Abstract
Parkinson’s disease (PD) is a currently incurable neurodegenerative disorder that affects the aging population. The loss of dopaminergic neurons in the substantia nigra is one of the pathological features of PD. The precise causes of PD remain unresolved but evidence supports both environmental and genetic contributions. Current efforts for the treatment of PD are directed toward the discovery of compounds that show promise in impeding age-dependent neurodegeneration in PD patients. Alpha-synuclein (α-Syn) is a human protein that is mutated in specific populations of patients with familial PD. Overexpression of α-Syn in animal models of PD replicates key symptoms of PD, including neurodegeneration. Here, we use the nematode C. elegans as a model system, whereby α-Syn toxicity causes dopaminergic neurodegeneration, to test the capacity of valproic acid (VA) to protect neurons. The results of our study showed that treatment of nematodes with moderate concentrations of VA significantly protects dopaminergic neurons against α-Syn toxicity. Consistent with previously established knowledge related to the mechanistic action of VA in the cell, we showed through genetic analysis that the neuroprotection conferred by VA is inhibited by cell-specific depletion of the C. elegans ortholog of the MAP extracellular signal-regulated kinase (ERK), MPK-1, in the dopaminergic neurons. These findings suggest that VA may exert its neuroprotective effect via ERK-MAPK, or alternately could act with MAPK signaling to additively provide dopaminergic neuroprotection.
Keywords: valproic acid, neurodegeneration, dopamine, RNAi, ERK-MAPK, Caenorhabditis
The degeneration of dopaminergic neurons in Parkinson’s disease (PD) patients represents one of the pathological features of PD. Risk factors for PD include both environmental and genetic factors [7]. The gene encoding the protein alpha-synuclein (α-Syn) is a well-studied genetic risk factor. α-Syn is a major constituent of the Lewy bodies found in PD patients, and is considered to play a key role in the pathogenesis of PD. For instance, it was found that point mutations as well as multiplication of the α-Syn locus cause familial forms of PD [24, 25]. Similarly, overexpression of α-Syn in mice and other model organisms mimic symptomatic features of PD, including accumulation of misfolded proteins, cellular toxicity, and neurodegeneration [26]. Current efforts for the treatment of PD are aimed at identifying compounds that exhibit potency in ameliorating age-dependent neurodegeneration.
Valproic Acid (VA) is an FDA approved compound that is normally prescribed for the treatment of epilepsy and bipolar disorder. Studies have shown that VA affects GABA transmission, voltage gated Na+ channels, T-type calcium channels, and histone deacetylases (HDACs) [4]. However, several studies revealed that VA can also activate ERK-MAPK both in vivo and in vitro. This action of VA, through ERK-MAPK, could have both neuroprotective and positive growth effects on neurons [4, 12]. For example, it has been reported that application of VA resulted not only in neuroprotection, but regeneration of injured retinal ganglion cells. This neuroprotective/regenerative effect was accompanied by prolonged activation of phosphorylated ERK 1/2 [1], suggesting that ERK-MAPK mediates both the neuroprotective and neuroregenerative effect of VA. In two other independent studies, VA was also shown to promote neurite growth through ERK [30], and positively affect cortical neuron growth and hippocampal neurogenesis in adult mice, also through the ERK pathway. As such, it was postulated that VA plays a role in promoting neurotrophic factors that positively regulate neuronal growth and maintenance to counteract neuronal cell death [9].
The neuroprotective effect of VA has also been documented in select models of PD. In one study, it was observed that chronic dietary administration of VA reduced dopaminergic cell death in neurodegenerative rats that were treated with rotenone [20]. In another model developed by the same group, VA was shown to confer neuroprotection in the degenerating brain cells of rats that were previously injected with the toxin 6-hydroxydopamine (6-OHDA) [21]. In a mouse model of PD, VA protected the nigrostriatal dopamine system against the toxin 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) [15]. These findings support the hypothesis that VA may have a neuroprotective effect on the dopaminergic neurons. Although such studies using acute neurotoxins have been insightful, it remains to be known whether or not VA can protect dopaminergic neurons impacted by the overproduction of α-Syn. Moreover, it has not been demonstrated if VA can exert its protective effect on the dopaminergic neurons through ERK-MAPK pathway.
Our laboratory had previously reported that overexpression of α-Syn in the dopaminergic neurons of C. elegans causes age-dependent neurodegeneration [3]. Despite its vast anatomical difference from humans, the C. elegans nervous system possesses important cellular and molecular features of mammalian neurons, which include conserved neurotransmitter systems (dopamine, GABA, acetylcholine, serotonin, etc.), receptors, axon guidance molecules, ion channels, and synaptic features. Moreover, the C. elegans genome contains homologs of many human genes including those that have been implicated in PD and other neurodegenerative diseases. Using this model system, we set out to test the hypothesis that VA may protect dopaminergic neurons of C. elegans against α-Syn toxicity via an ERK-MAPK-dependent mechanism.
We investigated this hypothesis using a combination of pharmacology and cell-specific RNA interference technology (RNAi). For the cell-specific RNAi experiments, the C. elegans ortholog of ERK-MAPK, MPK-1, and an upstream regulator MEK-2, were depleted in the dopaminergic neurons in the presence of overexpressed α-Syn. The cell-specific RNAi strain used in this study was created by introducing a sid-1 loss-of-function mutation in conjunction with SID-1 genomic DNA UA195 [sid-1(pk3321); baIn33 (Pdat-1::sid-1, Pmyo-2::mCherry)] into animals expressing α-Syn in the dopaminergic neurons UA44 [baIn11(Pdat-1:: α-syn, Pdat-1::GFP)]. The resulting strain UA196 [sid-1(pk3321); baIn11; baIn33], renders only the dopaminergic neurons susceptible to the effect of RNAi [10]. Using this strain, we monitored the loss of the dopaminergic neurons of C. elegans in adult animals. In this experimental paradigm, animals were treated with or without VA at various drug concentrations. All animals were cultured according to standard worm maintenance procedures [2]. A molten nematode growth medium (NGM) was used to dissolve VA inside conical flasks at 55-60 °C. Three independent mixtures were made containing 1mM, 2mM, and 3mM final concentrations. These mixtures were then poured into 60 mm Petri dishes and incubated at room temperature overnight. On the next day plates were seeded with bacteria (strain OP50), and incubated at 37 °C. Gravid adults were then transferred onto the plates to lay embryos and removed after 24 hours. The offspring were analyzed at day 7 post embryonically because significant neurodegeneration is reproducibly observed in populations at this time [9]. At day 7, 88.9% of α-syn animals displayed neurodegeneration in the dopaminergic neurons (11.1% animals with WT neurons) (Figures 1, 2).
Figure 1. Dopaminergic neurons (DA) of C. elegans in day-7 adult animals.
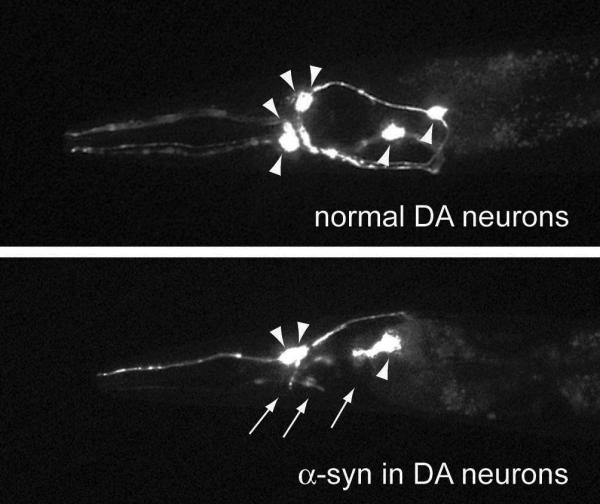
(A) The six anterior DA neurons remain intact in the absence of α-synuclein (α-Syn). (B) Anterior neurons undergoing neurodegeneration when α-Syn is overexpressed. Arrowheads indicate the cell bodies, and arrows depict the dying processes.
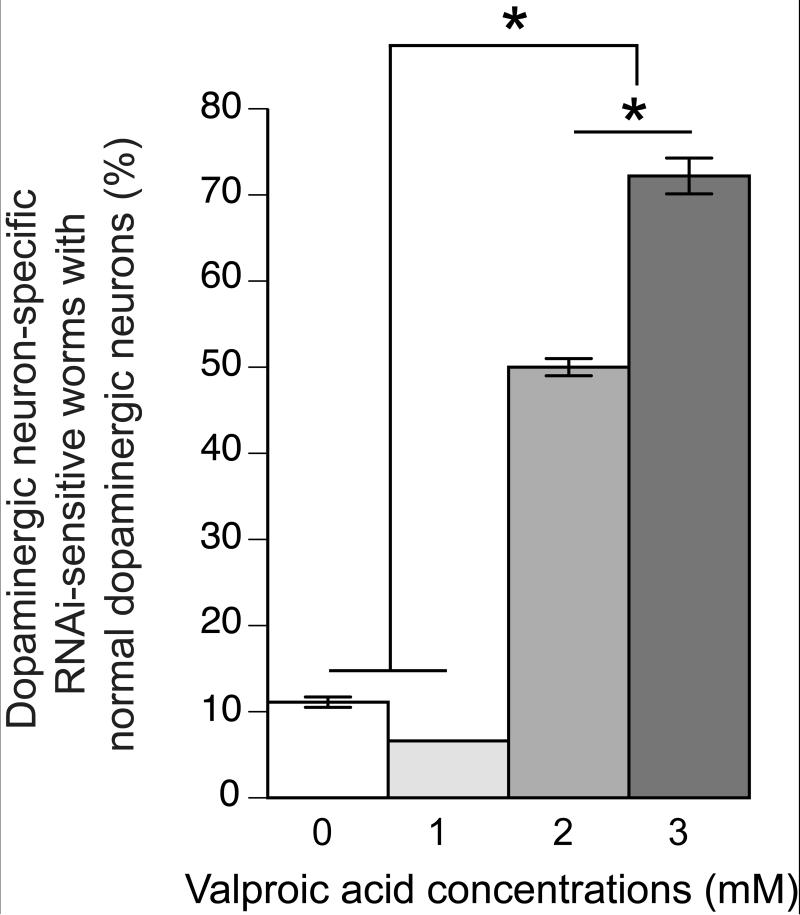
Figure 2. Treatment of α-Syn animals with Valproic Acid (VA) is neuroprotective.
Dopaminergic neuroprotection of α-Syn-expressing animals was commensurate with VA concentration, where 1mM was not protective, while 2mM and 3mM both displayed significant neuroprotection when compared to animals treated with vehicle alone. 3mM VA also provided significantly more neuroprotection than 2 mM VA. P < 0.05; One-way ANOVA with a Bonferroni post-hoc analysis.
To score dopaminergic neurons, animals were placed in a 3mM solution of levamisole and mounted with a coverslip on a two percent (2%) agarose pad. Animals were examined with a Nikon Eclipse E800 epifluorescence microscope at 400× magnification. The six anterior dopaminergic neurons of C. elegans were scored in our experiments. An animal with normal neurons is defined as having all intact dopaminergic neurons (i.e. cell bodies and processes). An animal with degenerating neurons is defined as having at least one missing neuron within that individual (i.e. missing cell body or dendrite). Statistical analyses were performed using either one-way ANOVA followed by the Bonferroni.post-hoc analysis for multiple comparisons (Figure 2) or Student’s t-test when comparing two sets of data/experiment involving control and treated animals (RNAi with VA treatments), as described in Figure 3. The mean values for all data sets were shown with standard deviations, where a p-value of less than 0.05 is considered significant. In total, 90 animals were examined, per experimental condition.
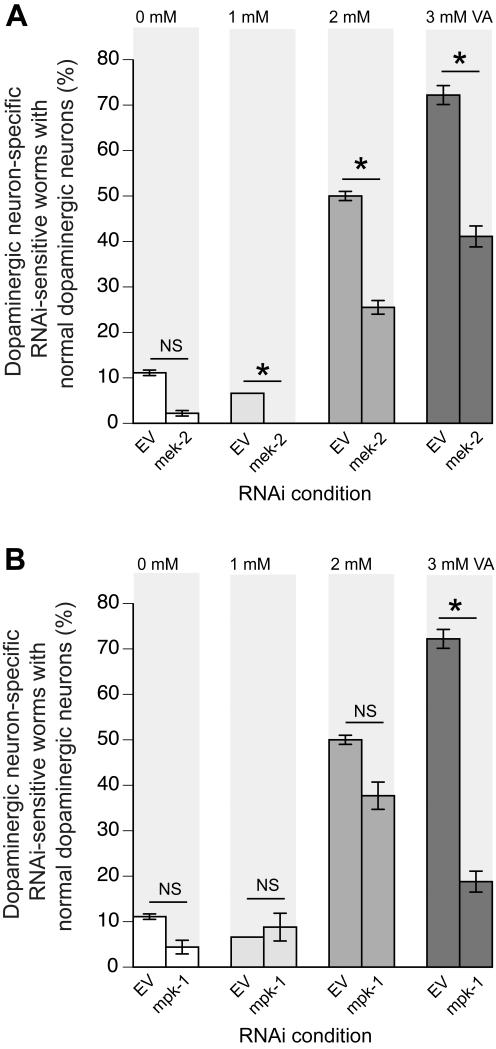
Figure 3. VA neuroprotection of α-Syn-overexpression in DA neurons is dependent on the ERK-MAPK signaling pathway.
(A) Cell-specific RNAi depletion of mek-2 negates the dopaminergic neuroprotection provided by VA at all concentrations of examined compared to empty vector (EV) control, where neuroprotection is still observed at 1, 2, and 3 mM VA. (B) Similar to A, mpk-1 depletion reverses neuroprotection of VA in dopaminergic neurons at 3 mM VA compared to EV control. P < 0.05; Student’s t-test.
Our results showed that VA is significantly neuroprotective against α-Syn neurodegeneration at 2mM and 3mM concentrations in day-7 adult animals. In untreated animals, only 11.1% of animals expressing α-Syn exhibit normal dopaminergic neurons (Figure 2). When animals were treated with 2mM of VA, the percentage of the population displaying the normal complement of dopamine neurons rose significantly to approximately 50%. There was an even greater increase when animals were treated with 3mM VA, as 72% of these animals displayed normal dopaminergic neurons at day 7 of development (Figure 2). There was a significant difference between the population of animals with normal neurons following treatment with 2 mM and 3 mM VA. In contrast, no significant protection was observed at 1mM (Figure 2). We could not analyze animals treated with 4mM VA (or higher) because 4 mM VA slowed the growth of animals to such an extent that at day 7 of development we were unable to obtain sufficient quantities of animals for analysis while at the lower concentrations there was an abundance of animals. Moreover, the size of the surviving animals treated with 4mM VA was significantly smaller than the non-treated animals, signifying a developmental delay. However, our results suggest that VA can protect the dopaminergic neurons of C. elegans against α-Syn toxicity at 2mM and 3mM concentrations.
We proceeded to ask whether VA protected the C. elegans dopaminergic neurons via ERK-MAPK. To answer this question we used cell-specific RNAi to deplete ERK-MAPK in the dopaminergic neurons. Until recently, it was difficult to achieve dopaminergic RNAi knockdown of target genes in C. elegans. However, new methods for neuronal-sensitive RNAi allow for selective knockdown of target genes in subsets of neurons. For example, the impact of candidates knocked down by RNAi can be examined exclusively in the dopaminergic neurons in our Pdat-1::α-syn + Pdat-1::GFP strain {UA196 [sid-1(pk3321); Pdat-1::α-syn, Pdat-1::GFP; Pdat-1::sid-1, Pmyo-2::mCherry]}. Worms expressing GFP without α-syn in the dopaminergic neurons {UA202 [sid-1(pk3321); Pdat-1::GFP; Pdat-1::sid-1, Pmyo-2::mCherry]} act as a control strain [10].
In C. elegans, the ortholog of ERK is encoded by the gene mpk-1 [16, 17, 27]. The direct upstream regulator of MPK-1 is MEK-2, which is a MAP2 kinase [5, 22, 28]. RNAi bacterial clones for mek-2 and mpk-1 were obtained from a comprehensive C. elegans RNAi library (MRC Cambridge) [14]. RNAi experiments were performed by growing RNAi bacteria (HT115) on agar plates containing 0.25% beta-lactose [19]. Gravid adult animals, UA196 α-Syn RNAi strain (described above), were transferred onto the RNAi plates and allowed to lay embryos for 24 hours. The offspring from these parental animals were left on the plates to eat HT115 bacteria containing the specified RNAi clones and were analyzed for neurodegeneration at day 7. The results of our RNAi experiments showed that depletion of mek-2 negates the protective effect of VA on the dopaminergic neurons of adult animals at all concentrations of VA tested (1, 2 and 3mM) compared to empty vector (EV) control (Figure 3A). In contrast, dopaminergic-selective RNAi against mpk-1 abolished neuroprotection only at the 3mM VA concentration in comparison to the EV control (Figure 3B). The difference in responses between mek-2 (RNAi) and mpk-1 (RNAi) could possibly have occurred because of differential levels of mpk-1 and mek-2 transcripts when worms are treated with different concentrations of VA. However, given that knockdown of both genes reverses the neuroprotective effect of VA compared to EV at one dose of VA implies that VA requires the presence of endogenous levels of MEK-2 and MPK-1 in order to protect the dopaminergic neurons from α-Syn toxicity. We therefore concluded that VA attenuates dopaminergic neurodegeneration via ERK-MAPK. However, we do not rule out the possibility that VA and ERK-MAPK may also act additively to protect the dopaminergic neurons.
What are the possible mechanisms and implications of our findings in relation to neuroprotection and PD? Treatment of neurons with VA can significantly enhance or prolong the phosphorylation of ERK-MAPK [1,9]. Such prolonged phosphorylation can lead to an increase in the amount of neurotrophic factors mediating positive neuronal growth through an unknown mechanism [9]. However, it has also been suggested that activation of p-ERK-MAPK by VA can impact downstream transcription factors resulting in altered nuclear gene expression and enhanced cell survival [6]. Interestingly, our data showed that VA significantly protected the dopaminergic neurons from the toxicity caused by α-Syn overexpression. Moreover, we showed that independent RNAi knockdowns of mek-2 and mpk-1 abolished the neuroprotective effect of VA on the dopaminergic neurons. This finding implicates ERK-MAPK as a plausible pathway mediating the neuroprotective effect of VA in the C. elegans dopaminergic neurons. Despite its pleiotropic roles in cells and tissues, ERK-MAPK is also an affirmed downstream component of D2 dopamine receptor signaling in the mammalian brain [29]. Such a role asserts that ERK-MAPK additionally plays a vital function in the regulation of various aspects of dopamine signaling and homeostasis. Coincidently, there is strong evidence that ERK-MAPK physically associates with α-Syn [13, 23], suggesting its potential role in PD. Moreover, transient overexpression of α-Syn in neuro2A cells suppressed the function of ERK-MAPK, resulting in decreased cell viability [13]. Such in vitro findings corroborate our findings in vivo, all of which affirm the notion that downregulation of ERK-MAPK mediates α-Syn induced cellular toxicity and neurodegeneration. In addition, studies using other cellular models of PD have also offered supporting evidence regarding the role of ERK-MAPK in cell viability. For instance, in one study it was reported that activation of ERK-MAPK is necessary for protection of dopaminergic cells against rotenone toxicity [11]. In another study, it was revealed that rapid activation of ERK-MAPK promoted the survival of dopaminergic neurons in the presence of 6-OHDA [18]. These findings corroborate the potential neuroprotective role of ERK-MAPK in the cell, and suggest that administration of VA may serve to enhance the neuroprotective function of this pathway. It is also important to note that cytoplasmic accumulation of phosphorylated ERK-MAPK has been detected in the substantia nigra of PD and Dementia Lewy Body (DLB) patients [8, 31], suggesting the potential link of this pathway to PD and other common Lewy Body diseases. Our finding that VA can ameliorate dopaminergic neurodegeneration via ERK-MAPK not only confirms VA as a neuroprotective compound, but also implicates ERK-MAPK signaling as the plausible molecular target of VA in the protection of the dopaminergic neurons.
HIGHLIGHTS.
Kautu et al., submission to Neuroscience Letters
The anti-epileptic drug, valproic acid, exhibits neuroprotective activity in an animal model of Parkinson’s disease
Valproic acid attenuates dopaminergic neurodegeneration associated with human α-synuclein overproduction in C. elegans
Neuroprotection by valproic acid is mediated through ERK-MAPK signaling in vivo
ACKNOWLEDGMENTS
We would like to acknowledge the outstanding collegiality of all members of the Caldwell lab. Funding for this study came from NIH grant 1R15NS075684-01 to GAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Giovanni SD, Lagrèze WA. Valproic Acid–Mediated Neuroprotection and Regeneration in Injured Retinal Ganglion Cells. Invest. Ophth. Vis. Sci. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- [2].Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-Mediated Protection from Cellular Stress in the Dopaminergic Neurons of Caenorhabditis elegans. J. Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Christian J, Ximenes M, Crisóstomo E, Verde1 L, Graça M, Viana NGSB. Valproic Acid, a Drug with Multiple Molecular Targets Related to Its Potential Neuroprotective Action. Neuroscience and Medicine. 2012;3:107–123. [Google Scholar]
- [5].Church DL, Guan KL, Lambie EJ. Three genes of the MAP kinase cascade, mek-2, mpk-1/sur-1 and let-60 ras, are required for meiotic cell cycle progression in Caenorhabditis elegans. Development. 1995;121:2525–2535. doi: 10.1242/dev.121.8.2525. [DOI] [PubMed] [Google Scholar]
- [6].Di Daniel E, Mudge AW, Maycox PR. Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disorders. 2005;7:33–41. doi: 10.1111/j.1399-5618.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- [7].Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and Models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- [8].Ferrer I, Blanco R, Carmona M, Puig B, Barrachina2 M, Gómez C, Ambrosio S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson’s disease and Dementia with Lewy bodies. J. Neural. Transm. 2001;108:1383–1396. doi: 10.1007/s007020100015. [DOI] [PubMed] [Google Scholar]
- [9].Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G. Mood Stabilizer Valproate Promotes ERK Pathway Dependent Cortical Neuronal Growth and Neurogenesis. J. Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harrington AJ, Yacoubian TA, Slone SR, Caldwell KA, Caldwell GA. Functional Analysis of VPS41-Mediated Neuroprotection in Caenorhabditis elegans and Mammalian Models of Parkinson’s Disease. J. Neurosci. 2012;32:2142–2153. doi: 10.1523/JNEUROSCI.2606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hsuan S, Klintworth HM, Xia Z. Basic Fibroblast Growth Factor Protects against Rotenone-Induced Dopaminergic Cell Death through Activation of Extracellular Signal-Regulated Kinases 1/2 and Phosphatidylinositol-3 Kinase Pathways. J. Neurosci. 2006;26:4481–4491. doi: 10.1523/JNEUROSCI.4922-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hunsberger J, Austin DR, Henter ID, Chen G. The neurotrophic and neuroprotective effects of psychotropic agents. Dialogues Clin. Neurosci. 2009;11:333–348. doi: 10.31887/DCNS.2009.11.3/jhunsberger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Iwata A, Maruyama M, Kanazawa I, Nukina N. alpha-Synuclein affects the MAPK pathway and accelerates cell death. J. Biol. Chem. 2001;276:45320–45329. doi: 10.1074/jbc.M103736200. [DOI] [PubMed] [Google Scholar]
- [14].Kamath SR, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapink A, Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- [15].Kidd SK, Schneider JS. Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2011;194:189–194. doi: 10.1016/j.neuroscience.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lackner MR, Kornfeld K, Miller LM, Horvitz HR, Kim SK. MAP kinase homolog, mpk-1, is involved in ras-mediated induction of vulval cell fates in Caenorhabditis elegans. Genes. Dev. 1994;8:160–173. doi: 10.1101/gad.8.2.160. [DOI] [PubMed] [Google Scholar]
- [17].Lee MH, Ohmachi S, Arur S, Nayak S, Franci R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germline development. Genetics. 2007;177:2039–2062. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J. Neurosci. Res. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- [19].Locke CJ, Kautu BB, Berry KP, Lee SK, Caldwell KA, Caldwell GA. Pharmacogenetic Analysis Reveals a Post-Developmental Role for Rac GTPases in Caenorhabditis elegans GABAergic Neurotransmission. Genetics. 2009;183:1357–1372. doi: 10.1534/genetics.109.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Monti B, Gatta V, Piretti F, Raffaelli SS, Virgili M, Contestabile A. Valproic Acid is Neuroprotective in the Rotenone Rat Model of Parkinson’s Disease, Involvement of α-Synuclein. Neurotox. Res. 2010;17:130–141. doi: 10.1007/s12640-009-9090-5. [DOI] [PubMed] [Google Scholar]
- [21].Monti B, Mercatelli D, Contestabile A. Valproic acid neuroprotection in 6-OHDA lesioned rat, a model for Parkinson’s disease. HOAJ Biology. 2012;1:1–4. [Google Scholar]
- [22].Okuyama T, Inoue H, Ookuma S, Satoh T, Kano K, Hisamoto S, Hisamoto N, Matsumoto K, Nishida E. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J. Biol. Chem. 2010:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J. Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- [25].Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- [26].Waxman EA, Giasson BI. Molecular mechanisms of α-synuclein neurodegeneration. Biochim. Biophys. Acta. 2009;1792:616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu Y, Han M. Suppression of activated let-60 ras protein defines a role of Caenorhabditis elegans Sur-1 MAP kinase in vulval differentiation. Genes. Dev. 1994;8:147–159. doi: 10.1101/gad.8.2.147. [DOI] [PubMed] [Google Scholar]
- [28].Wu Y, Han M, Guan KL. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes. Dev. 1995;9:742–755. doi: 10.1101/gad.9.6.742. [DOI] [PubMed] [Google Scholar]
- [29].Yan Z, Feng J, Fienburg AA, Greengard P. D2 dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc. Natl. Acad. Sci. U.S.A. 1999;196:11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yuan P, Huan L, Jian Y, Silvio J, Husseini G, Manji K, Chen G. The Mood Stabilizer Valproic Acid Activates Mitogen-activated Protein Kinases and Promotes Neurite Growth. J. Biol. Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- [31].Zhu J, Kulich SM, Oury TD, Chu CT. Cytoplasmic Aggregates of Phosphorylated Extracellular Signal-Regulated Protein Kinases in Lewy Body Diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]