Abstract
Objective
The objective of the study was to examine differences in labor patterns in a modern cohort compared with the 1960s in the United States.
Study Design
Data from pregnancies at term, in spontaneous labor, with cephalic, singleton fetuses were compared between the Collaborative Perinatal Project (CPP, n = 39,491 delivering 1959-1966) and the Consortium on Safe Labor (CSL; n = 98,359 delivering 2002-2008).
Results
Compared with the CPP, women in the CSL were older (26.8 ± 6.0 vs 24.1 ± 6.0 years), heavier (body mass index 29.9 ± 5.0 vs 26.3 ±4.1 kg/m2), had higher epidural (55% vs 4%) and oxytocin use (31% vs 12%), and cesarean delivery (12% vs 3%). First stage of labor in the CSL was longer by a median of 2.6 hours in nulliparas and 2.0 hours in multiparas, even after adjusting for maternal and pregnancy characteristics, suggesting that the prolonged labor is mostly due to changes in practice patterns.
Conclusion
Labor is longer in the modern obstetrical cohort. The benefit of extensive interventions needs further evaluation.
Keywords: labor, labor curve, labor duration
The original labor curve was created by Friedman in the 1950s.1,2 Zhang et al3 derived a labor curve from a much larger cohort of women from the 1960s using data from the National Collaborative Perinatal Project (CPP). Differences between these 2 labor curves are apparent, most notably that the nulliparous women in the CPP demonstrated amore gradual transition to active phase, that active phase labor in the multiparous women began around 5 cm cervical dilation, and that women in the CPP lacked the deceleration phase described by Friedman.3
More recently the Consortium on Safe Labor (CSL) assessed labor progression in a large, contemporary cohort of women who presented in spontaneous labor.4 The CSL analysis found that the rate of cervical dilation was slower in all parities compared with that described by Friedman, especially from 4 to 6 cm, with an acceleration point suggesting active phase that occurred much later (around 6 cm).
However, since the 1960s, both maternal characteristics and obstetric practices have changed considerably. Women are older and have higher body mass indices (BMI), oxytocin and epidural use are more common, and more women undergo induction of labor in current practice.5 Both increasing maternal age and BMI are associated with progressively longer labor.6,7 Epidurals and induction have also been shown to be associated with longer labor.8,9 Because maternal characteristics are less modifiable, it is important to know the effects of current obstetric practice on the changes in labor patterns. Thus, we used data from the contemporary CSL study and data from the CPP to examine the differences in labor patterns and outcomes in modern obstetrics compared with the 1960s in women with spontaneous labor. We hypothesized that longer labor in the modern cohort was due to both changes in obstetric practices as well as changes in maternal characteristics.
Materials and Methods
The course of labor and method of delivery were compared between the CPP and the CSL. The CPP was a prospective study of 54,390 births to 48,197 women recruited from 1959 to 1965 (last delivery in 1966).10,11 Women were enrolled at 12 university clinical centers across the United States, and detailed information on demographics, medical history, socioeconomic status, and behavior were collected via interviews. A physical examination and blood sample were also performed at the first visit. The women were followed up throughout pregnancy, and repeat interviews and physical examinations were performed. Upon admission to labor and delivery, a trained observer recorded the cervical examinations as well as all labor and delivery, postpartum, and neonatal events. A senior obstetrician also completed a summary of the pregnancy and labor and delivery. Children were followed up to age 7 years.
The CSL was a retrospective cohort study conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of child Health, and included women giving birth between 2002 and 2008, with the majority (87%) between 2005 and 2007.5 There were 228,668 deliveries from 12 clinical centers and 19 hospitals representing 9 American College of Obstetrics and Gynecology districts. Institutional review board approval was obtained by all participating institutions.
Data were extracted from the patient electronic medical records including demographic data, medical history, and labor and delivery information as well as obstetrical, postpartum, and neonatal outcomes. Data from the neonatal intensive care unit were collected and linked to the newborn record. Maternal and newborn discharge International Classification of Diseases, ninth revision, codes were also collected for each delivery. Data were transferred in electronic format from each site and were mapped to common categories for each predefined variable at the data coordinating center. Investigators at each site completed surveys on hospital and physician characteristics. Data inquiries, cleaning, and logic checking were performed. Validation studies were performed for 4 key variables and the electronic medical records were found to be an accurate representation of the medical charts.5
There were 54,304 observations in the CPP obstetrical data file, and we limited our analysis to each individual woman's first enrolled pregnancy (n = 44,669) to avoid an intraperson correlation. Only women who presented in spontaneous labor were included, leaving 39,491 for analysis. In the CSL database, there were 228,668 deliveries, and we included 208,695 observations from each individual woman's first enrolled pregnancy. Only women who presented in spontaneous labor and had a singleton gestation were included, for a total of 98,359 women. There were a total of 137,850 women in the combined CPP plus CSL dataset.
Statistical Methods
The maternal, labor, and neonatal characteristics of women by study were compared using χ2 and the Wilcoxon ranksum test where applicable (Table 1). To compare continuous demographic or neonatal characteristics and calculate the P values (Tables 2-4), we used a linear regression with a term for study (CPP or CSL). For binary variables, we used linear probability models (PROC GENMOD with binomial distribution and identity link in SAS; SAS Institute, Cary, NC) to obtain the adjusted mean differences and associated P values. When linear probability models failed to converge, we used instead a logistic regression to produce the corresponding P values. Labor progression transition times from 1 cm to another were estimated by fitting an interval censored regression (using PROC LIFEREG of SAS), as previously described by Zhang et al.12
Table 1. Maternal, labor, and neonatal characteristics of women by studya.
| Demographic information | CPP (n = 39,491) | CSL (n = 98,359) |
|---|---|---|
| Maternal age, y (mean ± SD) | 24.1 ± 6.0 | 26.8 ± 6.0 |
| Prepregnancy BMI, kg/m2 | 22.6 ± 4.2 | 24.6 ± 5.6 |
| Delivery BMI, kg/m2 | 26.3 ± 4.1 | 29.9 ± 5.0 |
| Maternal race, % | ||
| White | 43 | 50 |
| Black | 48 | 21 |
| Hispanic | 9 | 18 |
| Other | 0 | 11 |
| Gestational age at delivery (mean ± SD, wks) | 39.2 ± 3.4 | 38.5 ± 2.3 |
| Parity | ||
| 0 | 38 | 44 |
| 1 | 21 | 28 |
| ≥2 | 41 | 28 |
| Spontaneous ROM prior to admission, % | 14 | 39 |
| Labor characteristics | ||
| Epidural, % | 4 | 55 |
| Oxytocin augmentation, % | 12 | 31 |
| Forceps, % | 40 | 2 |
| Vacuum, % | 0.1 | 4 |
| Forceps or vacuum, % | 40 | 6 |
| Episiotomy, % | 68 | 18 |
| Third- or fourth-degree laceration | 4 | 2 |
| Intrapartum cesarean delivery, % | 3 | 12 |
| Neonatal characteristics | ||
| Birthweight, g (mean ± SD) | 3133 ±596 | 3232 ± 570 |
| Ethnicity | ||
| White | 3257 ± 584 | 3297 ± 515 |
| Black | 3022 ± 592 | 3085 ± 618 |
| Hispanic | 3129 ± 557 | 3240 ± 572 |
| Other | — | 3215 ± 542 |
P < .001 for all comparisons.
Table 2. Comparison between CPP and CSL in nulliparous women.
| Parameter | CPP (n = 14,791) | CSL (n = 43,576) | Adjusted median differencea | Adjusted P value |
|---|---|---|---|---|
| Maternal age, y (mean ± SD) | 20.4 ± 4.0 | 24.4 ± 5.7 | < .001 | |
| Delivery BMI, kg/m2 | 25.5 ± 3.4 | 29.6 ± 4.9 | < .001 | |
| Admission characteristics | ||||
| Gestational age at delivery, wks | 39.3 ± 3.4 | 38.6 ± 2.4 | <.001 | |
| Dilation on admission | ||||
| Median (10th, 90th percentiles) | 3(1,6.5) | 3.5 (1, 7) | ||
| Mean ± SD | 3.5 ± 2.2 | 3.8 ± 2.3 | 0.6b | < .001 |
| Effacement on admission | ||||
| Median (10th, 90th percentiles) | 85(40,100) | 90(60,100) | ||
| Mean ± SD | 77 ± 25 | 84 ± 18 | 7.3b | < .001 |
| Spontaneous ROM prior to admission, % | 16 | 41 | 18 | < .001 |
| Labor characteristics | ||||
| Epidural, % | 5 | 60 | 37 | < .001 |
| Oxytocin augmentation, % | 16 | 37 | 8 | < .001 |
| First-stage durations, h (median, 95th percentiles) | ||||
| From 4 cm to C/C | 3.9(18.5) | 6.5 (24.0) | 2.6 | < .001 |
| From 5 cm to C/C | 2.1 (11.8) | 3.6(15.1) | 1.3 | < .001 |
| From 6 cm to C/C | 1.2(8.2) | 2.2(10.0) | 0.7 | < .001 |
| Second-stage durations, h | ||||
| With spontaneous delivery (median, 95th percentiles) | 0.45 (2.0) | 0.90(3.1) | 0.22 | < .001 |
| With assisted delivery (median, 95th percentiles) | 0.75(3.1) | 1.65 (4.25) | 0.49 | < .001 |
| Forceps, % | 66 | 3 | −51 | < .001 |
| Vacuum, % | 0.2 | 6 | 4 | < .001 |
| Forceps or vacuum, % | 66 | 10 | −47 | < .001 |
| Episiotomy, % | 92 | 27 | −51 | < .001 |
| Third- or fourth-degree laceration, % | 8 | 5 | −4 | < .001 |
| Intrapartum cesarean delivery, % | 3 | 16 | 5 | < .001 |
| Neonatal characteristics | ||||
| Birthweight, g (mean ± SD) | 3077 ± 571 | 3190 ± 573 | ||
| Apgar score at 1 min (mean) | 7.5 ± 2.1 | 7.9 ± 1.4 | ||
| <4, % | 7 | 3 | ||
| 4-6 | 17 | 6 | ||
| ≥7 | 76 | 91 | ||
| Apgar score at 1 min <7, % | 24 | 9 | −13 | < .001 |
| Apgar score at 5 min (mean) | 8.9 ± 1.4 | 8.8 ± 0.8 | ||
| <4, % | 1.8 | 0.5 | ||
| 4-6 | 3.9 | 1.3 | ||
| ≥7 | 94.4 | 98.2 | ||
| Apgar score at 5 min <7, % | 6 | 2 | −4 | < .001 |
Median difference (except where noted) for parameter in the CSL minus the CPP, adjusted for maternal age, race, gestational age at delivery, BMI at delivery, spontaneous rupture of membranes, and birthweight;
Adjusted mean difference.
Table 4. Comparison between CPP and CSL in multiparous women.
| Parameter | CPP (n = 16,277) | CSL (n = 27,312) | Adjusted median differencea | Adjusted P value |
|---|---|---|---|---|
| Maternal age, y (mean ± SD) | 28.0 ± 5.7 | 30.1 ± 5.3 | < .001 | |
| Delivery BMI, kg/m2 | 27.1 ± 4.5 | 30.4 ± 5.1 | < .001 | |
| Admission characteristics | ||||
| Gestational age at delivery, wks | 39.1 ± 3.5 | 38.4 ± 2.2 | < .001 | |
| Dilation on admission | ||||
| Median (10th, 90th percentiles) | 4 (1.5,8) | 4 (2, 8) | ||
| Mean ± SD | 4.2 ± 2.4 | 4.7 ± 2.4 | 0.8b | < .001 |
| Effacement on admission | ||||
| Median (10th, 90th percentiles) | 75(25,100) | 80(50,100) | ||
| Mean ± SD | 68 ± 28 | 80 ± 20 | 12b | < .001 |
| Spontaneous ROM prior to admission, % | 13 | 38 | 19 | < .001 |
| Labor characteristics | ||||
| Epidural, % | 4 | 48 | 30 | < .001 |
| Oxytocin augmentation, % | 9 | 27 | 9 | < .001 |
| First-stage durations, h (median, 95th) | ||||
| From 5 cm to C/C | 1.0(8.6) | 2.8(15.5) | 2.0 | < .001 |
| From 6 cm to C/C | 0.5(5.1) | 1.3(8.9) | 0.9 | < .001 |
| Second-stage durations, h | ||||
| With spontaneous delivery (median, 95th percentiles) | 0.15(0.60) | 0.20(1.0) | 0.05 | < .001 |
| With assisted delivery (median, 95th percentiles) | 0.35(1.18) | 0.42(2.11) | 0.12 | < .001 |
| Forceps, % | 18 | 0.5 | -15 | < .001 |
| Vacuum, % | 0.1 | 1.7 | 2 | < .001 |
| Forceps or vacuum, % | 18 | 2.5 | -14 | < .001 |
| Episiotomy, % | 36 | 7 | -25 | < .001 |
| Third- or fourth-degree laceration, % | 1 | 0.3 | -1 | .1 |
| Intrapartum cesarean delivery, % | 2 | 7 | 3 | < .001 |
| Neonatal characteristics | ||||
| Birthweight, g (mean ± SD) | 3176 ± 625 | 3269 ± 577 | ||
| Apgar score at 1 min (mean) | 8.0 ± 1.9 | 8.2 ± 1.3 | ||
| < 4, % | 5 | 2 | ||
| 4-6 | 12 | 4 | ||
| ≥7 | 83 | 94 | ||
| Apgar score at 1 min <7, % | 17 | 6 | −11 | < .001 |
| Apgar score at 5 min (mean) | 9.0 ± 1.3 | 8.9 ± 0.8 | ||
| <4, % | 1.5 | 0.6 | ||
| 4-6 | 2.8 | 0.8 | ||
| ≥7 | 95.7 | 98.6 | ||
| Apgar score at 5 min <7, % | 4 | 1 | −3 | < .001 |
Median difference (except where noted) for parameter in the CSL minus the CPP, adjusted for maternal age, race, gestational age at delivery, BMI at delivery, spontaneous rupture of membranes, and birthweight;
Adjusted mean difference.
To determine whether length of labor was due to changes in practice patterns, multivariable regression models were used, and length of labor times were calculated after adjusting for potentially confounding factors related to the different maternal and pregnancy characteristics in the 2 cohorts (maternal age, gestational age at delivery, BMI at delivery, race, spontaneous rupture of membranes, and birthweight). For example, if increasing maternal BMI explained all of the differences in an increased length of labor, we would conclude that labor is longer in a modern cohort because of increased maternal BMI.
Average labor curves were created by parity and study cohort including only those women who reached 10 cm (full dilation) as documented by cervical examination in both studies. To estimate the duration of labor, we used an eighth-order polynomial (in time) regression using repeated cervical examination measures and evaluated at the average values of the combined population for maternal age, maternal race, BMI at delivery, spontaneous rupture of membranes, gestational age, and birthweight. The curves start at 4 cm for nulliparous women and 5 cm for multiparous women because many multiparous women were admitted at a more advanced dilation and did not have information before 4 cm dilation.
To confirm that the potential differences in labor between the 2 cohorts were not entirely due to changes in the maternal characteristics and also to account for the fact that variables may not have been defined or collected in the same fashion in the CSL compared with the CPP, labor curves were also created in a select subset of low-risk pregnancies with similar characteristics derived from each cohort: white race; maternal age 18-30 years; normal prepregnancy BMI (18.5 to <25.0 kg/m2); nulliparous; gestational age at delivery 37 to less than 42 weeks; birthweight 2500 to less than 4000 g; no oxytocin; and no gestational diabetes, pregestational diabetes, chronic hypertension, or preeclampsia.
Results
The characteristics of the women, their labors, and the neonates differed significantly between the CPP and CSL. Compared with women in the CPP study, women in the CSL study were older (26.8 ± 6.0 vs 24.1 ± 6.0 years), had a higher average prepregnancy BMI (24.6 ± 5.6 vs 22.6 ± 4.2 kg/m2), had a higher average BMI at delivery (29.9 ± 5.0 vs 26.3 ± 4.1 kg/m2), and were more racially diverse (P < .001 for all comparisons; Table 1). Even though the women in the CSL study delivered on average 0.7 weeks (4.9 days) earlier, their neonates weighed on average 99 g more. Epidural use and oxytocin augmentation were much more common in the CSL compared with the CPP (55% vs 4% and 31% vs 12%, respectively), whereas operative vaginal delivery was less common during the CSL compared with the CPP (6% vs 40%). Episiotomy was also far less common in the CSL, with 17% undergoing this procedure compared with 68% in the CPP. The intrapartum cesarean delivery rate was 4 times higher in the CSL compared with the CPP (12% vs 3%).
First stage of labor
The median time for first stage of labor increased significantly between the CPP and CSL for all parities, regardless of cervical dilation on admission. In nulliparas, even though the median cervical dilation and effacement were slightly more favorable on admission, the median time for first stage of labor from 4 cm to completely dilated was 2.6 hours longer in the CSL (Table 2). After adjusting for maternal age, race, BMI at delivery, gestational age at delivery, spontaneous rupture of membranes, and birthweight, the median time for first stage of labor was still 2.6 hours longer (Table 2). Both secundagravidas and multiparas had similar cervical dilation and slightly greater effacement on admission in the CSL compared with the CPP (Tables 3 and 4). From 5 cm to completely dilated, the median time for first stage of labor was 1.8 hours for secundagravidas (P1) and 1.7 hours for multiparas (P2 or greater) in the CSL (Tables 3 and 4). After adjusting, these times were slightly longer (2.0 hours for both).
Table 3. Comparison between CPP and CSL in secundagravida women.
| Parameter | CPP (n = 8337) | CSL (n = 27,471) | Adjusted median differencea | Adjusted P value |
|---|---|---|---|---|
| Maternal age, y (mean ± SD) | 23.1 ± 4.6 | 27.3 ± 5.6 | < .001 | |
| Delivery BMI, kg/m2 | 26.0 ± 3.9 | 29.9 ± 4.9 | < .001 | |
| Admission characteristics | ||||
| Gestational age at delivery, wks | 39.3 ± 3.3 | 38.5 ± 2.2 | < .001 | |
| Dilation on admission | ||||
| Median (10th, 90th percentiles) | 4 (2, 8) | 4 (2, 8) | ||
| Mean ± SD | 4.3 ± 2.4 | 4.6 ± 2.3 | 0.6b | < .001 |
| Effacement on admission | ||||
| Median (10th, 90th percentiles) | 80(40,100) | 90(50,100) | ||
| Mean ± SD | 75 ± 25 | 82 ± 20 | 7b | < .001 |
| Spontaneous ROM prior to admission, % | 13 | 36 | 19 | < .001 |
| Labor characteristics | ||||
| Epidural, % | 3 | 52 | 34 | < .001 |
| Oxytocin augmentation, % | 10 | 25 | 6 | < .001 |
| First-stage durations, h (median, 95th percentiles) | ||||
| From 5 cm to C/C | 1.2 (7.9) | 3.0 (15.0) | 2.0 | < .001 |
| From 6 cm to C/C | 0.6 (4.8) | 1.6 (8.9) | 0.9 | < .001 |
| Second-stage durations, h | ||||
| With spontaneous delivery (median, 95th percentiles) | 0.18(0.72) | 0.33(1.67) | 0.10 | < .001 |
| With assisted delivery (median, 95th percentiles) | 0.38(1.28) | 0.62 (3.08) | 0.26 | < .001 |
| Forceps, % | 36 | 0.8 | −29 | < .001 |
| Vacuum, % | 0.1 | 2 | 2 | .003 |
| Forceps or vacuum, % | 36 | 4 | −27 | < .001 |
| Episiotomy, % | 76 | 13 | −52 | < .001 |
| Third- or fourth-degree laceration, % | 3 | 1 | −2 | .001 |
| Intrapartum cesarean delivery, % | 2 | 10 | 3 | < .001 |
| Neonatal characteristics | ||||
| Birthweight, g (mean ± SD) | 3147 ± 571 | 3264 ± 553 | ||
| Apgar score at 1 min (mean) | 7.9 ± 1.9 | 8.1 ± 1.3 | ||
| < 4, % | 4.6 | 2.1 | ||
| 4-6 | 12.6 | 4.2 | ||
| ≥7 | 82.8 | 93.8 | ||
| Apgar score at 1 min <7, % | 17 | 6 | −11 | < .001 |
| Apgar score at 5 min (mean) | 9.1 ± 1.1 | 8.9 ± 0.7 | ||
| <4, % | 1.0 | 0.4 | ||
| 4-6 | 2.6 | 0.8 | ||
| ≥7 | 96.4 | 98.8 | ||
| Apgar score at 5 min <7, % | 4 | 1 | −3 | < .001 |
Median difference (except where noted) for parameter in the CSL minus the CPP, adjusted for maternal age, race, gestational age at delivery, BMI at delivery, spontaneous rupture of membranes, and birthweight;
Adjusted mean difference.
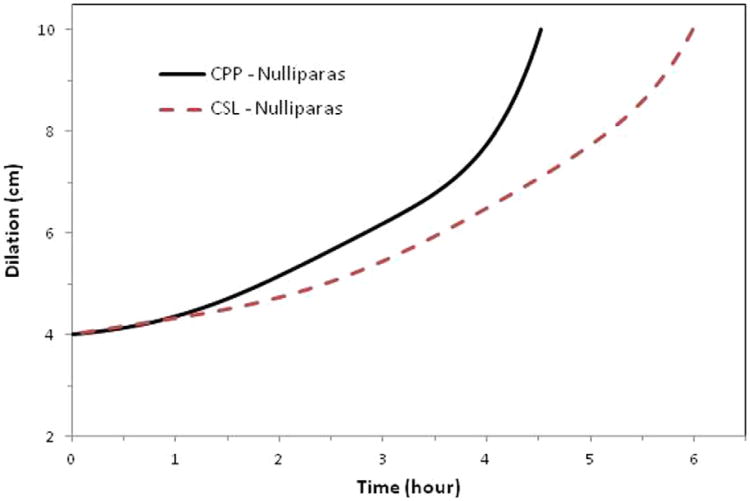
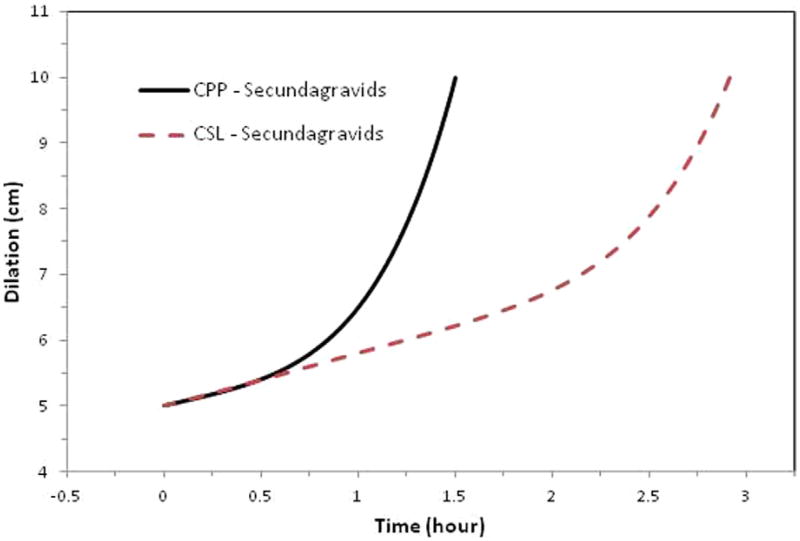
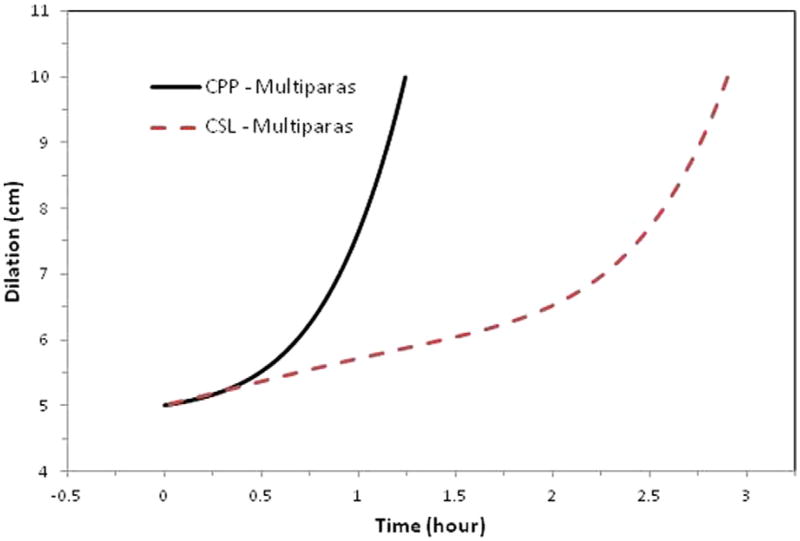
Nulliparous women in the CSL had an overall average slower rate for the first stage of labor with a less defined inflection point compared with the CPP (Figure 1). Multiparous women in the CSL also had an overall average slower rate for the first stage of labor, with a more prolonged latent phase and an inflection point that occurred later (around 6.5 cm) compared with closer to 5.5 cm in the CPP (Figures 2 and 3). When labor curves were compared in a select subset of pregnancies with similar characteristics, the results were similar to those of the overall comparison (data not shown).
Figure 1. Average labor curves for nulliparas (P0).
Average labor curves for women by study with singleton term pregnancies presenting in spontaneous labor with vaginal delivery for nulliparas (P0). Curves were evaluated at the average values of the combined population for maternal age, maternal race, BMI at delivery, spontaneous rupture of membranes, gestational age, and birthweight. The CPP was conducted from 1959 to 1966; the CSL was conducted from 2002 to 2008.
Figure 2. Average labor curves for secundagravidas (P1).
Average labor curves for women by study with singleton term pregnancies presenting in spontaneous labor with vaginal delivery for secundagravidas (P1). Curves were evaluated at the average values of the combined population for maternal age, maternal race, BMI at delivery, spontaneous rupture of membranes, gestational age, and birthweight. The CPP was conducted from 1959 to 1966; the CSL was conducted from 2002 to 2008.
Figure 3. Average labor curves for multiparas (P2).
Average labor curves for women by study with singleton term pregnancies presenting in spontaneous labor with vaginal delivery for multiparas (P2). Curves were evaluated at the average values of the combined population for maternal age, maternal race, BMI at delivery, spontaneous rupture of membranes, gestational age, and birthweight. The CPP was conducted from 1959 to 1966; the CSL was conducted from 2002 to 2008.
Second stage of labor
For the second stage of labor, 10% of nulliparous women had an operative vaginal delivery in the CSL compared with 66% in the CPP. Among women who delivered spontaneously, those in the CSL demonstrated a longer unadjusted and adjusted median time to delivery (0.45 hours [27 minutes] and 0.22 hours [13 minutes], respectively) (Table 2). Operative vaginal delivery occurred in 4% of secundagravidas (P1) and 2.5% of multigravidas (P2 or greater) in the CSL compared with 36% and18% in the CPP, respectively. The second stage labor was longer in secundagravidas (P1) and multigravidas (P2 or greater) in the CSL compared with CPP, but the median differences were of minimal clinical relevance (9 and 3 minutes, respectively; adjusted median differences 6 and 3 minutes, respectively) (Tables 3 and 4). The 95th percentile differences were more marked, at 1.0 hours and 0.4 hours, respectively, for secundagravidas (P1) and multigravidas (P2) in the CSL compared with CPP.
Neonatal Outcomes
Neonates weighed more in the CSL: 113 g more for nulliparas, 117 g for secundagravidas (P1), and 93 g for multiparous women (P2 or greater) (Tables 2-4). Neonatal Apgar scores at 1 and 5 minutes were higher in the CSL compared with CPP, regardless of parity (Tables 2-4). After adjusting for maternal and obstetrical characteristics, there was still a lower percentage of Apgar scores less than 7 at 5 minutes in the CSL compared with CPP for all parities (3-4% lower difference, P < .001 for all).
Comment
Labor patterns differ in contemporary practice in the CSL compared with approximately 50 years ago in the CPP. The first and second stages of labor were longer in the CSL, with an overall slower latent phase, a less obvious inflection point in nulliparas, and a later inflection point in multiparas. The population of women in contemporary obstetrics in the United States has changed compared with those in the CPP. Women in the CSL were older, had a higher BMI, and were more racially diverse. Their neonates also weighed more.
Obstetric practices also changed dramatically in the CSL compared with the CPP, with more women receiving oxytocin and epidurals, far less episiotomy and operative vaginal delivery use, and a cesarean section rate that was 4 times higher, regardless of parity. After adjusting for the differences in maternal and pregnancy characteristics, labor was still significantly longer in the modern CSL cohort compared with the older CPP cohort. In nulliparous women, changes in obstetric practice appear to have contributed the most to the longer median first stage of labor (from 4 to 10 cm cervical dilation) in the modern CSL cohort compared with the older CPP cohort. In secundagravidas (P1) and multiparous women (P2 or greater), changes in obstetric practice contributed to almost all of the difference to the longer median first stage of labor (from 5 cm to fully dilated).
Nulliparous women in the CSL had a longer median second stage for both spontaneous and operative vaginal delivery, and both maternal characteristics and neonatal birthweight and practice patterns contributed about evenly. For secundagravidas (P1) and multiparas (P2 or greater), the median differences in the length of the second stage for both spontaneous and operative vaginal delivery were slightly longer in the CSL, but the difference was of little clinical relevance. Comparisons of the second stage of labor should be interpreted with caution because of the high operative vaginal delivery and episiotomy rates in the CPP cohort. Perhaps the women who were allowed to deliver spontaneously or without an episiotomy in the CPP were different, with perceived faster progression or smaller neonate, which would bias the delivery time to be shorter.
Epidural use is one obstetric practice that is known to be associated with longer labor of 40-90 minutes but is strongly favored for maternal pain relief.13 In the overall CSL cohort, the rate of labor induction rate was 36.2% and that of prelabor cesarean 15.6%.5 The more liberal use of labor induction and prelabor cesarean deliveries in the overall CSL cohort resulted in only approximately one-half of the women who entered labor spontaneously. Conceivably, these women in the modern cohort may have had unmeasured differences that caused longer labor. However, even when we limited the labor curves to a select low-risk population with similar characteristics, labor in the CSL cohort was still longer. It would seem therefore that other changes in obstetric practices were likely influential.
Although we may not fully understand the factors contributing to longer labor, it is important to acknowledge that labor is longer in modern obstetrics and that routine interventions such as the use of oxytocin and timing of cesarean delivery may need to be thoughtfully reconsidered. For example, we noted that despite a 19% overall increase in oxytocin augmentation compared with women in the 1960s, women in contemporary practice who presented in spontaneous labor still had longer labor for all parities, and it may be that if women were allowed to progress in natural labor without oxytocin, the stages of labor could be even longer with the same odds for achieving vaginal delivery. Rouse et al14 demonstrated that 61% of women with a 2 hour arrest of labor in the active phase were able to deliver vaginally. Cesarean delivery also may be performed before the active phase, a conclusion supported by our previous study from the same CSL cohort that found a large percentage of women having a cesarean delivery during labor prior to 6 cm dilation.5
The length of labor has to be balanced with potential maternal and neonatal risks. In another study by Rouse et al15 in which women with spontaneous labor were allowed to continue beyond a 2 hour active phase arrest for up to 4 hours, there was an increased rate of chorioamnionitis but no severe adverse maternal or neonatal outcomes. Such findings also support consideration, or at least further investigation, of the optimal paradigm for the initiation of labor augmentation.
A study such as ours comparing clinical outcomes and practice between 2 data sets separated by some 40 years is bound to draw criticism. We would be the first to acknowledge that inherent limitations disallow the kind of firm conclusions that could be derived from a well-designed, prospectively performed, contemporaneous comparison. We limited our study to women who presented in spontaneous labor. Labor-related diagnoses may have changed over 50 years so that some variables are not coded in the same fashion today as in the past. In addition, the higher cesarean rate in the CSL causes an inherent selection bias from informative censoring. These likely obscure to some extent the true length of labor in contemporary practice because cesarean delivery occurred in 30.5% of women attempting vaginal delivery in the entire CSL cohort, and half of overall intrapartum cesarean deliveries were performed for failure to progress or cephalopelvic disproportion.5 Providers were using definitions of abnormal labor that were developed in a population of women that differ from the contemporary obstetrical population. Perhaps normal labor is even longer in modern obstetrics.
In addition, our study is limited because we could not compare neonatal outcomes because of the advances in neonatal care. Apgar scores at 5 minutes were lower in the CPP compared with the CSL, but it is difficult to attribute this to obstetrical practice differences because of the significant differences in preconception care. For example, screening for gestational diabetes, a condition that increases the risk of neonatal morbidity, was not routine during the CPP but was routine during the CSL. In addition, congenital anomalies were not detected before birth.
The limitations of our study notwithstanding, we are able to present a direct comparison between 2 uniquely large cohorts of women with detailed maternal demographic and labor characteristics. One plain and simple fact is that labor times are longer today than in the past in the United States, a fact with substantial cost implications. In 2010, Intermountain Healthcare obstetric facilities managed 5439 vaginal births in nulliparous women entering into spontaneous labor. A conservative estimate of the nursing cost alone for labor care in the Intermountain Healthcare system is $46.00 per hour (an average cost derived from analysis of women in active labor). Thus, in terms of the longer median time in labor for nulliparous women in the CSL study, the attributable cost is $110.40 per case, amounting to an annual cost of $600,466 within the Intermountain Healthcare system. The implications for health care systems and payors are obvious and should drive a reconsideration of modern-day labor process management with an eye toward process improvement.
In summary, for women who presented in spontaneous labor at term, the duration of labor from 4 cm in nulliparas and 5 cm in multiparas to complete dilation and the second stages of labor were longer in a contemporary population of labor patients than in a cohort from the 1960s. The longer overall median differences in the first stage of labor persisted after controlling for maternal and obstetric characteristics, indicating that modern labor differs from the older cohort largely because of changes in obstetric practices. Because labor times are longer today than in the past, the benefit of extensive interventions such as oxytocin and cesarean delivery in modern labor management needs further evaluation.
Acknowledgments
We thank James Troendle, PhD, at the National Institutes of Health National Heart, Lung, and Blood Institute, Zhen Chen, PhD, and Zhaohui Lu, MS, at the Eunice Kennedy Shriver National Institute of Child Health and Human Development for their assistance with the statistical analysis. Institutions involved in the consortium include, in alphabetical order: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH.; Summa Health System, Akron City Hospital, Akron, OH; the EMMES Corp, Rockville MD (data coordinating center); University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; and University of Texas Health Science Center at Houston, Houston, TX.
The data included in this paper were partly obtained from the Consortium on Safe Labor, supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract number HHSN267200603425C). The other institutions in the Consortium on Safe Labor are listed with the Acknowledgments section.
Footnotes
The authors report no conflict of interest.
The named authors alone are responsible for the views expressed in this article, which do not necessarily represent the decisions or stated policy of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Presented as a poster at the 31st annual meeting of the Society for Maternal-Fetal Medicine, San Francisco, CA, Feb. 7-12, 2011.
Reprints not available from the authors.
Contributor Information
Dr S. Katherine Laughon, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Dr D. Ware Branch, Intermountain Healthcare and University of Utah, Salt Lake City, UT.
Ms Julie Beaver, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Dr Jun Zhang, MOE and Shanghai Key Laboratory of Children's Environmental Health, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
References
- 1.Friedman EA. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6:567–89. doi: 10.1016/s0029-7844(02)02398-0. [DOI] [PubMed] [Google Scholar]
- 2.Friedman EA. Labor in multiparas: a graphicostatistical analysis. Obstet Gynecol. 1956;8:691–703. [PubMed] [Google Scholar]
- 3.Zhang J, Troendle J, Mikolajczyk R, Sundaram R, Beaver J, Fraser W. The natural history of the normal first stage of labor. Obstet Gynecol. 2010;115:705–10. doi: 10.1097/AOG.0b013e3181d55925. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Landy HJ, Branch DW, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116:1281–7. doi: 10.1097/AOG.0b013e3181fdef6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326.e1–10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treacy A, Robson M, O'Herlihy C. Dystocia increases with advancing maternal age. Am J Obstet Gynecol. 2006;195:760–3. doi: 10.1016/j.ajog.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Vahratian A, Zhang J, Troendle JF, Savitz DA, Siega-Riz AM. Maternal prepregnancy overweight and obesity and the pattern of labor progression in term nulliparous women. Obstet Gynecol. 2004;104:943–51. doi: 10.1097/01.AOG.0000142713.53197.91. [DOI] [PubMed] [Google Scholar]
- 8.Halpern SH, Leighton BL, Ohlsson A, Barrett JF, Rice A. Effect of epidural vs parenteral opioid analgesia on the progress of labor: a metaanalysis. JAMA. 1998;280:2105–10. doi: 10.1001/jama.280.24.2105. [DOI] [PubMed] [Google Scholar]
- 9.Osmundson SS, Ou-Yang RJ, Grobman WA. Elective induction compared with expectant management in nulliparous women with a favorable cervix. Obstet Gynecol. 116:601–5. doi: 10.1097/AOG.0b013e3181eb6e9b. [DOI] [PubMed] [Google Scholar]
- 10.Niswander KR, Gordon M, editors. The collaborative perinatal study of the National Institute of Neurological Diseases and Stroke: the women and their pregnancies. Philadelphia, PA: W. B. Saunders; 1972. [Google Scholar]
- 11.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13:303–11. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Troendle JF, Yancey MK. Reassessing the labor curve in nulliparous women. Am J Obstet Gynecol. 2002;187:824–8. doi: 10.1067/mob.2002.127142. [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetrics and Gynecology ACOG practice bulletin no. 36 Obstetric analgesia and anesthesia. Obstet Gynecol. 2002;100:177–91. doi: 10.1016/s0029-7844(02)02156-7. [DOI] [PubMed] [Google Scholar]
- 14.Rouse DJ, Owen J, Savage KG, Hauth JC. Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98:550–4. doi: 10.1016/s0029-7844(01)01516-2. [DOI] [PubMed] [Google Scholar]
- 15.Rouse DJ, Owen J, Hauth JC. Active-phase labor arrest: oxytocin augmentation for at least 4 hours. Obstet Gynecol. 1999;93:323–8. doi: 10.1016/s0029-7844(98)00448-7. [DOI] [PubMed] [Google Scholar]