and GluR4 were quantified for each subject as the distance between the PNGase F deglycosylated protein band of higher mobility (Band 4 in Figure 2B), and the same protein without PNGase F treatment (Band 3 in Figure 2B). Amount of ConA-reactive GluR2 after GluR2 immunoisolation was quantified as the ratio of intensity of the ConA-reactive GluR2 band to the intensity of total GluR2 in the each sample (Figure 4).
Figure 2. N-Glycosylation status of AMPA receptor subunits in DLPFC in schizophrenia.
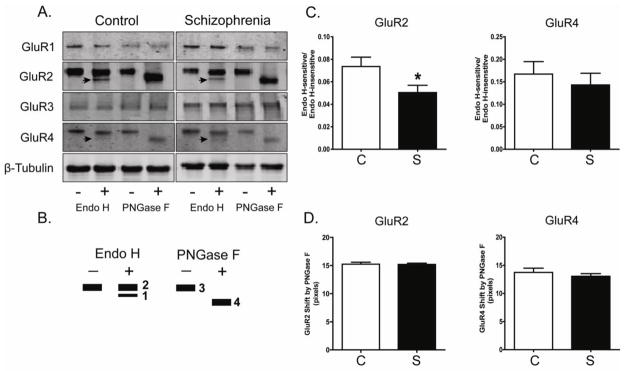
A. Representative immunoblots from DLPFC showing changes in electrophoretic mobility for GluR2 and GluR4, but not GluR1, GluR3 or β-tubulin following deglycosylation with Endo H or PNGase F. B. A schematic diagram of band patterns following deglycosylation with either Endo H or PNGase F. Endo H removes only immature, high mannose-containing or hybrid sugars, while PNGase F removes all N-linked sugars, thus leading to full N-linked deglycosylation. C. Quantification of Endo H-sensitive molecular mass shifts for GluR2 and GluR4. Graphs show the ratio of measured intensities of the Endo H-sensitive band to the Endo H-insensitive band (Bands 1 and 2, respectively, in Panel B). GluR2 was significantly less sensitive to Endo H in schizophrenia (S; N=35) versus the comparison group (C; N=31). *P<0.05. D. Quantification of PNGase F-sensitive molecular mass shifts for GluR2 and GluR4. Data are presented as the distance between the PNGase F deglycosylated protein band of higher mobility (Band 4 in Panel B), and the same protein without PNGase F treatment (Band 3 in Panel B). The molecular mass shift caused by PNGase F deglycosylation was not different between schizophrenia and comparison subjects.
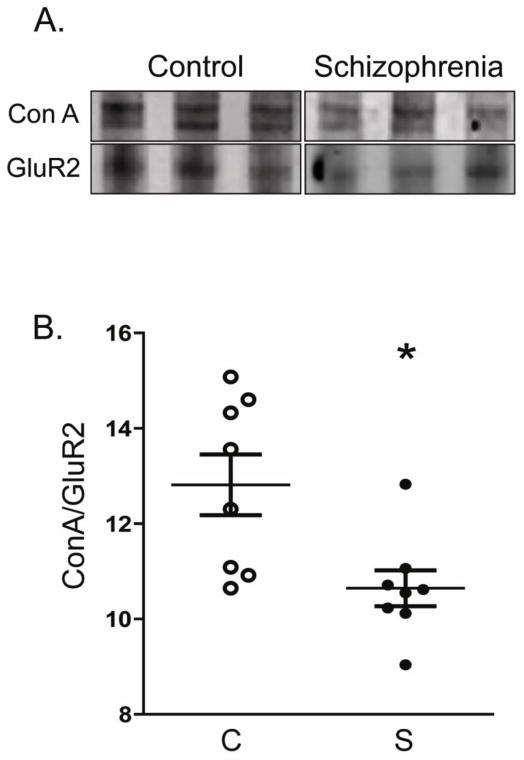
Figure 4. GluR2 immunoisolated from DLPFC of schizophrenia patients is less reactive with concanavalin A.
GluR2 was immunoisolated from DLPFC homogenates from a subset of subjects (schizophrenia (S), N=8; comparison subjects (C), N=8). A. Representative blots of immunoisolated GluR2 protein from DLPFC homogenates after probing with the lectin concanavalin A (ConA). B. Quantification of concanavalin A-reactive GluR2 normalized to the total amount of GluR2 in each sample. In schizophrenia, significantly less GluR2 is concanavalin A sensitive, consistent with a smaller proportion of this subunit containing mannose rich sugars. *P<0.05.
Data were analyzed using the Statistica software package (Statsoft, Tulsa, OK). All dependent measures were determined to be normally distributed. Correlation analyses were performed to determine associations between the dependent variables and age, PMI, and tissue pH. No correlations were found between these variables data and any dependent measures. Also, we have performed secondary analysis of medication status for the schizophrenia group of patients (receiving antipsychotics at the time of death vs. off medications for six weeks or more at the time of death). Antipsychotic medication did not affect expression or N-glycosylation status of AMPA receptor subunits in schizophrenia. Data were analyzed with one-way analysis of variance (ANOVA). For all statistical tests, α= 0.05. All assays were run with a single replicate.
Results
AMPA receptor subunit expression in DLPFC in schizophrenia
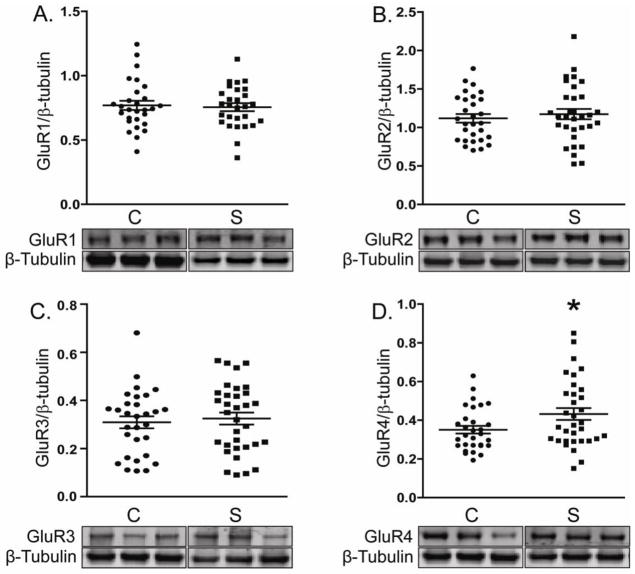
Using antibodies specific for each of the AMPA receptor subunits (GluR1-4), we measured by Western blotting protein expression levels of each in DLPFC in schizophrenia and a comparison group (Figure 1). All four AMPA receptor subunits were detected at apparent molecular weights of approximately 105 to 108 kDa. Only GluR4 protein expression was significantly altered in schizophrenia (F(1, 59)=4.0, P = 0.05) (Figure 1).
Figure 1. Expression of AMPA receptor subunits in DLPFC in schizophrenia.
Representative immunoblots from DLPFC showing expression levels of AMPA receptor subunits and β-tubulin. Data for each AMPA receptor subunit was normalized to β-tubulin as a loading control. Only GluR4 protein levels were different in schizophrenia (S; N=35) versus the comparison group (C; N=31). *P<0.05.
N-glycosylation of AMPA receptor subunits in schizophrenia
We next investigated the extent of N-glycosylation of the AMPA receptor subunits in DLPFC in schizophrenia. We determined N-glycosylation status of each subunit by incubating tissue with two different glycosidases: Endo H, which removes immature high mannose-containing or hybrid sugars, and PNGase F, which removes all N-linked sugars, leading to full N-linked deglycosylation of glycoproteins (Figure 2). A decrease in molecular mass of the AMPA receptor proteins indicates the removal of sugars from a given subunit. Both GluR2 and GluR4 were sensitive to Endo H and PNGase F treatment. Neither GluR1 nor GluR3 had altered electrophoretic mobility after deglycosylation, likely indicating lack of a significant population of N-glycosylated forms of either of these subunits in our postmortem samples. Treatment of GluR2 and GluR4 with Endo H resulted in two bands of 98 and 90 kDa (Note: Endo H-sensitive 90 kDa band is indicated by an arrow in Figure 2). PNGase F treatment yielded a single band of 90 kDa. GluR2 was significantly less sensitive to Endo H-driven deglycosylation in schizophrenia, suggesting fewer N-linked high mannose-containing and/or hybrid sugars attached to GluR2 (F(1, 57) = 4.6, P = 0.04).
Neither GluR2 or GluR4 were differentially shifted following PNGase F deglycosylation in schizophrenia.
GluR2 and GluR4 in human DLPFC contain N-linked mannose- and GlcNAc-containing residues
We further characterized glycosylation of each of the AMPA receptor subunits using a lectin affinity binding approach. AMPA receptor subunits from DLPFC were precipitated using biotin-conjugated lectins (Table 2) that bind carbohydrates with different specificities (Cummings, 1994). Lectins used included those that bind mannose-containing sugars (ConA, NPL and HHL), N-linked GlcNAc-containing sugars (LEL), and O-linked GalNAc-containing sugars (PNA) (Table 2). We found that there were mannose-containing sugars N-linked with GluR2 and GluR4 proteins based on their binding to ConA, NPL and HHL (Figure 3), consistent with our Endo H molecular mass shift data. In addition, we found that both GluR2 and GluR4 bind to LEL, which recognizes N-linked, GlcNAc-containing sugars.
Figure 3. GluR2 and GluR4 are the predominant AMPA receptor subunits that are glycosylated in human cortex.
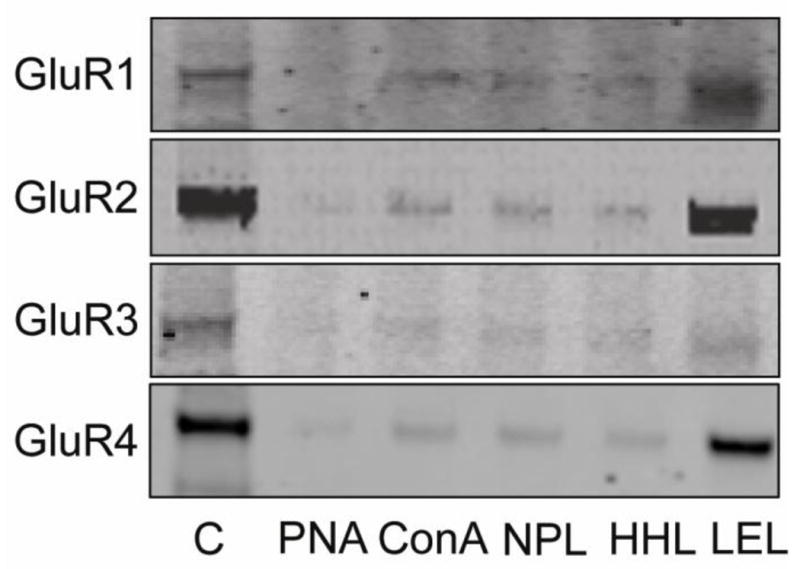
Each AMPA receptor subunit was precipitated from DLPFC homogenates from normal subjects using biotinylated lectins to isolate glycosylated proteins. Proteins that bound to lectins were separated by SDS–polyacrylamide electrophoresis, transferred to nitrocellulose membranes, and probed using specific antisera for each AMPA receptor subunit. Representative immunoblots show that most lectin binding was associated with the GluR2 and GluR4 subunits. Abbreviations: C, tissue homogenate. PNA, ConA, NPL, HHL, LEL are lectins as described in Table 2.
GluR2 is less reactive to ConA in schizophrenia
GluR2 protein was immunoisolated from DLPFC homogenates after membrane solubilization in a subset of schizophrenia (N=8) and comparison (N=8) subjects. GluR2 immunoisolated from schizophrenia was significantly less reactive to ConA, a lectin that recognizes mannose-containing sugars and (F(1, 16) = 8.6, P = 0.01) (Figure 4), consistent with our Endo H deglycosylation findings (Figure 2).
Discussion
In this study, protein levels of AMPA receptor subunits GluR1 - GluR4 were measured in the DLPFC in schizophrenia. N-linked glycosylation of the AMPA receptor subunits was also examined. GluR4 protein levels were increased in schizophrenia, whereas the other AMPA receptor subunits were unchanged. GluR2 and GluR4 were both found to be N-glycosylated as detected by an enzymatic deglycosylation assay. A smaller fraction of GluR2 subunit sensitive to Endo H was found in schizophrenia. GluR2 was also less reactive to ConA, lectin recognizing mannose-containing sugars and used extensively in studies of glutamate receptors (Bowie et al., 2003; Everts et al., 1997; Mathers and Usherwood, 1976).
Disturbances of glutamate neurotransmission, particularly underactivity of glutamate receptors, have been hypothesized to contribute to the pathophysiology of schizophrenia (Tsai and Coyle, 2002). While this hypothesis has typically focused on NMDA receptor abnormalities (Jentsch and Roth, 1999; Krystal et al., 1994; Tsai and Coyle, 2002), AMPA receptors have also been implicated in the dysregulation of glutamate neurotransmission in this illness (Dracheva et al., 2005; Meador-Woodruff et al., 2001; O’Connor et al., 2007). AMPA receptors are responsible for the primary depolarization in glutamate-mediated neurotransmission (Dingledine et al., 1999; Madden, 2002), which permits NMDA receptors to be activated by glutamate. AMPA receptors are hetero-tetramers comprised of GluR1-GluR4 subunits.
Numerous studies have measured AMPA receptors and associated subunits in postmortem brain in schizophrenia, yet no consistent pattern of abnormality has been identified from all of these studies (Beneyto and Meador-Woodruff, 2004, 2006; Breese et al., 1995; Corti et al., 2011; Deakin et al., 1989; Dracheva et al., 2005; Eastwood et al., 1997a; Eastwood et al., 1997b; Kerwin et al., 1990; Kerwin et al., 1988; Nishikawa et al., 1983). We found increased GluR4 protein expression, but normal protein levels for the other AMPA receptor subunits.
Recent work suggests dysregulation of AMPA receptor trafficking in cortical areas in schizophrenia. Proteins that associate with AMPA receptors and regulate forward trafficking or endosomal trafficking have been characterized, including stargazin, SAP97, GRIP1, and NSF (Beneyto and Meador-Woodruff, 2006; Mirnics et al., 2000; Toyooka et al., 2002). We recently demonstrated increased SAP97 and GRIP1 protein levels in brain and increased GluR1 in an early endosomal fraction in cortex in schizophrenia (Hammond et al., 2010; Hammond et al., 2011). These data are consistent with accelerated forward trafficking of GluR1-containing AMPA receptors or increased internalization of GluR1 subunit.
Trafficking of AMPA receptors begins with their exit from the ER, after assembly of heteroterameric complexes from monomeric subunits in the lumen of the ER. Native AMPA receptors co-assemble with transmembrane AMPA receptor regulatory proteins (TARPs), which act as as auxiliary subunits of AMPA receptors and regulate the ER transit, and modulate channel activity (Tomita, 2010; Vandenberghe and Bredt, 2004; Ziff, 2007). AMPA receptor subunits are N-glycosylated at a cognate sequence located in the extracellular portion of AMPA receptor subunit proteins (Greger et al. 2002). Initially, N-linked oligomannose glycans are trimmed and replaced by more elaborate glycans, first in the ER and subsequently in the Golgi apparatus, before AMPA receptors reach the plasma membrane (Clark et al., 1998; Kaplan et al., 1987; Silberstein and Gilmore, 1996).
Our findings indicate that a significantly smaller fraction of GluR2 contains high-mannose containing sugars in DLPFC in schizophrenia. Previously, an Endo H-sensitive form of GluR2 was found to be associated with the ER and was observed at much higher levels early in development (Greger et al., 2002). Given that Endo H sensitivity is associated with the ER, we interpret loss of Endo H sensitive GluR2 in schizophrenia as reflecting accelerated exit from the ER or forward trafficking of GluR2-containing AMPA receptors. Interestingly, the ER localization and trafficking of the GluR2 subunit is regulated by editing of glutamine (Glu607) into arginine (Arg607) residue in its transmembrane domain, creating the ER retention signal; edited GluR2 subunits are largely retained in the ER, whereas unedited GluR2 subunits readily tetramerize and traffic to synapses (Greger et al., 2003; Greger et al., 2002). Akbarian et. al. found tenfold increase in the unedited form of GluR2 in DLPFC (from 0.1% to 1.0%) (Akbarian et al., 1995). It remains, however, to be determined whether this imbalance of GluR2 editing may play a contributing factor in the amount of Endo H-insensitive form of GluR2 in schizophrenia patients. We have previously published similar findings for EAAT1 and EAAT2, members of the excitatory amino acid transporter (EAAT) family of molecules essential for regulation of synaptic glutamate levels. Both EAAT1 and EAAT2 had less N-glycosylation in schizophrenia (Bauer et al., 2010), suggesting that abnormalities in N-linked glycosylation involves multiple molecular components of the glutamate synapse.
We do not believe that there is a direct correlation between two major findings of this paper – increase in GluR4 levels and changes in N-glycosylation of GluR2 in DLPFC in schizophrenia. It is hard to predict whether observed changes will be colocalized to the same cell population in schizophrenia, therefore affecting trafficking or subunit composition of AMPA receptors. There are data to suggest that GluR4 subunit is the least expressed AMPA receptor subunit in human cerebral cortex (Dracheva et al., 2005). Nonetheless, in cells that express GluR4 protein increase in this subunit may potentially affect assembly and/or trafficking of AMPA receptors, shifting balance towards GluR4 containing and Ca2+-permeable AMPA receptors, that in turn resulting in population of cortical neurons prone to overestimation or excitotoxicity. Intriguingly, rat forebrain areas that preferentially express GluR4 have been reported to be highly susceptible to AMPA receptor-mediated neurotoxicity (Page and Everitt, 1995).
In summary, these data add to a growing literature implicating dysregulation of AMPA receptors in schizophrenia. These results suggest that the absolute level of AMPA receptors may not be critical, but rather changes in trafficking and activity of these receptors may contribute to schizophrenia pathophysiology. Thus, abnormalities of posttranslational modifications such as glycosylation may be the key points of disruption in schizophrenia, resulting in the abnormal subcellular localization and trafficking, and ultimately function of this receptor subtype in schizophrenia.
Acknowledgments
Role of funding source
Funding for this study was provided by the NIMH grants MH53327 and MH094445. The NIMH had no further role in study design; in collection, analysis and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
This work was supported by MH53327 (JMW), MH094445 (REM), MH064673 and MH066392 (VH).
Footnotes
Conflict of interest
All authors declared that they have not conflicts of interest.
Contributors
Drs. Meador-Woodruff and McCullumsmith design the study and provided intellectual contributions. Dr. Haroutunian provided the tissue for the study. Dr. Tucholski helped to design the study, performed the deglycosylation assays, performed the statistical analyses, and wrote the first draft of the manuscript. Mr. Simmons and Ms. Pinner performed the immunoisolation assay. All authors contributed and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Smith MA, Jones EG. Editing for an AMPA receptor subunit RNA in prefrontal cortex and striatum in Alzheimer’s disease, Huntington’s disease and schizophrenia. Brain Res. 1995;699(2):297–304. doi: 10.1016/0006-8993(95)00922-d. [DOI] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104(1–3):108–120. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117(1):92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J Comp Neurol. 2004;468(4):530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60(8):585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. Allosteric Regulation and Spatial Distribution of Kainate Receptors Bound to Ancillary Proteins. The Journal of Physiology. 2003;547(2):373–385. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Research. 1995;674(1):82–90. doi: 10.1016/0006-8993(94)01384-t. [DOI] [PubMed] [Google Scholar]
- Clark RA, Gurd JW, Bissoon N, Tricaud N, Molnar E, Zamze SE, Dwek RA, McIlhinney RA, Wing DR. Identification of lectin-purified neural glycoproteins, GPs 180, 116, and 110, with NMDA and AMPA receptor subunits: conservation of glycosylation at the synapse. J Neurochem. 1998;70(6):2594–2605. doi: 10.1046/j.1471-4159.1998.70062594.x. [DOI] [PubMed] [Google Scholar]
- Corti C, Xuereb JH, Crepaldi L, Corsi M, Michielin F, Ferraguti F. Altered levels of glutamatergic receptors and Na+/K+ ATPase-alpha1 in the prefrontal cortex of subjects with schizophrenia. Schizophr Res. 2011;128(1–3):7–14. doi: 10.1016/j.schres.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Cummings RD. [6] Use of lectins in analysis of glycoconjugates. In: William J, Lennarz GWH, editors. Methods in Enzymology. Academic Press; 1994. pp. 66–86. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, Losonczy MF, Keefe RS, Katz S, Frecska E. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry. 1995;152(2):197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Slater P, Simpson MD, Gilchrist AC, Skan WJ, Royston MC, Reynolds GP, Cross AJ. Frontal cortical and left temporal glutamatergic dysfunction in schizophrenia. J Neurochem. 1989;52(6):1781–1786. doi: 10.1111/j.1471-4159.1989.tb07257.x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Dracheva S, McGurk SR, Haroutunian V. mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J Neurosci Res. 2005;79(6):868–878. doi: 10.1002/jnr.20423. [DOI] [PubMed] [Google Scholar]
- Drummond JB, Simmons M, Haroutunian V, Meador-Woodruff JH. Upregulation of cornichon transcripts in the dorsolateral prefrontal cortex in schizophrenia. Neuroreport. 2012;23(17):1031–1034. doi: 10.1097/WNR.0b013e32835ad229. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Burnet PWJ, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Molecular Brain Research. 1997a;44(1):92–98. doi: 10.1016/s0169-328x(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Kerwin RW, Harrison PJ. Immunoautoradiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol Psychiatry. 1997b;41(6):636–643. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, McDonald B, Burnet PW, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Brain Res Mol Brain Res. 1995;29(2):211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-Glycosylation is not a prerequisite for glutamate receptor function but Is essential for lectin modulation. Mol Pharmacol. 1997;52(5):861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Funk AJ, Rumbaugh G, Harotunian V, McCullumsmith RE, Meador-Woodruff JH. Decreased expression of NMDA receptor-associated proteins in frontal cortex of elderly patients with schizophrenia. Neuroreport. 2009;20(11):1019–1022. doi: 10.1097/WNR.0b013e32832d30d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40(4):763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34(5):759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35(10):2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Endosomal trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Schizophr Res. 2011;130(1–3):260–265. doi: 10.1016/j.schres.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Davidson M, Powchik P, Parrella M, White L, Mohs RC. Assessment of dementia in elderly schizophrenics with structured rating scales. Schizophr Res. 1992;7(1):85–90. doi: 10.1016/0920-9964(92)90078-j. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148(10):1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20(3):201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kaplan HA, Welply JK, Lennarz WJ. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1987;906(2):161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39(1):25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- Kerwin RW, Patel S, Meldrum BS, Czudek C, Reynolds GP. Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet. 1988;1(8585):583–584. doi: 10.1016/s0140-6736(88)91371-2. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3(2):91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- Mathers DA, Usherwood PN. Concanavalin A blocks desensitisation of glutamate receptors on insect muscle fibres. Nature. 1976;259(5542):409–411. doi: 10.1038/259409a0. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Hogg AJ, Jr, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Res Bull. 2001;55(5):631–640. doi: 10.1016/s0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular Characterization of Schizophrenia Viewed by Microarray Analysis of Gene Expression in Prefrontal Cortex. Neuron. 2000;28(1):53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Takashima M, Toru M. Increased [3H]kainic acid binding in the prefrontal cortex in schizophrenia. Neurosci Lett. 1983;40(3):245–250. doi: 10.1016/0304-3940(83)90046-0. [DOI] [PubMed] [Google Scholar]
- O’Connor JA, Muly EC, Arnold SE, Hemby SE. AMPA receptor subunit and splice variant expression in the DLPFC of schizophrenic subjects and rhesus monkeys chronically administered antipsychotic drugs. Schizophr Res. 2007;90(1–3):28–40. doi: 10.1016/j.schres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KJ, Everitt BJ. The distribution of neurons coexpressing immunoreactivity to AMPA-sensitive glutamate receptor subtypes (GluR1–4) and nerve growth factor receptor in the rat basal forebrain. Eur J Neurosci. 1995;7(5):1022–1033. doi: 10.1111/j.1460-9568.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophr Bull. 1998;24(3):325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Silberstein S, Gilmore R. Biochemistry, molecular biology, and genetics of the oligosaccharyltransferase. Faseb J. 1996;10(8):849–858. [PubMed] [Google Scholar]
- Tamminga C. Glutamatergic aspects of schizophrenia. Br J Psychiatry. 1999;(Suppl 37):12–15. [PubMed] [Google Scholar]
- Tomita S. Regulation of Ionotropic Glutamate Receptors by Their Auxiliary Subunits. Physiology. 2010;25(1):41–49. doi: 10.1152/physiol.00033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Iritani S, Makifuchi T, Shirakawa O, Kitamura N, Maeda K, Nakamura R, Niizato K, Watanabe M, Kakita A, Takahashi H, Someya T, Nawa H. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. Journal of Neurochemistry. 2002;83(4):797–806. doi: 10.1046/j.1471-4159.2002.01181.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- Vandenberghe W, Bredt DS. Early events in glutamate receptor trafficking. Current Opinion in Cell Biology. 2004;16(2):134–139. doi: 10.1016/j.ceb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA Receptor Trafficking Paradox. Neuron. 2007;53(5):627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]