Abstract
Brain vasculature is a complex and interconnected network under tight regulatory control that exists in intimate communication with neurons and glia. Typically, hemodynamics are considered to exclusively serve as a metabolic support system. In contrast to this canonical view, we propose that hemodynamics also play a role in information processing through modulation of neural activity. Functional hyperemia, the basis of the functional MRI (fMRI) BOLD signal, is a localized influx of blood correlated with neural activity levels. Functional hyperemia is considered by many to be excessive from a metabolic standpoint, but may be appropriate if interpreted as having an activity-dependent neuro-modulatory function. Hemodynamics may impact neural activity through direct and indirect mechanisms. Direct mechanisms include delivery of diffusible blood-borne messengers and mechanical and thermal modulation of neural activity. Indirect mechanisms are proposed to act through hemodynamic modulation of astrocytes, which can in turn regulate neural activity. These hemo-neural mechanisms should alter the information processing capacity of active local neural networks. Here, we focus on analysis of neocortical sensory processing. We predict that hemodynamics alter the gain of local cortical circuits, modulating the detection and discrimination of sensory stimuli. This novel view of information processing—that includes hemodynamics as an active and significant participant— has implications for understanding neural representation and the construction of accurate brain models. There are also potential medical benefits of an improved understanding of the role of hemodynamics in neural processing, as it directly bears on interpretation of and potential treatment for stroke, dementia, and epilepsy.
INTRODUCTION
The brain contains a remarkably rich and interdependent network of neurons, whose activity is well correlated with information processing. The study of this network has been the focus of modern neuroscience (Cajal 1996). The brain also contains a rich and interdependent vascular network, whose “activity”— blood flow—is typically well correlated with neural activity. Based primarily on knowledge of anatomy, Aristotle believed that the vasculature was the biological system that manifested its activity in intelligence, emotion, and action (Gross 1998). In contrast, the standard modern view of blood flow is that it serves a physiological function unrelated to information processing, such as bringing oxygen to active neurons, eliminating “waste” generated by neural activity, or regulating temperature. Except in extreme cases of cell damage or death, current hypotheses do not include blood flow as a contributor to shaping neural activity. Realistic computational models of brain function do not include blood flow as a component, and neurophysiologists do not consider it as a regressor to explain variance in their data.
In contrast to this canonical position, we propose the hypothesis that hemodynamics play a role in information processing, through modulation of neural activity by blood flow. We predict that functional hyperemia, the “overflow” of blood to a brain region during neural activity, provides a spatially and temporally correlated source of regulation, modulating the excitability of the local circuit. This shaping of the neural response will, in turn, impact representation.
The goal of this paper is to describe this alternative view of information processing and to argue for the importance of incorporating this potential class of mechanism into our understanding of how different systems in the brain act in a synthetic manner. We focus our comments on the role that hemodynamics may play in sensory neocortex, because this brain region is widely studied with regard to its information processing role, and a great deal is known about its hemodynamic regulation. This focus is not meant to detract from the potential importance of hemo-neural interactions for processing in other brain areas, or in other contexts, as noted throughout.
A “TURING TEST” FOR BIOLOGICAL INFORMATION PROCESSING SYSTEMS
In what is commonly regarded as the “Turing test” (Turing 1950), the capacity of a computer for intelligent, autonomous thought is judged by whether the machine can lead a blinded human observer, unbiased by direct knowledge of the correspondent, to judge it as intelligent. Borrowing this logic to begin our argument, we provide a Turing test for biological information processing systems. We ask whether, given the following list of traits, a scientist would predict that this “anonymous” system plays a role in representation in the brain. The traits of this system are as follows:
-
●
The system possesses a complex anatomical network in the brain.
-
●
This system shows microscale structure in all brain areas.
-
●
This system shows regional anatomical specializations that reflect information processing, such as layer-specific localization of pathway density in different neocortical areas.
-
●
Activity in this system is co-modulated synergistically with activity in the known information processing network—the major neuromodulators such as acetylcholine (ACh), serotonin (5-HT), and norepinephrine (NE), for example, are key regulators of this system, and almost all factors that impact activity in this system also impact neural activity.
-
●
Fine regulation of activity in this network is not tightly coupled to metabolic need.
-
●
Fine regulation of activity in this network is tightly coupled to neural information processing.
-
●
The spatial and temporal pattern of activity in this system shifts with perceptual input, motor output, attention, and cognitive performance.
-
●
The spatial resolution of differential activity in this system can be as precise as single cortical columns or olfactory glomeruli.
-
●
The temporal onset of activity in this pathway is within hundreds of milliseconds of the onset of neural information processing, with a duration similar to the time scales for neural phenomena considered important for information processing such as synaptic depression and facilitation.
-
●
Localized damage in this system leads to specific and predictable information processing deficits.
Without the predisposition to view hemodynamics as only a mechanism for meeting metabolic need or other physiologic function, this anonymous description suggests that the vascular system, and its activity, expressed as blood flow, is poised to play a role in information processing through the directed modulation of neural activity.
The validity of this hypothesis depends on three points. First, whether the anatomy and physiological regulatory mechanisms can position hemodynamic signals spatially and temporally to have an impact on neural activity. Second, whether there are known correlations between information processing and changes in blood flow. Third, whether there are mechanisms by which changes in hemodynamics in the normal range of function can impact neural activity. In the following sections, we suggest that these basic criteria are met, and begin to present hypotheses as to the information processing role that such regulation may play.
INTERLEAVED ANATOMY AND PHYSIOLOGY OF HEMODYNAMIC AND NEURAL SYSTEMS
Anatomical specificity
The vascular pathways that regulate blood flow are finely articulated and interdigitated with neural architecture. These anatomical vascular patterns are not uniform, and in many cases reflect the information processing functionality of a given brain area. In the neocortex, capillary density shows specificity in the “vertical” dimension through enhanced concentration in specific layers, such as layer IV of primary sensory areas (Patel 1983; Woolsey et al. 1996; Zheng et al. 1991), in contrast to the flat laminar profile in the entorhinal cortex (Michaloudi et al. 2005). Further, this system shows “horizontal” specificity within a cortical area, such as the segmentation of capillary beds to the “barrels” of rodent somatosensory cortex or “blobs” and “stripes” of V1 (Patel 1983; Riddle et al. 1993; Woolsey et al. 1996; Zheng et al. 1991). Subcortical structures show similar apparent principles of distribution, as the striatum of the basal ganglia shows enhanced capillary density in the matrix as compared with the striosomes (Feekes and Cassell 2006). The olfactory bulb also shows localization of capillary density in the glomeruli (Borowsky and Collins 1989). Peripheral nerves and vessels are known to follow anatomically similar paths, with recent studies showing that nerves and peripheral vascular supply employ similar developmental guidance mechanisms (Carmeliet 2003; Eichmann et al. 2005).
Overlapping physiological regulation
When local populations of neurons are active, they recruit increased blood flow and volume to the activated region, a process known as “functional hyperemia” (Grubb et al. 1974; Hoge et al. 2005; Kong et al. 2004; Martin et al. 2006; Roy and Sherrington 1890). Functional hyperemia can be induced and modulated by a variety of mechanisms, with the relaxation of smooth muscle around arteries and arterioles a principal final common pathway leading to changes in blood flow and volume. As discussed later, astrocytes are believed to be a primary route for detecting neural activity and engaging the vasculature. Recent evidence has also implicated neurons in the direct control of blood flow. In neocortex, interneurons directly contact vascular processes, and intracellular electrical stimulation in vitro of interneurons adjacent to vessels can evoke dilation or constriction (Cauli et al. 2004; Hamel 2006; Vaucher et al. 2000).
The mechanisms that induce functional hyperemia typically also impact neural activity. Almost all chemical factors that are known to modulate blood flow directly or indirectly regulate neuronal function. Prime examples of this overlap are the major brain stem neuromodulatory inputs, such as ACh, 5-HT, and NE. Single cholinergic projections, for example, target pyramidal neurons, local interneurons (which in turn impact vasculature), astrocytes, and smooth muscles around vasculature and modulate activity in each within a restricted zone (for review, see Hamel 2006). Dopamine is believed to play a similar role in frontal and somatosensory neocortex, with contacts targeted to vasculature within the cortical mantle, implying local synergistic regulation (Krimer et al. 1998). Other factors known to directly regulate both networks include the freely diffusing molecule NO, which relaxes smooth muscle (Palmer et al. 1987) and modulates neural activity in a variety of ways. Processes from single interneurons and from astrocytes contact and impact neighboring neurons and nearby microvascular processes (Hamel 2006; Haydon and Carmignoto 2006; Oberheim et al. 2006; Zonta et al. 2003). These examples of local co-regulation imply fine-scale synergy in the function of neural and vascular networks.
These observations, that the anatomical organization of vascular networks map to known information processing subdivisions and that blood flow activity and neural activity share a wide range of local regulatory mechanisms, are obviously not arguments that compel an additional role for blood flow. Rather, they suggest that the anatomy and physiology of these two networks are positioned to function synergistically in information processing. As discussed in the next section, changes in blood flow correlate strongly with neural activity, but these correlations are not closely tied to fine-scale metabolic need.
FUNCTIONAL HYPEREMIA: SPATIAL AND TEMPORAL CORRELATION WITH INFORMATION PROCESSING
Spatial precision
The spatial resolution of functional hyperemia is widely debated and likely varies as a function of context and brain area. In the somatosensory and visual neocortex, a general consensus exists that the pattern of increased blood flow is similar to that of subthreshold neural activity, with a peak in signal that is localized to a cortical column (~400 µm) and an extent spanning several columns (Dunn et al. 2005; Hess et al. 2000; Lauritzen 2001; Sheth et al. 2004; Vanzetta et al. 2004; Yang et al. 1998). This correlation is strongest when better localized comparisons are made between the flow delivered to the neocortical layers through small arterioles and capillaries. Large vessels that run over the surface of the cortical mantle, which contribute prominently to many imaging techniques, will typically provide artifactually large estimates of the spread of blood delivered to the tissue (Disbrow et al. 2000; Dunn et al. 2005; Duong et al. 2001; Sheth et al. 2004; Vanzetta et al. 2004).
Vertical specificity in the cortical pattern of functional hyperemia has also been observed. In the somatosensory neocortex, the peak flow and volume changes evoked by sensory stimulation is localized to specific laminae (Gerrits et al. 2000; Norup Nielsen and Lauritzen 2001; Silva and Koretsky 2002). Evoked response amplitude and peak flow have also been correlated within cerebellar laminae (Akgoren et al. 1997). In other brain areas, evidence for more precise delivery has also been observed, because flow can be localized to a single glomerulus in the olfactory bulb during stimulus presentation (i.e., ≤100 µm) (Chaigneau et al. 2003; Yang et al. 1998). Several potential mechanisms exist for fast and localized regulation of this type, ranging from direct neural control caused by interneuron activation (Cauli et al. 2004; Hamel 2006) to pericyte control of vascular diameter in capillaries (Peppiatt et al. 2006).
Temporal precision
Functional hyperemia is also temporally locked to neural activity onset. In the somatosensory neocortex, blood flow increases measured using laser Doppler have been observed <200 ms after the onset of sensory-evoked neural responses (Matsuura et al. 1999; Norup Nielsen and Lauritzen 2001). Similarly, optical imaging techniques that integrate over local volumes at somewhat slower temporal resolution typically record a significant increase in flow within ≤500 ms of sensory stimulus presentation (Dunn et al. 2005; Malonek et al. 1997; Martin et al. 2006). The subsequent duration of these increases is often viewed as “poorly correlated” with neural activity, because functional hyperemia can sustain for seconds after the onset and offset of a stimulus. As discussed in a later section, this sustained temporal pattern may not be a mismatch between activity and flow, but rather may be consistent with the information processing role of blood flow.
Functional hyperemia is not well correlated with oxygen consumption
This increased supply of blood during functional hyperemia does not match most estimates of metabolic need. As first shown by Fox and Raichle (1986), the increase in oxygenated hemoglobin recruited by neural activity exceeds the oxygen delivery needs of the tissue. Although there is ongoing debate as to the mechanisms of energy use during neural activity and to the role that glucose demand plays in setting blood flow levels, there is general consensus that functional hyperemia, as the name suggests, overshoots the oxygen requirements of the tissue several fold (see Kida and Hyder 2006 and Raichle and Mintun 2006 for recent reviews).
Several hypotheses have been proposed to account for this significant uncoupling between the predicted metabolic need for blood and the actual levels observed, ranging from temperature regulation to metabolite (waste) removal (Hayward and Baker 1968; Woolsey et al. 1996; Yablonskiy et al. 2000; Zhu et al. 2006). While these alternative physiological processes may be one role of functional hyperemia, several recent reviews have concluded, in concurrence with the original conclusions of Fox and Raichle (1986), that a much stronger correlation exists between processes associated with signal processing, such as neurotransmitter release, and subthreshold electrophysiological activity, than with other metabolic or physiologic ends (Lauritzen 2001, 2005; Iadecola 2004).
POTENTIAL MECHANISMS LINKING FUNCTIONAL HYPEREMIA AND NEURAL ACTIVITY
We propose an alternative explanation for functional hyperemia, beyond an exclusively metabolic account. This spatially and temporally directed process may play an active role in modulating neural activity, and the observed changes in blood flow and volume may not be overly exuberant, but rather may meet a different goal. Under this hypothesis, functional hyperemia is not the overdelivery of oxygenated blood for metabolism but rather the targeted regulation of neural processing.
We emphasize that, while uncoupling of metabolic need and functional hyperemia is an argument for positing an alternative hypothesis, the proposed information processing role of functional hyperemia is not exclusive of other physiological roles for increased blood flow and volume. Even if oxygen supply were proportional to need in the brain, the localized process of functional hyperemia could still shape neural activity and impact representation and would still need to be included in any complete account of the processing machinery that supports information processing.
In this section, we discuss a variety of mechanisms through which functional hyperemia could impact neural activity. We have grouped these into two broad categories: indirect mechanisms, where astrocytes play a mediating role between vascular and neural networks, and direct mechanisms, in which astrocytes do not play an explicit role.
Direct hemo-neural interactions
DIFFUSIBLE FACTORS
Diffusible messengers that freely cross the blood–brain barrier represent one class of agent that can directly impact neural activity and that likely increase in concentration with increased blood volume. Within this class of modulators, NO is the best understood. This molecule diffuses across cellular membranes and can likely freely traverse the blood–brain barrier (Kitagami et al. 2003). There are at least two vascular sources of NO that could impact neural activity: transport from the blood and production in vascular tissue. The blood carries NO, much of which is bound to oxygenated hemoglobin. With deoxygenation under physiological conditions, the affinity of hemoglobin for NO decreases, leading to its release (Pawloski and Stamler 2002; Singel and Stamler 2005). While the impact of the blood-derived NO concentration on vasodilation is a topic of ongoing debate (Pawloski and Stamler 2002; Stamler et al. 1997), an increase in blood flow and/or blood volume should lead to an increase in the NO concentration in the surrounding tissues (Kitagami et al. 2003; Kubes et al. 1991; Vallance et al. 1989). Furthermore, NO is produced in endothelial cells that could be stimulated to release this factor by shear stress caused by enhanced flow (Charles 1999; Garthwaite et al. 2006; Iadecola et al. 1993).
The impact of increased NO concentration on neural activity could take many forms. In the presence of increased NO concentration, thalamic sensory relay neurons in vitro show depolarization and modulation of voltage-dependent metabotropic G protein–coupled channels, leading to dampened oscillatory activity and a more tonic mode of transmission (Pape and Mager 1992). In the somatosensory and visual thalamus in vivo, increased NO concentration leads to enhanced stimulusevoked firing rate, thought to occur through a distinct, N-methyl-d-aspartate (NMDA)-dependent mechanism (Cudeiro et al. 1994; Do et al. 1994; Shaw and Salt 1997). In the primary visual cortex, both suppression and facilitation of evoked firing rate have been observed following local increases in NO concentration, potentially indicating subpopulations of neurons with distinct responses in V1 (Kara and Friedlander 1999). Analyses based in signal detection theory suggest that the cumulative effect of these interactions is an enhanced signal-to-noise ratio of sensory transmission (Kara and Friedlander 1999). Various forms of synaptic plasticity such as long-term potentiation and depression are also modulated by NO (see Hardingham and Fox 2006 for a recent review). The presence of enhanced blood flow, when coupled with specific patterns of spike timing or bursting activity, could combine to induce modifications of synaptic efficacy.
Direct support for the view that vascular NO impacts neural activity comes from recent findings by Garthwaite et al. (2006). They provide evidence that endothelial NO production leads to a net depolarization in proximal nerve fibers. This tonic depolarization is thought to be mediated by guanylyl-cyclase–coupled receptors in the axons, whose production of cGMP engages hyperpolarization-dependent depolarizing ion channel receptors, leading to an increase in membrane potential. While these observations have yet to be tested in vivo, they provide strong initial reinforcement for the NO aspect of the hemoneural hypothesis.
MECHANICAL ENGAGEMENT
Functional hyperemia within a local region of cortex (e.g., a cortical column) could shape neural responses through its mechanical impact on the tissue. Increased flow, volume, pressure, and the local expansion or motion of vascular processes may lead to the deformation of neural membranes. In somatosensory cortex, sensory afferent stimulation evokes a 30–40% increase in the diameter of single pial arterioles, on average a net increase of 10–15 µm, with individual examples showing expansion in excess of 15 µm (Hughes and Barnes 1980; Koralek et al. 1990; Ngai and Winn 1996).
These local mechanical signals could modulate neural activity through the engagement of mechano-sensitive ion channels. Two channels positioned to translate hyperemia into depolarized membrane potentials are the amiloride-sensitive Na+ channel and stretch-activated cation channel, because both types are present in high concentration in the rodent neocortex (Lein et al. 2007). A further example of a neural channel positioned to sense these events in the neocortex is TRAAK, a stretch-activated potassium channel (Honore et al. 2006; Maingret et al. 1999). This channel is present in high concentrations across all neocortical layers, and shows peak density in the soma and proximal dendrites of neurons (Reyes et al. 2000; Talley et al. 2001). Mechanical engagement of this potassium channel would lead to hyperpolarization, presumably inducing a net suppression of the firing probability across a local neural population, or modulating firing modes of neurons, e.g., bursting in a subset of pyramidal cells (Silva et al. 1991).
TEMPERATURE CHANGE
Almost all cellular processes are sensitive to temperature. Ion channels show several varieties of temperature dependence, and the impact of temperature on the driving force across a membrane is predicted by the Nernst equation. Blood flow largely dictates brain temperature, transmitting changes in core body temperature to the brain and maintaining temperature in the context of the cooler extracranial environment (Baker et al. 1994; Hayward et al. 1966; Zhu et al. 2006). While there is substantial species-specific variation in the regulation of brain temperature (Baker et al. 1994), changes in behavioral and metabolic state regularly induce fluctuations in mammalian brain temperature of >2°C (Andersen and Moser 1995; Kiyatkin 2004; Kiyatkin et al. 2002). A dorso-ventral gradient of temperature (from cooler to warmer) exists across the brain, with higher temperatures in the ventral regions determined by the large basal arteries (Melzack and Casey 1967; Serota and Gerard 1938), and a steeper gradient in the neocortex (Zhu et al. 2006).
One hypothesis invoked to explain functional hyperemia is that it provides temperature regulation of the brain, removing heat caused by neural activity (Hayward and Baker 1968; Yablonskiy et al. 2000). Preliminary studies suggest that tactile stimulation (e.g., of vibrissae or skin) can induce increases in temperature from an unidentified source in somatotopically appropriate cortex or thalamus of >0.1°C (Gorbach 1993; Melzack and Casey 1967; Trubel et al. 2006), whereas different dynamics (and cooling) can be observed with sustained sensory stimulation (McElligott and Melzack 1967; Trubel et al. 2006; Yablonskiy et al. 2000). While a potentially important role for enhanced flow, the effects of functional hyperemia on temperature in a local region (e.g., a cortical column) have not been directly measured, and these effects may vary as a function of relative depth (Zhu et al. 2006). Recent studies using MRI as a probe for temperature change have reported decreases in temperature correlated with sensory-enhanced flow on the order of 1°C in visual cortices (Yablonskiy et al. 2000). Direct measurements of brain temperature changes during hypercapnia, a condition that transiently increases flow and arterial blood pressure, have shown decreased brain temperature, on the order of 0.25°C, in the nonhuman primate (Hayward and Baker 1968).
If functional hyperemia has even a relatively small impact on local temperature, there are several mechanisms through which it could affect neural activity. Evoked hippocampal field potential responses show increased latency with decreasing temperature changes of <2°C (see Andersen and Moser 1995 for a review), and decreasing temperature from 37 to 32°C doubles the amplitude of the afterhyperpolarization and enhances spike frequency adaptation (Thompson and Prince 1986; Thompson et al. 1985). Neurons in rodent parietal cortex show substantial temperature dependence in the physiological range. Decreases of 1°C from 36 to 35°C can lead to a complete suppression of ACh-induced action potential firing (Mednikova and Pasikova 2005). In layer II pyramidal neurons in the primary somatosensory cortex, a 2°C decrease in temperature (from 35 to 33°C) induces a 50% decrease in the mini–excitatory postsynaptic current (EPSC) rate, a 50% increase in the input resistance, and a decrease in the current-injection evoked firing rate (Simkus and Stricker 2002).
The changes in synaptic response observed with small changes in temperature in the physiological range in parietal cortices suggest that these effects could have a substantial impact on the net output of a cortical column. For example, within the rat barrel cortex, a difference in the rise time of excitatory conductances on the order of 1 ms can determine whether or not a vibrissa deflection will evoke a spike (Moore and Nelson 1998; Moore et al. 1999; Simons and Carvell 1989; Wilent and Contreras 2005). Many of these changes associated with temperature could modulate conductance and evoked release on this time scale, altering sensory signal transmission.
Indirect mechanisms: astrocyte-mediated hemo-to-neural signaling
Anatomically, astrocytes are positioned between neurons and vasculature and play a key role in neural-to-hemodynamic signaling. However, many recent studies have shown that astrocytes also modulate neural activity (for reviews, see Araque et al. 2001; Nedergaard et al. 2003; Newman 2003; Volterra and Meldolesi 2005). In this section, we propose that glia may mediate hemo-to-neural interactions, translating functional hyperemia-related vascular signals into changes in neural activity and, in turn, information processing.
ANATOMICAL INTERCONNECTIVITY AND RANGE OF IMPACT OF ASTROCYTES
Astrocytes have multiple processes that are interconnected with glial, neural, and vascular elements. Astrocytes are densely interconnected with other astrocytes, communicating through gap junctions and chemical signals. As first observed by Golgi (1885), astrocytic end-feet form a dense net of multiple discrete contacts with vessels (Nedergaard et al. 2003). Astrocytic end-feet also overlap axonal–dendritic connections in the CNS, forming the “tripartite synapse” (Araque et al. 1999; Nedergaard 1994; Nedergaard et al. 2003). In the hippocampus, 57% of synapses form this kind of anatomical and functional unit (Lehre and Danbolt 1998).
Individual astrocytes are proposed to have physiological impact over “microdomains” spanning −300–400 µm in diameter (Nedergaard et al. 2003; Oberheim et al. 2006). Within this extent, a given astrocyte contacts up to thousands of neurons (Fellin et al. 2004; Oberheim et al. 2006), and their end-feet line microvessels at the border of a putative microdomain (Simard et al. 2003). Signal transmission in astrocytes is triggered by traveling calcium oscillations that induce the release of several factors (Charles et al. 1991; Cornell-Bell et al. 1990). One support for the microdomain view is that spreading calcium waves and the related process of ATP diffusion extend approximately this distance. Evidence also exists for the selective induction of calcium waves in subzones within an astrocyte. Spontaneous calcium oscillations in hippocampal slices can occur in single astrocytic processes (Pasti et al. 1997; Taylor-Clarke et al. 2002) and electrical stimulation of the cerebellum can recruit activation in subregions of glia (Grosche et al. 1999, 2002). Local expression of calcium waves may provide a more precise and more rapid means of communication between vasculature and neurons via astrocytes.
ROLE OF ASTROCYTES IN NEURAL-TO-HEMODYNAMIC COMMUNICATION
Several theories predict that astrocytes play a major role in translating neural activity into enhanced blood flow (Araque et al. 1998a; Paulson and Newman 1987; Pellerin and Magistretti 1994; Rossi 2006; Takano et al. 2006; Zonta et al. 2003). Calcium signaling in astrocytes can be induced by a variety of factors released by neurons, including glutamate, GABA, and ACh (Fellin et al. 2006), and this increased calcium signal may trigger enhanced blood flow through vascular end foot contacts. Initial support for this view came from the observation that glutamate application in culture induces the production of vasodilatory substances in astrocytes (Fellin et al. 2006). Direct support comes from in vitro and in vivo studies, in which either electrical stimulation of local neurons or direct stimulation of astrocytes causes dilation of local vessels in a fashion correlated with increased astrocytic calcium concentration (Filosa et al. 2004; Mulligan and MacVicar 2004; Takano et al. 2006; Zonta et al. 2003).
ASTROCYTE-TO-NEURON COMMUNICATION
Astrocytes can regulate neural activity through a variety of mechanisms. A prominent mechanism is the release of neuro-active factors, including but not limited to steroids, neuropeptides, growth factors, aspartate, prostaglandin E2, and d-serine (Haydon and Carmignoto 2006; Volterra and Meldolesi 2005). The potentially most important member of this class is glutamate. In culture and slice preparations, glutamate release from astrocytes can cause large, NMDA-dependent depolarizations in adjacent neurons (Araque et al. 1998; Hassinger et al. 1995; Parpura et al. 1994; Parri et al. 2001; Pasti et al. 1997). Further, direct stimulation of adjacent astrocytes enhances the rate of spontaneous miniature EPSCs and net depolarization in neighboring neurons (Araque et al. 1998a). In some circumstances, the net effect of astrocytic stimulation is the potentiation of presynaptic neural inputs (Jourdain et al. 2007), whereas in others, the effect of the astrocyte-induced increase in spontaneous release is synaptic depression, decreased evoked response amplitudes, and increased failure rate (Araque et al. 1998a). At the neuromuscular junction, glial regulation is responsible for ~50% of the synaptic depression observed during repetitive stimulation (Newman 2003; Rochon et al. 2001). Similarly, astrocytic release of adenosine is believed to play a role in heterosynaptic suppression of transmission (Manzoni et al. 1994; Zhang et al. 2003). Some evidence also suggests a role for astrocytic glutamate release in maladaptive excitatory activity, specifically in the generation of epileptogenic-like activity in pyramidal neurons (Kang et al. 2005).
Astrocytic glutamate release can also impact local circuit function through influence on inhibitory interneurons. Release of caged calcium in hippocampal astrocytes increases the rate of spontaneous activity in inhibitory interneurons and the rate of inhibitory postsynaptic currents observed in neighboring neurons (Liu et al. 2004). Further, activation of interneurons by astrocytic glutamate facilitates the potentiation of inhibitory input to pyramidal neurons, leading to a decrease in the failure rate of presynaptic release from interneurons (Kang et al. 1998).
In addition to the direct release of factors onto neurons, other astrocytic mechanisms have been shown to modulate neural activity. The uptake of glutamate is a well-established role of astrocytes and provides a complementary mechanism for neural regulation. In the retina, the amplitude and duration of ganglion cell EPSCs are significantly increased when the glial glutamate transporter is blocked (Higgs and Lukasiewicz 1999), suggesting that glia can act to regulate the duration of excitatory signal transmission.
IMPACT OF FUNCTIONAL HYPEREMIA ON ASTROCYTE ACTIVITY
While it is accepted that astrocytes can impact neural activity, thereby providing a key step in the hemo-astro-neural progression proposed here, the second essential process, the impact of functional hyperemia on signaling in astrocytes, has not been established. Functional hyperemia could cause signaling in astrocytes relevant to neural information processing through a variety of mechanisms, including all of those described above for direct hemo-neural modulation.
Diffusible messengers carried by blood may modulate astrocytic function. Application of NO to astrocytes in vitro induces an influx of calcium (Li et al. 2003) and glutamate and ATP release (Bal-Price et al. 2002). Astrocytes and endothelial cells are known to interact closely in development, in cell signaling, and in the maintenance of the blood–brain barrier (see Abbott 2002 for a review). The dense apposition of astrocytic endfeet over capillary endothelial cells (Kacem et al. 1998) positions them to be modulated by endothelial NO release, which can be triggered by increased hemodynamic activity (Charles 1999; Garthwaite et al. 2006; Iadecola 1993).
Mechanical interactions are also a potential mechanism for vascular to astrocytic signaling leading to neural modulation. Several studies have shown the induction of calcium waves with mechanical deformation in astrocytes (Charles et al. 1991; Islas et al. 1993; Niggel et al. 2000; Stout and Charles 2003). Astrocytic end feet are, as described above, in an ideal position to detect the mechanical impact of functional hyperemia, as they ensheath cerebral microvessels, and endfeet have a high density of mechano-sensitive connexin channels that trigger intracellular calcium release (Simard et al. 2003).
IMPACT OF HEMO-TO-ASTROCYTIC ACTIVATION ON NEURAL PROCESSING
As described in the preceding review, neural activity is modulated by astrocytic activity, and astrocytes are in a key position to be modulated by changes in blood flow and volume. As such, functional hyperemia should, through this indirect mechanism, modulate neural activity. Anatomical and physiological considerations suggest that these proposed interactions will occur at least within a microdomain of ≤300–400 µm (Anderson and Nedergaard 2003; Oberheim et al. 2006). The calcium waves in astrocytic processes move slowly, on the order of microns per second (Mulligan and MacVicar 2004; Wang et al. 2006): however, calcium waves can occur in astrocytic processes locally opposed to neurons, and sensory stimulation recruits astrocytic processes on a faster time course (<1s) than cell bodies (Takano et al. 2006). These findings suggest that local interactions (<10 micron) may be crucial if indirect mechanisms contribute significantly to a more rapid hemodynamic impact on ongoing neural activity and more generally that the hemo-astro-neural pathway may impact longer time course state-related phenomena.
SUMMARY
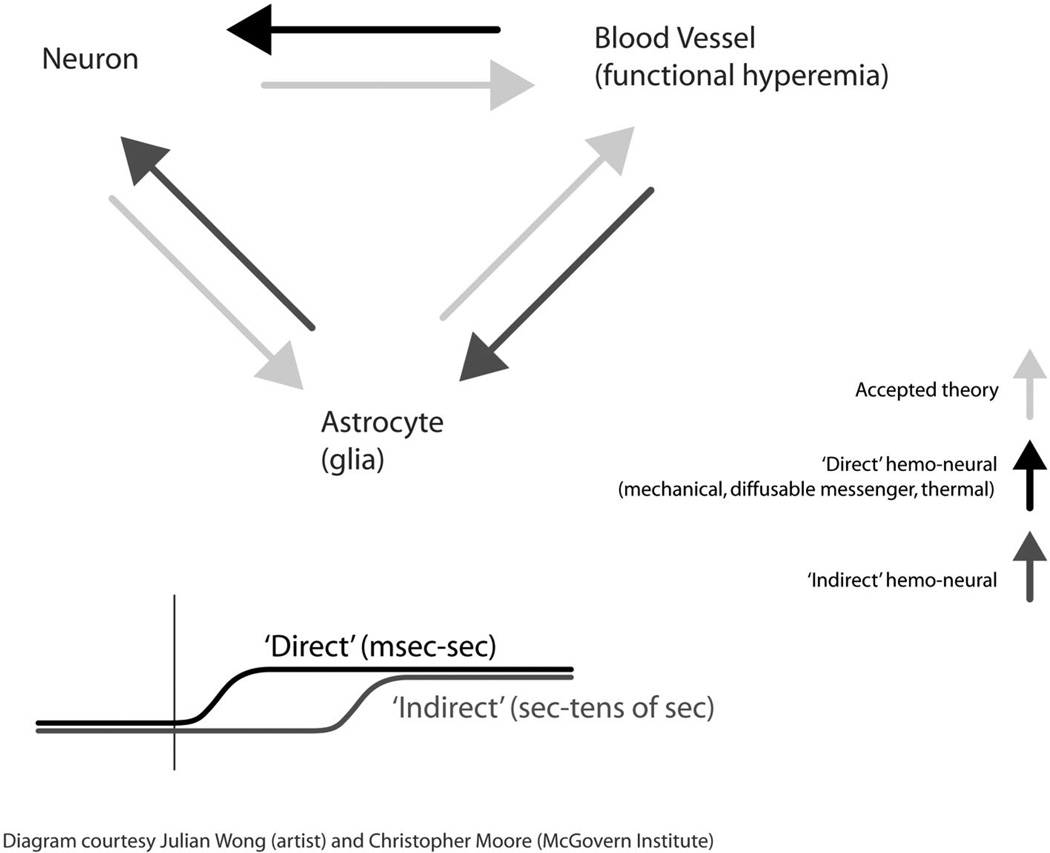
The pathways and time courses predicted by the hemo-neural hypothesis, and the related interactions between neurons, vasculature, and astrocytes, are summarized in Fig. 1. In the diagram, the gray arrows represent accepted interactions or those for which primary data currently exists. As reviewed above, neural activity is believed to induce functional hyperemia through direct contact with vasculature, and/or through astrocytic intermediaries. Astrocytic activity, in turn, has been shown to be able to drive vascular dilation and contraction and also to alter neural processing.
FIG 1.
The hemo-neural hypothesis: bidirectional interactions between hemodynamics, neurons, and glia. This figure shows the key concepts underlying the hemo-neural hypothesis. Neural activity is known to drive functional hyperemia and may do so through either neural inputs to the vasculature or through intermediary astrocytes (lighter gray arrows). The hemo-neural hypothesis proposes that hemodynamic changes can impact neural activity through direct and indirect pathways. Direct effects (black arrow) are hypothesized to occur through the release of diffusible messengers, mechanical interactions, and/or changes in temperature. Indirect effects (darker gray arrows) are hypothesized to occur through activation of astrocytes, which in turn modulate neural activity. As shown in the diagram on the bottom of the figure, direct interactions are predicted to occur within milliseconds to seconds following changes in the local hemodynamic environment and indirect interactions to occur on a time scale of seconds.
The darker arrows in Fig. 1 constitute this hypothesis. They represent the direct and indirect pathways through which hemodynamic modulation of neural information processing is proposed to occur. The time course at the bottom of Fig. 1 shows the predicted evolution of these effects. The direct pathway (black arrow) is predicted to cause rapid neuromodulation, occurring milliseconds to seconds after the onset of functional hyperemia. The indirect pathway that requires astrocytic transduction (dark gray arrows) is predicted to show delayed modulation, occurring seconds to tens of seconds after the onset of functional hyperemia.
ONE IMPACT OF BLOOD FLOW ON INFORMATION PROCESSING: CORTICAL DYNAMICS
The above evidence suggests that both direct and indirect mechanisms exist through which functional hyperemia can modulate neural activity. In this section, we propose one kind of impact of functional hyperemia on neural information processing: the dynamic modulation of neural excitability in sensory neocortex.
With regard to the primary somatosensory cortex, functional hyperemia has spatial structure on the order of a single cortical column (≤400 µm), its onset follows neural activity by ≤200 ms, and enhanced blood flow and volume sustain for seconds after the onset of neural activity. We predict that this signal provides a spatio-temporally targeted modulator of the gain state of neural transmission. The mechanisms defined above suggest that either enhancement or suppression of spontaneous and evoked activity can occur and that the directionality of modulation depends on brain area and context. Facilitation of neural activation could be engaged through recruitment of mechano-sensitive cation channels, through modulation by NO, or through astrocyte-mediated depolarization.
Depolarization or hyperpolarization of this type will impact the response of cortical neurons to a sensory stimulus, and dynamics of this kind have been proposed as a means of optimizing cortical function for perception. One hypothesis suggests that the transition from high cortical gain to low cortical gain represents a transition from states optimal for detection to those optimal for discrimination of sensory stimuli, respectively (for reviews, see Moore 2004; Moore et al. 1999). High gain modes in which a broader region of a cortical map is activated by a sensory stimulus are predicted to facilitate the detection of input, but may be suboptimal for precise stimulus representation. In contrast, low gain modes with sharper, more delimited lateral spread of signal are predicted to facilitate the precise representation and discrimination of sensory stimuli, but are less optimal for detection of weaker sensory signals. This transition could be modulated by the pattern of ongoing sensory stimulation, reflecting the information context in which processing is occurring. For example, high-frequency sensory input leading to an adapted state is predicted to impair detection and facilitate discrimination. Similarly, internal state properties may also modulate cortical activity levels and information processing, such as ongoing activity in the thalamo-cortical loop at α-band frequencies (Worden et al. 2000).
Perhaps the most important context in which to consider the Hemo-Neural hypothesis is during natural behavior. In many active sensing contexts, preparatory motor or cognitive behaviors will lead to an increase in blood flow in the cortical representation that is about to process incoming signals and may precondition neural processing by inducing enhanced blood flow before the receipt of meaningful information. One example of a motor behavior that is correlated with a shift to an attentive perceptual state is the “whisking” engaged by rodents during active sensing with the vibrissae. When a rat searches its environment, looking for an object to contact and identify, it actively whisks its vibrissae forward and backward at a rate of 5–12 Hz (Carvell and Simons 1990; Kleinfeld et al. 2006; Wi 1964). During search, whisking can begin up to seconds before contact with the surface. During whisking in air, both sensory feedback and input of signals from the motor system drive activity in SI (Fee et al. 1997; Ferezou et al. 2006; Kleinfeld et al. 2002), activity that should produce enhanced blood flow within two to three cycles of the onset of vibrissa motions (or ~250 ms). Under the present hypothesis, blood flow would anticipate the acquisition of information, helping transform cortical circuits to more optimally represent the incoming signals before surface contact.
This kind of anticipatory change in blood flow is not limited to cases where active motor exploration precedes acquisition. In many cognitive paradigms, blood flow modulation occurs in anticipation of or independent of the receipt of sensory input. One example of a context in which hemo-neural modulation of cortical dynamics may impact information processing is through enhancement of evoked responses during selective attention. A wide variety of studies has shown that attention to a region of input space (e.g., a retinotopic position or body area) is correlated with enhanced evoked action potential firing of cortical neurons with receptive fields overlapping the attended region (Bichot and Desimone 2006). These effects typically emerge 100–500 ms after the onset of attentional focus (Khayat et al. 2006; Khoe et al. 2006; Worden et al. 2000).
Functional hyperemia is positioned to modulate activity during attention, because enhanced blood flow is often localized to regions of the cortical map corresponding to the attended region of space. For example, in area V1, selective and divided attention to specific quadrants of the visual field is correlated with increased BOLD signal in the attended subregions of the retinotopic map (McMains and Somers 2004). Similarly, anticipation of contact in a specific body region is correlated with relatively greater blood volume in the corresponding part of the SI homunculus (e.g., the hand area if finger contact is predicted) compared with noncued body regions before stimulation onset (Drevets et al. 1995).
Ongoing blood flow and volume levels that impact representation may be determined by any of the many mechanisms that control functional hyperemia. Of particular interest given the current proposal is the regulation of hyperemia by neuromodulators already implicated in regulation of attention, such as ACh. A wide variety of studies have suggested that ACh release may be essential to the cortical dynamics mediating attention and working memory (Furey et al. 2000; Robbins et al. 2000; Sarter et al. 2005). Under the current hypothesis, ACh would have synergistic neuromodulatory and hemodynamic influences during enhanced attentional states, modulating the local vascular and neural network in the service of a common information processing goal.
Why engage functional hyperemia for neural modulation?
This proposal begs the question as to why functional hyperemia is a necessary aspect of neural modulation, given that other mechanisms are known to exist that recruit these dynamics. Put another way, why would such a mechanism evolve if it seems redundant to existing processes? There are several responses to this kind of teleological question applied to biology.
The emergence of a neuroregulatory role for functional hyperemia is not difficult to conceive. If one presumes any of the several possible metabolic needs for activity-driven increases in blood flow, in agreement with the existing theories for this phenomenon, a correlation is present between blood flow and neural activity. If an “overflow” of this supply also proved adaptive for the functionality of neural representation, one would predict its evolutionary emergence through the reinforcement of this correlation. Further, there is strong evidence from invertebrates for the vascular transmission of neuromodulatory substances that regulate neural firing, neural oscillations, and behavior, e.g., in control of the stomatogastric ganglion (Marder et al. 2005; Nusbaum and Beenhakker 2002).
There are also important physiological reasons why functional hyperemia may be used to suppress neural activity. Unchecked neural activation following stimulation is a hallmark of forms of epilepsy and schizophrenia (Blum and Mann 2002) and can be the cause of excitotoxic cell death (Tsuang et al. 2000). Metabolic control systems such as the hemodynamic pathways could therefore have evolved mechanisms for neural regulation targeted to the basic health of the system that have additional benefit for the efficient representation of information. The existence of severe maladies characterized by failed neural suppression suggests further that mechanisms of neural gain regulation are not sufficient in a significant fraction of the population. As such, the addition of a novel mechanism (functional hyperemia) is not redundant.
A further argument for a role for functional hyperemia in information processing is its unique character. Although there are several possible mechanisms through which functional hyperemia may differentially impact different neuron or synapse classes, a distinctive feature of this mechanism is that it acts on the neurons throughout a local area, “binding” their function. This kind of impact would be predicted to be of particular significance in representations, like a sensory map, where spatial position within a local network is important to representation. High-resolution sensory cortices such as primary visual cortex (Hubel and Wiesel 1977) and the vibrissa barrel cortex (Andermann and Moore 2006; Andermann et al. 2004; Moore and Andermann 2005) are characterized by clustering of neurons with similar tuning in an overlapping set of columns. These interdependent columnar systems may be ideally positioned to be regulated by a modulator acting on the spatial scale currently ascribed to hyperemia.
Recent hypotheses have emphasized that energetic efficiency in brain operation is a paramount constraint and therefore leads to sparse coding through the dynamic restriction of activity and the adaptability of networks (Laughlin and Sejnowski 2003). In such a situation, the interdependence of computation and metabolism would be adaptive. The synergistic engagement of the metabolic supply network to regulate coding is, in this context, a consistent proposal.
Perhaps the most appropriate answer to the question of why functional hyperemia needs to exist as an information processing mechanism is that this question is ill-posed. The attempt to delineate the evolutionary necessity of any given mechanism in an existing organism is almost always an open-ended endeavor. This practice is particularly difficult when judging the importance of a newly proposed mechanism, where the nature of its interactions is only hypothesized. Analysis of the neuromodulatory role of functional hyperemia is, at present, best focused on the study of the observable impact of hemodynamics on neural processing, reserving a further discussion of the evolutionary necessity of this mechanism until more is known about its basic biology and its impact on behaviorally relevant information processing.
CLINICAL IMPLICATIONS OF THE HEMO-NEURAL HYPOTHESIS
There are several potential clinical implications of the prediction that functional hyperemia modulates neural activity. Diseases that impact the vasculature and impact cognitive function may in part be operating through a failure in hemoneural interactions and the loss or alteration of neuromodulatory function that results.
The negative outcomes of stroke are commonly believed to arise only from localized cell death in the focus of the lesion and the metabolic losses and stresses associated with deprived flow. Under the current hypothesis, the altered blood supply in itself, and the subsequent loss of normal functionality in a given neural circuit, may be causal in behavioral symptoms. One cause of cell death in stroke is hyperexcitation of the tissue resulting from a variety of factors (Mattson 2003). A direct prediction of the hemoneural hypothesis is that decreased blood flow could induce an increase in the excitability of neural tissue and that this loss of a suppressive mechanism could contribute to this posttraumatic response. The loss of suppression also predicts a period of enhanced hyperexcitability at the edge of the lesioned area, sustained until homeostatic mechanisms can be engaged, and leaving the tissue beyond the predicted penumbra of obvious damage in a maladaptive state for normal information processing.
Recent advances suggest that Alzheimer’s disease is initially expressed as a vascular phenomenon, with impaired cholinergic dilation of blood vessels implicated in its pathophysiology (Cauli et al. 2004; de la Torre and Mussivand 1993; Geaney et al. 1990; Iadecola 2004; Sato and Sato 1995). As such, the hemo-neural hypothesis would predict an impact of vascular decline on hemo-neural interactions, taxing the information processing capacity of the system through loss of one form of normal modulatory control. A specific prediction is that prediagnosis Alzheimer’s patients could show enhanced facilitation and/or the failure of tonic and phasic levels of intracortical suppression, in agreement with recent transcranial magnetic stimulation studies (Liepert et al. 2001; Pierantozzi et al. 2004).
Epilepsy can arise through a broad variety of mechanisms, including a failure in the normal mechanisms of neural suppression (Duncan et al. 2006). The hemo-neural hypothesis predicts that hemodynamic abnormalities could lead to these symptoms. In agreement with this prediction, abnormal vascular anatomy is a hallmark of seizure disorders (Leutmezer et al. 2003; Zaidi et al. 2000), and a compromised blood–brain barrier predicts seizure onset (Oby and Janigro 2006). Furthermore, vagal nerve stimulation has recently been successfully used to suppress the onset of epileptic seizures (DeGiorgio et al. 2000; Schachter 2002) and could operate at least in part through vascular regulation.
A failure in cortical dynamics is also widely predicted to be a causal factor in schizophrenia. Schizophrenic subjects show several indications of failed cortical suppressive mechanisms, including decreased prominence of gamma oscillations (Lee et al. 2003; Spencer et al. 2003). While this symptomatology has many proposed neural causes (Lewis et al. 2005; Woo and Lu 2006), vascular abnormalities in resting blood volume distribution and presumed vascular anatomy have also been observed in schizophrenic subjects (Cohen et al. 1995) and could contribute.
OTHER LINKS BETWEEN HEMODYNAMICS AND INFORMATION PROCESSING
The interdependent nature of vascular and neural interactions suggests other hypotheses for their combined function in information processing, beyond the suggestion that functional hyperemia regulates neural dynamics. The role of hemodynamics in shaping oscillatory behaviors—such as those expressed in EEG and MEG signals—should be considered. This suggestion is particularly salient given the potential role of NO in regulating thalamic rhythms (Pape and Mager 1992) and astrocytes in unifying activity across disparate neurons (Angulo et al. 2004; Fellin et al. 2004). Vascular oscillations are also prominent features of the cerebral vasculature (van Helden et al. 2006), and changes in these hemodynamic state properties may interact with neural state properties. A further prediction mentioned above is that the combination of functional hyperemia and patterns of neural activity may facilitate the induction of longer-term neural plasticity. On a longer time scale, selective regulation of the blood–brain barrier, shaped by the recent history of flow to the region, could also serve an integrative role (Krizanac-Bengez et al. 2004; Leybaert 2005).
A more radical aspect of the hemo-neural hypothesis than the one elaborated above is that the vasculature also plays a direct role in carrying information, beyond just modulation of neurons. If neural activity in one region (e.g., a specific layer of the neocortex) was to produce a signal that was taken up by the vasculature, it could be selectively transmitted to a downstream target (e.g., other layers of the neocortex). At least two mechanisms could support this kind of communication. Peripheral and central vascular tissue is capable of electrical coupling (Segal and Duling 1987; Yamazaki and Kitamura 2003). Electrical signals can be transmitted, putatively through gap junction connections in endothelial and/or smooth muscle cells, from capillaries to their feeding arterioles, and may condition a vasodilatory response. These changes in membrane potential in vascular cells can be driven by neuromodulators (e.g., ACh) (McGahren et al. 1998) and by local changes in flow (e.g., the occlusion of a neighboring arteriole) (McGahren et al. 1997). These signals generated downstream could induce voltage-dependent interactions in upstream targets, from a capillary bed and the granular layers of the cortex, for example, to an arteriole and the supragranular layers. Moving in the other direction, the transport of diffusible messengers is also a possibility. Diffusible messengers produced in a superficial cortical layer could be transported down an arteriole and released in deeper layers. This view, while even further from currently accepted models of information processing, similarly bears further investigation.
FUTURE DIRECTIONS
The ideas presented have several direct implications for future research. The hemo-neural hypothesis argues that any computational model or broader theory that seeks an accurate and biophysical account of information processing in the brain should include hemodynamics as an explicit component. This view also suggests that the wealth of studies that have investigated hemodynamic patterns and neural-to-vascular coupling are not only informative with regard to the metabolic functioning of the brain but are also descriptions of signals that impact representation. As such, functional MRI and related techniques are not simply second-order localization tools, but probes of part of the process of information representation. If true, there are important considerations as to the logical interpretation of these hemodynamic signals and of how they synergistically interact with the variable that most of these studies would like to track, neural activity.
The hemo-neural hypothesis suggests several lines of experimentation to systematically test this proposal. Understanding the impact of hemodynamics on neural activity requires the independent regulation of blood flow in vivo. This kind of probe is essential for systematically dissociating these effects from alternative explanations and for ultimately addressing these predictions during active behavior. An informative frustration in attempting these experiments is the extensive overlap in factors that regulate hemodynamics that also directly or indirectly regulate neural activity through other pathways. The interdependent modulation of these two systems, while a strong argument for the hemo-neural hypothesis, also makes the discrete study of these predictions difficult. This challenge underscores the inter-related nature of central neural and hemodynamic processes and the likelihood that they function in tandem in the modulation of information processing.
ACKNOWLEDGMENTS
The authors thank several colleagues for input and careful reading of the manuscript and Thomas F. Peterson, the Mitsui Foundation, and the McGovern Institute for Brain Research for support. The illustration is by J. Wong.
REFERENCES
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgoren N, Mathiesen C, Rubin I, Lauritzen M. Laminar analysis of activity-dependent increases of CBF in rat cerebellar cortex: dependence on synaptic strength. Am J Physiol. 1997;273:H1166–H1176. doi: 10.1152/ajpheart.1997.273.3.H1166. [DOI] [PubMed] [Google Scholar]
- Andermann ML, Moore CI. A somatotopic map of vibrissa motion direction within a barrel column. Nat Neurosci. 2006;9:543–551. doi: 10.1038/nn1671. [DOI] [PubMed] [Google Scholar]
- Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron. 2004;42:451–463. doi: 10.1016/s0896-6273(04)00198-9. [DOI] [PubMed] [Google Scholar]
- Andersen P, Moser EI. Brain temperature and hippocampal function. Hippocampus. 1995;5:491–498. doi: 10.1002/hipo.450050602. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J Neurosci. 1998b;18:6822–6829. doi: 10.1523/JNEUROSCI.18-17-06822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KZ, Young WL, Stone JG, Kader A, Baker CJ, Solomon RA. Deliberate mild intraoperative hypothermia for craniotomy. Anesthesiology. 1994;81:361–367. doi: 10.1097/00000542-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Bal-Price A, Moneer Z, Brown GC. Nitric oxide induces rapid, calcium-dependent release of vesicular glutamate and ATP from cultured rat astrocytes. Glia. 2002;40:312–323. doi: 10.1002/glia.10124. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Desimone R. Finding a face in the crowd: parallel and serial neural mechanisms of visual selection. Prog Brain Res. 2006;155:147–156. doi: 10.1016/S0079-6123(06)55009-5. [DOI] [PubMed] [Google Scholar]
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–179. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- Borowsky IW, Collins RC. Metabolic anatomy of brain: a comparison of regional capillary density, glucose metabolism, and enzyme activities. J Comp Neurol. 1989;288:401–413. doi: 10.1002/cne.902880304. [DOI] [PubMed] [Google Scholar]
- Cajal RY. Recollections of My Life. Cambridge: MIT Press; 1996. p. 630. [Google Scholar]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci USA. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles A. Nitric oxide pumps up calcium signalling. Nat Cell Biol. 1999;1:E193–E195. doi: 10.1038/70221. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Cohen BM, Yurgelun-Todd D, English CD, Renshaw PF. Abnormalities of regional distribution of cerebral vasculature in schizophrenia detected by dynamic susceptibility contrast MRI. Am J Psychiatry. 1995;152:1801–1803. doi: 10.1176/ajp.152.12.1801. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cudeiro J, Grieve KL, Rivadulla C, Rodriguez R, Martinez-Conde S, Acuna C. The role of nitric oxide in the transformation of visual information within the dorsal lateral geniculate nucleus of the cat. Neuropharmacology. 1994;33:1413–1418. doi: 10.1016/0028-3908(94)90043-4. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Mussivand T. Can disturbed brain microcirculation cause Alzheimer’s disease? Neurol Res. 1993;15:146–153. doi: 10.1080/01616412.1993.11740127. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collins S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Labar D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Disbrow EA, Slutsky DA, Roberts TP, Krubitzer LA. Functional MRI at 1.5 tesla: a comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci USA. 2000;97:9718–9723. doi: 10.1073/pnas.170205497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Binns KE, Salt TE. Release of the nitric oxide precursor, arginine, from the thalamus upon sensory afferent stimulation, and its effect on thalamic neurons in vivo. Neuroscience. 1994;60:581–586. doi: 10.1016/0306-4522(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Jr, Raichle ME. Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature. 1995;373:249–252. doi: 10.1038/373249a0. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. Neuroimage. 2005;27:279–290. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution. Proc Natl Acad Sci USA. 2001;98:10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15:108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol. 1997;78:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- Feekes JA, Cassell MD. The vascular supply of the functional compartments of the human striatum. Brain. 2006;129:2189–2201. doi: 10.1093/brain/awl158. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Fellin T, Sul JY, D’Ascenzo M, Takano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp. 2006;276:208–217. doi: 10.1002/9780470032244.ch16. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, Garthwaite J. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci. 2006;26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geaney DP, Soper N, Shepstone BJ, Cowen PJ. Effect of central cholinergic stimulation on regional cerebral blood flow in Alzheimer disease. Lancet. 1990;335:1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- Gerrits RJ, Raczynski C, Greene AS, Stein EA. Regional cerebral blood flow responses to variable frequency whisker stimulation: an autoradiographic analysis. Brain Res. 2000;864:205–212. doi: 10.1016/s0006-8993(00)02142-9. [DOI] [PubMed] [Google Scholar]
- Golgi C. Sulla fina anatomia degli organi centrali del sistema nervoso. Riv Sper Fremiat Med Leg Alienazioni Ment. 1885:72–123. [Google Scholar]
- Gorbach AM. Infrared imaging of brain function. Adv Exp Med Biol. 1993;333:95–123. doi: 10.1007/978-1-4899-2468-1_11. [DOI] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res. 2002;68:138–149. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- Gross CG. Brain Vision Memory: Tales in the History of Neuroscience. Cambridge: MIT Press; 1998. p. 247. [Google Scholar]
- Grubb RL, Jr, Hernandez-Perez MJ, Raichle ME, Phelps ME. The effects of iodinated contrast agents on autoregulation of cerebral blood flow. Stroke. 1974;5:155–160. doi: 10.1161/01.str.5.2.155. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hardingham N, Fox K. The role of nitric oxide and GluR1 in presynaptic and postsynaptic components of neocortical potentiation. J Neurosci. 2006;26:7395–7404. doi: 10.1523/JNEUROSCI.0652-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassinger TD, Atkinson PB, Strecker GJ, Whalen LR, Dudek FE, Kossel AH, Kater SB. Evidence for glutamate-mediated activation of hippocampal neurons by glial calcium waves. J Neurobiol. 1995;28:159–170. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Baker MA. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol. 1968;215:389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Hayward JN, Smith E, Stuart DG. Temperature gradients between arterial blood and brain in the monkey. Proc Soc Exp Biol Med. 1966;121:547–551. doi: 10.3181/00379727-121-30827. [DOI] [PubMed] [Google Scholar]
- Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci. 2000;20:3328–3338. doi: 10.1523/JNEUROSCI.20-09-03328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs MH, Lukasiewicz PD. Glutamate uptake limits synaptic excitation of retinal ganglion cells. J Neurosci. 1999;19:3691–3700. doi: 10.1523/JNEUROSCI.19-10-03691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Creso J, Singleton M, Bartho P, Buzsaki G. Two-photon imaging of brain pericytes in vivo using dextran-conjugated dyes. Glia. 2004;46:95–100. doi: 10.1002/glia.10295. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Franceschini MA, Covolan RJ, Huppert T, Mandeville JB, Boas DA. Simultaneous recording of task-induced changes in blood oxygenation, volume, and flow using diffuse optical imaging and arterial spin-labeling MRI. Neuroimage. 2005;25:701–707. doi: 10.1016/j.neuroimage.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proc Natl Acad Sci USA. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hughes M, Barnes C. Neural Control of Circulation. New York: Academic Press; 1980. [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Beitz AJ, Renno W, Xu X, Mayer B, Zhang F. Nitric oxide synthase-containing neural processes on large cerebral arteries and cerebral microvessels. Brain Res. 1993;606:148–155. doi: 10.1016/0006-8993(93)91583-e. [DOI] [PubMed] [Google Scholar]
- Islas L, Pasantes-Morales H, Sanchez JA. Characterization of stretch-activated ion channels in cultured astrocytes. Glia. 1993;8:87–96. doi: 10.1002/glia.440080204. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005;94:4121–4130. doi: 10.1152/jn.00448.2005. [DOI] [PubMed] [Google Scholar]
- Kara P, Friedlander MJ. Arginine analogs modify signal detection by neurons in the visual cortex. J Neurosci. 1999;19:5528–5548. doi: 10.1523/JNEUROSCI.19-13-05528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat PS, Spekreijse H, Roelfsema PR. Attention lights up new object representations before the old ones fade away. J Neurosci. 2006;26:138–142. doi: 10.1523/JNEUROSCI.2784-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoe W, Freeman E, Woldorff MG, Mangun GR. Interactions between attention and perceptual grouping in human visual cortex. Brain Res. 2006;1078:101–111. doi: 10.1016/j.brainres.2005.12.083. [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F. Physiology of functional magnetic resonance imaging: energetics and function. Methods Mol Med. 2006;124:175–195. doi: 10.1385/1-59745-010-3:175. [DOI] [PubMed] [Google Scholar]
- Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA. Brain hyperthermia during physiological and pathological conditions: causes, mechanisms, and functional implications. Curr Neurovasc Res. 2004;1:77–90. doi: 10.2174/1567202043480233. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Brown PL, Wise RA. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci. 2002;16:164–168. doi: 10.1046/j.1460-9568.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Ahissar E, Diamond ME. Active sensation: insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Sachdev RN, Merchant LM, Jarvis MR, Ebner FF. Adaptive filtering of vibrissa input in motor cortex of rat. Neuron. 2002;34:1021–1034. doi: 10.1016/s0896-6273(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Kong Y, Zheng Y, Johnston D, Martindale J, Jones M, Billings S, Mayhew J. A model of the dynamic relationship between blood flow and volume changes during brain activation. J Cereb Blood Flow Metab. 2004;24:1382–1392. doi: 10.1097/01.WCB.0000141500.74439.53. [DOI] [PubMed] [Google Scholar]
- Koralek KA, Olavarria J, Killackey HP. Areal and laminar organization of corticocortical projections in the rat somatosensory cortex. J Comp Neurol. 1990;299:133–150. doi: 10.1002/cne.902990202. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Muly EC, III, Williams GV, Goldman-Rakic PS. Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci. 1998;1:286–289. doi: 10.1038/1099. [DOI] [PubMed] [Google Scholar]
- Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostasis and pathophysiology. Neurol Res. 2004;26:846–853. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M. Relationship of spikes, synaptic activity, and local changes of cerebral blood flow. J Cereb Blood Flow Metab. 2001;21:1367–1383. doi: 10.1097/00004647-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Lauritzen M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat Rev Neurosci. 2005;6:77–85. doi: 10.1038/nrn1589. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Breakspear M, Gordon E. Synchronous gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev. 2003;41:57–78. doi: 10.1016/s0165-0173(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Leutmezer F, Podreka I, Asenbaum S, Pietrzyk U, Lucht H, Back C, Benda N, Baumgartner C. Postictal psychosis in temporal lobe epilepsy. Epilepsia. 2003;44:582–590. doi: 10.1046/j.1528-1157.2003.32802.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- Li N, Sul JY, Haydon PG. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J Neurosci. 2003;23:10302–10310. doi: 10.1523/JNEUROSCI.23-32-10302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Bar KJ, Meske U, Weiller C. Motor cortex disinhibition in Alzheimer’s disease. Clin Neurophysiol. 2001;112:1436–1441. doi: 10.1016/s1388-2457(01)00554-5. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Kang J, Nedergaard M. Astrocyte activation of presynaptic metabotropic glutamate receptors modulates hippocampal inhibitory synaptic transmission. Neuron Glia Biol. 2004;1:307–316. doi: 10.1017/S1740925X05000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA. Release of adenosine by activation of NMDA receptors in the hippocampus. Science. 1994;265:2098–2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Fujita H, Seki C, Kashikura K, Yamada K, Kanno I. CBF change evoked by somatosensory activation measured by laser-Doppler flowmetry: independent evaluation of RBC velocity and RBC concentration. Jpn J Physiol. 1999;49:289–296. doi: 10.2170/jjphysiol.49.289. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- McElligott JG, Melzack R. Localized thermal changes evoked in the brain by visual and auditory stimulation. Exp Neurol. 1967;17:293–312. doi: 10.1016/0014-4886(67)90108-2. [DOI] [PubMed] [Google Scholar]
- McGahren ED, Beach JM, Duling BR. Capillaries demonstrate changes in membrane potential in response to pharmacological stimuli. Am J Physiol. 1998;274:H60–H65. doi: 10.1152/ajpheart.1998.274.1.H60. [DOI] [PubMed] [Google Scholar]
- McGahren ED, Dora KA, Damon DN, Duling BR. A test of the role of flow-dependent dilation in arteriolar responses to occlusion. Am J Physiol. 1997;272:H714–H721. doi: 10.1152/ajpheart.1997.272.2.H714. [DOI] [PubMed] [Google Scholar]
- McMains SA, Somers DC. Multiple spotlights of attentional selection in human visual cortex. Neuron. 2004;42:677–686. doi: 10.1016/s0896-6273(04)00263-6. [DOI] [PubMed] [Google Scholar]
- Mednikova YS, Pasikova NV. The temperature sensitivity of the cholinergic responses of cortical neurons in the guinea pig brain. Neurosci Behav Physiol. 2005;35:615–621. doi: 10.1007/s11055-005-0101-6. [DOI] [PubMed] [Google Scholar]
- Melzack R, Casey KL. Localized temperature changes evoked in the brain by somatic stimulation. Exp Neurol. 1967;17:276–292. doi: 10.1016/0014-4886(67)90107-0. [DOI] [PubMed] [Google Scholar]
- Michaloudi H, Grivas I, Batzios C, Chiotelli M, Papadopoulos GC. Areal and laminar variations in the vascularity of the visual, auditory, and entorhinal cortices of the developing rat brain. Brain Res Dev Brain Res. 2005;155:60–70. doi: 10.1016/j.devbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Moore CI, Andermann ML. The vibrissa resonance hypothesis. In: Ebner FF, editor. Neural Plasticity in Adult Somatic Sensory-Motor Systems. Boca Raton, FL: Taylor and Francis Publishing Group, CRC; 2005. pp. 21–59. [Google Scholar]
- Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol. 1998;80:2882–2892. doi: 10.1152/jn.1998.80.6.2882. [DOI] [PubMed] [Google Scholar]
- Moore CI, Nelson SB, Sur M. Dynamics of neuronal processing in rat somatosensory cortex. Trends Neurosci. 1999;22:513–520. doi: 10.1016/s0166-2236(99)01452-6. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Winn HR. Estimation of shear and flow rates in pial arterioles during somatosensory stimulation. Am J Physiol. 1996;270:H1712–H1717. doi: 10.1152/ajpheart.1996.270.5.H1712. [DOI] [PubMed] [Google Scholar]
- Niggel J, Sigurdson W, Sachs F. Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells and C6 glioma cells. J Membr Biol. 2000;174:121–134. doi: 10.1007/s002320001037. [DOI] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pape HC, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U. Non-random distribution of blood vessels in the posterior region of the rat somatosensory cortex. Brain Res. 1983;289:65–70. doi: 10.1016/0006-8993(83)90006-9. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawloski JR, Stamler JS. Nitric oxide in RBCs. Transfusion. 2002;42:1603–1609. doi: 10.1046/j.1537-2995.2002.00278.x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt C, Attwell D. Neurobiology: feeding the brain. Nature. 2004;431:137–138. doi: 10.1038/431137a. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantozzi M, Panella M, Palmieri MG, Koch G, Giordano A, Marciani MG, Bernardi G, Stanzione P, Stefani A. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin Neurophysiol. 2004;115:2410–2418. doi: 10.1016/j.clinph.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Reyes R, Lauritzen I, Lesage F, Ettaiche M, Fosset M, Lazdunski M. Immunolocalization of the arachidonic acid and mechanosensitive baseline traak potassium channel in the nervous system. Neuroscience. 2000;95:893–901. doi: 10.1016/s0306-4522(99)00484-4. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–4213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Mehta MA, Sahakian BJ. Neuroscience. Boosting working memory. Science. 2000;290:2275–2276. doi: 10.1126/science.290.5500.2275. [DOI] [PubMed] [Google Scholar]
- Rochon D, Rousse I, Robitaille R. Synapse-glia interactions at the mammalian neuromuscular junction. J Neurosci. 2001;21:3819–3829. doi: 10.1523/JNEUROSCI.21-11-03819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ. Another BOLD role for astrocytes: coupling blood flow to neural activity. Nat Neurosci. 2006;9:159–161. doi: 10.1038/nn0206-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol. 1890;11:85–158. doi: 10.1113/jphysiol.1890.sp000321. 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y. Cholinergic neural regulation of regional cerebral blood flow. Alzheimer Dis Assoc Disord. 1995;9:28–38. doi: 10.1097/00002093-199505000-00007. [DOI] [PubMed] [Google Scholar]
- Schachter SC. Vagus nerve stimulation: where are we? Curr Opin Neurol. 2002;15:201–206. doi: 10.1097/00019052-200204000-00013. [DOI] [PubMed] [Google Scholar]
- Segal SS, Duling BR. Propagation of vasodilation in resistance vessels of the hamster: development and review of a working hypothesis. Circ Res. 1987;61:II20–II25. [PubMed] [Google Scholar]
- Serota HM, Gerard RW. Localized thermal changes in the cat’s Brain. J Neurophysiol. 1938;1:115–124. [Google Scholar]
- Shaw PJ, Salt TE. Modulation of sensory and excitatory amino acid responses by nitric oxide donors and glutathione in the ventrobasal thalamus of the rat. Eur J Neurosci. 1997;9:1507–1513. doi: 10.1111/j.1460-9568.1997.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, Toga AW. Columnar specificity of microvascular oxygenation and volume responses: implications for functional brain mapping. J Neurosci. 2004;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci USA. 2002;99:15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkus CR, Stricker C. Properties of mEPSCs recorded in layer II neurones of rat barrel cortex. J Physiol. 2002;545:509–520. doi: 10.1113/jphysiol.2002.022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, Carvell GE. Thalamocortical response transformation in the rat vibrissa/barrel system. J Neurophysiol. 1989;61:311–330. doi: 10.1152/jn.1989.61.2.311. [DOI] [PubMed] [Google Scholar]
- Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- Stout C, Charles A. Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia. 2003;43:265–273. doi: 10.1002/glia.10257. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clarke M, Kennett S, Haggard P. Vision modulates somatosensory cortical processing. Curr Biol. 2002;12:233–236. doi: 10.1016/s0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985;5:817–824. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Prince DA. Activation of electrogenic sodium pump in hippocampal CA1 neurons following glutamate-induced depolarization. J Neurophysiol. 1986;56:507–522. doi: 10.1152/jn.1986.56.2.507. [DOI] [PubMed] [Google Scholar]
- Trubel HK, Sacolick LI, Hyder F. Regional temperature changes in the brain during somatosensory stimulation. J Cereb Blood Flow Metab. 2006;26:68–78. doi: 10.1038/sj.jcbfm.9600164. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Faraone SV. Toward reformulating the diagnosis of schizophrenia. Am J Psychiatry. 2000;157:1041–1050. doi: 10.1176/appi.ajp.157.7.1041. [DOI] [PubMed] [Google Scholar]
- Turing AM. Computing machinery and intelligence. Mind Qtly Rev Psychol Philosophy. 1950;59:433–460. [Google Scholar]
- Vallance P, Collier J, Moncada S. Nitric oxide synthesised from L-arginine mediates endothelium dependent dilatation in human veins in vivo. Cardiovasc Res. 1989;23:1053–1057. doi: 10.1093/cvr/23.12.1053. [DOI] [PubMed] [Google Scholar]
- van Helden DF, Hosaka K, Imtiaz MS. Rhythmicity in the microcirculation. Clin Hemorheol Microcirc. 2006;34:59–66. [PubMed] [Google Scholar]
- Vanzetta I, Slovin H, Omer DB, Grinvald A. Columnar resolution of blood volume and oximetry functional maps in the behaving monkey; implications for FMRI. Neuron. 2004;42:843–854. doi: 10.1016/j.neuron.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol. 2000;421:161–171. [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- Wi W. Analysis of sniffing in the albino rat. Behavior. 1964;12:223–244. [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Rovainen CM, Cox SB, Henegar MH, Liang GE, Liu D, Moskalenko YE, Sui J, Wei L. Neuronal units linked to microvascular modules in cerebral cortex: response elements for imaging the brain. Cereb Cortex. 1996;6:647–660. doi: 10.1093/cercor/6.5.647. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci USA. 2000;97:7603–7608. doi: 10.1073/pnas.97.13.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Kitamura K. Intercellular electrical coupling in vascular cells present in rat intact cerebral arterioles. J Vasc Res. 2003;40:11–27. doi: 10.1159/000068934. [DOI] [PubMed] [Google Scholar]
- Yang X, Renken R, Hyder F, Siddeek M, Greer CA, Shepherd GM, Shulman RG. Dynamic mapping at the laminar level of odor-elicited responses in rat olfactory bulb by functional MRI. Proc Natl Acad Sci USA. 1998;95:7715–7720. doi: 10.1073/pnas.95.13.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A, Clough P, Cooper P, Scheepers B, Fitzpatrick AP. Misdiagnosis of epilepsy: many seizure-like attacks have a cardiovascular cause. J Am Coll Cardiol. 2000;36:181–184. doi: 10.1016/s0735-1097(00)00700-2. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zheng D, LaMantia AS, Purves D. Specialized vascularization of the primate visual cortex. J Neurosci. 1991;11:2622–2629. doi: 10.1523/JNEUROSCI.11-08-02622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Ackerman JJ, Sukstanskii AL, Yablonskiy DA. How the body controls brain temperature: the temperature shielding effect of cerebral blood flow. J Appl Physiol. 2006;101:1481–1488. doi: 10.1152/japplphysiol.00319.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]