Abstract
Context:
Knee injuries are prevalent, and the associated knee pain is linked to disability. The influence of knee pain on movement biomechanics, independent of other factors related to knee injuries, is difficult to study and unclear.
Objective:
(1) To evaluate a novel experimental knee-pain model and (2) better understand the independent effects of knee pain on walking and running biomechanics.
Design:
Crossover study.
Setting:
Biomechanics laboratory.
Patients or Other Participants:
Twelve able-bodied volunteers (age = 23 ± 3 years, height = 1.73 ± 0.09 m, mass = 75 ± 14 kg).
Intervention(s):
Participants walked and ran at 3 time intervals (preinfusion, infusion, and postinfusion) for 3 experimental conditions (control, sham, and pain). During the infusion time interval for the pain and sham conditions, hypertonic or isotonic saline, respectively, was continuously infused into the right infrapatellar fat pad for 22 minutes.
Main Outcome Measure(s):
We used repeated-measures analyses of variance to evaluate the effects of time and condition on (1) perceived knee pain and (2) key biomechanical characteristics (ground reaction forces, and joint kinematics and kinetics) of walking and running (P < .05).
Results:
The hypertonic saline infusion (1) increased perceived knee pain throughout the infusion and (2) reduced discrete characteristics of each component of the walking ground reaction force, walking peak plantar-flexion angle (range = 62°–67°), walking peak plantar-flexion moment (range = 95–104 N·m), walking peak knee-extension moment (range = 36–49 N·m), walking peak hip-abduction moment (range = 62–73 N·m), walking peak support moment (range = 178–207 N·m), running peak plantar-flexion angle (range = 38°–77°), and running peak hip-adduction angle (range = 5–21°).
Conclusions:
This novel experimental knee pain model consistently increased perceived pain during various human movements and produced altered running and walking biomechanics that may cause abnormal knee joint-loading patterns.
Key Words: gait, infusion, hypertonic saline, kinematics, kinetics
Key Points.
Our novel experimental knee-pain model caused notable, consistent, participant-perceived anterior knee pain over 22 minutes during walking and running.
The participant-perceived pain altered certain lower extremity mechanics during walking and running.
The observed biomechanical alterations appeared to reflect an effort from the participants to unload the painful knee.
Knee injuries will likely affect 1 in 2 Americans who reach the age of 85 years, and the related annual costs are approaching $20 billion.1,2 Knee injuries usually alter lower extremity biomechanics and eventually result in functional disability.3 Signs and symptoms of knee pathologies are numerous and often include pain,4 swelling,5 quadriceps muscle inhibition and atrophy,6,7 and related decreased neuromuscular control.8 Knee pain is a prevalent factor that limits function for individuals who suffer from knee injuries4 and has even been described as the latest musculoskeletal epidemic.9
The independent effects (ie, effects that are due to pain only) of knee pain on lower extremity biomechanics during walking and running are unclear and difficult to study. Some scientists10 have argued that independent knee pain is insignificant relative to other knee signs and symptoms; however, researchers who used a single injection of hypertonic saline to cause experimental knee pain (EKP) reported that knee pain independently changed quadriceps activation patterns11 and knee biomechanics immediately after the injection (approximately 5 minutes).12,13 These neuromechanical changes likely cause abnormal knee-loading patterns during human movement. If such changes persist, articular cartilage health may be affected, as articular cartilage is sensitive to load characteristics.14 Some limitations related to these EKP studies11–13 are that perceived pain levels peaked relatively quickly (approximately 2–5 minutes), were not consistent over time, and completely resolved relatively quickly (approximately 15 minutes). It is unknown whether EKP that is maintained over a longer duration (>5 minutes) affects knee biomechanics similarly.
Specific biomechanical measures have been associated with knee-joint load during human gait. The ground reaction force (GRF) heavily influences knee-joint load.15 Internal net hip-, knee-, and ankle-joint moments (ie, the net joint torques that are produced by internal structures such as muscles) also influence knee-joint load; eg, the net internal hip extension,16 hip abduction,17,18 knee extension,19 plantar flexion,19 and overall support moment20 (the algebraic sum of the hip-extension, knee-extension, and plantar-flexion moments) each contribute to vertical GRF and the corresponding tibiofemoral joint load. Peak external knee-adduction torque has also been associated with knee-joint load.21 In addition, joint kinematics that are associated with the aforementioned GRF and net joint moments affect knee-joint load during human gait.
The purpose of this study was to answer the following research question: Does consistent knee pain, experienced over an extended duration (relative to previous studies: >5 minutes), independently alter knee-joint biomechanics during walking and running? We hypothesized that knee pain, experienced for an extended duration, does alter knee biomechanics during walking and running in a way that suggests abnormal loading of the involved knee. Specifically, we hypothesized that a novel EKP model would produce consistent knee pain over an extended period of time and that extended knee pain would cause the following changes to biomechanical variables that have been directly related to knee-joint load: (1) reduced peak impact and push-off vertical GRF, peak posterior GRF, and peak lateral GRF during walking; (2) reduced net hip-flexion, hip-abduction, knee-extension, internal plantar-flexion, and support moments during walking; (3) altered relative contributions from the net internal hip, knee, and ankle, moments to the support moment during walking; and (4) altered sagittal-plane hip, knee, and ankle and frontal-plane hip kinematics during walking and running.
METHODS
Participants and General Procedures
We obtained approval from the appropriate institutional review board before conducting this study. Twelve volunteers gave informed consent and participated (6 males and 6 females; age = 23 ± 3 years; height = 1.73 ± 0.09 m; mass = 75 ± 14 kg). Individuals with current knee pain or a history of lower extremity surgery were excluded from the study. Participants attended 3 data-collection sessions that were separated by 2 days. Each data-collection session corresponded to 1 of 3 experimental conditions: control (no infusion), sham (physiologic saline infusion), or pain (hypertonic saline infusion). Conditions were administered in a counterbalanced order. Within each condition, 3 times were considered: preinfusion, infusion, and postinfusion.
Data Collection.
For all 3 experimental conditions, participants wore spandex shorts, athletic shoes, and socks. Women also wore a sports bra. Twenty reflective markers were applied to each participant. Rigid clusters of 4 markers were attached to the distal-lateral right thigh and shank. Reflective markers were applied over the sacrum, left and right anterior-superior iliac spine, right greater trochanter, medial and lateral right knee, medial and lateral right ankle, and the heel, dorsal midfoot, lateral foot, and toe (between the second and third metatarsals) of the right foot.
For all 3 experimental conditions, a static video was recorded after reflective-marker application. For this static video, participants stood in anatomical position, which was considered neutral alignment. Participants then performed the preinfusion trials, which consisted of performing 3 walking trials across the laboratory walkway and then running on a treadmill for 30 seconds (for all times and conditions, walking trials were performed first, and running trials were performed second). Next, participants lay in a supine position. For the sham and pain experimental conditions, a 20-gauge flexible catheter (Becton Dickinson Medical Systems, Sandy, UT) was inserted into the right infrapatellar fat pad. The catheter was inserted from the lateral side at an angle of 45° in the inferior-medial direction to a depth of 1 cm to the middle of the infrapatellar fat pad under the patellar tendon (Figure 1). To ensure that the catheter was inserted into the correct location, catheter location was confirmed using dynamic ultrasound immediately after the insertion. Using a 100-cm extension tube (B. Braun, Bethlehem, PA), the catheter was connected to a 30-mL syringe that was attached to a portable infusion pump (Graseby Medical, Hertfordshire, UK). This pump produced a continuous saline flow of 0.3 mL·min−1 for 22 minutes (6.6 mL of saline). For the sham and pain experimental conditions, respectively, an infusion of sterile isotonic (0.9% sodium chloride; Hospira, Inc, Lake Forest, IL) or hypertonic (5.0% sodium chloride; B. Braun, Irvine, CA) saline was initiated. Once the infusion was initiated, participants lay supine for 3 minutes, sat upright for 3 minutes, and stood for 2 minutes (to maintain consistency, they were required to lie, sit, and stand for equivalent durations during the control condition). These 8 minutes helped them become familiar with the effects of the infusion. Participants then performed the infusion trials. The infusion trials for the control condition did not involve a catheter or infusion. Other than the infusion (for the sham and pain conditions), the infusion trials were the same as the preinfusion trials (ie, 3 walking trials, followed by the running trials).
Figure 1.
Catheter insertion.
For the sham and pain experimental conditions, the catheter was removed 22 minutes after the infusion began (Figure 2). For all 3 experimental conditions, participants then sat quietly in a chair for 15 minutes; for those in the pain condition, this time allowed for pain reduction (Figure 2). After 15 minutes, for each condition, participants performed the postinfusion trials, which also involved 3 walking trials and 30 seconds of treadmill running. All walking trials were performed at a self-selected speed that was determined by the average horizontal velocity of the sacral marker. Running trials were performed at a speed that was normalized to leg length; using a previously recommended normalization technique,22 the dimensionless velocity for all running trials was 1:
|
|
Figure 2.
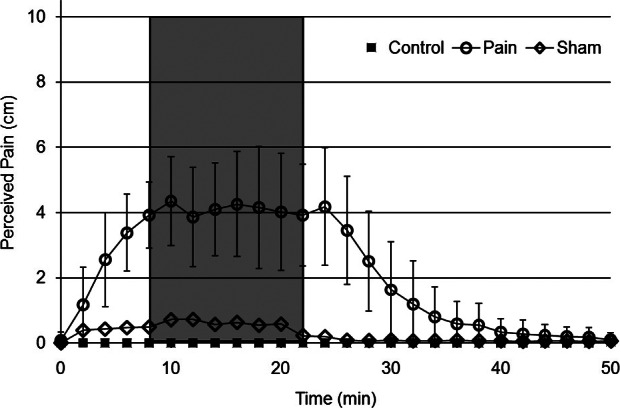
Perceived pain level for 3 experimental conditions (control, sham, and pain). Variance is only shown for the pain condition to increase clarity. Preinfusion trials were performed prior to time 0 (time of catheter insertion). Treatment trials were performed during the time indicated by the shaded area. Postinfusion trials were performed beginning at 37 minutes.
Self-perceived pain level was assumed to be zero during the preinfusion trials because current knee pain was an exclusion criterion for this study. Using a 10-cm visual analog scale, perceived pain was evaluated just before catheter insertion and every 2 minutes thereafter, until 10 minutes after the completion of the postinfusion trials (Figure 2). For all walking trials, synchronized motion and GRF data were collected using 6 high-speed digital video cameras (100 Hz; VICON, Santa Rosa, CA) and 1 force platform that was embedded in the laboratory walkway (1500 Hz; AMTI, Watertown, MA). Only motion data were collected during the running trials.
Data Reduction.
Spatial trajectories for the reflective markers were tracked using VICON Nexus software and then imported into Visual 3D software (C-Motion, Germantown, MD). These trajectories were then smoothed using a fourth-order, low-pass Butterworth filter; cutoff frequencies (determined using residual analyses23) of 6 and 10 Hz were used for the walking and running trials, respectively. The smoothed marker coordinates were then used to calculate 3-dimensional hip-, knee-, and ankle-joint kinematics for walking and running. A static model was created for each participant using previously described methods.24 Joint angles were calculated using a Cardan rotation sequence of flexion-extension, abduction-adduction, and internal-external rotation. For the walking trials, 3-dimensional joint moments were calculated using a standard inverse dynamics approach that involved the synchronized joint kinematic and GRF data and anthropometric data.25 The joint moments reported here are internal moments. Calculated joint kinematics and kinetics were exported to MATLAB (The MathWorks, Natick, MA), and the discrete dependent variables were identified using custom algorithms. The kinetic dependent variables related to the walking trials are shown in Table 1. The kinematic dependent variables related to the walking and running trials are shown in Tables 2 and 3, respectively.
Table 1.
Kinetic Variables During Walking for 3 Conditions (Control, Sham, and Pain) and Times (Preinfusion, Infusion, and Postinfusion)a (Mean ± SD)
| Dependent Variable |
Session |
||||||||
| Control |
Sham |
Pain |
|||||||
| Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
|
| Peak impact GRF (BW)a | 1.13 ± 0.04 | 1.14 ± 0.04 | 1.15 ± 0.04 | 1.15 ± 0.05 | 1.15 ± 0.05 | 1.15 ± 0.05 | 1.14 ± 0.07 | 1.10 ± 0.07 | 1.15 ± 0.06 |
| Peak push-off GRF (BW)a | 1.13 ± 0.0.5 | 1.14 ± 0.05 | 1.14 ± 0.05 | 1.14 ± 0.06 | 1.13 ± 0.05 | 1.14 ± 0.05 | 1.12 ± 0.06 | 1.06 ± 0.08 | 1.13 ± 0.06 |
| Peak breaking GRF (BW)a | 0.20 ± 0.0.3 | 0.20 ± 0.03 | 0.20 ± 0.03 | 0.19 ± 0.02 | 0.19 ± 0.03 | 0.20 ± 0.03 | 0.20 ± 0.03 | 0.17 ± 0.04 | 0.19 ± 0.03 |
| Peak lateral GRF (BW × 10−2) | 4.12 ± 1.68 | 4.09 ± 1.54 | 4.13 ± 1.44 | 3.89 ± 1.20 | 3.71 ± 1.25 | 3.83 ± 1.26 | 3.71 ± 1.08 | 2.86 ± 1.09 | 4.18 ± 1.30 |
| Peak support moment (Nm) | 202.5 ± 54.5 | 206.9 ± 58.3 | 203.9 ± 54.3 | 201.5 ± 53.8 | 199.2 ± 53.9 | 201.7 ± 56.6 | 195.0 ± 49.0 | 178.0 ± 50.5 | 197.7 ± 49.0 |
| Hip contribution, % | 26 ± 4 | 27 ± 5 | 27 ± 5 | 25 ± 5 | 24 ± 4 | 25 ± 4 | 26 ± 5 | 26 ± 6 | 27 ± 5 |
| Knee contribution, % | 22 ± 5 | 22 ± 6 | 21 ± 6 | 22 ± 6 | 23 ± 7 | 22 ± 7 | 21 ± 8 | 20 ± 8 | 20 ± 8 |
| Ankle contribution, % | 52 ± 5 | 51 ± 5 | 51 ± 4 | 53 ± 4 | 53 ± 5 | 53 ± 5 | 53 ± 5 | 54 ± 5 | 53 ± 5 |
| Peak hip-extension moment (Nm) | 53.6 ± 19.7 | 56.6 ± 19.6 | 56.1 ± 19.4 | 50.2 ± 14.5 | 47.3 ± 12.1 | 48.8 ± 13.1 | 50.6 ± 15.7 | 46.9 ± 19.8 | 52.4 ± 16.4 |
| Peak hip-flexion moment (Nm) | 119.3 ± 26.3 | 116.2 ± 23.5 | 114.0 ± 24.3 | 124.0 ± 33.2 | 119.6 ± 33.2 | 124.0 ± 35.1 | 120.2 ± 25.3 | 110.5 ± 31.6 | 123.5 ± 24.6 |
| Peak hip-abduction moment (Nm)a | 70.7 ± 15.3 | 71.2 ± 16.8 | 68.1 ± 18.3 | 70.9 ± 22.0 | 66.2 ± 19.1 | 68.5 ± 23.2 | 72.5 ± 12.9 | 61.6 ± 15.1 | 67.4 ± 12.8 |
| Peak knee-extension moment (Nm)a | 45.4 ± 18.1 | 46.6 ± 22.2 | 44.6 ± 20.1 | 46.8 ± 22.8 | 48.6 ± 23.4 | 47.5 ± 24.5 | 42.5 ± 20.8 | 36.1 ± 17.5 | 41.8 ± 22.6 |
| Peak knee-abduction moment (Nm) | 27.7 ± 7.9 | 28.7 ± 10.0 | 29.7 ± 7.8 | 27.3 ± 7.3 | 26.3 ± 6.5 | 28.4 ± 6.6 | 28.1 ± 8.4 | 26.7 ± 11.3 | 31.2 ± 11.0 |
| Peak plantar-flexion moment (Nm)a | 103.5 ± 22.0 | 103.7 ± 22.4 | 103.2 ± 22.3 | 104.4 ± 23.3 | 103.3 ± 23.4 | 105.4 ± 23.5 | 101.9 ± 22.5 | 95.0 ± 24.3 | 103.6 ± 22.0 |
Abbreviations: BW, body weight; GRF, ground reaction force.
Condition × time interactions and between-time differences for the pain condition. Peak support, hip-abduction, knee-extension, and plantar-flexion moments were less as a result of experimental knee pain.
Table 2.
Kinematic Variables During Walking for 3 Conditions (Control, Sham, and Pain) and Times (Preinfusion, Infusion, and Postinfusion)a (Mean ± SD)
| Dependent Variable |
Session |
||||||||
| Control |
Sham |
Pain |
|||||||
| Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
|
| Peak hip-extension angle (°) | 16.7 ± 3.1 | 16.0 ± 5.0 | 15.5 ± 5.8 | 18.0 ± 3.1 | 16.8 ± 2.4 | 18.1 ± 2.4 | 17.1 ± 4.1 | 16.8 ± 5.7 | 18.7 ± 4.8 |
| Peak hip-flexion angle (°) | 23.8 ± 4.0 | 25.3 ± 5.0 | 25.5 ± 5.4 | 22.5 ± 2.8 | 22.7 ± 3.2 | 22.6 ± 4.1 | 22.5 ± 3.9 | 21.7 ± 4.8 | 21.6 ± 5.1 |
| Peak hip-adduction angle (°) | 8.7 ± 3.3 | 8.9 ± 3.8 | 9.6 ± 4.3 | 8.3 ± 4.5 | 7.9 ± 4.5 | 8.4 ± 4.6 | 9.8 ± 3.0 | 8.4 ± 2.7 | 9.7 ± 3.3 |
| Peak knee-flexion angle (°) | 14.8 ± 4.1 | 17.1 ± 4.9 | 17.5 ± 5.5 | 14.2 ± 4.6 | 15.9 ± 4.5 | 15.4 ± 4.6 | 13.0 ± 5.5 | 14.1 ± 6.7 | 14.1 ± 6.4 |
| Peak knee-adduction angle (°) | 1.9 ± 2.0 | 1.9 ± 1.9 | 1.9 ± 2.2 | 1.7 ± 4.0 | 1.6 ± 4.5 | 1.7 ± 4.5 | 2.3 ± 3.4 | 2.2 ± 3.6 | 3.4 ± 3.2 |
| Peak dorsiflexion angle (°) | 94.1 ± 2.5 | 93.6 ± 4.4 | 93.9 ± 3.7 | 94.2 ± 3.5 | 94.0 ± 3.5 | 94.4 ± 3.1 | 93.3 ± 3.3 | 94.4 ± 5.4 | 93.6 ± 4.3 |
| Peak plantar-flexion angle (°)a | 63.2 ± 6.3 | 62.7 ± 6.3 | 61.8 ± 5.1 | 64.8 ± 4.3 | 64.7 ± 3.9 | 63.0 ± 4.0 | 62.9 ± 4.6 | 66.8 ± 8.9 | 62.1 ± 4.6 |
| Stance time (s) | 0.68 ± 0.05 | 0.67 ± 0.06 | 0.68 ± 0.05 | 0.68 ± 0.05 | 0.68 ± 0.04 | 0.68 ± 0.04 | 0.70 ± 0.05 | 0.69 ± 0.05 | 0.69 ± 0.04 |
| Walking speed (m/s) | 1.41 ± 0.15 | 1.43 ± 0.16 | 1.41 ± 0.14 | 1.41 ± 0.13 | 1.41 ± 0.12 | 1.42 ± 0.12 | 1.38 ± 0.13 | 1.35 ± 0.19 | 1.40 ± 0.10 |
Condition × time interactions and between-time differences for the pain condition. Peak plantar flexion was less as a result of experimental knee pain.
Table 3.
Kinematic Variables During Running for 3 Conditions (Control, Sham, and Pain) and Times (Preinfusion, Infusion, and Postinfusion)a (Mean ± SD)
| Dependent Variable |
Session |
||||||||
| Control |
Sham |
Pain |
|||||||
| Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
Preinfusion |
Infusion |
Postinfusion |
|
| Peak hip-extension angle (°) | 14.9 ± 5.4 | 14.0 ± 6.1 | 13.9 ± 6.1 | 16.4 ± 4.2 | 15.3 ± 3.3 | 16.5 ± 3.7 | 16.7 ± 3.7 | 15.9 ± 5.3 | 18.2 ± 4.6 |
| Peak hip-flexion angle (°) | 31.5 ± 3.3 | 31.4 ± 4.4 | 31.5 ± 4.5 | 30.3 ± 4.0 | 28.2 ± 3.8 | 29.1 ± 4.1 | 29.3 ± 3.6 | 27.8 ± 4.2 | 27.5 ± 4.8 |
| Peak hip-adduction angle (°)a | 12.7 ± 4.4 | 12.8 ± 4.9 | 12.6 ± 4.3 | 12.8 ± 5.0 | 12.2 ± 5.3 | 11.7 ± 4.9 | 14.3 ± 3.3 | 12.3 ± 4.4 | 13.9 ± 4.2 |
| Peak knee-flexion angle (°) | 40.0 ± 4.1 | 41.6 ± 4.0 | 41.5 ± 5.0 | 38.9 ± 3.5 | 39.9 ± 3.9 | 39.3 ± 3.9 | 38.2 ± 4.4 | 37.4 ± 5.3 | 38.5 ± 5.2 |
| Peak knee-adduction angle (°) | 0.8 ± 1.7 | 0.8 ± 2.1 | 1.0 ± 2.0 | 0.8 ± 3.7 | 0.8 ± 4.0 | 0.9 ± 4.0 | 1.1 ± 2.2 | 1.8 ± 2.7 | 2.0 ± 2.8 |
| Peak dorsiflexion angle (°) | 103.6 ± 2.4 | 103.7 ± 3.2 | 104.0 ± 2.9 | 104.5 ± 2.8 | 104.0 ± 3.3 | 104.8 ± 2.5 | 104.3 ± 3.6 | 103.3 ± 4.0 | 104.7 ± 5.2 |
| Peak plantar-flexion angle (°)a | 55.3 ± 6.3 | 53.7 ± 6.9 | 53.7 ± 5.9 | 55.7 ± 5.8 | 55.4 ± 5.5 | 54.3 ± 5.6 | 54.2 ± 4.8 | 57.7 ± 7.5 | 53.9 ± 7.0 |
| Stance time (s) | 0.34 ± 0.07 | 0.36 ± 0.07 | 0.35 ± 0.06 | 0.37 ± 0.05 | 0.39 ± 0.05 | 0.38 ± 0.04 | 0.38 ± 0.06 | 0.38 ± 0.05 | 0.39 ± 0.05 |
Condition × time interactions and between-time differences for the pain condition. Peak hip-adduction and plantar-flexion angles were less as a result of experimental knee pain.
Statistical Analysis.
The independent variables were experimental condition (control, sham, and pain) and time (preinfusion, infusion, and postinfusion). Means for the dependent variables (Tables 1–3) were calculated and compared across condition and time using repeated-measures analyses of variance (P < .05). Condition × time interactions were evaluated for each dependent variable and Tukey post hoc comparisons were used to determine the nature of detected interactions (P < .05). Effect sizes were also calculated ([X̄1 − X̄2]/σpooled)26 to better interpret the between-time differences for each variable for each condition (Supplemental Table S1 is available at http://dx.doi.org/10.4085/1062-6050-48.2.02.S1 (64.1KB, pdf) ).
Results
We found a condition × time interaction for self-perceived pain that lasted as long as the hypertonic infusion. The related post hoc comparisons showed that, for the pain condition only, self-perceived pain was greater for the infusion trials than for the preinfusion and postinfusion trials (Figure 2). Perceived pain remained elevated throughout the hypertonic infusion (Figure 2). Similarly, a condition × time interaction was detected for each of the 4 observed GRF characteristics during walking (Table 1). For the pain condition only, post hoc comparisons indicated that (1) peak vertical impact GRF was 3% and 4% less for the infusion trials than for the preinfusion and postinfusion trials, respectively; (2) peak vertical push-off GRF was 5% and 6% less for the infusion trials than for the preinfusion and postinfusion trials, respectively; (3) peak braking GRF was 12% and 13% less for the infusion trials than for the preinfusion and postinfusion trials, respectively; and (4) peak lateral GRF was 23% and 32% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05). No observed GRF characteristic changed between any of the times for the control or sham conditions (Table 1).
We found condition × time interactions for 4 joint kinetic variables during the stance phase of walking. Similar to the GRF variables, the post hoc comparisons revealed between-time differences for the pain condition only for these joint kinetic variables (Table 1). Peak support moment was 9% and 10% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05). Peak plantar-flexion moment was 7% and 8% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05). Peak knee-extension moment during early stance was 15% and 14% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05). Peak hip-abduction moment during early stance was 15% and 9% less for the infusion trials (P < .05) than for the preinfusion and postimpact trials, respectively. No between-time difference was observed for any joint kinetic variable during the control or sham conditions (Table 1).
We found a significant condition × time interaction for 1 kinematic variable during walking (Table 2) and 2 kinematic variables during running (Table 3). Similar to the observed kinetic variables, the post hoc comparisons indicated that between-time differences existed for the pain condition only for these 3 kinematic variables. Peak plantar-flexion angle during walking was 6% and 8% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05; Table 2). Peak plantar-flexion angle during running was 6% and 7% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05; Table 3). Peak hip-adduction angle during running was 14% and 12% less for the infusion trials than for the preinfusion and postinfusion trials, respectively (P < .05; Table 3). Self-selected walking speed was not influenced by the EKP (P = .45). No between-time difference was observed for any joint kinematic variable (walking or running) during the control or sham conditions (Tables 2 and 3).
DISCUSSION
The purpose of this study was to learn more about the independent effects of knee pain on lower extremity biomechanics during walking and running. Our results show that the novel EKP model produced consistent EKP over an extended duration during various human movements. Also, the present findings corroborate the idea that knee pain does independently affect certain lower extremity biomechanics during walking and running. Perceived pain and the corresponding biomechanical alterations persisted as long as the hypertonic saline was being infused. When the infusion was terminated, the EKP diminished and pre-EKP lower extremity biomechanics were again demonstrated by the participants. This indicates that the observed biomechanical alterations were caused by the EKP. The EKP resulted in a reduction of (1) peak vertical, lateral, and posterior GRF (Table 1), (2) several peak moments (hip abduction, knee extension, plantar flexion, and support; Table 1), and (3) peak plantar-flexion and hip-adduction angles (Tables 2 and 3). Because these biomechanical variables have been associated with support during human locomotion, the observed alterations appear to be related to an attempt by the participants to facilitate some level of unloading for the involved knee and lower extremity. These biomechanical differences should not be attributed to walking speed, as speed did not differ across time for any of the experimental conditions (Table 2). The present data are comparable to similar previously collected data,12,27–30 indicating that our data-collection and -analysis methods were appropriate.
One of the most important parts of our results is that EKP was consistently perceived by the participants for a relatively extended duration (ie, greater than other tested durations12,31,32) during different movements (walking and running). During this study, the average perceived pain level of 4.29 cm (Figure 2) was maintained for 22 minutes. This pain level is greater than most of the previously reported mean perceived pain levels (2.58 and 3.20 cm),12,32 and the duration is longer than previously reported durations.12,13,31 These differences in pain level and duration are likely due to the different volume of utilized hypertonic saline and extended nature of the infusion. Whereas previous researchers used single hypertonic saline injections of varying volumes, including 0.7512 and 1.00 mL,32 we used a continuous infusion (0.3 mL·min−1 for 22 minutes) to deliver 6.6 mL of hypertonic saline into the infrapatellar fat pad. Rather than declining over time after a single injection, the present pain levels were consistent as long as the infusion continued (Figure 2). The relatively extended duration of the present EKP allowed us to study the independent effects of knee pain on walking and running biomechanics over a relatively extended duration (compared with previous EKP models). This new approach may be a valuable tool in the study of the biomechanical effects of chronic knee pain. Although the single-injection method appears to be an effective way to induce EKP, perhaps the constant infusion is more effective because it produces EKP over an increased duration at a consistent intensity. Additionally, the increased saline volume alone had no effect on our dependent variables, as evidenced by the lack of between-time changes for the sham condition, in which the same volume (6.6 mL) of isotonic saline was infused.
Important biomechanical alterations occurred as a result of the EKP. Some of these alterations are consistent with reports from investigators12 who previously used EKP and similar research designs to evaluate independent effects of EKP on movement biomechanics. Experimental knee pain altered various walking biomechanics, including reducing internal knee-extension moment12 and decreasing strength for muscles crossing the knee joint.32 The decreased vertical and braking GRF we observed are likely related to a reduced internal knee-extension moment and decreased quadriceps strength. The internal knee-extension moment is created largely by the knee extensors (quadriceps) and counters the external knee-flexion moment that is created, in large part, by the GRF. The present findings may also support those of previous researchers11,33 who reported that EKP, due to hypertonic saline in the infrapatellar fat pad, altered quadriceps activation characteristics, because quadriceps activity contributes to upward center-of-mass acceleration during walking. In speculation, quadriceps inhibition, due to EKP, may have occurred during the present study, resulting in reduced peak push-off vertical GRF, knee-extension moment, and upward acceleration of the center of mass.
The net joint moments that were reduced as a result of the knee pain during walking have been associated with GRF (transmitted to the involved lower extremity) and knee-joint load. Specifically, reduced peak plantar-flexion and knee-extension moments (Table 1) are likely related to the reduction in GRF, as well as previously reported reduced GRF.34 Reduced plantar-flexion and knee-extension moments have also been associated with decreased axial knee-joint contact forces.19,35 Axial knee-joint contact force peaks twice during the stance phase of gait, at approximately 15% and 50% of the gait cycle.19,35 The vastii and gastrocnemius muscles are the primary contributors to the first and second peaks, respectively.15,19 As the vastii are associated with the first knee-extension moment peak,15,19 the observed decrease for knee-extension moment may indicate reduced vastii activity in an attempt to decrease knee-joint load. Similarly, the gastrocnemius contributes to peak plantar-flexion moment at approximately 50% of the gait cycle, and this corresponds to the second axial knee-contact force peak.15,19 The observed reduction in peak plantar-flexion moment may be related to reduced gastrocnemius activity in an attempt to reduce knee-joint load. Additionally, the hip abductors have been identified as important contributors to support (the vertical GRF) and knee-joint load during midstance.36 The reduced hip-abduction moment (Table 1) could indicate reduced gluteus medius activity, in an attempt to reduce GRF and knee-joint load. Knee pain appears to alter motor strategies in order to facilitate unloading.
In speculation, various negative effects could result if the observed biomechanical alterations persisted over an extended duration. Over time, the observed unloading strategy could result in disuse-related weakness of the involved musculature (hip abductors, knee extensors, and plantar flexors). These biomechanical alterations would also likely affect articular cartilage health, as articular cartilage health depends upon appropriate knee joint-loading patterns.14,37 An unloading strategy might also result in chronic bilateral strength asymmetry38 because this potential unloading strategy implies altered loading characteristics for the uninvolved lower extremity. For example, if impulse due to vertical GRF is decreased for the involved lower extremity, the same impulse must increase for the uninvolved lower extremity to adequately support the center of mass during walking. Because we did not collect bilateral data, we can only speculate as to the effects on the uninvolved lower extremity, and this should be considered a limitation to the present study.
The observed decreased support moment also reflects an overall unloading of the involved lower extremity. The support moment has been quantified in gait research in various contexts, is typically less variable than individual joint moments, and provides an indication of how effectively the lower extremity is supporting the center of mass against gravitational forces.20,39,40 A reduced support moment indicates a decreased capability, or at least willingness, of the involved lower extremity to resist gravity and prevent collapse (1 of 2 important functions of the lower extremities during gait). This reduced support moment reflects a decreased GRF applied to the involved lower extremity.34
Although the peak support moment decreased as a result of EKP, the relative contribution to the support moment from the hip-, knee-, and ankle-joint moments did not change (Table 1). This finding implies that muscles contributing to hip-extension, knee-extension, and ankle plantar-flexion moments were influenced in similar ways by knee pain; ie, all of the aforementioned muscles decreased proportionately in an attempt to unload the involved knee. These consistent relative contributions contradicted our hypothesis but they fit with a previous report.41 A decreased support moment for the involved lower extremity (posterior cruciate ligament injury) was observed during squatting exercises; however, no corresponding change in relative contribution to the support moment from the hip-, knee-, and ankle-joint moments was observed.41 We speculate that these consistent percentage contributions for hip-extension, knee-extension, and ankle plantar-flexion, moments may be partially due to the relatively low intensity of the task (walking). Perhaps a more intense task requiring increased support (eg, sprinting, jumping, or landing) would induce disproportionate changes for relative contributions to the support moment from the hip, knee, and ankle. Such was the case for patients with tendinopathy who were required to hop on the involved lower extremity.40
Although some joint kinematics were altered, our participants generally exhibited kinematic invariance42 (ie, relatively little change in kinematic variables) among the 3 experimental conditions (control, pain, and sham). In speculation, this may be related to the kinematic invariance that has been documented for able-bodied43 and amputee participants.42,44 The present data support the idea that walking with consistent kinematics is an implicit or explicit goal for humans.42–44 Altering lower extremity kinetics in an attempt to maintain consistent lower extremity kinematics might be accomplished using various motor strategies. One such strategy might involve a shift of body weight (the trunk) toward the involved side. This type of shift may have contributed to the decreased hip-adduction angle we observed during the running trials (Table 3). Researchers have suggested that such a shift likely reduces necessary contributions from the involved-leg musculature to support body weight45 and acutely alters knee-joint loading patterns.46 Unfortunately, we did not measure upper body kinematics and are unable to quantitatively support this idea; however, future investigators could clarify this issue.
The present approach to studying the influence of knee pain on movement biomechanics is novel, and some aspects are unclear. We are unsure how closely the EKP experienced by our participants resembles knee pain that is caused by actual knee injury. Although the perceived pain levels appeared to be sufficient (Figure 2), whether other characteristics of this EKP pain are representative is unknown. Previous authors47–50 supported the idea that EKP induced by hypertonic saline is representative of joint pain related to knee injury. Investigators47 have reported that the nature, quality, and distribution of pain elicited by hypertonic saline is similar to clinical anterior knee joint pain. Also, results49,50 from animal experiments suggest that group III and IV nociceptive afferents are active due to hypertonic saline, providing more evidence that the pathways involved in EKP are similar to musculoskeletal pain. Researchers48 have also reported that hypertonic saline induces the release of substance P from C-fiber neurons, which is consistent with musculoskeletal pain. We assume that C-fiber sensory information is responsible for the biomechanical alterations we observed. Without data that directly describe neuromuscular activation characteristics, however, we are unsure about the related neural mechanism(s). Although the exact pain pathways involved in the present study are unclear, our data do provide an idea of how knee pain may independently alter walking and running biomechanics. Additionally, the contribution of knee pain to movement alterations, relative to other knee-injury factors, remains unclear. Future authors will need to determine the contributions of all knee-injury factors to movement alterations. Finally, our small sample size and associated limitations should be noted. We performed several power analyses using our own pilot data (GRF and joint kinematics and kinetics) and previously reported joint kinematic data.51 Results of these power analyses varied, depended on the variability of the data, and indicated that 13 to 135 participants would be necessary to achieve 80% power at a significance level of .05. We chose a feasible sample size that would allow us to study the same number of condition orders and counterbalance the condition order. Investigators11,46,52 in other similar experimental pain studies have used comparably small samples; however, the small size of our sample does increase the likelihood of committing a type II error.
In summary, there were 2 major findings of this study. First, the present pain model can be used to produce and maintain a consistent level of perceived pain for a relatively extended duration while participants perform various movements. Second, this knee pain influences numerous lower extremity biomechanical variables (GRF, hip-abduction angle and moment, knee-extension moment, and plantar-flexion angle and moment) during walking and running, independent of other knee pathology signs and symptoms. This is the first study to evaluate the independent effects of knee pain on human movement biomechanics using a continuous infusion of hypertonic saline. The infusion allowed us to study the effects of hypertonic saline administered over a relatively extended duration (>5 minutes). Perceived pain levels were consistent and the observed biomechanical alterations remained as long as the pain persisted. Generally, lower extremity kinetics (peak GRF and net joint moments) appeared to be affected more by the EKP than by lower extremity joint kinematics (peak angles). Many of the biomechanical variables that were altered as a result of EKP have been associated with knee-joint load, and these biomechanical alterations may have reflected an attempt to unload the affected knee.
SUPPLEMENTAL DATA
Table S1. Cohen Effect Sizes for Between-Time Comparisons (Preinfusion to Infusion and Infusion to Postinfusion) for Each Condition (Control, Pain, and Sham)
REFERENCES
- 1.Gottlob CA, Baker CL, Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Rel Res. 1999;(367):272–282. [PubMed] [Google Scholar]
- 2.Murphy L, Schwartz TA, Helmick CG et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59(9):1207–1213. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurley M, Scott D, Rees J, Newham D. Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis. 1997;56(11):641–648. doi: 10.1136/ard.56.11.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merle-Vincent F, Couris CM, Schott AM et al. Cross-sectional study of pain and disability at knee replacement surgery for osteoarthritis in 299 patients. Joint Bone Spine. 2007;74(6):612–616. doi: 10.1016/j.jbspin.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Jones DW, Jones DA, Newham DJ. Chronic knee effusion and aspiration: the effect on quadriceps inhibition. Br J Rheumatol. 1987;26(5):370–374. doi: 10.1093/rheumatology/26.5.370. [DOI] [PubMed] [Google Scholar]
- 6.Hurly MV, Newham DJ. The influence of arthrogenous muscle inhibition of quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993;32(2):127–131. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- 7.Wood L, Ferrell WR, Baxendale RH. Pressures in normal and acutely distended human knee joints and effects on quadriceps maximal voluntary contractions. Q J Exp Physiolo. 1988;73(3):305–314. doi: 10.1113/expphysiol.1988.sp003147. [DOI] [PubMed] [Google Scholar]
- 8.Beard DJ, Kyberg PJ, Fergussion CM, Dodd CA. Proprioception after rupture of the anterior cruciate ligament. J Bone Joint Surg. 1993;75(2):311–315. doi: 10.1302/0301-620X.75B2.8444956. [DOI] [PubMed] [Google Scholar]
- 9.Symmons D. Knee pain in older adults: the latest musculoskeletal “epidemic.”. Ann Rheum Dis. 2001;60(2):89–90. doi: 10.1136/ard.60.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty: the contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87(5):1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges PW, Mellor R, Crossley K, Bennell K. Pain induced by injection of hypertonic saline into the infrapatellar fat pad and effect on coordination of the quadriceps muscles. Arthritis Rheum. 2009;61(1):70–77. doi: 10.1002/art.24089. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen M, Graven-Nielsen T, Aaboe J, Andriacchi TP, Bliddal H. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care Res (Hoboken) 2010;62(4):501–509. doi: 10.1002/acr.20033. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen M, Rosager S, Aaboe J, Graven-Nielsen T, Bliddal H. Experimental knee pain reduces muscle strength. J Pain. 2011;12(4):460–467. doi: 10.1016/j.jpain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Roemhildt ML, Coughlin KM, Peura GD et al. Effects of increased chronic loading on articular cartilage material properties in the Lapine tibiofemoral joint. J Biomech. 2010;43(12):2301–2308. doi: 10.1016/j.jbiomech.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelburne KB, Torry MR, Pandy MG. Contributions of muscles, ligaments, and the ground-reaction force to tibiofemoral joint loading during normal gait. J Orthop Res. 2006;24(10):1983–1990. doi: 10.1002/jor.20255. [DOI] [PubMed] [Google Scholar]
- 16.Liu MQ, Anderson FC, Pandy MG, Delp SL. Muscles that support the body also modulate forward progression during walking. J Biomech. 2006;39(14):2623–2630. doi: 10.1016/j.jbiomech.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Chang A, Hayes K, Dunlop D et al. Hip abduction moment and protection against medial tibiofemoral osteoarthritis progression. Arthritis Rheum. 2005;52(11):3515–3519. doi: 10.1002/art.21406. [DOI] [PubMed] [Google Scholar]
- 18.Kutzner I, Heinlein B, Graichen F et al. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 2010;43(11):2164–2173. doi: 10.1016/j.jbiomech.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki K, Neptune RR. Individual muscle contributions to the axial knee joint contact force during normal walking. J Biomech. 2010;43(14):2780–2784. doi: 10.1016/j.jbiomech.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter DA. Overall principle of lower limb support during stance phase of gait. J Biomech. 1980;13(11):923–927. doi: 10.1016/0021-9290(80)90162-1. [DOI] [PubMed] [Google Scholar]
- 21.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 22.Hof AL. Scaling gait data to body size. Gait Posture. 1996;4(3):222–223. [Google Scholar]
- 23.Jackson KM. Fitting of mathematical functions to biomechanical data. IEEE Trans Biomed Eng. 1979;26(2):122–124. doi: 10.1109/tbme.1979.326551. [DOI] [PubMed] [Google Scholar]
- 24.Ford KR, Shapiro R, Myer GD, Van den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sport Exer. 2010;42(10):1923–1931. doi: 10.1249/MSS.0b013e3181dc99b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempster W. Space requirements of the seated operator. WADC Technical Report (TR-55-159) Wright-Patterson Air Force Base, OH. 1955 [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates;; 1988. [Google Scholar]
- 27.Barrios JA, Davis IS, Higginson JS, Royer TD. Lower extremity walking mechanics of young individuals with asymptomatic varus knee alignment. J Orthop Res. 2009;27(11):1414–1419. doi: 10.1002/jor.20904. [DOI] [PubMed] [Google Scholar]
- 28.Bovi G, Rabuffetti M, Mazzoleni P, Ferrarin M. A multiple-task gait analysis approach: kinematic, kinetic and EMG reference data for healthy young and adult subjects. Gait Posture. 2011;33(1):6–13. doi: 10.1016/j.gaitpost.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Bowsher KA, Vaughan CL. Effect of foot-progression angle on hip-joint moments during gait. J Biomech. 1995;28(6):759–762. doi: 10.1016/0021-9290(94)00123-l. [DOI] [PubMed] [Google Scholar]
- 30.Dierks TA, Manal KT, Hamill J, Davis IS. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38(8):448–456. doi: 10.2519/jospt.2008.2490. [DOI] [PubMed] [Google Scholar]
- 31.Hodges PW, Mellor R, Crossley K, Bennell K. Pain induced by injection of hypertonic saline into the infrapatellar fat pad and effect on coordination of the quadriceps muscles. Arthritis Rheum. 2009;61(1):70–77. doi: 10.1002/art.24089. [DOI] [PubMed] [Google Scholar]
- 32.Henriksen M, Rosager S, Aaboe J, Graven-Nielsen T, Bliddal H. Experimental knee pain reduces muscle strength. J Pain. 2011;12(4):460–7. doi: 10.1016/j.jpain.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Long B, Knight K, Hopkins J, Feland J, Parcell A, Schaalje B. Arthrogenic muscle inhibition occurs with pain and is removed with cryotherapy. 2009; San Antonio, TX Paper presented at: Annual Meeting and Clinical Symposia of the National Athletic Trainers; Association. [Google Scholar]
- 34.Seeley M, Park J, King D, Hopkins JT. Ground reaction force alterations due to experimentally induced anterior knee pain during walking. 2010 Paper presented at: Annual Meeting of the American College of Sports Medicine; June 5. Baltimore, MD. [Google Scholar]
- 35.Kim HJ, Fernandez JW, Akbarshahi M, Walter JP, Fregly BJ, Pandy MG. Evaluation of predicted knee-joint muscle forces during gait using an instrumented knee implant. J Orthop Res. 2009;27(10):1326–1331. doi: 10.1002/jor.20876. [DOI] [PubMed] [Google Scholar]
- 36.Anderson FC, Pandy MG. Individual muscle contributions to support in normal walking. Gait Posture. 2003;17(2):159–169. doi: 10.1016/s0966-6362(02)00073-5. [DOI] [PubMed] [Google Scholar]
- 37.Hinterwimmer S, Krammer M, Krotz M et al. Cartilage atrophy in the knees of patients after seven weeks of partial load bearing. Arthritis Rheum. 2004;50(8):2516–2520. doi: 10.1002/art.20378. [DOI] [PubMed] [Google Scholar]
- 38.Salem GJ, Salinas R, Harding FV. Bilateral kinematic and kinetic analysis of the squat exercise after anterior cruciate ligament reconstruction. Arch Phys Med Rehabil. 2003;84(8):1211–1216. doi: 10.1016/s0003-9993(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 39.Winter DA. Kinematic and kinetic patterns in human gait: variability and compensating effects. Hum Mov Sci. 1984;3((1–2)):51–76. [Google Scholar]
- 40.Souza RB, Arya S, Pollard CD, Salem G, Kulig K. Patellar tendinopathy alters the distribution of lower extremity net joint moments during hopping. J Appl Biomech. 2010;26(3):249–255. doi: 10.1123/jab.26.3.249. [DOI] [PubMed] [Google Scholar]
- 41.Liu MF, Chou PH, Liaw LJ, Su FC. Lower-limb adaptation during squatting after isolated posterior cruciate ligament injuries. Clin Biomech (Bristol, Avon) 2010;25(9):909–913. doi: 10.1016/j.clinbiomech.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Selles RW, Bussmann JB, Klip LM, Speet B, Van Soest AJ, Stam HJ. Adaptations to mass perturbations in transtibial amputees: kinetic or kinematic invariance? Arch Phys Med Rehabil. 2004;85(12):2046–2052. doi: 10.1016/j.apmr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Skinner HB, Barrack RL. Ankle weighting effect on gait in able-bodied adults. Arch Phys Med Rehabil. 1990;71(2):112–115. [PubMed] [Google Scholar]
- 44.Hillery SC, Wallace ES, McIlhagger R, Watson P. The effect of changing the inertia of a trans-tibial dynamic elastic response prosthesis on the kinematics and ground reaction force patterns. Prosthet Orthot Int. 1997;21(2):114–123. doi: 10.3109/03093649709164539. [DOI] [PubMed] [Google Scholar]
- 45.Mundermann A, Asay JL, Mundermann L, Andriacchi TP. Implications of increased medio-lateral trunk sway for ambulatory mechanics. J Biomech. 2008;41(1):165–170. doi: 10.1016/j.jbiomech.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Henriksen M, Aaboe J, Simonsen EB, Alkjær T, Bliddal H. Experimentally reduced hip abductor function during walking: implications for knee joint loads. J Biomech. 2009;42(9):1236–1240. doi: 10.1016/j.jbiomech.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 47.Bennell K, Hodges P, Mellor R, Bexander C, Souvlis T. The nature of anterior knee pain following injection of hypertonic saline into the infrapatellar fat pad. J Orthop Res. 2004;22(1):116–121. doi: 10.1016/S0736-0266(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 48.Garland A, Jordan JE, Necheles J et al. Hypertonicity, but not hypothermia, elicits substance P release from rat C-fiber neurons in primary culture. J Clin Invest. 1995;95(5):2359–2366. doi: 10.1172/JCI117928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paintal AS. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960;152(2):250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torry MR, Decker MJ, Viola RW, O'Connor DD, Steadman JR. Intra-articular knee joint effusion induces quadriceps avoidance gait patterns. Clin Biomech (Bristol, Avon) 2000;15(3):147–159. doi: 10.1016/s0268-0033(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 52.Henriksen M, Alkjaer T, Simonsen EB, Bliddal H. Experimental muscle pain during a forward lunge: the effects on knee joint dynamics and electromyographic activity. Br J Sports Med. 2009;43(7):503–507. doi: 10.1136/bjsm.2008.050393. [DOI] [PubMed] [Google Scholar]