Abstract
Purpose
Lennox–Gastaut syndrome (LGS) is an epileptogenic disorder that arises in childhood and is typically characterized by multiple seizure types, slow spike-and-wave complexes on EEG and cognitive impairment. If medical treatment fails, patients can proceed to one of two palliative surgeries, vagus nerve stimulation (VNS) or corpus callosotomy (CC). Their relative seizure control rates in LGS have not been well studied. The purpose of this paper is to compare seizure reduction rates between VNS and CC in LGS using meta-analyses of published data.
Methods
A systematic search of Pubmed, Ovidsp, and Cochrane was performed to find articles that met the following criteria: (1) prospective or retrospective study, (2) at least one patient diagnosed with Lennox–Gastaut syndrome, and (3) well-defined measure of seizure frequency reduction. Seizure reduction rates were divided into seizure subtypes, as well as total seizures, and categorized as 100%, >75%, and >50%. Patient groups were compared using chi-square tests for categorical variables and t-test for continuous measures. Pooled proportions with 95% confidence interval (95% CI) of seizure outcomes were estimated for total seizures and seizure subtypes using random effects methods.
Results
17 VNS and 9 CC studies met the criteria for inclusion. CC had a significantly better outcome than VNS for >50% atonic seizure reduction (80.0% [67.0–90.0%] vs. 54.1% [32.1–75.4%], p < 0.05) and for >75% atonic seizure reduction (70.0% [48.05–87.0%] vs. 26.3% [5.8–54.7%], p < 0.05). All other seizure types, as well as total number of seizures, showed no statistically significant difference between VNS and CC.
Conclusions
CC may be more beneficial for LGS patients whose predominant disabling seizure type is atonic. For all other seizure types, VNS offers comparable rates to CC.
Keywords: Lennox–Gastaut syndrome, Vagus nerve stimulation, Corpus callosotomy, Seizure frequency, Atonic seizures
1. Introduction
Lennox–Gastaut syndrome (LGS) is an epileptogenic disorder arising in childhood with seizure control a therapeutic challenge. 1,2 Its characteristics can include multiple seizure types, slow spike-and-wave complexes on EEG and cognitive impairment.3,4 LGS usually persists through adulthood and has a poor prognosis, despite extensive pharmacological treatment.4,5 The most common seizure types include tonic, atonic, and atypical absence, but generalized tonic–clonic (GTC), myoclonic and complex partial seizures (CPS) can also be present.2,6
When medications fail and there is no resectable seizure focus, patients can proceed to one of two palliative surgeries, vagus nerve stimulation (VNS) or corpus callosotomy (CC). Although VNS was approved by the FDA in 1997 for treatment of refractory partial-onset seizures,7 its efficacy for other seizure-types is recognized.8 CC was first introduced as an experimental procedure in 19409 and has since been accepted in clinical practice to decrease the frequency and severity of generalized seizures, primarily atonic seizures,10 although its efficacy in the treatment of other seizure types has also been recognized.11
VNS is usually recommended first because it is a reversible procedure and is thought to have seizure reduction rates comparable to those of CC but without the risks of a craniotomy. 1,12 However, no study has attempted to directly compare these rates with large samples of LGS patients. Furthermore, information on the two interventions’ effectiveness for the different specific seizure types in LGS is widely scattered. We hypothesized that CC would prove more effective in reducing generalized epilepsy types, which include atonic, tonic, GTC, and myoclonic seizures.
2. Methods
2.1. Search strategy
Three authors (GL, MV, THS) independently performed a systematic search of PubMed, Ovidsp, and Cochrane for English-language studies published through December 2010. Search terms included all combinations of (1) Lennox–Gastaut syndrome, LGS, Lennox, and (2) VNS, vagal nerve stimulation, vagus nerve stimulation, callosotomy, corpus callosum, commisurotomy. The investigators identified potentially relevant articles by reviewing abstracts and then thoroughly reviewed references.
2.2. Selection of studies
Inclusion criteria for this meta-analysis consisted of the following: (1) prospective or retrospective study, (2) case reports or group study, (3) at least one patient diagnosed with Lennox–Gastaut syndrome, and (4) well-defined measure of seizure frequency reduction, either in numbers or ranges, after VNS or CC. Studies were excluded if seizure frequency data for LGS could not be extracted from the study population’s data, which sometimes included other primarily generalized epilepsies. Three authors (GL, MV, THS) independently reviewed studies that met inclusion criteria to determine their suitability and quality and unanimously agreed upon the studies to be included in this meta-analysis.
2.3. Data collection
Data were collected on the following: first author, year of study, retrospective or prospective study, type of treatment, number of LGS patients in study, gender, age at surgery, age at epilepsy onset, duration of epilepsy, etiology, VNS parameters, whether VNS patients had previous CC, partial vs. full CC, time of follow-up, complications, and seizure reduction rates for each seizure subtype and all seizures combined (“total seizures”), categorized as 100%, >75%, >50%, and <50%. In studies where patients were listed individually, means and sum totals were used to represent the study in the final analysis. Duplicate data between different studies were identified and excluded from the analyses. The corresponding author of the You et al. paper13 was directly contacted regarding the CC complications in his paper, which he stated were all transient. If there were multiple follow-up points in a study with declining numbers of patients, and the data could not be extracted individually, then the latest follow-up point which maintained a large proportion of the initial sample was used, as agreed upon by the authors. Not all data were available in every study.
2.4. Statistical analysis
Characteristics of patients groups (VNS vs. CC) were compared using chi-square tests for categorical variables and t-test for continuous measures. p values less than 0.05 were considered significant. Pooled proportions with 95% confidence interval (95% CI) of seizure outcomes were estimated for total seizures and seizure subtypes by VNS and CC. To account for heterogeneity across studies, random effects models were used for the estimation. The differences between two pooled proportions were tested by evaluating the overlap of the 95% CI. The formula in the Wolfe and Hanley paper was followed to decide significance.14 Publication bias was tested with Begg–Mazumdar bias indicator. Analyses were conducted in StatsDirect version 2.7.8 and STATA version 11. This study was approved by the Institutional Review Board of the New York Presbyterian-Weill Cornell Medical Center.
3. Results
3.1. Eligible articles
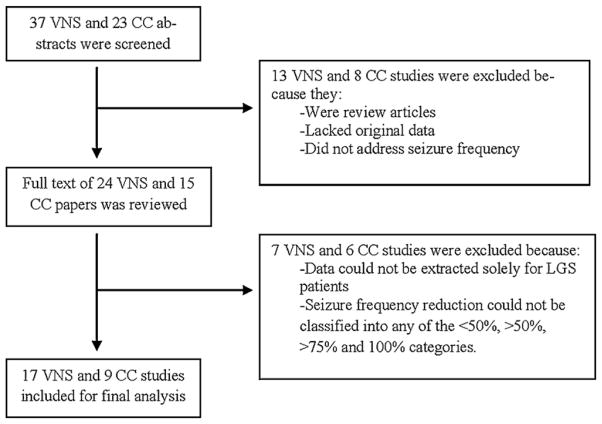
The literature search yielded 37 VNS and 23 CC abstracts, of which 13 VNS and 8 CC papers were excluded because they were review articles, lacked original data, or did not address seizure frequency (Fig. 1). After careful review and consideration of the remaining full text articles, 17 VNS studies13,15–30 and 9 CC studies13,31–38 were included. One study contained data on both VNS and CC patients independently.13 Additionally, one CC study was split into groups for the analysis based upon data presentation. 34 All studies were published between 1990 and 2010, of which 14 were prospective and 12 were retrospective, resulting in a total of 203 VNS patients and 145 CC patients.
Fig. 1.
Flow chart of study selection.
3.2. Group comparisons
VNS patients did not differ significantly from CC patients with respect to gender, mean age at epilepsy surgery, mean age of seizure onset and mean seizure duration. CC patients had longer follow-up (p = 0.018) than VNS patients (Table 1).
Table 1.
Descriptive data reported as mean (standard deviation) (26 studies).
| Number of studies with data available | Vagus nerve stimulation | Corpus callosotomy | p | |
|---|---|---|---|---|
| Follow-up time (months) | 19 | 16.23 (12.39) | 37.76 (24.05) | 0.018 |
| Percentage of female | 18 | 0.38 (0.15) | 0.30 (0.24) | 0.445 |
| Mean age at surgery (years) | 21 | 14.91 (6.98) | 11.76 (8.11) | 0.357 |
| Duration of epilepsy (years) | 15 | 11.90 (4.47) | 7.97 (5.81) | 0.169 |
| Percentage of cryptogenic or Idiopathic | 15 | 0.45 (0.22) | 0.27 (0.21) | 0.194 |
| Age at epilepsy onset (years) | 15 | 1.86 (1.39) | 3.17 (3.53) | 0.310 |
3.3. VNS
Of the 103 patients whose etiologies were reported, 47 (46%) had cryptogenic or idiopathic etiology. The other etiologies included hypoxic–ischemic encephalopathy (14), unspecified cerebral malformations (11), tuberous sclerosis (5), lissencephaly (3), trauma (3), meningoencephalitis (3), encephalitis (2), corpus callosum agenesis (2), immunization-induced encephalopathy (2), vitamin b12 deficiency (2), and 1 each of unspecified perinatal injury, unspecified CNS infection, prenatal rubella, NF1, measles, post-radiotherapy encephalopathy, cortical dysplasia, double cortex syndrome, and microcephaly. 15 had previous corpus callosotomies.
3.4. VNS parameters
All sixteen studies that reported VNS parameters used 30 s stimulation (one study did not report VNS parameters). The “OFF” time was 5 min in twelve studies, 3 min in two studies, between 3 and 5 min in one study, and 10 min in one study. 8/9 studies reported a pulse width of 500 μs while 1/9 used 250 μs. 11/12 studies used an initial output current of 0.25 mA while 1/12 used 0.50 mA. The target output current varied among studies and within studies but was always between 0.5 mA and 3.0 mA. 7/16 studies reported trying rapid cycling (7 s ON, 14–18 s OFF) in some patients if the standard parameters were not effective.
3.5. CC
Of the 81 patients whose etiologies were reported, 25 (31%) had cryptogenic or idiopathic etiology, while 25 had hypoxic–ischemic encephalopathy, 15 had encephalitis, 8 had unspecified cerebral malformations, 6 had trauma, 1 had prolonged febrile convulsions, and 1 had an unspecified intracranial birth injury. 24/129 (19%) underwent total callosal section while 105 (81%) had partial callosotomies (ranging from 1/2 to 5/6). Despite increase in heterogeneity, these groups were all pooled together to be able to increase sample size.
4. Results of meta analyses
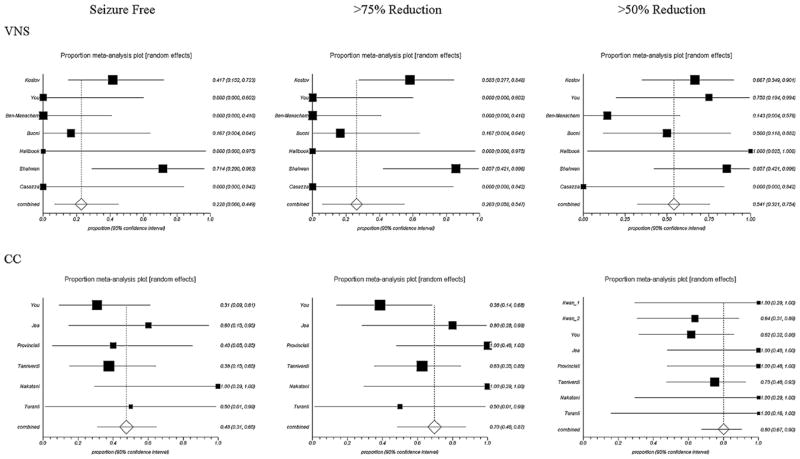
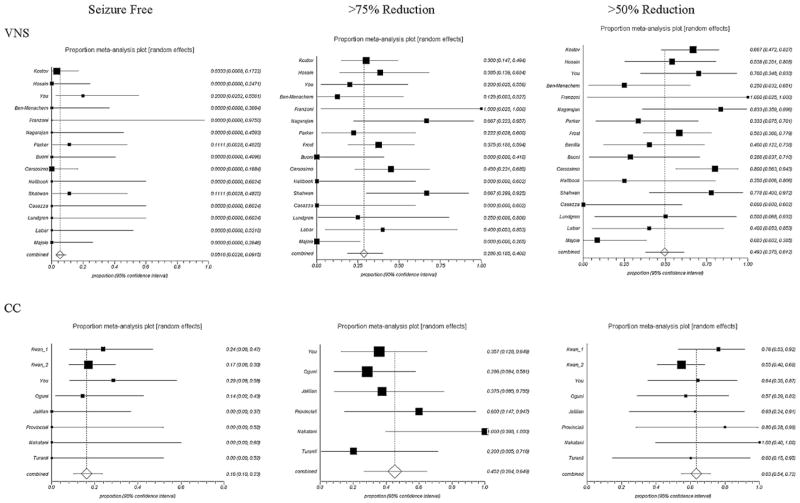
Table 2 shows the number of studies and patients for each seizure and treatment type and the pooled proportion of seizure reduction with 95% confidence intervals. There were insufficient data to analyze outcomes for atypical absence seizures. CC had a significantly better outcome than VNS for >50% atonic seizure reduction (p < 0.05) and for >75% atonic seizure reduction (p < 0.05). Although there was also a large mean difference between CC and VNS for patients who became free of atonic seizures (48.0% CC vs. 22.8% VNS), this was not found to be significant in the analysis. All other seizure types, as well as total number of seizures, showed no statistically significant difference between VNS and CC. Forest plots for atonic seizure reduction and total seizure reduction are presented in Figs. 2 and 3. Heterogeneity between studies was present especially for the pooled estimation of atonic seizure and total seizures with VNS treatment. No publication bias was found for the majority of the pooled analysis.
Table 2.
Data summary comparing VNS and CC for all seizure types. First row is number of studies–number of patients, second row is the pooled proportion with 95% confidence intervals.
| Seizure type | Treatment type | Seizure free | >75% reduction | >50% reduction |
|---|---|---|---|---|
| Atonic | VNS | 7–39 | 7–39 | 7–39 |
| 22.8% [6.6–44.9%] | 26.3% [5.8–54.7%] | 54.1% [32.1–75.4%] | ||
| CC | 6–44 | 6–44 | 8–58 | |
| 48.0% [31.0–65.0%] | 70.0% [48.05–87.0%] | 80.0% [67.0–90.0%] | ||
| Tonic | VNS | 5–29 | 5–29 | 5–29 |
| 14.2% [4.7–27.8%] | 28.2% [14.6–44.3%] | 34.8% [16.4–55.9%] | ||
| CC | No data | No data | 3–28 | |
| 62.0% [29.0–90.0%] | ||||
| GTC | VNS | 6–34 | 6–34 | 6–34 |
| 13.6% [5.0–25.7%] | 22.1% [10.9–35.9%] | 44.0% [24.6–64.5%] | ||
| CC | 5–24 | 5–24 | 7–63 | |
| 35.0% [9.0–68.0%] | 60.1% [42.3–76.6%] | 65.0% [53.6–75.6%] | ||
| CPS | VNS | 5–10 | 5–10 | 5–10 |
| 15.0% [1.0–42.0%] | 40.0% [9.0–77.0%] | 54.0% [19.0–87.0%] | ||
| CC | 3–6 | 3–6 | 5–11 | |
| 50.4% [9.0–91.4%] | 50.4% [9.0–91.4%] | 56.9% [26.7–84.4%] | ||
| Myoclonic | VNS | 2–12 | No data | 2–12 |
| 46.6% [0.6–98.0%] | 66.0% [40.0–88.0%] | |||
| CC | No data | No data | 3–37 | |
| 65.0% [49.0–78.0%] | ||||
| Total | VNS | 15–142 | 16–166 | 17–176 |
| 5.2% [2.3–9.2%] | 28.6% [18.5–40.0%] | 49.3% [37.5–61.2%] | ||
| CC | 8–124 | 6–50 | 8–124 | |
| 16.0% [10.0–23.0%] | 45.2% [26.4–64.9%] | 63.0% [54.0–72.0%] |
Significant differences between VNS and CC are in bold (test using the methods in Wolfe and Hanley14).
Fig. 2.
Forest plots of atonic seizure frequency reduction. VNS and CC are significantly different at the >75% (p < 0.05) and >50% (p < 0.05) levels. Note: Squares indicate point estimates for proportion; horizontal lines indicate 95% CIs; diamonds indicate pooled estimates with 95% CIs.
Fig. 3.
Forest plots of total seizures. No differences between VNS and CC are significant. Note: Squares indicate point estimates for proportion; horizontal lines indicate 95% CIs; diamonds indicate pooled estimates with 95% CIs.
5. Complications
5.1. VNS
Based on the available data, 5/134 (3.7%) VNS patients had complications, which included 2 infections at the incision site, 1 vocal cord paralysis, 1 persistent cough, and 1 case of aspiration. Many patients experienced the standard side effects of VNS treatment, including hoarseness, coughing, tingling in the throat, drooling, and voice alteration. These occurred mainly while the stimulator was on and were considered transient and tolerable.
5.2. CC
Data on CC complications are limited relative to the sample size; 3/36 (8.3%) suffered complications, including 1 subgaleal fluid collection, 1 meningitis with prolonged seizures, and 1 respiratory complication. Some patients experienced transient neurological deficits, which included akinesia, mutism, hemiparesis, disconnection syndrome, and constructional apraxia. All of these improved rapidly and disappeared a few days after surgery.
6. Discussion
This meta-analysis found that CC is significantly more effective than VNS in achieving a 50% and 75% atonic seizure frequency reduction in LGS patients. Furthermore, patients in this analysis were more likely to be completely free of atonic seizures if they underwent CC (48.0% vs. 22.8% with VNS), although this was not significant.
Tonic, GTC, complex-partial, and myoclonic seizures showed no statistical difference in response to the two treatments, which may be due in part to small sample sizes, since patients undergoing CC showed higher response rates for tonic, GTC, and complex-partial seizures. Atypical absence seizures, which are particularly difficult to measure accurately, were not reported often enough in the literature to conduct any analysis.
The medical literature is rife with debate about the risks and benefits of CC and VNS for patients with medically intractable epilepsy and no resectable seizure focus. The general consensus remains that VNS should be tried first because its perceived risk is lower and it is reversible.1,12 However, this question is especially complex in LGS patients who have multiple seizure types and frequent disabling drop attacks. If the primary goal is significant reduction of these atonic drop attacks, then CC appears to be the better option. However, if other seizure types are present, then VNS can offer roughly comparable reduction rates to CC.
The risks and side effects of VNS and CC are well-established in the literature. VNS can cause hoarseness, coughing, tingling in the throat, and voice alteration during stimulation, in addition to a small risk of infection after implantation of the device.39 CC carries the risks of intracranial surgery including infection, hemorrhage, and stroke, as well as usually transient post-operative neurological morbidities including lower extremity weakness, disconnection syndrome, and decreased verbal output.10 In this meta-analysis, however, serious complications for both procedures were infrequent. The often-cited “disconnection syndrome” was only reported in one CC patient and improved after a few days. Overall, the benefits of both procedures, which are usually a last resort to long-suffering LGS patient, outweigh the risks.
This study has many limitations. The most important is the seizure count reliability, as most studies relied on the patient or caregiver to keep a complete seizure diary and to accurately identify the different seizure types. In the case of the caregivers, this necessitated being well-informed about seizure characteristics and being in constant supervision of the patient. Myoclonic and atypical absence seizures are especially difficult to count, and even atonic and tonic seizures can be brief and easy to miss. Since most tonic seizures in LGS occur during sleep,6 they were likely not counted in these studies.
Many studies had missing variables or incomplete data. We were not able to differentiate between the 15 VNS patients who had previous callosotomy and the rest who did not, nor were we able to compare the results of symptomatic vs. cryptogenic etiologies or partial vs. complete CC. Although it is likely that seizure control would have been better had all patients undergone a complete callosotomy, the risk of lasting neurological morbidity may also have increased. Follow-up time was significantly greater in CC patients, which may have affected the analysis because VNS seizure control tends to improve over time.40,41 In our study, VNS patients with >12 months of follow-up had a significantly greater 50% seizure reduction rate for all seizures combined vs. <12 months of follow-up, while 75% and 100% reduction rates did not show significant differences. Meta-regression analysis showed that follow-up time and age at surgery were not significantly associated with any seizure outcomes in CC patients (data not shown).
This study does not address the question of whether there is an additive effect of CC and VNS, which might argue for performing both procedures simultaneously to maximize seizure control. Lastly, the sample size was small for many seizure types. Although a randomized-controlled trial comparing the two procedures is impossible, future multi-center studies could prospectively follow large groups of LGS patients receiving either VNS or CC and thoroughly chart their seizure outcomes.
Acknowledgments
Center for Education and Research in Therapeutics (CERTs) (AHRQ RFA-HS-05-14) and Clinical Translational Science Center (CTSC) (NIH UL1-RR024996) are acknowledged for Dr. Mazumdar’s effort.
Footnotes
Conflicts of interest
None of the authors has any conflict of interest to disclose.
References
- 1.Schmidt D, Bourgeois B. A risk-benefit assessment of therapies for Lennox–Gastaut syndrome. Drug Safety. 2000;22(June 6):467–77. doi: 10.2165/00002018-200022060-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hancock EC, Cross HH. Treatment of Lennox–Gastaut syndrome. Cochrane Database of Systematic Reviews. 2009;3:CD003277. doi: 10.1002/14651858.CD003277.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Dulac O, N’Guyen T. The Lennox–Gastaut syndrome. Epilepsia. 1993;34(Suppl 7):S7–17. doi: 10.1111/j.1528-1157.1993.tb04593.x. [DOI] [PubMed] [Google Scholar]
- 4.Wheless JW. Managing severe epilepsy syndromes of early childhood. Journal of Child Neurology. 2009;24(August 8 Suppl):24S–32S. doi: 10.1177/0883073809338153. quiz 33S–26S. [DOI] [PubMed] [Google Scholar]
- 5.Arzimanoglou A, French J, Blume WT, Cross HJ, Ernst JP, Feucht M, et al. Lennox–Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. The Lancet Neurology. 2009;8(January 1):82–93. doi: 10.1016/S1474-4422(08)70292-8. [DOI] [PubMed] [Google Scholar]
- 6.Crumrine PK. Management of seizures in Lennox–Gastaut syndrome. Paediatric Drugs. 2011;13(April 2):107–18. doi: 10.2165/11536940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term followup on first 67 patients exiting a controlled study. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(May–June 3):637–43. doi: 10.1111/j.1528-1157.1994.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 8.Elliott RE, Morsi A, Kalhorn SP, Marcus J, Selin J, Kang M, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy & Behavior. 2011;20(January 1):57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Van Wagenen WP, Herren RY. Surgical division of commisural pathways in the corpus callosum: relation to spread of an epileptic attack. Archives of Neurology & Psychiatry. 1940;44(October 4):740–59. [Google Scholar]
- 10.Asadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy & Behavior. 2008;13(August 2):271–8. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Sunaga S, Shimizu H, Sugano H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure. 2009;18(March 2):124–8. doi: 10.1016/j.seizure.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 12.van Rijckevorsel K. Treatment of Lennox–Gastaut syndrome: overview and recent findings. Journal of Neuropsychiatric Disease and Treatment. 2008;4(December 6):1001–19. doi: 10.2147/ndt.s1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You SJ, Kang HC, Ko TS, Kim HD, Yum MS, Hwang YS, et al. Comparison of corpus callosotomy and vagus nerve stimulation in children with Lennox–Gastaut syndrome. Brain and Development. 2008;30(March 3):195–9. doi: 10.1016/j.braindev.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe R, Hanley J. If we’re so different, why do we keep overlapping? When 1 plus 1 doesn’t make 2. CMAJ Canadian Medical Association Journal. 2002;166(January 1):65–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Labar D, Nikolov B, Tarver B, Fraser R. Vagus nerve stimulation for symptomatic generalized epilepsy: a pilot study. Epilepsia. 1998;39(February 2):201–5. doi: 10.1111/j.1528-1157.1998.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 16.Lundgren J, Amark P, Blennow G, Stromblad LG, Wallstedt L. Vagus nerve stimulation in 16 children with refractory epilepsy. Epilepsia. 1998;39(August 8):809–13. doi: 10.1111/j.1528-1157.1998.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Menachem E, Hellstrom K, Waldton C, Augustinsson LE. Evaluation of refractory epilepsy treated with vagus nerve stimulation for up to 5 years. Neurology. 1999;52(April 6):1265–7. doi: 10.1212/wnl.52.6.1265. [DOI] [PubMed] [Google Scholar]
- 18.Parker AP, Polkey CE, Binnie CD, Madigan C, Ferrie CD, Robinson RO. Vagal nerve stimulation in epileptic encephalopathies. Pediatrics. 1999;103(April 4 Pt 1):778–82. doi: 10.1542/peds.103.4.778. [DOI] [PubMed] [Google Scholar]
- 19.Hosain S, Nikalov B, Harden C, Li M, Fraser R, Labar D. Vagus nerve stimulation treatment for Lennox–Gastaut syndrome. Journal of Child Neurology. 2000;15(August 8):509–12. doi: 10.1177/088307380001500803. [DOI] [PubMed] [Google Scholar]
- 20.Frost M, Gates J, Helmers SL, Wheless JW, Levisohn P, Tardo C, et al. Vagus nerve stimulation in children with refractory seizures associated with Lennox–Gastaut syndrome. Epilepsia. 2001;42(September 9):1148–52. doi: 10.1046/j.1528-1157.2001.23900.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagarajan L, Walsh P, Gregory P, Lee M. VNS therapy in clinical practice in children with refractory epilepsy. Acta Neurologica Scandinavica. 2002;105(Janusry 1):13–7. doi: 10.1034/j.1600-0404.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- 22.Buoni S, Mariottini A, Pieri S, Zalaffi A, Farnetani MA, Strambi M, et al. Vagus nerve stimulation for drug-resistant epilepsy in children and young adults. Brain and Development. 2004;26(April 3):158–63. doi: 10.1016/S0387-7604(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 23.Hallbook T, Lundgren J, Stjernqvist K, Blennow G, Stromblad LG, Rosen I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behaviour and mood. Seizure. 2005;14(October 7):504–13. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Majoie HJ, Berfelo MW, Aldenkamp AP, Renier WO, Kessels AG. Vagus nerve stimulation in patients with catastrophic childhood epilepsy, a 2-year followup study. Seizure. 2005;14(January 1):10–8. doi: 10.1016/j.seizure.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Benifla M, Rutka JT, Logan W, Donner EJ. Vagal nerve stimulation for refractory epilepsy in children: indications and experience at The Hospital for Sick Children. Childs Nervous System. 2006;22(Auguat 8):1018–26. doi: 10.1007/s00381-006-0123-6. [DOI] [PubMed] [Google Scholar]
- 26.Casazza M, Avanzini G, Ferroli P, Villani F, Broggi G. Vagal nerve stimulation: relationship between outcome and electroclinical seizure pattern. Seizure. 2006;15(April 3):198–207. doi: 10.1016/j.seizure.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kostov K, Kostov H, Tauboll E. Long-term vagus nerve stimulation in the treatment of Lennox–Gastaut syndrome. Epilepsy & Behavior. 2009;16(October 2):321–4. doi: 10.1016/j.yebeh.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 28.Shahwan A, Bailey C, Maxiner W, Harvey AS. Vagus nerve stimulation for refractory epilepsy in children: more to VNS than seizure frequency reduction. Epilepsia. 2009;50(May 5):1220–8. doi: 10.1111/j.1528-1167.2008.01940.x. [DOI] [PubMed] [Google Scholar]
- 29.Franzoni E, Gentile V, Colonnelli MC, Brunetto D, Cecconi I, Iero L, et al. VNS in drug resistant epilepsy: preliminary report on a small group of patients. Italian Journal of Pediatrics. 2010;36:30. doi: 10.1186/1824-7288-36-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cersosimo RO, Bartuluchi M, De Los Santos C, Bonvehi I, Pomata H, Caraballo RH. Vagus nerve stimulation: effectiveness and tolerability in patients with epileptic encephalopathies. Childs Nervous System. 2011;27(May 5):787–92. doi: 10.1007/s00381-010-1314-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakatani S, Nii Y, Ikejiri Y, Tanabe H, Mogami H. Partial callosotomy for Lennox–Gastaut syndrome – first cases in Japan. Neurologia Medico-Chirurgica. 1990;30(Novomber 12):930–9. doi: 10.2176/nmc.30.930. [DOI] [PubMed] [Google Scholar]
- 32.Provinciali L, Del Pesce M, Censori B, Quattrini A, Paggi A, Ortenzi A, et al. Evolution of neuropsychological changes after partial callosotomy in intractable epilepsy. Epilepsy Research. 1990;6(July 2):155–65. doi: 10.1016/0920-1211(90)90091-9. [DOI] [PubMed] [Google Scholar]
- 33.Oguni H, Olivier A, Andermann F, Comair J. Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Annals of Neurology. 1991;30(September 3):357–64. doi: 10.1002/ana.410300307. [DOI] [PubMed] [Google Scholar]
- 34.Kwan SY, Lin JH, Wong TT, Chang KP, Yiu CH. A comparison of seizure outcome after callosotomy in patients with Lennox–Gastaut syndrome and a positive or negative history for West syndrome. Seizure. 2006;15(October 7):552–7. doi: 10.1016/j.seizure.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Turanli G, Yalnizoglu D, Genc-Acikgoz D, Akalan N, Topcu M. Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nervous System. 2006;22(October 10):1322–7. doi: 10.1007/s00381-006-0045-3. [DOI] [PubMed] [Google Scholar]
- 36.Jea A, Vachhrajani S, Johnson KK, Rutka JT. Corpus callosotomy in children with intractable epilepsy using frameless stereotactic neuronavigation: 12-year experience at the Hospital for Sick Children in Toronto. Neurosurgical Focus. 2008;25(September 3):E7. doi: 10.3171/FOC/2008/25/9/E7. [DOI] [PubMed] [Google Scholar]
- 37.Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. Journal of Neurosurgery. 2009;110(February 2):332–42. doi: 10.3171/2008.3.17570. [DOI] [PubMed] [Google Scholar]
- 38.Jalilian L, Limbrick DD, Steger-May K, Johnston J, Powers AK, Smyth MD. Complete versus anterior two-thirds corpus callosotomy in children: analysis of outcome. Journal of Neurosurgery Pediatrics. 2010;6(September 3):257–66. doi: 10.3171/2010.5.PEDS1029. [DOI] [PubMed] [Google Scholar]
- 39.Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35(May–June 3):627–36. doi: 10.1111/j.1528-1157.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 40.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Group E01–E05. Neurology. 1999;53(November 8):1731–5. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 41.Uthman BM, Reichl AM, Dean JC, Eisenschenk S, Gilmore R, Reid S, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63(September 6):1124–6. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]