Abstract
Background
In many resource-limited settings monitoring of combination antiretroviral therapy (cART) is based on the current CD4 count, with limited access to HIV RNA tests or laboratory diagnostics. We examined whether the CD4 count slope over 6 months could provide additional prognostic information.
Methods
We analyzed data from a large multi-cohort study in South Africa, where HIV RNA is routinely monitored. Adult HIV-positive patients initiating cART between 2003 and 2010 were included. Mortality was analyzed in Cox models; CD4 count slope by HIV RNA level was assessed using linear mixed models.
Results
44,829 patients (median age 35 years, 58% female, median CD4 count at cART initiation 116 cells/mm3) were followed-up for a median of 1.9 years, with 3,706 deaths. Mean CD4 count slopes per week ranged from 1.4 (95% CI: 1.2, 1.6) cells/mm3 when HIV RNA was <400 copies/mL to −0.32 (95% CI: −0.47, −0.18) cells/mm3 with >100,000 copies/mL. The association of CD4 slope with mortality depended on current CD4 count: the adjusted hazard ratio (aHRs) comparing a >25% increase over 6 months with a >25% decrease was 0.68 (95% CI 0.58-0.79) at <100 cells/mm3 but 1.11 (95% CI 0.78-1.58) at 201-350 cells/mm3. In contrast, the aHR for current CD4 count, comparing >350 with <100 cells/mm3, was 0.10 (95% CI 0.05-0.20).
Conclusions
Absolute CD4 count remains a strong risk for mortality with a stable effect size over the first four years of cART. However, CD4 count slope and HIV RNA provide independently added to the model.
Background
Monitoring changes in CD4 T-cell lymphocyte count (CD4 count) is a cornerstone of managing patients prior to combination antiretroviral therapy (cART) initiation and during cART. It is used for guiding timing of cART initiation and assessing risk of opportunistic illnesses and evaluating for possible treatment failure 1. However, CD4 count decline prior to cART initiation is highly variable and depends on multiple host and viral factors. For example, HIV RNA level and current CD4 count both help to predict CD4 count decline prior to cART initiation 2;3. Likewise, CD4 count increase after starting cART is highly heterogeneous and is also influenced by multiple factors 4-6. This mismatch between CD4 count change and HIV RNA level limits the clinical value of using CD4 counts as a surrogate for HIV RNA suppression 7-9. As a result, where the resources are available, both CD4 count and HIV RNA are typically monitored and are used in concert for making clinical decisions.
Absolute CD4 count is valuable for patient management because of the strong association between CD4 count and opportunistic illness and death, prior to cART use and during cART 10-12. Although HIV RNA value may be associated with mortality given other variables, including CD4 count,13 it is frequently not routinely available in resource-limited settings. A better understanding of the information provided by routine CD4 count testing may help with developing prognostic tools and to provide further insight into mortality in resource limited settings. For example, during the pre-ART period, beyond the current CD4 count and HIV RNA level, CD4 slope has been reported to provide additional information regarding mortality risk 14. However, during cART, it is unclear what additional prognostic information is provided by the CD4 count slope beyond that of the current CD4 count. In addition, while the association between risk of death and CD4 count around cART initiation is well documented, it is unclear if the effect size persists over time on cART and data are lacking from a resource-limited-setting 15;16. Thus, several questions remain regarding CD4 count slope and survival during cART, particularly in resource-limited-settings.
We analyzed the South African cohorts from a large multi-cohort collaboration with extensive longitudinal CD4 count and HIV RNA data to assess change in CD4 count by HIV RNA level on cART and the associations between mortality and current CD4 count, CD4 count slope and HIV RNA.
Methods
Cohorts and eligibility criteria
The International epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA, www.iedea-sa.org) is a collaboration of HIV treatment cohorts in southern Africa 17;18. Each cohort regularly provides data to data centers at the Universities of Cape Town, South Africa and Bern, Switzerland through a specific standardized data sharing system. This study only included cohorts from South Africa, where regular assessment of CD4 cell count and HIV RNA is part of routine clinical care. All laboratory tests are performed by accredited clinical laboratories 19.
The included cohorts were from the Aurum Institute, Africa Centre for Health and Population Studies, Themba Lethu Clinic, Khayelitsha, Gugulethu, Tygerberg, and Masiphumelele and represented urban, peri-urban, and rural populations20. Patients were eligible if they were HIV-positive, ART-naïve at the time of entry into the HIV care programs, aged ≥16 years and <85 years, initiated ART between 2003 and 2010, and had at least 6 months of potential follow-up time. In addition, we excluded women known to be pregnant at the time of ART initiation due to potential effects of pregnancy on CD4 count and on ART response. Patients with no CD4 data were excluded from analysis. All IeDEA-SA sites obtained ethical approval from relevant local institutions before contributing anonymised patient data to IeDEA-SA.
Definitions
Baseline characteristics (measured within 6 months prior to ART initiation) included age, sex, CD4 count, HIV RNA, calendar year of cART initiation, and cohort. Longitudinal characteristics included absolute CD4 counts and HIV RNA values during the course of ART. We stratified current CD4 count into four levels: ≤100, 100-200, 201-350, >350 cells/mm3. Current CD4 count or current HIV RNA was defined as the most recent value, which was carried forward until the next value became available for a maximum of 180 days. We calculated CD4 count slope as the change in CD4 count between two consecutive CD4 values. The slope was expressed as the change in CD4 cells per week or the percentage change over 6 months in three categories: >25% decrease, 25% decrease to 25% increase, and >25% increase. We used a 25% increase or decrease because the inter-test variability of CD4 count measures is approximately 20% 21 ; we referred to the <25% decline and <s35% increase category as “no change.” We considered HIV RNA as suppressed (<400 copies/mL), unsuppressed (≥400 copies/mL) and in four levels : <400, 400-999, 1000-39,999, 50,000-100,000, and >100,000 copies/mL. The cut-off of 400 copies/mL was used as this was the upper bound of the limit of detection for HIV RNA for assays used by some of the cohorts. The four levels were selected for convenience and consistency with the literature. Immunologic failure was defined as failure to achieve a CD4 count increase >50 cells/mm3 by 6 months and >100 cells/mm3 by 12 months on cART.
Ascertainment of deaths
Deaths were ascertained as per clinic protocols. To address the under-ascertainment of deaths from routine clinic data 22-25, we linked civil identification (ID) numbers, when available, to the South African Department of Home Affairs vital status registry. To address incomplete data on ID numbers, we used inverse probability weighting to correct for missing deaths among those defined as lost to follow-up 24;26. Briefly, patients who were lost to follow-up and who had ID numbers were up-weighted to represent all patients lost to follow-up, enabling more accurate estimates of mortality. The weights were based on the inverse modeled probabilities of having an ID given cohort, gender, age and CD4 count addressing potential differences between patients with and without ID numbers among those lost to follow-up.
Multiple imputation of missing CD4 count and HIV RNA values
To address gaps in CD4 count and HIV RNA data due to missed clinic visits or laboratory values we created “expected visits” for any gap of more than 300 days. We then used multiple imputation to create complete longitudinal data for these “expected visit days” and also imputed baseline CD4 count and HIV RNA values, when missing. We created 10 imputed datasets using a specialized software package 27. The imputation model included sex, age, CD4 count, HIV RNA, year of cART initiation, duration on cART, and mortality 28;29. All results of our multivariable analyses are based on the imputed datasets and were combined with Rubin’s rules 30.
Statistical analyses
Baseline characteristics were summarized using medians with interquartile ranges (IQR) or proportions. Age, CD4 count, and HIV RNA were described as continuous and categorical variables and used as categorical variables in modeling. CD4 count change by HIV RNA level was assessed using a linear mixed model. Because our primary interest was immune reconstitution and not the initial rise in CD4 count we excluded CD4 counts from the first 6 months of cART 31. We included sex and age as fixed effects and cohort, patient, and each time interval within a given HIV RNA category as random effects. We displayed the distributions of CD4 count slope graphically by HIV RNA level, plotting the distribution of CD4 count slopes (cells/mm3/week) within each HIV RNA category. We also described the CD4 count slope strata of >25% decrease over 6 months, no change, and >25% increase in relation to the HIV RNA level.
Cox’s proportional hazards regression models were used to assess crude and adjusted associations between baseline and time-updated characteristics and mortality. Time was measured from the start of cART to the first of death, loss to follow-up, analysis closure, or four years on cART. Loss to follow-up was defined as at least 6 months without a visit (and no subsequent visits) with time censored at the last clinic visit. Analysis closure was at 6 months before database closure to allow for full loss to follow-up assignment. For patients who started ART but had no further contact, we added one day of follow-up to allow for their inclusion in survival analyses.
All available variables were considered potential confounders and were included in initial multivariable models. We examined interactions between duration on cART (split at 12 months) and all statistically significant variables to test the proportional hazard assumption. We also assessed for interactions between current CD4 count and CD4 count slope, current CD4 count and current HIV RNA, and CD4 count slope and sex. The final Cox model included statistically significant covariates and CD4 count, CD4 count slope, and the interaction between current CD4 count and CD4 count slope. Stratification by cohort was used to adjust for potential cohort differences. We included CD4 count slope and CD4 count in a non-linear manner using polynomials. The hazard by current CD4 count, CD4 count slope, and interaction between the two was visualized by means of a three-dimensional plot. In an attempt to assess whether the added value of CD4 count slope in the model was independent of absolute CD4 count, or may have provided a more precise estimate of CD4 count at the time of death, we repeated the analysis, limiting follow-up time to 30 days after the last CD4 count. In addition, to evaluate the effect size of an association between CD4 count slope and mortality in the absence of HIV RNA data, we repeated the analysis without including HIV RNA. Finally, we assessed mortality following 6 months on cART or 12 months on cART using immunologic failure at those two time points as one of the covariates. For this analysis, entry occurred at 6 or 12 months on cART and patients were censored 6 months after entry (at 12 or 18 months on cART).
Data were analyzed using STATA 12.0 (STATA Corporation, College Station, Texas, USA) and R 2.12 (The R Development Core Team).
Results
The cohorts included a total of 47,320 patients receiving cART. Thirty-nine (0.1%) patients were excluded because their age was <16 or >85 years at cART initiation, 547 (1.2%) were excluded because they initiated cART before 2002 or after 2010, 660 (1.4%) were excluded because they lacked a potential for 6 months follow-up on cART, and 1245 (2.6%) were excluded because they were pregnant. This left a total of 44,829 patients meeting eligibility criteria. The number of patients per cohort ranged from 517 to 16,415. Of these patients, 26,171 (58%) were female, the median age was 35 years (interquartile range (IQR): 30-42), the median CD4 count at cART initiation was 116 cells/mm3 (IQR: 52-178; Table 1). Median duration of follow-up was 1.9 (IQR: 1.2-3.0) years. The median number of CD4 count measurements while on cART was 3 (IQR: 2-5), obtained a median of 166 (IQR: 106-196) days apart. There were 293,192 actual CD4 count values present and 34,463 (10%) time points with missing CD4 counts and 270,832 actual HIV RNA results present and 56,823 (17%) time points with missing HIV RNA values. During follow-up, there were 3,706 (8.3%) deaths in the weighted analysis.
Table 1.
Baseline Characteristics (total population: 44,829)
| N (%) or median (IQR) | |
|---|---|
| Sex | |
| Men | 18,658 (42) |
| Women | 26,171 (58) |
|
| |
| Age | |
| 16-24 | 2,771 (6) |
| 25-34 | 17,833 (40) |
| 35-44 | 15,419 (34) |
| >44 | 8,806 (20) |
|
| |
| CD4 COUNT at cART initiation | |
| Median | 116 (52, 178) |
|
| |
| HIV RNA at cART initiation | |
| Median | 51,000 (13,000, 170,000) |
|
| |
| Year of cART initiation | |
| 2003 | 1,317 (3) |
| 2004 | 4,614 (10) |
| 2005 | 7.988 (18) |
| 2006 | 11,405 (25) |
| 2007 | 9,606 (21) |
| 2008 | 6,677 (15) |
| 2009 | 3,222 (7) |
|
| |
| Civil identification number present | |
| Yes | 21,033 (46) |
| No | 23,796 (53) |
|
| |
| Cohort | |
| Aurum | 16,415 (37) |
| Gugulethu | 2,198 (5) |
| Hlabisa | 7,411 (17) |
| Khayelitsha | 5,845 (13) |
| Masiphumelele | 517 (1) |
| McCord | 2,791 (6) |
| Thembalethu | 8,563 (19) |
| Tygerberg | 1,089 (2) |
CD4 count change by HIV RNA suppression
CD4 count increased an average of 1.3 (95% confidence interval (CI): 1.1, 1.5) cells/mm3 per week between 6 and 48 months on cART. The steepest rise was among those with HIV RNA <400 copies/mL with 1.4 (95% CI: 1.2, 1.6) cells/mm3 per week, followed by 0.41 (95% CI: 0.18, 0.60) cells/mm3 per week for HIV RNA 400-1000 copies/mL, 0.-0.012 (95% CI: −0.17, 0.14) cells/mm3 per week for HIV RNA of 1000-50,000 copies/mL, −1.12(95% CI: −3.6, 1.4) for HIV RNA 50,000-100,000 copies/mL, and −0.48 (95% CI: −0.96, 0.007) cells/mm3 per week for HIV RNA >100,000 copies/mL (Figure 1).
Figure 1.
CD4 count slope distribution density by HIV RNA level. Y-axis represents density of CD4 count slopes.
However, even amongst individuals with evidence of persistent HIV RNA suppression, the CD4 count response was heterogeneous with both increases and marked decreases in CD4 count. During 6 month periods with HIV RNA <400 copies/mL, there was a decline in CD4 count of more than 25% among 9.0% of patients compared to such a decline among 14% of patients when HIV RNA was 400-1000 copies/mL, 24% of patients when HIV RNA was 1000-50,000 copies/mL, and 44% of patients when HIV RNA was >50,000 copies/mL.
Mortality
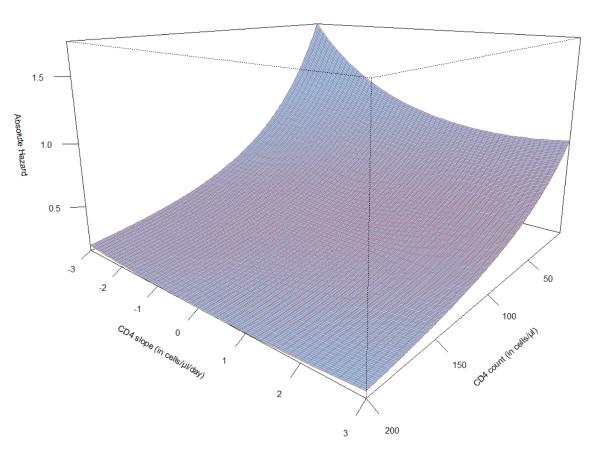
All-cause mortality during the 48 months of follow-up was 4.1 per 100 person-years (95% CI: 3.9-4.2). Mortality risk was associated with current CD4 count and with the slope in CD4 count (Table 2). In assessing the proportional hazards assumption we found that there was no interaction between either current CD4 count or CD4 count slope and duration on cART (p>0.1). We also found no interaction between sex and CD4 count slope (p=0.9). However, CD4 count slope interacted with time-updated absolute CD4 count, losing association with mortality when the time-updated CD4 count was >200 cells/mm3 (p<0.001 from test of interaction). This is graphically represented in Figure 2. In multivariable modeling we included sex, age, current CD4 count, CD4 count trend, and HIV RNA category. In this model, compared to a 25% decline, the mortality hazard was lower for a non-decreasing CD4 count with a current CD4 count between 100 and 200 cells/mm3 (0.76, 95% CI: 0.62-0.94 for no change and 0.77, 95% CI: 0.63-0.94 for a 25% increase). Thus, the risk of dying was lower with an increasing CD4 count, independent of current CD4 count and HIV RNA. HIV RNA level was also associated with mortality independent of CD4 count and CD4 count slope, with mortality hazard increasing consistently with increasing HIV RNA level (Table 2).
Table 2.
Univariable and multivariable associations with mortality up to 3 years on cART
| Mortality per 100 PYRs |
HR | p | aHR | p | |
|---|---|---|---|---|---|
| Sex | |||||
| Men | 5.1 (4.8, 5.4) | Referent | <0.001 | Referent | 0.009 |
| Women | 3.4 (3.2, 3.5) | 0.75 (0.69, 0.81) | 0.89 (0.82, 0.97) | ||
|
| |||||
| Age (years) | |||||
| 16-24 | 3.3 (2.8, 3.9) | Referent | <0.001 | Referent | <0.001 |
| 25-34 | 3.3 (3.1, 3.5) | 0.96 (0.80, 1.15) | 1.02 (0.85, 1.22) | ||
| 35-44 | 4.3 (4.0, 4.6) | 1.16 (0.97, 1.39) | 1.26 (1.05, 1.52) | ||
| >44 | 5.4 (5.0, 5.8) | 1.43 (1.18, 1.72) | 1.58 (1.30, 1.91) | ||
| Weight at ART initiation (kg) | |||||
| <50 | 7.1 (6.6, 7.7) | Referent | Referent | ||
| 50-80 | 3.7 (3.5, 3.8) | 0.52 (0.48, 0.57) | 0.55 (0.50, 0.61) | ||
| >80 | 2.9 (2.5, 3.4) | 0.37 (0.30, 0.46) | 0.46 (0.38, 0.57) | ||
|
| |||||
| Current CD4 count (cells/mm3) | |||||
| ≤100 | 14 (14, 15) | Referent | <0.001 | Referent | <0.001 |
| 100-200 | 5.0 (4.7, 5.4) | 0.32 (0.29, 0.35) | 0.38 (0.32, 0.46) | ||
| 201-350 | 2.5 (2.3, 2.8) | 0.15 (0.14, 0.17) | 0.18 (0.13, 0.25) | ||
| >350 | 1.4 (1.2, 1.6) | 0.09 (0.07, 0.10) | 0.10 (0.05, 0.20) | ||
|
| |||||
| CD4 count trend* | <0.001 | <0.001 | |||
| CD4 count: <100 | |||||
| >25% decrease | 30 (27, 33) | Referent | Referent | ||
| no change | 20 (17, 23) | 0.66 (0.56, 0.79) | 0.75 (0.63, 0.89) | ||
| >25% increase | 16 (14, 18) | 0.52 (0.45, 0.60) | 0.68 (0.58, 0.79) | ||
| CD4 count: 100-200 | |||||
| >25% decrease | 8.2 (6.9, 9.7) | Referent | Referent | ||
| no change | 5.2 (4.4, 6.0) | 0.70 (0.57, 0.86) | 0.79 (0.64, 0.98) | ||
| >25% increase | 4.3 (3.7, 5.0) | 0.65 (0.53, 0.80) | 0.79 (0.63, 0.98) | ||
| CD4 count: 201-350 | |||||
| >25% decrease | 2.4 (1.8, 3.4) | Referent | Referent | ||
| no change | 2.1 (1.8, 2.5) | 0.79 (0.56, 1.09) | 0.87 (0.63, 1.21) | ||
| >25% increase | 2.8 (2.5, 3.2) | 1.00 (0.71, 1.43) | 1.11 (0.78, 1.58) | ||
| CD4 count: >350 | |||||
| >25% decrease | 0.91 (0.46, 2.0) | Referent | Referent | ||
| no change | 1.0 (0.83, 1.3) | 0.88 (0.41, 1.88) | 0.95 (0.45, 2.03) | ||
| >25% increase | 1.6 (1.3, 1.8) | 1.25 (0.60, 2.62) | 1.31 (0.63, 2.76) | ||
|
| |||||
| HIV RNA (c/mL) | |||||
| <400 | 2.8 (2.7, 3.0) | Referent | <0.001 | Referent | <0.001 |
| 400-4999 | 3.6 (3.3, 4.0) | 1.95 (1.69, 2.26) | 1.63 (1.41, 1.89) | ||
| 5000-50,000 | 9.7 (8.8, 11) | 3.34 (2.94, 3.80) | 2.18 (1.92, 2.48) | ||
| >50,000 | 22 (20, 23) | 6.51 (5.89, 7.20) | 3.16 (2.82, 3.55) | ||
HR, crude hazard ratio; aHR, adjusted hazard ratio
interaction between updated CD4 count and CD4 count slope; p<0.001
Figure 2.
Absolute mortality hazard from a Cox proportional hazards model of CD4 count slope, (cells/mm3/day), current CD4 count, and the interaction between the two for a reference population of men, aged <24 years, with HIV RNA <400 c/mL.
When we repeated the analysis excluding HIV RNA from the model the effect size of the association between CD4 count slope increased slightly: for CD4 count ≤100 cells/mm3 with >25% decrease as the referent group, the hazard ratio for no change was 0.65 (95% CI: 0.55, 0.78) and for >25% increase was 0.51 (95% CI: 0.44, 0.60); for CD4 count 100-200 cells/mm3 the hazard ratios were 0.70 (95% CI: 0.57, 0.86) and 0.65 (95% CI: 0.53, 0.80), respectively; for CD4 counts 201-350 cells/mm3 the hazard ratios were 0.84 (95% CI: 0.56, 1.09) and 1.0 (95% CI: 0.70, 1.4), respectively; and finally for CD4 counts >350 cells/mm3, the hazard ratios were 0.87 (95% CI: 0.41, 1.9) and 1.2 (95% CI: 0.59, 2.6), respectively.
To further assess the role of CD4 count trend, we undertook an analysis censoring time 30 days after the last CD4 count and repeating the adjusted analysis. In this analysis, the association between CD4 count trend and mortality remained with a slight but non-significant increase in effect sizes.
We also assessed for an association between CD4 count response/failure at 6 and 12 months and mortality. In multivariable modeling, adjusting for current CD4 count, current HIV RNA, sex, and age, both 6 and 12 month CD4 count response was associated independently with reduced mortality with an adjusted hazard ratio of 0.63 (95% CI: 0.51-0.80, p<0.001) for CD4 count response by 6 months and an adjusted hazard ratio of 0.37 (95% CI: 0.20-0.69, p=0.002) for CD4 count response by 12 months.
Discussion
Using a large multi-cohort ART population with robust mortality estimates and availability of routine laboratory monitoring, we have demonstrated that a lower current CD4 count, CD4 count slope with >25% decline averaged over six months, and a higher current HIV RNA level each are independently associated with increased mortality among people receiving cART. Furthermore, although mortality declined with time from cART initiation, the effect size between CD4 count and mortality remained constant over time on cART, consistent with a study from Northern cohorts 15. Thus we reconfirm the strong association between CD4 count and mortality, demonstrate that it remains constant for up to four years on ART, and have added findings on the associations both of current HIV RNA level and CD4 count slope in estimating mortality hazard. In addition, patients with a poor initial CD4 response in spite of virologic suppression were at an even greater hazard of death than suggested by the other parameters, including current CD4 count.
Our findings of an association between CD4 count slope and mortality build on the overall understanding of mortality risk during cART, adding the independent value of the CD4 count slope 22;23;32-37. The finding raises the question of whether the CD4 count decline is a result of an ongoing illness or whether the declining CD4 count predisposes to a potentially life-threatening illness. Another possibility is that the CD4 count slope provides a better measure of the true CD4 count at the time of an event. However, our finding of association between CD4 count slope and mortality, even when limiting follow-up to 30 days after the last CD4 count, makes this a less likely explanation. Whether this finding can be translated into clinical use will require further study; however, seeing a decline in CD4 count should raise a clinician’s concern for the potential of increased mortality risk.
HIV RNA category was independently associated with mortality with a consistent increase in hazard with increasing HIV RNA. It is notable that the mortality hazard was 64% higher for patients with even modestly elevated HIV RNA between 400-4999 c/mL versus <400. Prior studies from resource-limited-settings have focused on the association between suppressed versus not suppressed in assessing for associations with mortality and have not assessed multiple gradation of viremia 23;32;33. Studies from high-income settings that have assessed for association between gradations of HIV RNA and mortality during cART have reported disparate findings. Several studies found no association between HIV RNA level if CD4 count was included in the model 38-41. Whereas others reported an association between HIV RNA and either mortality, mortality and AIDS defining illnesses, or AIDS defining illness with an effect size of 1.0-1.8 for HIV RNA 1000-10,000 copies/mL and 1.8-3.9 for HIV RNA ≥10,000 copies/mL, each compared to either <80 or <500 copies/mL 42-45. These studies did not specifically assess the 400-4999 copies/mL range and either identified no or minimal association among participants with HIV RNA between 1000-10,000 copies/mL. It is plausible that the larger size of our study, with 3,848 deaths, allowed us to detect an association that was not found in some prior studies. Another plausible explanation is that causes of mortality in South Africa, such as tuberculosis, may be more closely associated with HIV RNA level than causes or behaviors associated with mortality in Europe 13;46-48.
Our estimates of CD4 change by HIV RNA level build on prior reports. For example, studies from high income countries have estimated CD4 count increase among individuals on cART with suppressed HIV RNA to range from 32 and 127 cells/mm3/year4;49;50; our slope for HIV RNA <400 c/mL of 72 cells/mm3/year fits in the middle of this range. In addition, the variability in CD4 count change during cART is well described 6;51.
Although this study has the strength of a large dataset generated from routine HIV care programs, it also has several limitations. Firstly, because we used routinely collected data, missing data are inevitable. We used multiple imputation to address this limitation, generating datasets with imputed missing values for up to 17% of laboratory results. However, multiple imputation assumes that missingness can be predicted from observed variables. It is possible that individuals were missing CD4 counts or HIV RNA values because they were sick and at increased risk for death, in excess of what can be predicted from other available covariates. This could introduce bias, attenuating the observed associations with mortality. In addition, although our dataset was large, the number of patients contributing outcomes to specific combinations of characteristics may be small and may have contributed to the wide confidence intervals in our adjusted model as we observed with hazard ratio point estimates for CD4 count slope with higher time updated CD4 counts. While the effect of uncontrolled HIV viremia on the risk of opportunistic illnesses and other conditions is known, our finding of an independent association between viremia and mortality could be partially confounded by behaviors or circumstances which result in both poor medication adherence and increased risk of illness or death.
Measuring absolute CD4 count remains the cornerstone of ART management in resource-limited settings, and our study confirms the strong association between absolute CD4 count and mortality. We have, however, described a strong independent association between HIV RNA and mortality in a treatment context typical of many in southern Africa, and have additionally highlighted the contribution of CD4 count trajectory in hazard of future mortality. The next steps are to better understand the relationships between each phenomenon and severe illness, and to identify optimal clinical and monitoring interventions to reduce mortality among those at highest risk.
Acknowledgements
This study was supported by the US National Institute of Allergy and Infectious Disease (NIAID, grant U01-AI069924–05). We are grateful to all patients and staff at the HIV care programs included in this analysis and to the staff at the data centers.
Funding: National Institute of Allergy and Infectious Diseases (NIAID), Grant 2U01-AI069924. CJH was supported NIAID AI083099. MPF was supported by NIAID K01AI083097. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID or the National Institutes of Health.
Footnotes
Conflicts of Interest: All authors: none
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.World Health Organization . Antiretroviral therapy for HIV infection in adults and adolescents. WHO; Austria: 2010. [PubMed] [Google Scholar]
- 2.Mellors JW, Rinaldo CR, Jr., Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–70. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez B, Sethi AK, Cheruvu VK, Mackay W, Bosch RJ, Kitahata M, Boswell SL, Mathews WC, Bangsberg DR, Martin J, Whalen CC, Sieg S, Yadavalli S, Deeks SG, Lederman MM. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296:1498–506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44:441–46. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 5.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328–37. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 6.Tuboi SH, Brinkhof MW, Egger M, Stone RA, Braitstein P, Nash D, Sprinz E, Dabis F, Harrison LH, Schechter M. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 7.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. AIDS. 2008;22:1971–77. doi: 10.1097/QAD.0b013e32830e4cd8. [DOI] [PubMed] [Google Scholar]
- 8.Rawizza HE, Chaplin B, Meloni ST, Eisen G, Rao T, Sankale JL, Dieng-Sarr A, Agbaji O, Onwujekwe DI, Gashau W, Nkado R, Ekong E, Okonkwo P, Murphy RL, Kanki PJ. Immunologic criteria are poor predictors of virologic outcome: implications for HIV treatment monitoring in resource-limited settings. Clin Infect Dis. 2011;53:1283–90. doi: 10.1093/cid/cir729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keiser O, MacPhail P, Boulle A, Wood R, Schechter M, Dabis F, Sprinz E, Egger M. Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Trop Med Int Health. 2009;14:1220–1225. doi: 10.1111/j.1365-3156.2009.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–63. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hanson DL, Chu SY, Farizo KM, Ward JW. Distribution of CD4+ T lymphocytes at diagnosis of acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. The Adult and Adolescent Spectrum of HIV Disease Project Group. Arch Intern Med. 1995;155:1537–42. [PubMed] [Google Scholar]
- 12.Gebo KA, Gallant JE, Keruly JC, Moore RD. Absolute CD4 vs. CD4 percentage for predicting the risk of opportunistic illness in HIV infection. J Acquir Immune Defic Syndr. 2004;36:1028–33. doi: 10.1097/00126334-200408150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, Nzunetu G, Gabillard D, Rouet F, Sorho S, Chaix ML, Eholie S, Menan H, Sauvageot D, Bissagnene E, Salamon R, Anglaret X. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–89. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 14.Mellors JW, Margolick JB, Phair JP, Rinaldo CR, Detels R, Jacobson LP, Munoz A. Prognostic value of HIV-1 RNA, CD4 cell count, and CD4 Cell count slope for progression to AIDS and death in untreated HIV-1 infection. JAMA. 2007;297:2349–50. doi: 10.1001/jama.297.21.2349. [DOI] [PubMed] [Google Scholar]
- 15.CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9:e1001194. doi: 10.1371/journal.pmed.1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 17.Cornell M, Technau K, Fairall L, Wood R, Moultrie H, Van CG, Giddy J, Mohapi L, Eley B, MacPhail P, Prozesky H, Rabie H, Davies MA, Maxwell N, Boulle A. Monitoring the South African National Antiretroviral Treatment Programme, 2003-2007: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:653–60. [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, Hartwell T, Graber C, Chi BH, Boulle A, Dabis F, Wools-Kaloustian K. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2011 doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glencross DK, Janossy G, Coetzee LM, Lawrie D, Aggett HM, Scott LE, Sanne I, McIntyre JA, Stevens W. Large-scale affordable PanLeucogated CD4+ testing with proactive internal and external quality assessment: in support of the South African national comprehensive care, treatment and management programme for HIV and AIDS. Cytometry B Clin Cytom. 2008;74(Suppl 1):S40–S51. doi: 10.1002/cyto.b.20384. [DOI] [PubMed] [Google Scholar]
- 20.Cornell M, Grimsrud A, Fairall L, Fox MP, Van CG, Giddy J, Wood R, Prozesky H, Mohapi L, Graber C, Egger M, Boulle A, Myer L. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24:2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lathey JL, Hughes MD, Fiscus SA, Pi T, Jackson JB, Rasheed S, Elbeik T, Reichman R, Japour A, D’Aquila RT, Scott W, Griffith BP, Hammer SM, Katzenstein DA. Variability and prognostic values of virologic and CD4 cell measures in human immunodeficiency virus type 1-infected patients with 200-500 CD4 cells/mm(3) (ACTG 175). AIDS Clinical Trials Group Protocol 175 Team. J Infect Dis. 1998;177:617–24. doi: 10.1086/514250. [DOI] [PubMed] [Google Scholar]
- 22.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, Wood R, Laurent C, Sprinz E, Seyler C, Bangsberg DR, Balestre E, Sterne JA, May M, Egger M. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 23.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–13. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–7. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, Avalos A, Friedman H, Dickinson D, Frank I, Ndwapi N. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boulle A, Van CG, Hilderbrand K, Cragg C, Abrahams M, Mathee S, Ford N, Knight L, Osler M, Myers J, Goemaere E, Coetzee D, Maartens G. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 27.Honaker J, King G, Blackwell M. Amelia II: A program for missing data. Version 1.5 2012.
- 28.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honaker J. What to do about missing values in time-series cross-section data. American Journal of Political Science. 2010;54:561–81. [Google Scholar]
- 30.Rubin DB. Multiple imputation after 18+ years. Journal of the American Statistical Association. 1996;91:473–89. [Google Scholar]
- 31.Bosch RJ, Wang R, Vaida F, Lederman MM, Albrecht MA. Changes in the slope of the CD4 cell count increase after initiation of potent antiretroviral treatment. J Acquir Immune Defic Syndr. 2006;43:433–35. [PubMed] [Google Scholar]
- 32.Hoffmann CJ, Katherine FL, Victoria J, Salome C, Craig I, Moore RD, Chaisson RE, Grant AD, Churchyard GJ. Changing predictors of mortality over time from cART start: implications for care. J Acquir Immune Defic Syndr. 2011 doi: 10.1097/QAI.0b013e31823219d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, Wood R. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, Abo Y, Salamon R, Bissagnene E, Seyler C, Eholie S, Anglaret X. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–91. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 35.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, Harries AD. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–60. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 36.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, Keiser O, Sterne JA, Dabis F, Egger M. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, Dabis F, Pascoe M, Egger M. Mortality of HIV-infected patients starting antiretroviral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastburn A, Scherzer R, Zolopa AR, Benson C, Tracy R, Do T, Bacchetti P, Shlipak M, Grunfeld C, Tien PC. Association of low level viremia with inflammation and mortality in HIV-infected adults. PLoS One. 2011;6:e26320. doi: 10.1371/journal.pone.0026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghani AC, de WF, Ferguson NM, Donnelly CA, Coutinho R, Miedema F, Goudsmit J, Anderson RM. Surrogate markers for disease progression in treated HIV infection. J Acquir Immune Defic Syndr. 2001;28:226–31. doi: 10.1097/00042560-200111010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Lewden C, Raffi F, Cuzin L, Cailleton V, Vilde JL, Chene G, Allavena C, Salamon R, Leport C. Factors associated with mortality in human immunodeficiency virus type 1-infected adults initiating protease inhibitor-containing therapy: role of education level and of early transaminase level elevation (APROCO-ANRS EP11 study). The Antiproteases Cohorte Agence Nationale de Recherches sur le SIDA EP 11 study. J Infect Dis. 2002;186:710–714. doi: 10.1086/342047. [DOI] [PubMed] [Google Scholar]
- 41.Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, Mussini C, Wit F, d’Arminio MA, Weber R, Fusco G, Staszewski S, Law M, Hogg R, Lampe F, Gill MJ, Castelli F, Phillips AN. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 42.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, d’Arminio MA, Hermans P, Goebel FD, Blaxhult A, Kirk O, Phillips AN. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185:178–87. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 43.Ferry T, Raffi F, Collin-Filleul F, Dupon M, Dellamonica P, Waldner A, Strady C, Chene G, Leport C, Moing VL. Uncontrolled viral replication as a risk factor for non-AIDS severe clinical events in HIV-infected patients on long-term antiretroviral therapy: APROCO/COPILOTE (ANRS CO8) cohort study. J Acquir Immune Defic Syndr. 2009;51:407–15. doi: 10.1097/QAI.0b013e3181acb65f. [DOI] [PubMed] [Google Scholar]
- 44.Reekie J, Gatell JM, Yust I, Bakowska E, Rakhmanova A, Losso M, Krasnov M, Francioli P, Kowalska JD, Mocroft A. Fatal and nonfatal AIDS and non-AIDS events in HIV-1-positive individuals with high CD4 cell counts according to viral load strata. AIDS. 2011;25:2259–68. doi: 10.1097/QAD.0b013e32834cdb4b. [DOI] [PubMed] [Google Scholar]
- 45.Anastos K, Barron Y, Cohen MH, Greenblatt RM, Minkoff H, Levine A, Young M, Gange SJ. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140:256–64. doi: 10.7326/0003-4819-140-4-200402170-00007. [DOI] [PubMed] [Google Scholar]
- 46.Smurzynski M, Wu K, Benson CA, Bosch RJ, Collier AC, Koletar SL. Relationship between CD4+ T-cell counts/HIV-1 RNA plasma viral load and AIDS-defining events among persons followed in the ACTG longitudinal linked randomized trials study. J Acquir Immune Defic Syndr. 2010;55:117–27. doi: 10.1097/QAI.0b013e3181e8c129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng CY, Chen MY, Hsieh SM, Sheng WH, Sun HY, Lo YC, Liu WC, Hung CC. Risk of pneumocystosis after early discontinuation of prophylaxis among HIV-infected patients receiving highly active antiretroviral therapy. BMC Infect Dis. 2010;10:126. doi: 10.1186/1471-2334-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costiniuk CT, Fergusson DA, Doucette S, Angel JB. Discontinuation of Pneumocystis jirovecii pneumonia prophylaxis with CD4 count <200 cells/microL and virologic suppression: a systematic review. PLoS One. 2011;6:e28570. doi: 10.1371/journal.pone.0028570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt PW, Deeks SG, Rodriguez B, Valdez H, Shade SB, Abrams DI, Kitahata MM, Krone M, Neilands TB, Brand RJ, Lederman MM, Martin JN. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17:1907–15. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 50.Ledergerber B, Lundgren JD, Walker AS, Sabin C, Justice A, Reiss P, Mussini C, Wit F, d’Arminio MA, Weber R, Fusco G, Staszewski S, Law M, Hogg R, Lampe F, Gill MJ, Castelli F, Phillips AN. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364:51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 51.Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One. 2011;6:e21795. doi: 10.1371/journal.pone.0021795. [DOI] [PMC free article] [PubMed] [Google Scholar]