Abstract
Background
The mu opioid receptor (MOR) has previously been found to regulate ethanol-stimulated dopamine release under some, but not all, conditions. A difference in ethanol-evoked dopamine release between male and female mixed background C57BL/6J-129SvEv mice led to questions about its ubiquitous role in these effects of ethanol. Using congenic C57BL/6J MOR knockout (KO) mice and C57BL/6J mice pretreated with an irreversible MOR antagonist, we investigated the function of this receptor in ethanol stimulated dopamine release
Methods
Microdialysis was used to monitor dopamine release and ethanol clearance in MOR −/−, +/+ and +/− male and female mice after intraperitoneal (i.p.) injections of 1.0, 2.0 and 3.0 g/kg ethanol (or saline). We also measured the increase in dopamine release after 5 mg/kg morphine (i.p.) in male and female MOR +/+ and −/− mice. In a separate experiment, male C57BL/6J mice were pretreated with either the irreversible MOR antagonist beta funaltrexamine (BFNA) or vehicle, and dopamine levels were monitored after administration of 2 g/kg ethanol or 5 mg/kg morphine.
Results
Although ethanol stimulated dopamine release at all the three doses of alcohol tested, there were no differences between MOR +/+, −/− and +/− mice in these effects. Female mice had a more prolonged effect compared to males at the 1 g/kg dose. Administration of 2 g/kg ethanol also caused a similar increase in dopamine levels in both saline pretreated and BFNA pretreated mice. Five mg/kg morphine caused a significant increase in dopamine levels in MOR +/+ mice but not in MOR −/− mice, and in saline pretreated mice but not in BFNA pretreated mice. Intraperitoneal saline injections had a significant, albeit small and transient, effect on dopamine release when given in a volume equivalent to the ethanol doses, but not in a volume equivalent to the 5 mg/kg morphine dose. Ethanol pharmacokinetics were similar in all genotypes and both sexes at each dose and in both pretreatment groups.
Conclusions
MOR is not involved in ethanol stimulated dopamine release in the ventral striatum of C57BL/6J mice.
Keywords: MOR, Beta funaltrexamine, Microdialysis, Dopamine, C57BL/6J mice
Introduction
The mesolimbic dopamine pathway, from the ventral tegmental area (VTA) to the nucleus accumbens (Weiss and Porrino, 2002), has been hypothesized to play an important role in the reinforcing and rewarding effects of ethanol (Gonzales et al., 2004; Pierce and Kumaresan, 2006). Systemic ethanol administration (Ramachandra et al., 2007; Tang et al., 2003; Yim et al., 2000) and voluntary consumption (Howard et al., 2009; Weiss et al., 1993) caused an increase in dopamine release in mesolimbic regions of the brain. In addition, altering dopamine receptor function either genetically (Risinger et al., 2000) or pharmacologically (Hodge et al., 1992; Samson et al., 1992) causes changes in ethanol consumption.
While the exact mechanisms underlying ethanol induced dopamine release are unclear, one potential mediator is the opioid system. Rats and mice pretreated with the nonselective opioid receptor antagonist, naltrexone, do not show elevated dopamine levels during ethanol self-administration and consumption compared to saline pretreated controls (Gonzales and Weiss, 1998; Middaugh et al., 2003). Under specific conditions, mice pretreated with naltrexone also showed reduced ethanol intake (Kamdar et al., 2007; Middaugh et al., 1999; Phillips et al., 1997) compared to controls.
Of the three main opioid receptor subtypes, there is the greatest evidence implicating the mu opioid receptor (MOR) as a mediator of ethanol intake and mesolimbic dopamine release induced by ethanol. Voluntary ethanol consumption and self administration were reduced in MOR −/− mice when compared to MOR +/+ controls (Hall et al., 2001; Roberts et al., 2000), and also in HAD (High-Alcohol-Drinking) rats pretreated with the irreversible MOR antagonist, beta funaltrexamine (BFNA) (Krishnan-Sarin et al., 1998). MOR agonists microinjected into the paraventricular nucleus of the hypothalamus increased dopamine concentrations in the nucleus accumbens, while an antagonist decreased accumbal dopamine levels (Rada et al., 2010). Also, MOR activation in the VTA led to an increase in somatodendritic dopamine levels (Chefer et al., 2009).
With respect to ethanol-induced dopamine release, systemic administration of the mu1 receptor antagonist, naloxonazine, prevented increases in extracellular dopamine in rats that were injected with ethanol (Tanda and Di Chiara, 1998). In a previous study we also demonstrated that congenic C57BL/6J and mixed background C57BL/6J-129SvEv MOR −/− mice had a blunted dopamine response compared to MOR +/+ controls when injected intraperitoneally (i.p.) with a 2 g/kg dose of ethanol, with female mice showing a greater effect in animals on the mixed background (Job et al., 2007). Additionally, in the same study a pharmacological experiment using C57BL/6J-129SvEv MOR +/+ male and female mice pretreated with the mu1 receptor antagonist naloxonazine showed a blunted dopamine response to ethanol only in female mice. These initial studies were limited in several respects, including the dose range examined; nonetheless, from these observations it would appear that although mu opioid receptors regulate ethanol-induced dopamine release under some conditions, this is not the sole mechanism by which ethanol acts to elevate dopamine levels. Mu opioid receptor mediation of ethanol-induced dopamine release may be dependent on both sex and strain (e.g. genetic background).
Therefore, to further explore and extend these findings, we examined the effects of sex and two additional ethanol doses (1 and 3 g/kg i.p.) on ventral striatal dopamine release in MOR +/+, +/− and −/− mice from the congenic C57BL/6J KO line. To complement this genetic study, we examined the effect of 2 g/kg i.p. ethanol injection on mesolimbic dopamine release in male C57BL/6J mice after pharmacological blockade of mu receptors with the non-competitive mu receptor antagonist beta funaltrexamine (BFNA) (Portoghese et al., 1980; Ward et al., 1985).
Materials and Methods
Subjects
Congenic C57BL/6J +/+, +/− and −/− MOR KO mice (male and female, 8 – 24 months old, 19–39 g) were bred at the University of Texas at Austin, while mice used in the pharmacological experiments (male only, 2–3 months old, 20–26 g) were obtained from Jackson Laboratories (Bar Harbor, ME). The congenic line consisted of mice that were created by backcrossing the C57BL/6J strain onto the mixed background C57BL/6J-129SvEv MOR KO strain for 10–12 generations as previously described (Job et al., 2007). All mice were housed in groups of 3 – 5 per cage prior to surgery and singly housed after surgery. The vivarium was maintained at a constant temperature (23 °C), on a 12 h light/dark cycle (lights on 7:00 AM), with food and water available ad libitum. All procedures used were conducted under protocols approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin and in accordance with guidelines described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Genotyping
Genotyping was performed using Polymerase chain reaction with 2 mm ear tissue digests exactly as described in Job et al. (2007). Ear punch tissue was incubated at 55° C overnight with a mixture of Proteinase K and Direct PCR Lysis Reagent (Ear) (Viagen Biotech, Inc., Los Angeles, CA). The digests were then placed in an 85° C heat block for 45 min and the supernatant was analyzed using PCR. The primers used were as follows: mu-1 (5′CTGGATGAGCTGTAAGAATAGG3′), mu-2 (5′CAGCCAACACAATATCACATTC3′) and mu-neo (5′CGGACAGGTCGGTCTTGAC3′). This produced 550 and 800 bp PCR products for the wildtype and knockout genotypes, respectively. Tfl polymerase (Epicentre, Madison, WI) was used under the following conditions: 2 minutes at 94° C, 40 cycles of (a) 94° C for 30 seconds, (b) 55° C for 1 minute, (c) 72° C for 2 minutes; followed by an extension at 72° C for 7 minutes and 4° C hold. Amplicons were separated by gel electrophoresis on 2% MetaPhor agarose gels and bands were visualized under UV illumination.
Microdialysis
Surgery and microdialysis procedures were performed as previously described in Tang et al. (2003) and Ramachandra et al. (2007). Briefly, the animals were anesthetized with 2.5% isoflurane gas and then implanted with a 10 mm long, stainless steel guide cannula (21 gauge) over the left ventral striatum using the following coordinates: +1.7 mm anterior to bregma, −0.8 mm lateral of the midline, and −2.0 mm ventral to the surface of the brain. Animals were allowed at least 3 days for recovery after surgery and received once daily injections of 0.1 ml saline during this time to habituate them to the injection procedure. Between 14 – 18 hours prior to sample collection, microdialysis probes (active area 1 mm, constructed as described in Ramachandra et al. 2007) were inserted into the guide cannulae while mice were under isoflurane sedation. The overnight flow rate was set to 0.2 μl/min; at least two hours prior to sample collection, the flow rate was increased to 1 μl/min. Microdialysis experiments, brain extraction and histological analysis were then performed exactly as described in Ramachandra et al. (2007).
Experimental Design
The effects of 1, 2 and 3 g/kg ethanol on dopamine release in congenic C57BL/6J MOR KO mice
The number, sex and genotype of mice used at each dose are listed in Table 1. Mice were injected i.p. with a dose of 1, 2 or 3 g/kg ethanol (15% v/v in normal saline) in addition to volume equivalent saline injections at each ethanol dose, in a within subject control design, as described previously in Ramachandra et al. (2007). Briefly, 4 basal samples were collected at 15-minute intervals after which all animals received volume equivalent saline injections (i.p.). Six more samples were then collected, after which all subjects received an injection of ethanol (1, 2 or 3 g/kg i.p., 15% ethanol/saline w/v). Four more samples were then collected after the injection of 1 g/kg, or eight samples after the 2 and 3 g/kg doses of ethanol. Finally, to ensure dopamine concentrations were due to exocytotic release, the perfusate was switched to calcium free ACSF for at least one hour, after which time two more samples were collected to determine the calcium dependency of the dopamine measured in dialysates.
Table 1.
Basal dialysate dopamine concentrations and peak dialysate ethanol concentrations in MOR +/+, −/− and +/− male (M) and female (F) C57BL/6J mice. Values represent mean concentrations ± SEM.
| Experiment | Sex | Genotype | Basal DA (nM) | Peak EtOH (mM) | n |
|---|---|---|---|---|---|
|
| |||||
| 1g/kg | M | +/+ | 1.2 ± 0.1 | 4.1 ± 0.8 | 5 |
| −/− | 1.1 ± 0.3 | 4.9 ± 0.6 | 3 | ||
| +/− | 1.4 ± 0.2 | 4.7 ± 0.6 | 6 | ||
|
| |||||
| F | +/+ | 1.4 ± 0.1 | 5.4 ± 0.4 | 5 | |
| −/− | 1.6 ± 0.4 | 5.0 ± 0.6 | 6 | ||
| +/− | 1.5 ± 0.2 | 4.5 ± 0.4 | 6 | ||
|
| |||||
| 2g/kg | M | +/+ | 1.2 ± 0.4 | 11.1 ± 1.1 | 6 |
| −/− | 1.6 ± 0.5 | 10.5 ± 1.5 | 5 | ||
| +/− | 1.3 ± 0.2 | 10.4 ± 0.8 | 7 | ||
|
| |||||
| F | +/+ | 1.2 ± 0.4 | 10.5 ± 0.3 | 5 | |
| −/− | 1.2 ± 0.3 | 10.9 ± 1.1 | 6 | ||
| +/− | 1.3 ± 0.2 | 9.5 ± 0.8 | 6 | ||
|
| |||||
| 3g/kg | M | +/+ | 1.1 ± 0.3 | 14.6 ± 1.0 | 5 |
| −/− | 1.4 ± 0.5 | 14.7 ± 1.3 | 4 | ||
| +/− | 1.5 ± 0.3 | 14.6 ± 0.5 | 8 | ||
|
| |||||
| F | +/+ | 1.1 ± 0.3 | 14.9 ± 0.8 | 6 | |
| −/− | 1.3 ± 0.3 | 13.7 ± 1.4 | 8 | ||
| +/− | 1.2 ± 0.3 | 16.7 ± 1.9 | 7 | ||
|
| |||||
| Morphine | +/+ | 1.2 ± 0.3 | — | 9 | |
| — | (5M, 4F) | ||||
|
| |||||
| −/− | 0.9 ± 0.2 | — | 7 | ||
| — | (5M, 2F) | ||||
The effects of 5 mg/kg morphine in congenic C57BL/6J MOR KO mice
The number and sex of the mice in each genotype is listed in Table 1. Similar to the previous experiment, a within subject saline control design was used to examine congenic C57BL/6J MOR +/+ and −/− mice. After four basal samples were collected at 15-minute intervals, animals were injected with a volume equivalent bolus of saline (i.p.). Four more 15 minute samples were collected after which animals received a dose of 5 mg/kg morphine (1 mg/mL solution in normal saline, i.p.). We then collected three 30 minute samples. After a 1.5 hour interval two additional 15 minute samples were collected to establish a new baseline for assessment of calcium dependency as described above.
The effects of BFNA pretreatment on ethanol and morphine induced dopamine release in male C57BL/6J mice
Mice were pretreated with either 40 mg/kg BFNA (2 mg/mL solution in normal saline) or volume equivalent saline 20 – 22 hours prior to the start of sample collection. This dose of BFNA was chosen based on studies of BFNA antagonism of morphine antinociception (Narita et al., 2002, Pick et al., 1991). On the day of microdialysis testing, mice received either 5 mg/kg morphine or 2 g/kg ethanol. Experiments were conducted with a within subject saline control condition in a time-line similar to that described above.
Dopamine and Ethanol Analysis
Dopamine in dialysate samples was analyzed using reverse phase HPLC. For approximately half the experimental sample sets, an autosampler system was used as described in Howard et al. (2008). The rest of the sample sets were analyzed using a manual injector. Here, 1.5 – 2 μL ascorbate oxidase was added to 7 – 10 μL of each dialysate sample. 5 – 6 μL of this mixture was then injected using an 8125 manual injector onto a 50x1, C18 column (Phenomenex). Dopamine was detected using a 2 mm glassy carbon working electrode with either a salt-bridge Ag/AgCl reference set at a potential of 450 mV, or an in situ Ag/AgCl (ISAAC) reference electrode set at a potential of 345 mV . The signal to noise ratio (S/N) for our lowest basal dialysate sample was no less than 7, while the S/N for our lowest dopamine standard was no less than 10. Dialysate ethanol concentrations were investigated exactly as described in Doyon et al. (2003) and Tang et al. (2003). Briefly, 2 μL of each sample taken after ethanol administration was immediately analyzed using gas chromatography with flame ionization detection. External dopamine and ethanol standards were used for quantification.
Statistical Analysis
Repeated measures analysis of variance (ANOVA) was used on percent basal dopamine values to test for significance of main effects as well as interactions. We defined the basal dopamine concentration as the last two pre-injection samples. The effect of saline injection was analyzed separately from the effects of ethanol and morphine. In all analyses that used repeated measures ANOVA, time after injection was the within subject variable.
Genotype and sex were the between subject variables for congenic animals that received 1, 2 or 3 g/kg i.p. ethanol administration. For congenic animals that received 5 mg/kg morphine, the data were first analyzed with only sex as the between subject variable. When no significant effect of sex was found, the data were reanalyzed with only genotype as the between subject variable. For mice used in the BFNA experiments, pretreatment was the between subject variable. For the analyses of basal dopamine concentrations and ethanol clearance, dose was an additional between subject variable.
If significant interactions involving the time factor were found, post hoc tests compared the last two pre-injection time points to each subsequent post-injection time point. One subject was excluded as a statistical outlier for an abnormal dopamine response to saline injection.
Univariate ANOVA was used to analyze basal dopamine concentrations (average of the last two basal samples), to ensure no differences existed between congenic MOR +/+, −/− and +/− mice. Dopamine concentration was the dependent variable. A separate analysis was carried out on vendor obtained C57BL/6J mice used for the BFNA experiment.
For the analysis of ethanol levels in dialysates, time was the repeated measure. We also analyzed a measure of the ethanol clearance rates by calculating the slope of the linear portion of the dialysate ethanol time-course in individual animals. Univariate ANOVA was then used to determine statistical differences, with this measure of clearance as the dependent variable.
We defined the criterion for statistical significance as p < 0.05 for all analyses. Bonferroni corrections were used for post hoc tests, and values are reported as mean ± S.E.M. Missing points were accounted for by averaging the values of the two adjacent points, and adjusting the degrees of freedom for the within subjects error term accordingly.
Results
The basal dialysate dopamine concentration, peak ethanol concentration and number of animals used in each experimental design are listed in Table 1. Basal dopamine concentrations in congenic C57BL/6J mice did not differ between genotypes [F(2,105) = 0.56, p > 0.05] or sexes [F(1,105) = 0.003, p > 0.05].
The effects of 1, 2 and 3 g/kg ethanol on dopamine release in congenic C57BL/6J MOR KO mice
Microdialysis probe placements for male and female congenic C57BL/6J MOR KO mice (+/+, +/− and −/−) are shown in Figures 1A thru D, respectively. Only those subjects that had at least 50% of the active dialysis membrane (1.0 mm) in the ventral striatum and at least a 40% decrease from baseline dopamine levels when perfused with calcium-free ACSF were included in the study.
Figure 1.
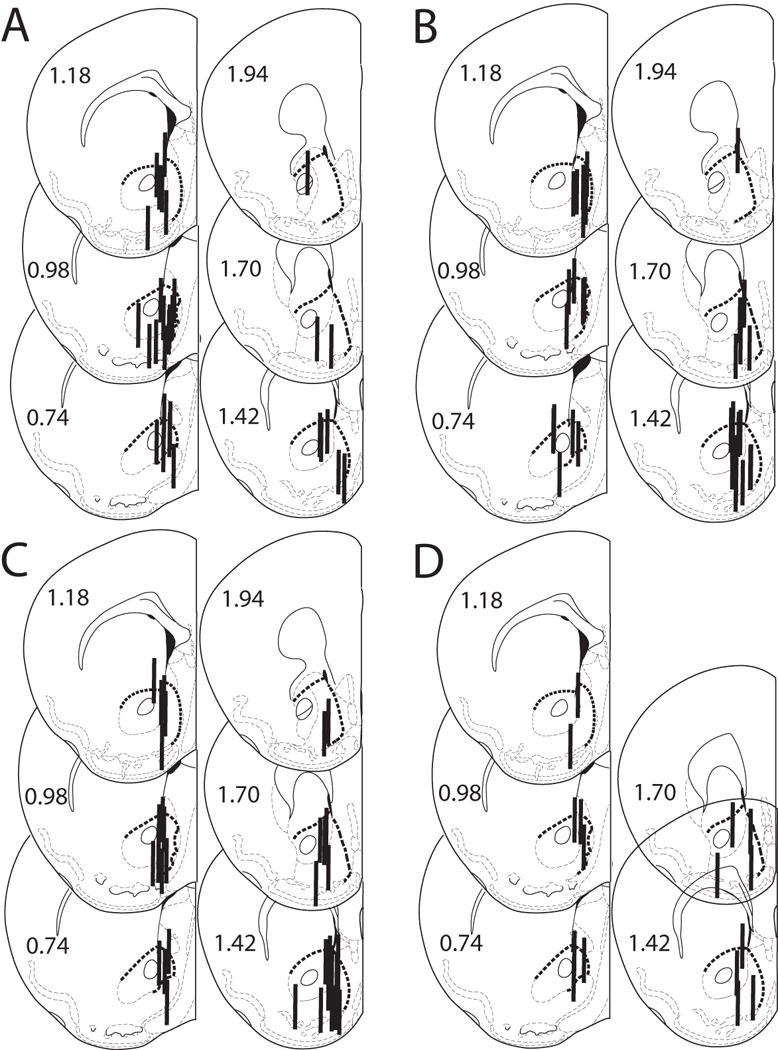
A – D. Histological analysis of probe placements in the ventral striatum of MOR +/+, −/− and +/− male and female mice. Numbers beside each slice represent the AP position relative to bregma (mm). The heavy dashed line indicates the border of the ventral striatum. Probe length is 1 mm. A. 1 g/kg ethanol, n = 31. B. 2 g/kg ethanol, n = 35. C. 3 g/kg ethanol, n = 38. D. 5 mg/kg morphine, n = 16.
At the 1 g/kg dose, ethanol injection significantly increased dopamine levels by 10 – 28% above basal concentrations (Figure 2A, B). There was no genotype x time interaction; however, a significant sex x time interaction at this dose was observed (Table 2). Post hoc tests revealed that female mice had a significantly elevated dopamine response for the entire hour after the ethanol injection [F(2,125) ≥ 9.1, p < 0.05], while in male mice only the first 15 minute time point after the injection was significantly elevated above baseline [F(2,125) = 27.1, p < 0.05]. Although there were differences in the time course of dopamine release, the ethanol time-course at this dose was similar across genotypes and sexes (Figure 2C, D) (Table 3).
Figure 2.
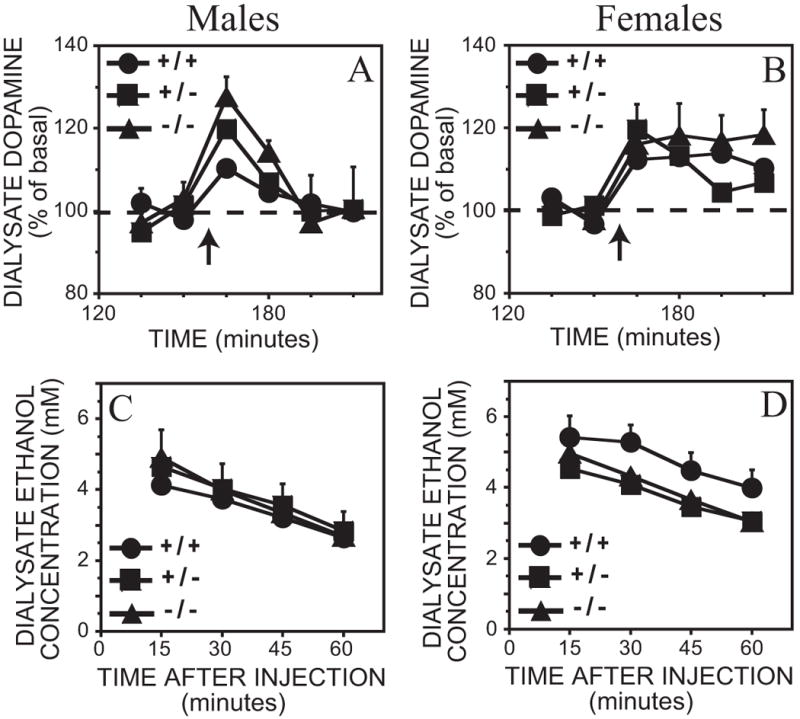
A – D. Effect of 1 g/kg ethanol intraperitoneal injection on dialysate dopamine and ethanol concentrations from the ventral striatum in MOR +/+, −/− and +/− mice. Each point represents the mean ± representative SEM. In the interests of clarity, only error bars portraying the greatest amount of variation are included. A. Dopamine levels in male mice. B. Dopamine levels in female mice. Time of ethanol injection is indicated by the arrow. The ethanol content from the above dialysate samples are shown in C. Male mice and D. Female mice.
Table 2.
Results from repeated measures ANOVA for dopamine response after 1, 2 and 3 g/kg ethanol and 5 mg/kg morphine. * p < 0.05.
| Type of factor | Source of variation | 1 g/kg | 2 g/kg | 3 g/kg | Morphine | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| df | F | df | F | df | F | df | F | ||
| Between | Error | 25 | 29 | 32 | 14 | ||||
| Sex | 1 | 1.5 | 1 | 1.0 | 1 | 1.9 | 1 | 0.1 | |
| Genotype | 2 | 1.2 | 2 | 0.4 | 2 | 0.04 | 1 | 3.2 | |
| Sex by genotype | 2 | 0.7 | 2 | 0.05 | 2 | 3.5* | |||
|
| |||||||||
| Within | Error | 125 | 257 | 287 | 56 | ||||
| Time | 5 | 17.5* | 9 | 35.4* | 9 | 39.0* | 4 | 9.9* | |
| Time by sex | 5 | 4.0* | 9 | 0.5 | 9 | 1.1 | 4 | 0.1 | |
| Time by genotype | 10 | 1.7 | 18 | 0.8 | 18 | 1.1 | 4 | 3.2* | |
| Time by sex by genotype | 10 | 1.2 | 18 | 1.0 | 18 | 1.5 | |||
Table 3.
Results from repeated measures ANOVA for ethanol time course after 1, 2 and 3 g/kg ethanol. * p < 0.05.
| Type of factor | Source of variation | 1 g/kg | 2 g/kg | 3 g/kg | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| df | F | df | F | df | F | ||
| Between | Error | 25 | 29 | 32 | |||
| Sex | 1 | 1.6 | 1 | 0.8 | 1 | 0.2 | |
| Genotype | 2 | 0.5 | 2 | 0.6 | 2 | 0.8 | |
| Sex by genotype | 2 | 1.4 | 2 | 0.3 | 2 | 0.9 | |
|
| |||||||
| Within | Error | 74 | 201 | 223 | |||
| Time | 3 | 100* | 7 | 153* | 7 | 130* | |
| Time by sex | 3 | 0.6 | 7 | 2.4* | 7 | 0.5 | |
| Time by genotype | 6 | 1.4 | 14 | 0.4 | 14 | 0.7 | |
| Time by sex by genotype | 6 | 0.1 | 14 | 0.6 | 14 | 0.4 | |
The 2 g/kg ethanol dose caused a significant 36 – 58% increase in dopamine levels above basal values with no significant genotype or sex x time interactions (Figure 3A, B and Table 2). The ethanol time course at this dose was similar across genotypes but a sex difference was observed (Figure 3C, D and Table 3). Although the effect of sex was significant overall, post hoc tests revealed that there was no significant difference between ethanol concentrations at any individual time-point between male and female subjects [F(1,230) ≤ 3.7, p > 0.05].
Figure 3.
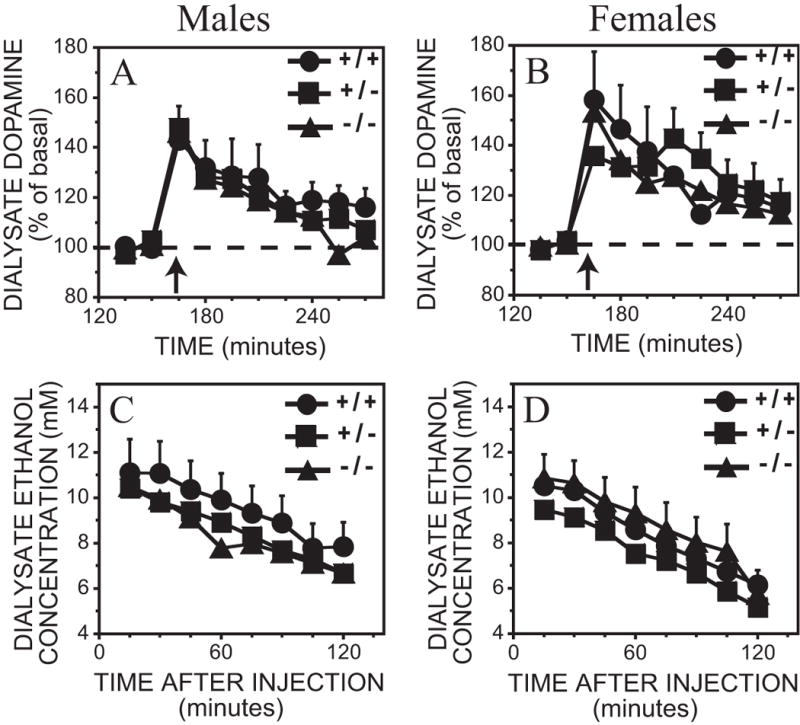
A – D. Effect of 2 g/kg ethanol intraperitoneal injection on dialysate dopamine and ethanol concentrations from the ventral striatum in MOR +/+, −/− and +/− mice. Each point represents the mean ± representative SEM. In the interests of clarity, only error bars portraying the greatest amount of variation are included. A. Dopamine levels in male mice. B. Dopamine levels in female mice. Time of ethanol injection is indicated by the arrow. The ethanol content from the above dialysate samples are shown in C. Male mice and D. Female mice.
At the highest ethanol dose, 3 g/kg, dopamine levels increased by 37 – 61% above basal values (Figure 4A, B). There were no significant genotype or sex by time interactions; however, a significant effect of sex x genotype was observed (Table 3). Post hoc tests revealed a sex difference in heterozygous mice only [F(1,32) = 10.1, p < 0.05]; male MOR +/− mice had less of a dopamine response to 3 g/kg ethanol than female MOR +/− mice. However, when the data were analyzed using raw dopamine concentrations as opposed to percent basal values, no significant main or interaction effects were observed. The ethanol time-course at this dose was also similar across genotypes and sex (Figure 4C, D and Table 3).
Figure 4.
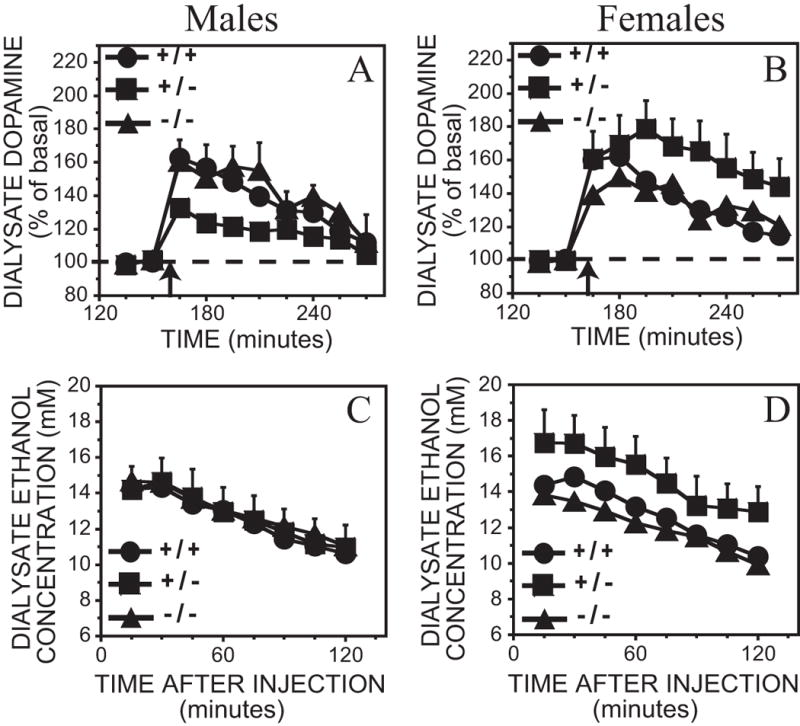
A – D. Effect of 3 g/kg ethanol intraperitoneal injection on dialysate dopamine and ethanol concentrations from the ventral striatum in MOR +/+, −/− and +/− mice. Each point represents the mean ± representative SEM. In the interests of clarity, only error bars portraying the greatest amount of variation are included. A. Dopamine levels in male mice. B. Dopamine levels in female mice. Time of ethanol injection is indicated by the arrow. The ethanol content from the above dialysate samples are shown in C. Male mice and D. Female mice.
Saline injections caused a slight, and short-lasting dopamine increase (5 – 20%) in all groups of mice, observed only at the first 15 minute time point [F(2,122) = 5.3, p < 0.05 for the 1 g/kg dose; F(2,142) = 13.0, p < 0.05 for the 2 g/kg dose; F(2,157) = 17.1, p < 0.05 for the 3 g/kg dose]. No genotype or sex differences were observed after saline injection (data not shown). Dialysate ethanol clearance also did not differ between genotypes [F(2,86) = 0.40, p > 0.05] or sex [F(1,86) = 0.03, p > 0.05].
The effects of 5 mg/kg morphine on dopamine release in congenic C57BL/6J MOR KO mice
To ensure that our MOR −/− mice had a functional gene deletion, we conducted positive control experiments using i.p. morphine injections. The 5 mg/kg morphine injection caused a 66 ± 20% increase in dopamine concentrations in MOR +/+ mice and a non-significant 22 ± 11% increase above basal dopamine concentrations in MOR −/− mice. This was confirmed by a significant genotype x time interaction. No sex differences were observed (Figure 5 and Table 2). Post hoc tests revealed that the last two post-morphine time points were significantly higher than basal concentrations in MOR +/+ mice [F(2,56) ≥ 17.0, p < 0.05] , while MOR −/− mice did not show a significant elevation in dopamine levels compared to baseline values [F(4,56) = 0.9, p > 0.05]. Saline did not significantly affect dopamine concentrations compared with basal levels [F(5,70) = 2.0, p > 0.05] (data not shown).
Figure 5.
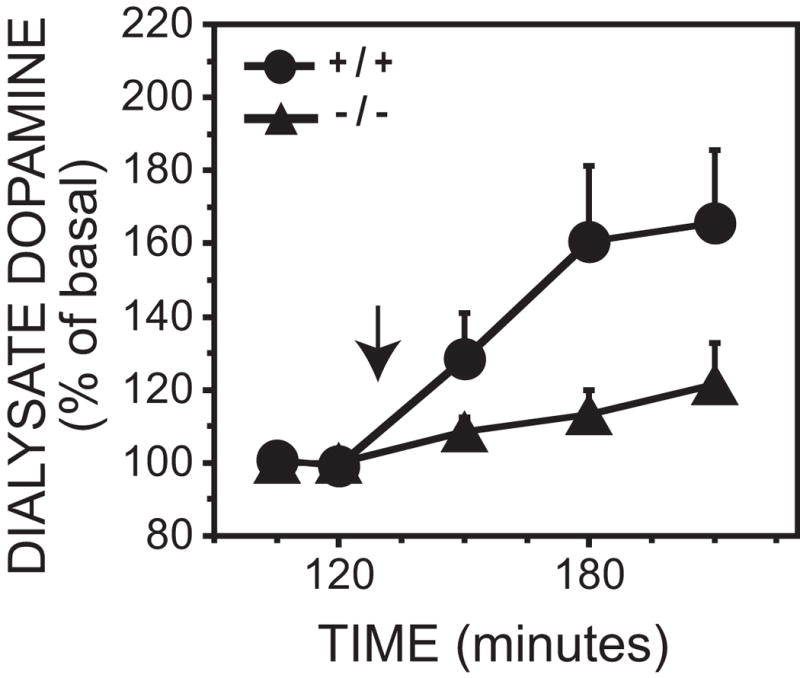
Effect of 5 mg/kg morphine intraperitoneal injection on dialysate dopamine concentrations from the ventral striatum in MOR +/+ and −/− mice. Each point represents the mean ± SEM. Time of morphine injection is indicated by the arrow.
The effects of BFNA on ethanol and morphine induced dopamine release in male C57BL/6J mice
Microdialysis probe placements for BFNA pretreated male C57BL/6J mice are shown in Figures 6A and B. The criteria for inclusion were the same as those stated previously.
Figure 6.
Histological analysis of probe placements in the ventral striatum of male mice pretreated with BFNA. Numbers beside each slice represent the AP position relative to bregma (mm). The heavy dashed line indicates the border of the ventral striatum. Probe length is 1 mm. A. 5 mg/kg morphine, n = 14. B. 2 g/kg ethanol, n = 11.
BFNA did not affect basal dopamine concentrations [F(1,21) = 0.70, p > 0.05]. Morphine injection (i.p.) caused a 54 ± 20% increase in dopamine levels in saline pretreated animals while only a small and non-significant increase was observed in BFNA pretreated animals, resulting in a significant pretreatment x time interaction [F(4,47) = 3.3, p < 0.05] (Figure 7A). Post hoc tests showed that saline pretreated mice had elevated extracellular dopamine levels at the 60 and 90 minute time points after the morphine injection [F(2,47) ≥ 14.7, p < 0.05], while BFNA pretreated mice did not show a significant increase in extracellular dopamine levels at any time-point [F(4,47) = 0.44, p > 0.05]. Saline injection did not cause an increase in extracellular dopamine levels in either group [F(5,59) = 0.9, p > 0.05] (data not shown).
Figure 7.
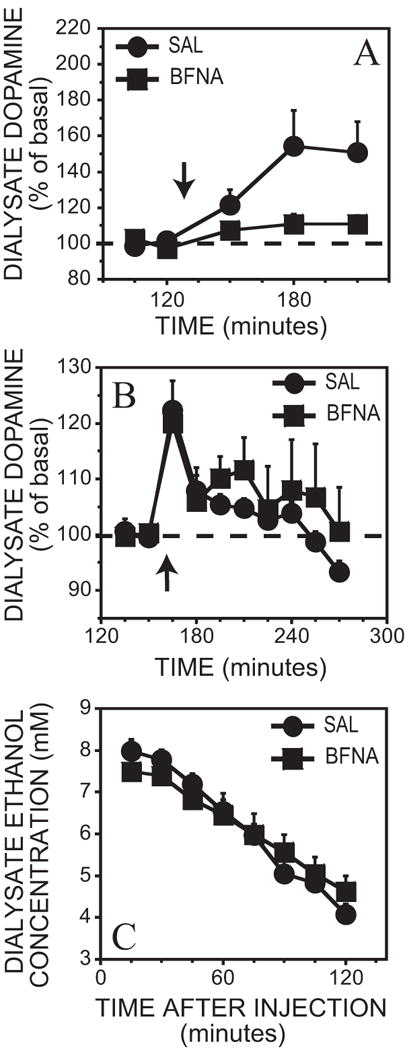
A. Effect of 5 mg/kg morphine intraperitoneal injection on dialysate dopamine concentrations from the ventral striatum in BFNA pretreated (n = 6; average basal = 1.2 ± 0.2 nM) and saline pretreated mice (n = 8; average basal = 0.9 ± 0.2 nM). Time of morphine injection is indicated by the arrow.
B – C. Effect of 2 g/kg ethanol intraperitoneal injection on dialysate dopamine and dialysate ethanol concentrations from the ventral striatum in BFNA pretreated (n = 5; average basal = 0.9 ± 0.2 nM) and saline pretreated mice (n = 6; average basal = 0.9 ± 0.1 nM). Each point represents the mean ± SEM. Time of ethanol injection is indicated by the arrow.
The 2 g/kg ethanol injection (i.p.) caused a significant increase in dopamine levels in both saline and BFNA pretreated mice [F(5,45) = 12.7, p < 0.05]: a 22 ± 5% increase in saline animals and a 20 ± 3% increase in BFNA animals (Figure 7B). No significant pretreatment x time effect was observed [F(5,45) = 0.8, p > 0.05]. Saline did not significantly affect dopamine concentrations compared to basal levels [F(2,62) ≤ 4.2, p > 0.05] (data not shown). With respect to the dialysate ethanol time course (Figure 7C), although an overall effect of pretreatment x time was observed [F(7,63) = 5.52, p < 0.05], post hoc analysis revealed that there was no significant difference in ethanol concentrations at each individual time point between the two pretreatment groups. Dialysate ethanol clearance rate also did not differ between the two groups [F(1,9) = 3.51, p > 0.05].
Discussion
Our results indicate that MOR is not involved in ethanol-stimulated dopamine release in C57BL/6J mice. Similar increases in extracellular dopamine concentrations were observed between congenic C57BL/6J MOR +/+, +/− and −/− mice at all three doses of alcohol tested. Furthermore, in C57BL/6J male mice, pretreatment with the irreversible MOR antagonist BFNA did not affect dopamine release after the administration of 2 g/kg ethanol. Taken together, the studies presented here provide strong evidence that MOR is not a critical link in the mechanism by which ethanol stimulates dopamine release in the ventral striatum.
These findings are surprising considering the results of the previous study of ethanol stimulated dopamine release in MOR −/− mice (Job et al., 2007). In that study, a blunted dopamine response to ethanol was observed in both male and female C57BL/6J MOR −/− mice and in female mixed background C57BL/6J-129SvEv MOR −/− mice compared to their MOR +/+ littermates. Pretreatment with the mu1 receptor antagonist, naloxonazine, abolished ethanol stimulated dopamine release in female C57BL/6J-129SvEv MOR +/+ mice, but not in males, consistent with the sex-dependent effects of the knockout in that strain. Therefore, to ensure the functional deficiency of the congenic C57BL/6J MOR −/− mice in the present experiments and to verify that the dose of BFNA used antagonized MORs in C57BL/6J male mice, positive control experiments were conducted using 5 mg/kg (i.p.) morphine. This dose of morphine was unable to evoke an increase in dopamine release in MOR −/− mice or in C57BL/6J male mice pretreated with 40 mg/kg BFNA (s.c.). Although we cannot exclude the possibility that BFNA may have different effects in female C57BL/6J mice or that there may be slight genetic variations between wild type mice in our MOR knockout colony and C57BL/6J mice, the data we present here support our major conclusion that MOR does not play a critical role in the mechanism of ethanol-induced dopamine release in the mesolimbic dopamine system.
There are several potential explanations for the differences between the present results and these earlier observations. Firstly, differences in experimental design could have contributed to these divergent results. Job et al. (2007) used between subject saline controls, while the current study used a within subject saline control design. To test this hypothesis, we conducted an additional experiment (n = 4–5) comparing MOR +/+ and MOR −/− mice after injection with only 2 g/kg ethanol (data not shown). No differences in ethanol-induced dopamine release were observed. It must be noted that this is the same strain as the congenic strain reported in the Job et al. (2007) study.
Although this is the same congenic strain that was used in the Job et al. (2007) study, environmental or genetic factors may have altered the characteristics of the strain in the intervening 4–5 years between the present study and the previous one. The initial subjects for the current experiments were derived from the colony of congenic C57BL/6J mice generated as described in Hall et al. (2003), but were 3 generations removed from the original experiments. During this intervening time, environmental changes may have led to epigenetic changes in our MOR −/− strain of mice (for review, see Whitelaw and Whitelaw, 2006). In particular, we experienced a change in rodent chow distributor for our vivarium at the start of the experiments. Changes in diet may in turn lead to changes in DNA methylation which in turn promote changes in gene expression and animal phenotype (Cooney et al., 2002, Waterland et al., 2006, Waterland and Jirtle, 2003). Therefore, we cannot discount the possibility that epigenetic influences in our line of MOR KO mice led to the differences between our findings and those described in Job et al. (2007).
To minimize genetic drift or effects of selection, two additional backcrosses were performed during the course of the present experiments, by breeding MOR +/− males to C57BL/6J females; subsequent mice were then bred from heterozygous crosses of the offspring. The original congenic C57BL/6J mice, although technically “congenic”, in all likelihood retained a certain amount of “flanking” genetic material from the original 129 ES cells around the site of the transgene (for review, see Gerlai, 1996). In order for the phenotype to have altered between the 10th and 12th backcrosses there must have been a recombination event near the site of the transgene, which would then alter the phenotype from a 129-like phenotype to a C57-like phenotype. This implies that the mechanism controlling ethanol-induced dopamine release is different in the two strains (opioid dependent and independent respectively) and that in the original study (Job et al., 2007) the effects of the knockout and these flanking genes were confounded. In addition, there has been at least one other case in the literature where a particular ethanol-related phenotype in knockout animals cannot be replicated (i.e., increased ethanol consumption in 5-HT1B −/− mice on the mixed 129 substrain background, for review, see Crabbe et al., 2006).
Although the spectre of flanking gene effects has always been a major possible confound in transgenic studies, there has been surprisingly little experimental work on this problem. Perhaps this is in part because the probability of such a confounding effect is low given that genes which are involved in the same trait tend to be distributed across the genome rather than next to each other. For example, genome wide association studies of addiction repeatedly identify particular chromosomal regions that are involved in addiction, but these are spread across the genome (Johnson et al., 2006; Uhl et al., 2009). However, recently an example of this phenomenon was characterized precisely because the gene of interest (KEPI; Ppp1r14c) was being examined for a phenotype (morphine-induced analgesia) that was known to be substantially determined by a neighboring gene (MOR) (Drgonova et al., 2010). In the initial analysis of the original KEPI −/− mice in which the transgene was expressed on a mixed 129S6-C57BL/6J genetic background, substantial phenotypic differences were observed. However, in recombinant KEPI −/− mice that were generated to have minimal amounts of 129S6 DNA surrounding the transgene, some of the effects observed in the original strain were shown to have resulted from 129 flanking genes, while others remained, and were likely to have indeed been the result of the transgene.
Although there were a number of differences, both the current study and the Job et al. (2007) study identified particular responses that differed between male and female subjects. When collapsed across genotype, female mice had a prolonged elevation in dopamine levels, while male mice only had a brief increase in dopamine release after 1 g/kg ethanol injection. This significant difference in dopamine response between sexes was confirmed when statistics were performed on raw dopamine concentrations. We also report a sex x genotype interaction effect at the 3 g/kg ethanol dose. The sex difference at this dose was only seen in heterozygous animals and only when the analysis was performed on the percent basal dopamine data. No difference in dopamine response between male and female mice at the 3 g/kg dose was observed when statistics were performed on the raw dopamine concentrations. Therefore, caution must be used when interpreting this particular finding. However, we cannot discount the possibility of an “overdominance” effect in our heterozygous mice, which is defined as a behavioral effect that is greater in the heterozygous animal than in either of the homozygous parental strains. For example, studies have shown that the hybrid offspring of C57BL/6J x FVB/NJ mice consume significantly more ethanol than either parental strain (Blednov et al., 2010, 2005; Phillips et al., 2010). This heightened phenotype in the hybrid offspring may be due to epistatic interactions between alleles that are unique to each of the parental strains. In our experimental data, this epistatic interaction may have been mediated by sex in addition to genotype, and the effect may only have been visible at the highest dose of ethanol used.
Differences in ethanol-induced dopamine release between males and females at low doses of alcohol have been seen previously in rats (Blanchard et al., 1993). However, the significant decrease in dopamine levels at the 2 g/kg ethanol dose found in this study has yet to be replicated. Also, Yim et al. (2000) found no sex difference between male and female rats after 1 g/kg ethanol administration (i.p.); however, a larger sampling size may have revealed an effect, albeit in the opposite direction to the present study (males showing a greater response than females). To the best of our knowledge, our study is the first showing a difference in ethanol-evoked dopamine release at the 1 g/kg dose between male and female C57BL/6J mice.
Thus, when this pattern of results across the current and previous studies is considered together, we conclude that MOR is not exclusively involved in mediating ethanol stimulated dopamine release under all conditions. Other genetic factors, including some related to the sex of the subject, may contribute to determining whether ethanol-stimulated dopamine release is MOR-dependent or independent. This suggestion may have important considerations for the treatment of alcoholism. Currently a number of pharmacological treatments are being used in the treatment of alcoholism; this includes drugs from a variety of classes, including opiate antagonists. Given the present results, it would appear that the effectiveness of these treatments might differ with the opioid-dependence of the effects of ethanol in individuals. Variants in MOR have been associated with alcoholism (Deb et al., 2010), but more importantly, other variants are predictive of the therapeutic response to opiate antagonists in alcoholics (Oroszi et al., 2009). Recently, evidence has emerged that a single point mutation allelic variant (OPRM1 A118G polymorphism) results in a much greater ventral striatal dopamine response to ethanol challenge compared to homozygous 118A allele carriers (Ramchandani et al., 2011). Consideration of this and the present results suggests that there is a genetic basis for MOR-dependent, and MOR-independent, mechanisms of ethanol-induced dopamine release that are each likely to be relevant to the rewarding effects of ethanol. For any particular individual, however, it may be that the effects of ethanol are primarily mediated by one or the other mechanism. These differences in the underlying mechanisms of ethanol’s effects would consequently impact on the utility of opiate antagonists as treatments in individual alcoholics. The ability to model this human variation in transgenic mice may aid the development of ways to predict which alcoholic individuals will be effectively treated by opiate antagonists, or by other medications.
Acknowledgments
We wish to thank Tim Ward, Christina Schier and Julie Wu for their technical assistance. We also thank the NIDA Drug Supply Program for providing the morphine and the beta funaltrexamine.
Supported by Grant RO1 AA 14874 from the National Institute on Alcohol Abuse and Alcoholism (RAG) and by funding from the Intramural Research Program of the National Institute on Drug Abuse (GRU, FSH).
References
- Blanchard BA, Steindorf S, Wang S, Glick SD. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–973. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J × FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alcohol consumption. Behav Genet. 2010;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Denoroy L, Zapata A, Shippenberg TS. Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci. 2009;30:272–278. doi: 10.1111/j.1460-9568.2009.06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393–2400. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Deb I, Chakraborty J, Gangopadhyay PK, Choudhury SR, Das S. Single-nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu-opioid receptor and may contribute to the genetic risk for addiction. J Neurochem. 2010;112:486–496. doi: 10.1111/j.1471-4159.2009.06472.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Drgonova J, Zimonjic DB, Hall FS, Uhl GR. Effect of KEPI (Ppp1r14c) deletion on morphine analgesia and tolerance in mice of different genetic backgrounds: when a knockout is near a relevant quantitative trait locus. Neuroscience. 2010;165:882–895. doi: 10.1016/j.neuroscience.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Sora I, Ugl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hall FS, Li XF, Goeb M, Roff S, Hoggatt H, Sora I, Uhl GR. Congenic C57BL/6 mu opiate receptor (MOR) knockout mice: baseline and opiate effects. Genes Brain Behav. 2003;2:114–121. doi: 10.1034/j.1601-183x.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Haraguchi M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol Biochem Behav. 1992;43:249–254. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154:1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Tang A, Hall FS, Sora I, Uhl GR, Bergeson SE, Gonzales RA. Mu (mu) opioid receptor regulation of ethanol-induced dopamine response in the ventral striatum: evidence of genotype specific sexual dimorphic epistasis. Biol Psychiatric. 2007;62:627–634. doi: 10.1016/j.biopsych.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Drgon T, Liu QR, Walther D, Edenberg H, Rice J, Foroud T, Uhl GR. Pooled association genome scanning for alcohol dependence using 104,268 SNPs: validation and use to identity alcoholism vulnerability loci in unrelated individuals from the collaborative study on the genetics of alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:844–853. doi: 10.1002/ajmg.b.30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Wand GS, Li XW, Portoghese PS, Froehlich JC. Effect of mu opioid receptor blockade on alcohol intake in rats bred for high alcohol drinking. Pharmacol Biochem Behav. 1998;59:627–635. doi: 10.1016/s0091-3057(97)00474-7. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Jr, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res. 1999;23:456–464. [PubMed] [Google Scholar]
- Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- Narita M, Imai S, Itou Y, Yajima Y, Suzuki T. Possible involvement of mu1-opioid receptors in the fentanyl- or morphine-induced antinociception at supraspinal and spinal sites. Life Sci. 2002;70:2341–2354. doi: 10.1016/s0024-3205(01)01550-8. [DOI] [PubMed] [Google Scholar]
- Oroszi G, Anton RF, O’Malley S, Swift R, Pettinati H, Couper D, Yuan Q, Goldman D. OPRM1 Asn40Asp predicts response to naltrexone treatment: a haplotype-based approach. Alcohol Clin Exp Res. 2009;33:383–393. doi: 10.1111/j.1530-0277.2008.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Reed C, Burkhart-Kasch S, Li N, Hitzemann R, Yu CH, Brown LL, Helms ML, Crabbe JC, Belknap JK. A method for mapping intralocus interactions influencing excessive alcohol drinking. Mamm Genome. 2010;21:39–51. doi: 10.1007/s00335-009-9239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Wenger CD, Dorow JD. Naltrexone effects on ethanol drinking acquisition and on established ethanol consumption in C57BL/6J mice. Alcohol Clin Exp Res. 1997;21:691–702. [PubMed] [Google Scholar]
- Pick CG, Paul D, Pasternak GW. Comparison of naloxonazine and beta-funaltrexamine antagonism of mu 1 and mu 2 opioid actions. Life Sci. 1991;48:2005–2011. doi: 10.1016/0024-3205(91)90155-5. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE. A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem. 1980;23:233–234. doi: 10.1021/jm00177a002. [DOI] [PubMed] [Google Scholar]
- Rada P, Barson JR, Leibowitz SF, Hoebel BG. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res. 2010;1312:1–9. doi: 10.1016/j.brainres.2009.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, Grandy DK. Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl) 2000;152:343–350. doi: 10.1007/s002130000548. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Samson HH, Hodge CW, Tolliver GA, Haraguchi M. Effect of dopamine agonists and antagonists on ethanol-reinforced behavior: the involvement of the nucleus accumbens. Brain Res Bull. 1992;30:133–141. doi: 10.1016/0361-9230(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Tanda G, Di Chiara G. A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci. 1998;10:1179–1187. doi: 10.1046/j.1460-9568.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Tang A, George MA, Randall JA, Gonzales RA. Ethanol increases extracellular dopamine concentration in the ventral striatum in C57BL/6 mice. Alcohol Clin Exp Res. 2003;27:1083–1089. doi: 10.1097/01.ALC.0000075825.14331.65. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Liu QR. Addiction genetics and pleiotropic effects of common haplotypes that make polygenic contributions to vulnerability to substance dependence. J Neurogenet. 2009;23:272–282. doi: 10.1080/01677060802572929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Fries DS, Larson DL, Portoghese PS, Takemori AE. Opioid receptor binding characteristics of the non-equilibrium mu antagonist, beta-funaltrexamine (beta-FNA) Eur J Pharmacol. 1985;107:323–330. doi: 10.1016/0014-2999(85)90257-2. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44:401–406. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. How lifetimes shape epigenotype within and across generations. Hum Mol Genet. 2006;15:R131–137. doi: 10.1093/hmg/ddl200. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Robinson DL, White ML, Jaworski JN, Randall PK, Lancaster FE, Gonzales RA. Dissociation between the time course of ethanol and extracellular dopamine concentrations in the nucleus accumbens after a single intraperitoneal injection. Alcohol Clin Exp Res. 2000;24:781–788. [PubMed] [Google Scholar]


