Abstract
Oligozoospermia (low sperm count) is a common semen deficiency. However, to date, few genetic defects have been identified to cause this condition. Moreover, even fewer molecular genetic diagnostic tests are available for patients with oligozoospermia in the andrology clinic. Based on animal and gene expression studies of oligozoospermia, several molecular pathways may be disrupted in post-meiotic spermatozoa. One of the disrupted pathways is protein ubiquitination and cell apoptosis. A critical protein involved in this pathway is the ubiquitin-conjugating enzyme 2B, UBE2B. Absence of Ube2b in male mice causes spermatogenic meiotic disruption with increased apoptosis, leading to infertility. To examine the association between messenger RNA defects in UBE2B and severe oligozoospermia (0.1–10 × 106 cells/ml), sequencing of sperm cDNA in 326 oligozoospermic patients and 421 normozoospermic men was performed. mRNA alterations in UBE2B were identified in sperm in 4.6% (15 out of 326) of the oligozoospermic patients, but not found in control men, suggesting strong association between mRNA defects and oligozoospermia (χ2 = 19, P = 0.0001). Identified UBE2B alterations include nine splicing, four missense and two nonsense alterations. The follow-up screen of corresponding DNA regions did not reveal causative DNA mutations, suggesting a post-transcriptional nature of identified defects. None of these variants were reported in the dbSNP database, although other splicing abnormalities with low level of expression were present in 11 out of 421 (2.6%) controls. Our findings suggest that two distinct molecular mechanisms, mRNA editing and splicing processing, are disrupted in oligozoospermia. We speculate that the contribution of post-transcriptional mRNA defects to oligozoospermia could be greater than previously anticipated.
Keywords: male infertility, oligozoospermia, UBE2B alterations, abnormal splicing, post-transcriptional errors
Introduction
Infertility is a major health problem for propagation of the germline. Fertility defects affect nearly 15% of couples, half of which are attributed to a male factor (Anderson et al., 2009; Sigman et al., 2009). However, a routine semen analysis cannot predict male infertility unless a man is azoospermic; infertile patients may be normozoospermic, and likewise spontaneous pregnancies can occur in men with abnormal sperm parameters (Guzick et al., 2001; Nallella et al., 2006). Among the possible abnormal diagnoses in the andrology clinic, azoospermia (absence of sperm in the semen) and oligozoospermia (low sperm count of <20 × 106/ml) (WHO, 1999) are well-established causes of infertility. The etiologies of these conditions remain largely unknown (Lipshultz and Lamb, 2007; Anderson et al., 2009; Barratt et al., 2011). The increasing use of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) without adequate knowledge of genetic defects in patients with idiopathic male infertility raises additional safety concerns (Pinborg et al., 2004; Olson et al., 2005; Davies et al., 2012).
To date, the most common genetic defects are structural and numerical chromosome abnormalities that are diagnosed in up to 14 and 10% of azoospermic and oligozoospermic patients, respectively (Egozcue et al., 1983; Retief et al., 1984; Clementini et al., 2005; Yatsenko et al., 2010). Our understanding of the genetics of male infertility has been greatly advanced by studies of mouse models with targeted gene deletions, but progress in deciphering the cause of human male infertility is modest (Matzuk and Lamb, 2002, 2008; Jamsai and O'Bryan, 2011). Many studies of known mouse model candidate genes for oligozoospermia and azoospermia could not yield significant results. With the exception of a few genes, such as CFTR and AURKC in patients of North African heritage, none of the other mutated genes identified are considered in the evaluation of the infertile men (Riordan et al., 1989; Anguiano et al., 1992; Dieterich et al., 2007; Dieterich et al., 2009). Such modest progress is likely due to the fact that male infertility is genetically heterogeneous and complex; there are literally thousands of genes expressed in the testis (Schultz et al., 2003), as well as a plethora of non-genetic factors that potentially might affect male reproductive function.
The targeted deletion of Ube2b in mice disrupted ubiquitination, causing aberrations in synaptonemal complex structure, histone modifications and chromatin structure with abnormal meiotic recombination in the spermatocytes, abnormal post-meiotic histone modifications, X chromosome derepression and misregulated transcription (Baarends and Grootegoed, 2003; Baarends et al., 2003; Escalier et al., 2003; Baarends et al., 2007; Mulugeta et al., 2010). Multiple ubiquitins bind to abnormal or short-lived proteins, labeling them for degradation by the proteasome. Three classes of proteins [ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2) and ubiquitin-protein ligases (E3)] are involved in ubiquitination, with 32 different ubiquitin-conjugating enzymes present in the E2 class (van Wijk and Timmers, 2010). UBE2B catalyzes the covalent linkage between ubiquitin and other proteins and is a key protein in the DNA damage repair pathway after replication (van Wijk and Timmers, 2010). In this role, UBE2B most likely has a dynamic role in chromatin reorganization in meiotic and post-meiotic germ cells and is most active during spermiogenesis (Roest et al., 1996; Baarends et al., 2003). As a result, Ube2b null male mice display low sperm counts with abnormal morphology and decreased motility (oligoasthenoteratozoospermia) (Roest et al., 1996; Baarends et al., 2003; Escalier et al., 2003).
Recently, two studies have reported association of non-coding single nucleotide polymorphisms (SNPs) in UBE2B with oligozoospermia and azoospermia in infertile Indian and American men (Huang et al., 2008; Suryavathi et al., 2008). The first study reported significant association for two SNPs in 5′- and 3′-untranslated region sites (UTRs) in azoospermic men, whereas the second study revealed association for three novel SNPs (in intron 3, exon 4 and 3′-UTR) in non-obstructive azoospermic and oligozoospermic males. Thus, UBE2B is a strong candidate for mutation screening in oligozoospermic patients and controls. Herein, we present a mutation screening of the UBE2B gene in 326 oligozoospermic patients and 421 normozoospermic controls by direct sequencing of spermatozoal cDNA and show the association of UBE2B changes in sperm mRNA with severe oligozoospermia. This approach was based upon evidence that RNA from semen provides a reliable source of genetic information (Ostermeier et al., 2004; Yatsenko et al., 2006).
Materials and Methods
Patient specimens obtained during the course of their evaluation were provided by the Special Procedures Laboratory in the Scott Department of Urology at Baylor College of Medicine. Samples were de-identified and collected under IRB protocols (H-12083 and H-19753). The study was approved and overseen by the Institutional Review Board for the Protection of Human Subjects at Baylor College of Medicine. Semen samples were provided for routine semen analyses performed according to the 4th Edition of the WHO guidelines (WHO, 1999). Semen samples were selected as severe oligozoospermic, if their sperm count was ≤10 × 106/ml and normozoospermic if sperm count was >34 × 106/ml. The experimental study population consisted of severe oligozoospermic men (n = 423) and normozoospermic controls (n = 545). Oligozoospermic patients and normozoospermic controls were collected by the Special Procedures Laboratory from the Greater Houston area. Because specimens were de-identified, ethnicity of individual samples could not be obtained. However, ethnicity distribution in patient and control populations were estimated previously (Yatsenko et al., 2006).
Extraction of semen RNA and DNA was performed as previously described (Yatsenko et al., 2006; Yatsenko et al., 2012). Freshly ejaculated semen specimens were incubated for 30 min at 37°C to liquefy. Specimens were washed with an equal volume of Sperm Washing Medium (Irvine Science, Santa Ana, CA, USA) and spun at 25°C for 10 min at 652g. The sperm pellet was resuspended in residual medium and lyzed in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was extracted from the aqueous phase and DNA from the phenol phase according to the manufacturer's protocol. The RNAs were resuspended in diethylpyrocarbonate (DEPC)-treated water (Ambion, Austin, TX, USA), and the DNAs were resuspended in sterile 10 mM Tris/1 mM EDTA solution, pH 7.0 (Ambion, Austin, TX, USA). The total RNA was treated with DNase I (Invitrogen), and its concentration was measured. RNA samples were arranged in 96 well plates.
For first strand cDNA synthesis, we used total sperm RNA, MMLV Reverse Transcriptase (Invitrogen) and random primers. RT–PCR for UBE2B was performed with at least 40 ng of cDNA and high fidelity KAPA HiFi HotStart DNA Polymerase (Kapa Biosystems, Woburn, MA, USA). We used a set of PCR primers, forward: ATTGCAGGGTTGTTTGTCAGTC and reverse: TGGCACTTAAAATTTGTTAGCC with Ta 62°C to amplify a single 688 bp cDNA fragment encompassing the UBE2B open reading frame (ORF). The RT–PCR products were sequenced using BigDye V3.1 sequencing reagent and ABI Prism Sequencer 3130XL (Applied Biosystems, Foster City, CA, USA). To estimate transcript ‘dropout rate’ due to selective amplification of long products, we performed RT–PCR amplification of shorter cDNA fragments of interest with designed primers that flanked abnormal splicing products (see Supplementary data, Table SI). For RT–PCR, we used uniform amount of RNA (∼50–70 ng), a limited number of cycles (35), estimated yield of abnormal splicing via gel density and performed subsequent cDNA sequencing. To sequence UBE2B exons, we designed primers that cover exons and ∼50–100 bp of flanking intronic regions (see Supplementary data, Table SII). We analyzed sequences with Sequencher4.2 (Gene Codes, Ann Arbor, MI, USA). Protein multiple alignments were performed using the online software ClustalW (www.ebi.ac.uk/clustalw). All SNPs were checked against the dbSNP database (build 37.1). The potential damaging effect of amino acid missense changes was predicted using Polyphen-2 and SIFT softwares. To test for an association between mutations in UBE2B and oligozoospermia, a likelihood ratio Chi-square test compared the frequencies of SNPs in oligozoospermic patients and normozoospermic controls. All tests were two sided with an alpha level ≤0.05 indicating statistical significance. Statistical analysis was performed using JMP Start Statistics software (SAS Institute Inc., Cary, NC, USA).
Results
To study messenger RNA mutations in UBE2B, the validated cDNA approach was employed using total sperm RNA. RT–PCR conditions were optimized allowing the amplification of the entire 459 bp coding region as one 688 bp RT–PCR product. Under these experimental conditions, UBE2B RT–PCR products were generated from 77% (326 out of 423) of oligozoospermic patients and 77% (421 out of 545) of normozoospermic controls. These results confirm that our method detects pre-meiotically expressed genes in ∼75% of patients tested (Yatsenko et al., 2006). Importantly, the mRNA detection rate did not differ significantly between oligozoospermic patients and normozoospermic controls.
Alterations in mRNA were identified in sperm specimens from 20 out of 326 (6.1%) severe oligozoospermic patients. Five patients were excluded after further analysis as described below, resulting in 15 patients with novel mRNA defects (4.6%) see Table 1. The initial findings included 14 splicing, 4 missense and 2 nonsense UBE2B changes (see Fig. 1 and Supplementary data, Fig. S1). Using shorter RT–PCR, we observed a variable ‘dropout rate’ for long RT–PCR products. Due to this variability of abnormal splicing fractions, we excluded five patients with abnormal splicing (four patients with c.127_131delCCAGA and one patient with c.del125 5′UTR, p.M1-L9del, see Table 2 numbers 9 and 10) who have defects also observed in controls (see Table 3 numbers 6 and 8). Most of the detected alterations translate to premature termination codons (PTC) directly (patients 4, 6, nonsense mutations) or indirectly via abnormal splicing isoforms with mRNA frameshift deletions and eventual downstream PTC (patients 8–10, 13–15) (Figs 1 and 2). Sequence analysis revealed that the majority of the single nucleotide changes and splicing mutations identified were heterozygous (i.e. affecting nearly half of transcripts). We identified only two homozygous missense (p.A38T and p.I87T) and one homozygous splicing alteration (expression level of abnormal splicing is ∼100% of the wild-type RT–PCR product) (patients 1, 2 and 12, Table 1). Notably, this splicing defect leads to an in-frame deletion and shorter protein product by 39 amino acids (Fig. 2).
Table I.
UBE2B messenger RNA mutations identified in 326 oligozoospermic patients.
| Patient | Volume | Density | Motility | FP | cDNA change, nt | Protein change, aa | Type, splicing %, zygosity |
|---|---|---|---|---|---|---|---|
| 1 | 4 | 2.5 | 20 | 2 | c.112GCA>ACA | p.A38T | Missense/homo |
| 2 | 2.5 | 3.5 | 25 | 2.5 | c. 260ATA>ACA | p.I87T | Missense/homo |
| 3 | 2.5 | 2.5 | 30 | 2.5 | c.298TAT>CAT | p.Y100H | Missense/hetero |
| 4 | 1.5 | 3.5 | 25 | 2 | c.298_299delAT | p.Y100X | Nonsense/hetero |
| 5 | 6 | 6.5 | 45 | 2.5 | c.308TCT>TTT | p.S103F | Missense/hetero |
| 6 | 0.6 | 1 | 15 | 2 | c.391CAG>TAG | p.Q131X | Nonsense/hetero |
| 7 | 1.5 | 7.5 | 25 | 2.5 | spl ex2, c.45del81bp | p. L16del27aa, in-frame | ∼50% |
| 8 | 3.5 | 7.5 | 45 | 2.5 | spl ex3, c.126del26 bp | p.P43fs, PTC | ∼50% |
| 9 | 4.5 | 2 | 5 | 2 | spl ex3-4, c.126del116 bp | p.P43fs, PTC | ∼50% |
| 10 | 3 | 3.6 | 5 | 1.5 | spl ex3-4, c.126del116 bp | p.P43fs, PTC | ∼50% |
| 11 | 4.5 | 1.5 | 5 | 1.5 | spl ex4, c.152del90bp | p.G51Vdel30aa, in-frame | ∼50% |
| 12 | 1.5 | 7.5 | 35 | 2 | spl ex4-5, c.152del117bp | p. G51Ddel39aa, in-frame | ∼90% |
| 13 | 4.5 | 4.5 | 20 | 2 | spl ex5, c.242del89bp | p.V81fs, PTC | ∼50% |
| 14 | 6 | 0.1 | 0 | 0 | spl c.242dell31bp, ex5 del89bp, intr5 ins58bp, | p. V81fs, PTC | ∼50% |
| 15 | 1.5 | 0.5 | 10 | 1.5 | spl ex5-6, c.320del133bp, ex5 del11bp, ex6 del122bp, | p.T107Iins7aaX8 | ∼50% |
FP, forward progression; PTC, premature termination codon; nt, nucleotide; aa, amino acid.
Figure 1.
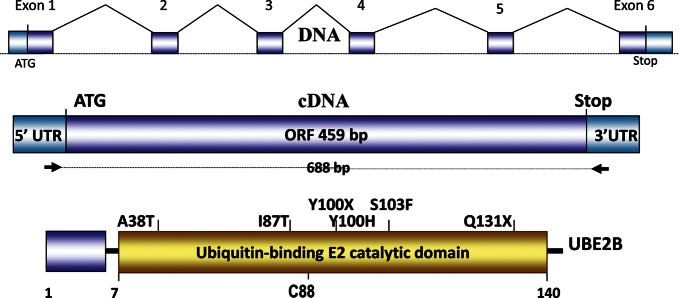
UBE2B gene, cDNA and point alterations identified in severe oligozoospermic patients. Schematic structure of the UBE2B gene, cDNA amplification strategy and single nucleotide mutations in the gene are shown. The upper diagram depicts the normal splicing pattern. The positions of the single nucleotide mutations and variants in the UBE2B protein are shown. The position of the cysteine residue (C) is shown at the bottom. The UBC E2 domain is predicted at 7–140 amino acids. The protein structure is based on Interproscan, Prosite, Pfam and Blocks algorithm predictions.
Table II.
Polymorphic, rare and low-expressed splicing defects in UBE2B mRNA identified in 23 out of 326 (7%) of oligozoospermic patients.
| Number | cDNA variant | Protein change | Zygosity, splicing % | Frequency |
|---|---|---|---|---|
| 1 | c.-31T>C | 5′UTR | Homo | 1 out of 326 (0.3%) |
| 2 | c.48TTA>TTT | p.L16L | Hetero | 1 out of 326 (0.3%) |
| 3 | c.-10G>T | 5′UTR | Homo | 3 out of 326 (0.9%) |
| 4 | c.-10G>T | 5′UTR | Hetero | 9 out of 329 (2.7%) |
| 5 | c.-28C>T | 5′UTR | Hetero | 1 out of 326 (0.6%) |
| 6 | c.406CGA>AGA | p.R134R | Hetero | 1 out of 326 (0.3%) |
| 7 | c.57GAC>GAT | p.D19D | Hetero | 1 out of 326 (0.3%) |
| 8 | c.426TCG>TCA | p.S142S | Hetero | 1 out of 326 (0.3%) |
| 9 | c.-125_8del133bp | del125 5′UTR, p.M1-L9del | ∼70% | 1 out of 326 (0.3%) |
| 10 | c.127_131delCCAGA, spl ex3 | p.P43Rins2X | ∼50% | 4 out of 326 (1.2%) |
Table III.
Polymorphic and rare UBE2B mRNA changes identified in 17 out of 421 (4%) of normozoospermic controls.
| N | cDNA variant | Protein change | Zygosity, splicing % | Frequency |
|---|---|---|---|---|
| 1 | c.-9C>T | 5′UTR | Hetero | 1 out of 421 (0.2%) |
| 2 | spl ex1 -125_-25bp | del100 5′UTR | ∼90% | 1 out of 421 (0.2%) |
| 3 | spl ex1 -120_-15bp | del105 5′UTR | ∼90% | 1 out of 421 (0.2%) |
| 4 | spl ex1 -123_-15bp | del108 5′UTR | ∼90% | 2 out of 421 (0.4%) |
| 5 | c.42AAG>AAA | p.K14K | hetero | 1 out of 421 (0.2%) |
| 6 | c.-125_8del133bp | del125 5′UTR, p.M1-L9del | ∼50% | 2 out of 421 (0.4%) |
| 7 | c.391CAG>TAG | p.Q131H | ∼30% | 1 out of 421 (0.2%) |
| 8 | c.127_131delCCAGA, spl ex3 | p.P43Rins2X | ∼30% | 3 out of 421 (0.7%) |
| 9 | spl ex3,4&5 out | del 205bp, p.50fs, PTS | ∼30% | 5 out of 421 (1.1%) |
Figure 2.
Schematic diagrams for UBE2B splicing mutations identified in oligozoospermic patients. The schematics show abnormal splicing defects of UBE2B exons in yellow. Patient's number (P#) is shown on the left side; these numbers follow numbering from Table 1. Patients 9 and 10 have identical splicing errors. The starting position of splicing mutations was sorted in the downstream direction. UBE2B exons involved are shown above. Normal splicing is shown in blue, and abnormally spliced exons are shown in yellow. Information describing splicing in-frame deletions or frameshifts is shown on the right side.
All mutations identified were located in the active ubiquitin-binding catalytic (UBC) domain of the UBE2B protein (Figs 1 and 2), presumably having a significant damaging effect on protein function. Whereas nonsense mutations are normally implicated as being deleterious for protein function, the splicing mutations also lead to frame shifts and PTCs in a fraction of protein products that would disrupt overall protein function. The missense mutations are predicted by SIFT and Polyphen-2 to cause milder damage to protein function (Supplementary data, Fig. S2). However, because the missense mutations affect highly conserved residues located in the UBC domain (∼100% conserved sequence; see Supplementary data, Fig. S3), it is likely that these missense changes have significant damaging effects as well. Our analysis of the abnormally spliced products revealed that these aberrations span the entire UBE2B coding region, and most abnormally spliced mRNAs introduce frame shift and protein PTCs (Fig. 2).
Our study of UBE2B mRNA changes in 421 normozoospermic controls did not reveal identified missense or highly expressed splicing alterations, providing statistical significance for the UBE2B association with severe oligozoospermia (χ2 = 26, P = 0.0001). In addition, we detected rare polymorphic missense and low-expressed splicing abnormalities (expression approximately 25–30% of normal transcripts) in 23 out of 326 (7%) patients (Table 2) and 17 out of 421 (4%) normozoospermic controls (Table 3). Because these abnormalities were detected at a low concentration (∼30% of the normal product), they presumably lead to milder mRNA defects. Finally, we attempted to find causative DNA changes in corresponding genomic regions in 13 available DNA specimens from selected oligozoospermic patients. We sequenced all coding exons and at least 50 bp of flanking intronic regions. Interestingly, none of the mRNA missense and splicing defects were identified in corresponding DNA specimens.
Discussion
The initial clinical evaluation of the nearly 4 million American men with infertility includes routine semen analysis (Anderson et al., 2009). Decreased sperm count is a common diagnosis and accounts for about half of all male infertility cases (WHO, 2010). It is estimated that nearly 50% of infertility can be attributed to a genetic factor (Lipshultz and Lamb, 2007). Because IVF and ICSI introduce an increased risk of transmission of genetic defects to offspring, a comprehensive preconception genetic test of parents at risk would help to mitigate the risk of transmission of known defective genes to the offspring.
Progress in understanding the genetic basis of human infertility lags behind the advances in basic research, where over 500 mouse models with male fertility have been discovered to date (Matzuk and Lamb, 2008; Jamsai and O'Bryan, 2011). One plausible gene candidate for severe oligozoospermia in men is UBE2B. The gene encodes ubiquitin conjugating enzyme 2B and is involved in DNA damage repair, histone modifications, synaptonemal complex and chromatin structure in meiosis and spermiogenesis (Roest et al., 1996; Baarends et al., 2003a; Baarends et al., 2003b; Baarends et al., 2007). During spermiogenesis, male germ cells go through a morphological transformation as they become mature spermatozoa, eventually developing polarity, a fully formed head, tail, an acrosomal cap and are finally released from Sertoli cells into the seminiferous tubules. Ube2b knockout mice are infertile due to meiotic arrest, decreased sperm concentration and abnormal sperm morphology (Roest et al., 1996). Recent human studies also reported association of UBE2B SNPs with oligozoospermia (Huang et al., 2008; Suryavathi et al., 2008).
Here, we investigated a large cohort of oligozoospermic patients and normozoospermic controls for UBE2B mRNA alterations. For this study, we used a cDNA approach that facilitates efficient detection of germ-cell mRNA mutations predominantly located in the gene coding region (Yatsenko et al., 2006; Yatsenko et al., 2012). Our study identified novel mRNA defects in UBE2B in 4.6% (15 out of 326) of oligozoospermic patients that were not found in normozoospermic controls (0 out of 421). A following Chi-square test confirmed the association of UBE2B mRNA alterations with severe oligozoospermia in infertile males. The majority of the defects (six splicing and two nonsense mutations, Table 1) were predicted to lead to protein premature stop codons and considered to be deleterious. This provides evidence that post-transcriptional editing and processing errors identified in sperm RNA have a damaging effect on the mature mRNAs and ultimately on protein function. Interestingly, there is growing evidence that certain post-transcriptional missense mRNA errors (DNA–RNA mismatches) occur repeatedly at certain non-random sites, highlighting the importance of such epigenetic processes in protein diversity in normal and likely abnormal cells (Li et al., 2011). However, the nature and magnitude of post-transcriptional defects (editing and processing) in mRNA is not fully understood (Gordon et al., 2009; Li et al., 2011). Furthermore, among few studies that looked at mRNA defects in semen deficiencies, one showed severe expression disruption of the ubiquitin-proteosome pathway (Platts et al., 2007; Lalancette et al., 2009; Heyn et al., 2012; Krausz et al., 2012).
Notably, our cDNA sequence analysis revealed the presence of identical splicing defects in patients and normozoospermic controls, highlighting a spectrum of mRNA defects ranging from highly expressed defects in patients, to low-expressed frameshift splicing defects in 2.6% of the controls. One likely explanation for this finding could be that UBE2B mRNA defects lead to a more complex phenotype than oligozoospermia (i.e. oligoteratozoospermia) as suggested by the Ube2b mouse knockout phenotype. This would explain the presence of abnormal splicing products in 2.6% of controlsbecause semen morphology defects were not analyzed in the study controls. An alternative explanation is that systemic abnormal splicing errors may occur naturally in normal and abnormal male germ cells, but are tolerated up to a certain minimal non-damaging level (i.e. the ‘threshold splicing noise’ model) (Pickrell et al., 2010). Although the latter model needs substantial support and extensive studies of RNA presence in different semen categories, we cannot rule out this possibility.
In summary, we identified novel splicing and missense mRNA defects in UBE2B that are associated with oligozoospermia. Our findings suggest that post-transcriptional editing and processing errors in UBE2B are associated with severe low sperm count. Importantly, until recently, a role of post-transcriptional mRNA editing errors in semen pathologies was largely unknown. Both mouse and our human studies show that genetic and post-transcriptional defects in UBE2B are associated with oligozoospermia, suggesting that UBE2B is critically important for spermiogenesis in mammals (Roest et al., 1996; Baarends et al., 2003). Our study supports the recent notion that sperm cDNAs can be an efficient non-invasive screening biomarker of genetic and epigenetic defects and male germ cells quality (Hamatani, 2012). Although our study reports missense and splicing errors in sperm mRNA, it is possible that the study reveals only a fraction of a larger systemic phenomenon of abnormal post-transcriptional defects in sperm mRNA editing and splicing in oligozoospermic patients. To investigate such systemic errors in mRNA editing and splicing, modern high-throughput approaches like next generation RNA sequencing should be utilized in the future. We believe that future systematic investigations of post-transcription errors in mRNA editing and splicing in oligozoospermia could bring great progress to our understanding of oligozoospermia pathology in the context of genetics–environment interactions.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Acknowledgements
We thank Dr Larry I. Lipshultz for clinical evaluation of the patients. We thank Dr Greg Buchold (NIH/NIEHS) for useful critical comments to our manuscript.
Authors' roles
A.N.Y. and M.M.M. designed the study and prepared the manuscript, to which other authors added their comments. Laboratory work was undertaken by A.N.Y., A.P.G., and L.J.M. D.J.L. was responsible for oversight of all the andrology testing performed and sample acquisition.
Funding
These studies were supported by a Mentored Clinical Scientist Development Award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K08HD058073 to A.N.Y.) and the Program Project grant on the Molecular Basis of Male Infertility (P01HD36289 to D.J.L. and M.M.M.).
Conflict of interest
None declared.
References
- Anderson JE, Farr SL, Jamieson DJ, Warner L, Macaluso M. Infertility services reported by men in the United States: national survey data. Fertil Steril. 2009;91:2466–2470. doi: 10.1016/j.fertnstert.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Anguiano A, Oates RD, Amos JA, Dean M, Gerrard B, Stewart C, Maher TA, White MB, Milunsky A. Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA. 1992;267:1794–1797. [PubMed] [Google Scholar]
- Baarends WM, Grootegoed JA. Chromatin dynamics in the male meiotic prophase. Cytogenet Genome Res. 2003a;103:225–234. doi: 10.1159/000076808. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Wassenaar E, Hoogerbrugge JW, van Cappellen G, Roest HP, Vreeburg J, Ooms M, Hoeijmakers JH, Grootegoed JA. Loss of HR6B ubiquitin-conjugating activity results in damaged synaptonemal complex structure and increased crossing-over frequency during the male meiotic prophase. Mol Cell Biol. 2003b;23:1151–1162. doi: 10.1128/MCB.23.4.1151-1162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarends WM, Wassenaar E, Hoogerbrugge JW, Schoenmakers S, Sun ZW, Grootegoed JA. Increased phosphorylation and dimethylation of XY body histones in the Hr6b-knockout mouse is associated with derepression of the X chromosome. J Cell Sci. 2007;120:1841–1851. doi: 10.1242/jcs.03451. [DOI] [PubMed] [Google Scholar]
- Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK. Diagnostic tools in male infertility–the question of sperm dysfunction. Asian J Androl. 2011;13:53–58. doi: 10.1038/aja.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementini E, Palka C, Iezzi I, Stuppia L, Guanciali-Franchi P, Tiboni GM. Prevalence of chromosomal abnormalities in 2078 infertile couples referred for assisted reproductive techniques. Hum Reprod. 2005;20:437–442. doi: 10.1093/humrep/deh626. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Moore V, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;2012:1–11. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- Dieterich K, Soto Rifo R, Faure AK, Hennebicq S, Ben Amar B, Zahi M, Perrin J, Martinez D, Sele B, Jouk PS, et al. Homozygous mutation of AURKC yields large-headed polyploid spermatozoa and causes male infertility. Nat Genet. 2007;39:661–665. doi: 10.1038/ng2027. [DOI] [PubMed] [Google Scholar]
- Dieterich K, Zouari R, Harbuz R, Vialard F, Martinez D, Bellayou H, Prisant N, Zoghmar A, Guichaoua MR, Koscinski I, et al. The aurora kinase C c.144delC mutation causes meiosis I arrest in men and is frequent in the North African population. Hum Mol Genet. 2009;18:1301–1309. doi: 10.1093/hmg/ddp029. [DOI] [PubMed] [Google Scholar]
- Egozcue J, Templado C, Vidal F, Navarro J, Morer-Fargas F, Marina S. Meiotic studies in a series of 1100 infertile and sterile males. Hum Genet. 1983;65:185–188. doi: 10.1007/BF00286660. [DOI] [PubMed] [Google Scholar]
- Escalier D, Bai XY, Silvius D, Xu PX, Xu X. Spermatid nuclear and sperm periaxonemal anomalies in the mouse Ube2b null mutant. Mol Reprod Dev. 2003;65:298–308. doi: 10.1002/mrd.10290. [DOI] [PubMed] [Google Scholar]
- Gordon AJ, Halliday JA, Blankschien MD, Burns PA, Yatagai F, Herman C. Transcriptional infidelity promotes heritable phenotypic change in a bistable gene network. PLoS Biol. 2009;7:e44. doi: 10.1371/journal.pbio.1000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Hamatani T. Human spermatozoal RNAs. Fertil Steril. 2012;97:275–281. doi: 10.1016/j.fertnstert.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Heyn H, Ferreira HJ, Bassas L, Bonache S, Sayols S, Sandoval J, Esteller M, Larriba S. Epigenetic disruption of the PIWI pathway in human spermatogenic disorders. PLoS One. 2012;7:e47892. doi: 10.1371/journal.pone.0047892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang I, Emery BR, Christensen GL, Griffin J, Peterson CM, Carrell DT. Novel UBE2B-associated polymorphisms in an azoospermic/oligozoospermic population. Asian J Androl. 2008;10:461–466. doi: 10.1111/j.1745-7262.2008.00386.x. [DOI] [PubMed] [Google Scholar]
- Jamsai D, O'Bryan MK. Mouse models in male fertility research. Asian J Androl. 2011;13:139–151. doi: 10.1038/aja.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz C, Sandoval J, Sayols S, Chianese C, Giachini C, Heyn H, Esteller M. Novel insights into DNA methylation features in spermatozoa: stability and peculiarities. PLoS One. 2012;7:e44479. doi: 10.1371/journal.pone.0044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette C, Platts AE, Johnson GD, Emery BR, Carrell DT, Krawetz SA. Identification of human sperm transcripts as candidate markers of male fertility. J Mol Med. 2009;87:735–748. doi: 10.1007/s00109-009-0485-9. [DOI] [PubMed] [Google Scholar]
- Li M, Wang IX, Li Y, Bruzel A, Richards AL, Toung JM, Cheung VG. Widespread RNA and DNA sequence differences in the human transcriptome. Science. 2011;333:53–58. doi: 10.1126/science.1207018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshultz LI, Lamb DJ. Risk of transmission of genetic diseases by assisted reproduction. Nat Clin Pract Urol. 2007;4:460–461. doi: 10.1038/ncpuro0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4:s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14:1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta AE, Wassenaar E, Hoogerbrugge JW, Sleddens-Linkels E, Ooms M, Sun Z-W, van Ijcken WFJ, Grootegoed JA, Baarends WM. The ubiquitin-conjugating enzyme HR6B is required for maintenance of X chromosome silencing in mouse spermatocytes and spermatids. BMC Genomics. 2010;11:367. doi: 10.1186/1471-2164-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–634. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Olson CK, Keppler-Noreuil KM, Romitti PA, Budelier WT, Ryan G, Sparks AE, Van Voorhis BJ. In vitro fertilization is associated with an increase in major birth defects. Fertil Steril. 2005;84:1308–1315. doi: 10.1016/j.fertnstert.2005.03.086. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature. 2004;429:154. doi: 10.1038/429154a. [DOI] [PubMed] [Google Scholar]
- Pickrell JK, Pai AA, Gilad Y, Pritchard JK. Noisy splicing drives mRNA isoform diversity in human cells. PLoS Genet. 2010;6:e1001236. doi: 10.1371/journal.pgen.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Nyboe AA. Neonatal outcome in a Danish national cohort of 8602 children born after in vitro fertilization or intracytoplasmic sperm injection: the role of twin pregnancy. Acta Obstet Gynecol Scand. 2004;83:1071–1078. doi: 10.1111/j.0001-6349.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Platts AE, Dix DJ, Chemes HE, Thompson KE, Goodrich R, Rockett JC, Rawe VY, Quintana S, Diamond MP, Strader LF, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16:763–773. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- Retief AE, Van Zyl JA, Menkveld R, Fox MF, Kotze GM, Brusnicky J. Chromosome studies in 496 infertile males with a sperm count below 10 million/ml. Hum Genet. 1984;66:162–164. doi: 10.1007/BF00286592. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Roest HP, van Klaveren J, de Wit J, van Gurp CG, Koken MH, Vermey M, van Roijen JH, Hoogerbrugge JW, Vreeburg JT, Baarends WM, et al. Inactivation of the HR6B ubiquitin-conjugating DNA repair enzyme in mice causes male sterility associated with chromatin modification. Cell. 1996;86:799–810. doi: 10.1016/s0092-8674(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Schultz N, Hamra FK, Garbers DL. A multitude of genes expressed solely in meiotic or postmeiotic spermatogenic cells offers a myriad of contraceptive targets. Proc Natl Acad Sci USA. 2003;100:12201–6. doi: 10.1073/pnas.1635054100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Lipshultz LI, Howards SI. Office evaluation of the subfertile male. In: Lipshultz LI, Howards SI, Niederberger CS, editors. Infertility in the Male. Cambridge: Cambridge University Press; 2009. pp. 153–176. [Google Scholar]
- Suryavathi V, Khattri A, Gopal K, Rani DS, Panneerdoss S, Gupta NJ, Chakravarty B, Deenadayal M, Singh L, Thangaraj K. Novel variants in UBE2B gene and idiopathic male infertility. J Androl. 2008;29:564–571. doi: 10.2164/jandrol.107.004580. [DOI] [PubMed] [Google Scholar]
- van Wijk SJ, Timmers HT. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization Laboratory Manual for the Examination Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- WHO. WHO Laboratory Manual for Examination and Processing of Human Semen. 5th edn. 2010. pp. 56–102. Geneva, Switzerland: WHO Press. [Google Scholar]
- Yatsenko AN, Roy A, Chen R, Ma L, Murthy LJ, Yan W, Lamb DJ, Matzuk MM. Non-invasive genetic diagnosis of male infertility using spermatozoal RNA: KLHL10 mutations in oligozoospermic patients impair homodimerization. Hum Mol Genet. 2006;15:3411–3419. doi: 10.1093/hmg/ddl417. [DOI] [PubMed] [Google Scholar]
- Yatsenko AN, Yatsenko SA, Weedin JW, Lawrence AE, Patel A, Peacock S, Matzuk MM, Lamb DJ, Cheung SW, Lipshultz LI. Comprehensive 5-year study of cytogenetic aberrations in 668 infertile men. J Urol. 2010;183:1636–1642. doi: 10.1016/j.juro.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsenko AN, O'Neil DS, Roy A, Arias-Mendoza PA, Chen R, Murthy LJ, Lamb DJ, Matzuk MM. Association of mutations in the zona pellucida binding protein 1 (ZPBP1) gene with abnormal sperm head morphology in infertile men. Mol Hum Reprod. 2012;18:14–21. doi: 10.1093/molehr/gar057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



