Abstract
Interactions of hyaluronan with CD44 in tumor cells play important cooperative roles in various aspects of malignancy and drug resistance. Emmprin (CD147; basigin)is a cell surface glycoprotein of the immunoglobulin superfamily that is highly up-regulated in malignant cancer cells and stimulates hyaluronan production, as well as several downstream signaling pathways. Emmprin also interacts with various monocarboxylate transporters (MCT). Malignant cancer cells use the glycolytic pathway and require MCTs to efflux lactate that results from glycolysis. Glycolysis and lactate secretion contribute to malignant cell behaviors and drug resistance in tumor cells. In the present study, we find that perturbation of endogenous hyaluronan, using small hyaluronan oligosaccharides, rapidly inhibits lactate efflux from breast carcinoma cells; down-regulation of emmprin, using emmprin small interfering RNA, also results in decreased efflux. In addition, we find that CD44 coimmunoprecipitates with MCT1, MCT4, and emmprin and colocalizes with these proteins at the plasma membrane. Moreover, after treatment of the cells with hyaluronan oligosaccharides, CD44, MCT1, and MCT4 become localized intracellularly whereas emmprin remains at the cell membrane. Together, these data indicate that constitutive interactions among hyaluronan, CD44, and emmprin contribute to regulation of MCT localization and function in the plasma membrane of breast carcinoma cells.
Introduction
Emmprin (CD147), a multifunctional glycoprotein and member of the immunoglobulin superfamily, was originally characterized as an inducer of matrix metalloproteinase synthesis and shown to be identical to basigin (1). Emmprin is highly expressed on the surface of numerous types of malignant cancer cells (2), and experimental up-regulation markedly enhances tumor growth and invasion in vivo (3). However, it is now clear that emmprin interacts with a wide range of binding partners (2), including monocarboxylate transporters (MCT; ref. 4). At least 14 members of this family have been cloned and distinguished by their kinetic properties and tissue distribution. MCT1, MCT3, and MCT4 require association with emmprin in the endoplasmic reticulum for trafficking to the plasma membrane. The MCTs transport lactate across the plasma membrane, and emmprin-MCT interaction is required for MCT activity, as well as trafficking to the plasma membrane, in muscle and retinal cells (5–7). Furthermore, MCT activity is fundamental to the glycolytic phenotype that characterizes most malignant cancers, wherein glycolysis is increased even in the presence of oxygen: the so-called “Warburg effect.” Increased glycolysis in cancers is associated with various conditions, such as hypoxia, acidosis, and mitochondrial defects, which result in enhanced drug resistance and malignancy (8–11). An outcome of increased glycolysis is production of lactate, which must be pumped across the plasma membrane via the proton-coupled MCTs to avoid cytotoxic intracellular accumulation of lactate. Lactate efflux at the leading edge of tumor cells acidifies the surrounding microenvironment, which can enhance cell invasion (12), metastasis (13), and drug resistance (11). MCT1 is the most widely expressed member of this family and is elevated in a variety of cancers, including neuroblastoma (14), high-grade gliomas (15), and colorectal carcinomas (9). MCT4 is expressed preferentially in tissues that require high levels of glycolysis. Recently, MCT4 was shown to be colocalized with emmprin in the plasma membrane of metastatic MDA-MB231 breast cancer cells, wherein trafficking of these two proteins to the plasma membrane is mutually interdependent. Moreover, suppressed expression of MCT4 resulted in decreased migratory capacity in these cells, most likely due to inhibition of emmprin function (16).
Studies in our laboratory have revealed that emmprin stimulates production of the extracellular and cell surface–associated polysaccharide, hyaluronan, in breast carcinoma cells (17). In addition to its structural role in extracellular matrix assembly and in hydrodynamic properties of tissues, hyaluronan has an instructive, cell signaling function that is activated in malignant cancer cells (18, 19). Hyaluronan signals through multivalent interaction with several cell surface receptors, with CD44 being the best characterized of these receptors in cancer cells. Variant forms of CD44 with several exon combinations are commonly up-regulated in carcinomas and have been implicated in numerous aspects of cancer progression (20, 21). Many of the prooncogenic, downstream effects of hyaluronan-CD44 interaction are also induced by increased emmprin expression; correspondingly, treatment with antagonists of constitutive hyaluronan-CD44 interactions reverses these effects of emmprin (17, 22, 23). One means of antagonizing hyaluronan-CD44 interactions is treatment with small hyaluronan oligosaccharides (oligomers) that act by competitively replacing endogenous, multivalent hyaluronan polymer with a monovalent ligand (24) or by inhibiting hyaluronan production (25).
Two sets of observations indicate a possible relationship of hyaluronan to MCT activity. The first of these, discussed above, is that emmprin stimulates hyaluronan production and its downstream effects. Second, lactate stimulates hyaluronan synthesis and expression of CD44 variants in fibroblasts (26) and melanoma cells (27), and lactate response elements are present in several hyaluronan-related genes, e.g., CD44 and the hyaluronidase HYAL1 (28). Thus, we have examined the relationship of hyaluronan, CD44, and emmprin to MCT expression and activity in breast carcinoma cells. We have found that antagonizing hyaluronan interactions by treatment with hyaluronan oligomers and knockdown of emmprin with small interfering RNA (siRNA) both result in a decrease in lactate secretion. Furthermore, we found that both emmprin and CD44 form complexes with MCT1 and MCT4 at the plasma membrane and that treatment with hyaluronan oligomers leads to intracellular localization of CD44 and the MCTs. Together, the data indicate that interactions among hyaluronan, CD44, and emmprin contribute to MCT localization and function at the plasma membrane.
Materials and Methods
Cells and reagents
Human breast adenocarcinoma cell lines MCF-7, MDA-MB231, and MDA-MB436 were obtained from American Type Culture Collection. Fetal bovine serum (FBS) was purchased from Atlas Biologicals. RPMI 1640 was purchased from Sigma. The following antibodies were obtained for these studies: MCT1, MCT4, and CD44 (“pan-CD44” antibody: HCAM, DF1485; Santa Cruz Biotechnology), FITC-tagged CD44 (Cedarlane Laboratories), emmprin (BD Pharmingen), CD44v3 (Calbiochem), β-actin (Sigma-Aldrich), goat anti-mouse horseradish peroxidase (HRP) and goat anti-rabbit HRP (Chemicon), and Alexa Fluor 488, 555, 647 (Invitrogen). AlexaFluor phalloidin was also purchased from Invitrogen. DRAQ5 nuclear stain was obtained from Biostatus Limited. Western blotting detection reagent (enhanced chemiluminescence) was purchased from Pierce. Hyaluronan oligosaccharides (oligomers) used in this study were a mixed fraction of average molecular weight ~2.5 × 103 composed of 3 to 10 disaccharide units that were fractionated from testicular hyaluronidase (Sigma, type 1-S) digests of hyaluronan polymer (Sigma, sodium salt); fractionation was by trichloroacetic acid precipitation followed by serial dialysis, using membranes of Amicon Ultra Ultracel 5,000 MWCO (Millipore) and Spectra/Por Membrane 1,000 MWCO (Spectrum Laboratories). All other chemicals were of reagent grade or higher.
Cell culture
MCF7, MDA-MB231, and MDA-MB436 cells were cultured in RPMI 1640 (R-8755) with 2.38 g/L HEPES, 2 g/L sodium bicarbonate, and 10% FBS (pH 7.4) and maintained at 37°C in a humidified 5% CO2-95% air incubator. For preparation of lysates from oligomer-treated cells, cells were seeded in 10-cm dishes 48 h before oligomer treatment. For preparation of confocal microscopy slides from cells treated with oligomer, cells were seeded in eight-well multichamber culture slides (BD Biosciences) 48 h before oligomer treatment.
siRNA silencing
At 24 h after plating, cells were transfected with control or emmprin siRNA (Santa Cruz Biotechnologies) according to the manufacturer’s recommendations in serum-free medium for 24 h before cell fixation or lactate efflux analysis.
Immunoprecipitation and immunoblot analysis
After a 1-h incubation with hyaluronan oligomers, whole-cell lysates were prepared for immunoprecipitation or direct immunoblotting using a modified immunoprecipitation assay buffer provided with the Catch and Release immunoprecipitation kit (Upstate Biotechnology) modified to contain 1 mmol/L phenylmethylsulfonyl fluoride, 10 µg/mL aprotinin and leupeptin, 2 mmol/L sodium orthovanadate, and 10 mmol/L sodium fluoride. Protein content was determined by bicinchoninic acid assay (Pierce), and samples containing 50 µg or 500 µg of protein were solubilized in SDS sample buffer or used in immunoprecipitation, respectively. Immunoprecipitation was carried out with the Catch and Release immunoprecipitation kit, according to the manufacturer’s specifications, with resultant bound protein solubilized in SDS sample buffer. Samples so collected were resolved on Pierce 4% to 20% reducing polyacrylamide gels, transferred to nitrocellulose (Osmonics) with a Pierce apparatus, and subject to immunoblot analysis. Immunoblots were blocked, probed, and visualized, as previously described (23), with antibodies as indicated.
Lactate efflux analysis
The XF Extracellular Flux Analyzer (Seahorse Bioscience) is a 24-well instrument that continuously measures the uptake and excretion of metabolic end products. The XF analyzer contains a sensor cartridge, embedded with 24 pairs of fluorescent biosensors (O2 and pH), which monitor substrate concentration changes by a fiber optic waveguide. Lactate secretion was measured by analysis of extracellular acidification rate (ECAR), which is caused by proton release and primarily reflects lactate excretion (29) when repeated measurements are taken over a period of time, i.e., ~1 h (Seahorse Biosciences).
Cells were seeded in XF24 cell culture microplates (Seahorse Biosciences) at 5.0 to 6.0 × 104 per well in 100 AL serum medium and incubated at 37°C in 5% CO2 for 24 h. The assay is initiated by aspirating the serum medium, replacing it with 700 to 900 µL of prewarmed (37°C) assay medium (DMEM without bicarbonate) containing 5 mmol/L glucose and 100 nmol/L insulin, and then incubating the plate at 37°C. The assay medium is mixed by the XF24 analyzer every 3 to 5 min to restore normal oxygen tension and pH in the wells. After baseline ECAR measurements are taken, 70 to 100 AL of a test compound prepared in assay media are injected into each well to final working concentration. After injection, the solution in the wells is mixed, after which the pH and O2 tension are measured every 22 s for ~2 min, during which time the O2 tension drops ~30 mm Hg and the pH of the medium declines up to 0.4 pH units. ECAR is calculated from the slope of pH versus time, and it is reported in milli-pH per minute.
Confocal microscopy
Cells plated at low density on tissue culture-treated glass multichamber culture slides (BD Falcon) and treated as indicated were fixed with 3% paraformaldehyde for 10 min at 23°C, permeabilized by treatment with PBS containing 0.02% Triton X-100 for 10 min at 23°C, and blocked for nonspecific binding by incubation for 12 h at 4°C with PBS–3% bovine serum albumin (BSA). Cells were incubated with antibodies as indicated in PBS–3% BSA overnight at 4jC. After three 5-min washes with PBS, cells were incubated with AlexaFluor (Molecular Probes) secondary antibodies and Draq5 nuclear stain (Biostatus Limited) or AlexaFluor phalloidin (Molecular Probes) for 2 h. After three additional 5-min washes with PBS, mounting medium (Biomeda Corp.) was added and coverslips were applied. Slides were viewed on a Leica Total Confocal System, Spectral Prism 2, Acoustic Optical Beam Splitter (TCS SP2 AOBS) in the Molecular Morphology and Imaging Center in the Department of Cell Biology and Anatomy.
Results
Inhibition of lactate efflux by emmprin siRNA and by hyaluronan oligomers
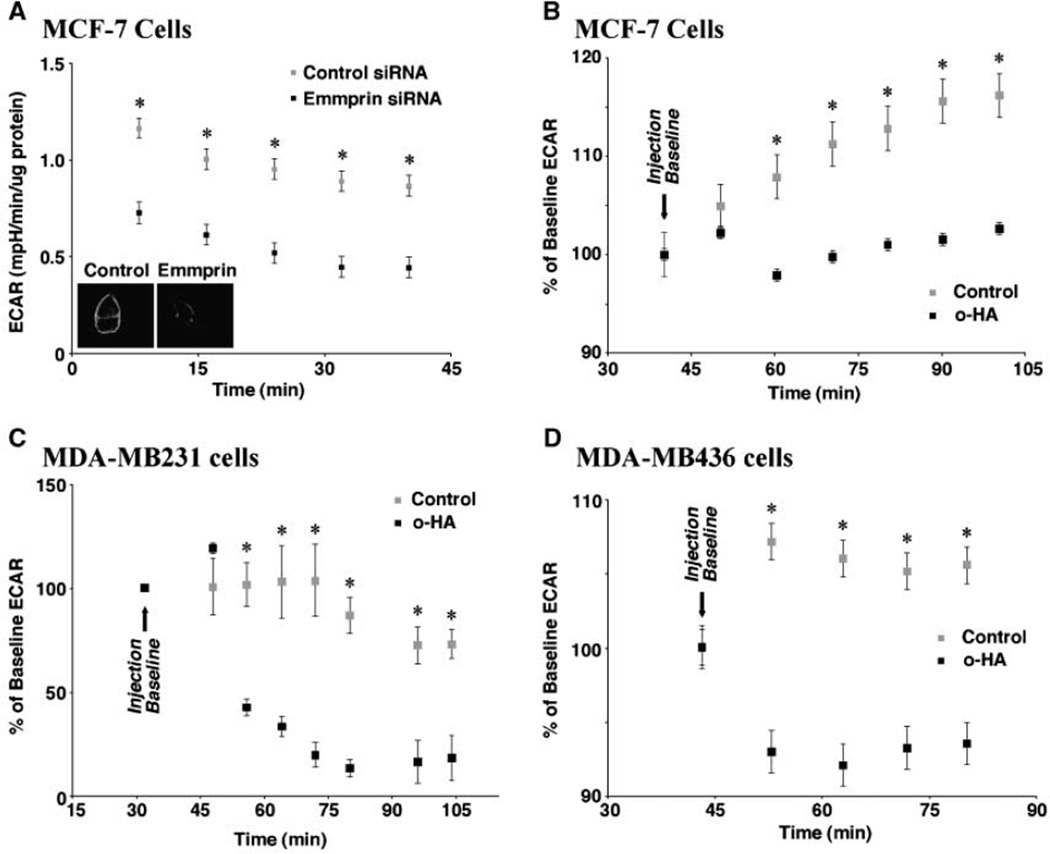
As discussed in Introduction, emmprin is a chaperone or cofactor for MCT1, MCT3, and MCT4 and is essential for their trafficking to the plasma membrane and for their function in various nontumor cell types. Emmprin-MCT interactions also have been shown in human breast carcinoma cells (16). Moreover, interaction of emmprin with MCT4 promotes expression of both protein partners at the plasma membrane of these cells (16). We show here that emmprin is also essential for MCT function in breast carcinoma cells in that siRNA knockdown of emmprin inhibits lactate efflux, as measured by analysis of ECAR (Fig. 1A).
Figure 1.
Inhibition of lactate efflux by emmprin siRNA and hyaluronan oligomers. A, emmprin is necessary for lactate efflux. MCF7 human breast carcinoma cells were grown to 70%confluence , transfected for 24 h with control or emmprin siRNA, and subject to ECAR analysis as described in Materials and Methods. Equal cell density was adjusted according to µg protein per well. The inset shows the decreased level of emmprin associated with cells treated with emmprin siRNA (right) compared with control siRNA (left); emmprin was visualized by immunolabeling. B–D, hyaluronan oligomers decrease lactate efflux. MCF-7 (B), MDA-MB231 (C), and MDA-MB436 (D) human breast carcinoma cells were grown to 70%confluence. After baseline acquisition, cells were treated with 100 µg/mL hyaluronan oligomers (o-HA) and subject to ECAR analysis, as described in Materials and Methods. Error bars, SDs. Significant differences were observed (*, P < 0.05, compared with control). A–D, representative of three or more independent experiments.
Because emmprin and hyaluronan activities interact at many levels, we investigated whether an antagonist of constitutive hyaluronan interactions also affects lactate efflux. Small hyaluronan oligomers are competitive inhibitors of polymeric hyaluronan-CD44 interaction (24) and attenuate constitutive hyaluronan-CD44–induced signaling (23, 30). In addition, they have been found to inhibit hyaluronan production (25). We found that treatment with hyaluronan oligomers inhibits lactate efflux in three different human breast carcinoma cell lines. This inhibition occurred as quickly as within 10 to 20 minutes of treatment and continued for the 1-hour to 2-hour duration of the experiment (Fig. 1B–D).
Interaction of CD44 with emmprin, MCT1, and MCT4
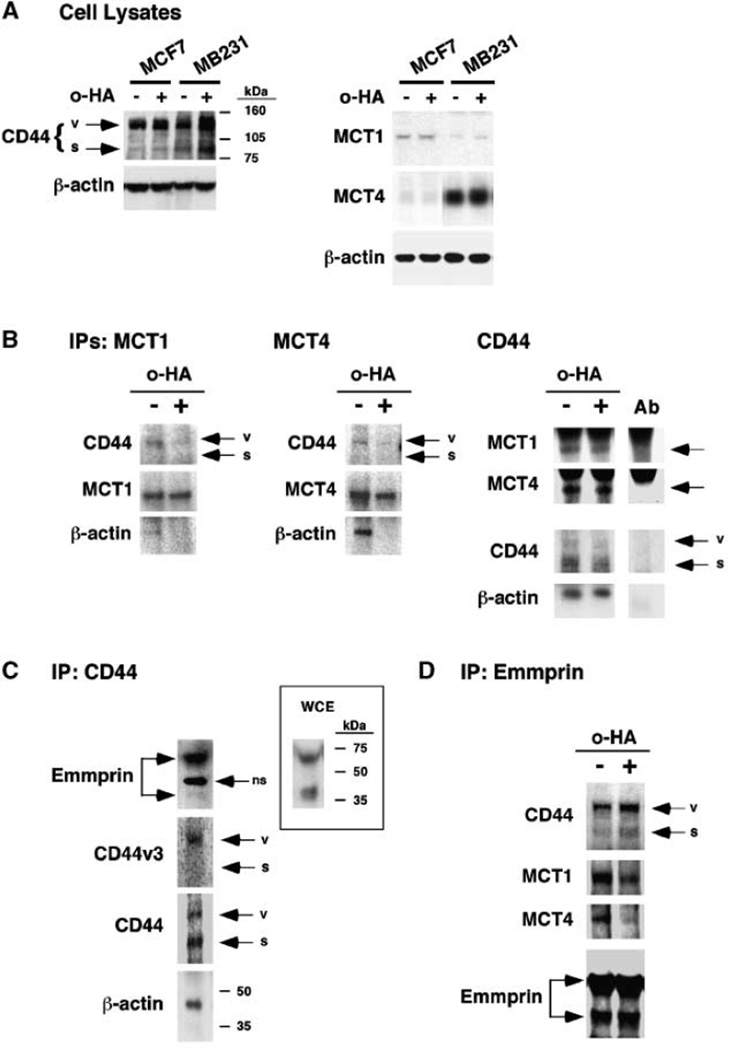
The functional results shown in Fig. 1 suggest that constitutive hyaluronan-CD44 interactions influence MCT activity. Therefore, it is possible that CD44, as well as emmprin, may interact directly or indirectly with one or more of the MCTs. To begin investigating this possibility, we analyzed lysates of MCF-7 and MDA-MB231 cells for the presence of CD44 and MCTs. Both cell types express standard (~85 kDa) and variant (~130 kDa) forms of CD44 (Fig. 2A, left). We chose to examine MCT1 and MCT4 because they are the most common MCTs found in cancer cells and they are known to interact with emmprin. MB231 cells express both MCT1 and MCT4, although the latter is predominant as found previously for these cells (16); MCF-7 cells express mainly MCT1 (Fig. 2A, right). We also examined the effect of treatment of the cells with hyaluronan oligomers on expression of CD44 and the MCTs, but no significant effects were obtained (Fig. 2A).
Figure 2.
CD44 forms complexes with MCTs and emmprin. A, MCF-7 and MDA-MB231 cells express CD44 and MCTs. Cells were grown to 70%conf luence (48 h). After treatment with and without 100 µg/mL hyaluronan oligomers for 1 h, whole-cell extracts were prepared, as described in Materials and Methods, and 50 µg lysates were resolved on a 4%to 20% SDS-gel and processed for Western blot analysis, as described in Materials and Methods. Left, MCF-7 and MB231 cells express standard CD44 (s, molecular weight of ~85 kDa) and CD44 splice variants (v, molecular weight of ~130 kDa). Right, MCF-7 cells express predominantly MCT1; MB231 cells express predominantly MCT4. Multiple experiments showed that treatment of the cells with hyaluronan oligomers (o-HA) had no significant effect on MCT1 or MCT4 expression. B, CD44 forms complexes with MCT1, MCT4, and β-actin. Whole-cell extracts were prepared identically to the blots in A from MB231 cells that had been pretreated with and without 100 µg/mL hyaluronan oligomers for 1 h. The extracts were immunoprecipitated (IP) with antibodies to MCT1 (left), MCT4 (middle), or CD44 (“pan-CD44”; right), then processed for Western blotting as indicated. Multiple experiments showed that mainly variant CD44 coimmunoprecipitated with the MCTs and that treatment with hyaluronan oligomers reproducibly reduced the amount of this CD44 in the MCT immunoprecipitates (left and middle). Note that standard CD44 was immunoprecipitated preferentially with the “pan-CD44” antibody (right), although more variant CD44 than standard is present in these extracts (A, left); also note that cross-reaction occurred between MCT secondary antibodies and this “pan-CD44” antibody (lane Ab). The hyaluronan oligomers decreased interaction of actin with the MCTs (left and middle) but not with CD44 (right). C, CD44 interacts with emmprin. MB231 cells were treated, and whole-cell extracts immunoprecipitated with antibody against CD44 as in B, then processed for immunoblotting as indicated. Although less variant CD44 than standard CD44 was immunoprecipitated, the ~130-kDa variant form included the v3 exon product. Emmprin, mainly the high molecular form, was coimmunoprecipitated with CD44 (ns, nonspecific band). The immunoblot in the inset shows high and low molecular forms of emmprin in a whole cell extract (WCE) from nontreated cells. D, emmprin forms complexes with MCTs and CD44. Whole-cell extracts from MB231 cells, prepared as in A, were immunoprecipitated with an antibody to emmprin. Mainly variant CD44 was coimmunoprecipitated with emmprin. Hyaluronan oligomer treatment of the cells decreased emmprin-MCT interaction but did not affect emmprin-CD44 interaction significantly. A–D, representative of three or more independent experiments.
To determine whether CD44 interacts with MCT1 or MCT4, we performed immunoprecipitations from lysates of MDA-MB231 cells using antibodies against MCT1 and MCT4, then immunoblotted with antibody against CD44. In both cases, we observed a single band of CD44 (Fig. 2B, left and middle) that corresponds with the ~130 kDa variant form that was observed in immunoblots of whole-cell lysates (Fig. 2A); although the amounts of CD44 that coimmunoprecipitated were relatively small, they were consistent in several experiments. In addition, we found that MCT1 and CD44, mainly variant CD44, coimmunoprecipitate from MCF-7 cell lysates on treatment with antibody against MCT1 (data not shown). We also performed immunoprecipitations with antibody against CD44 and then immunoblotted for MCT1 and MCT4. Both MCTs were found in the CD44 immunoprecipitates (Fig. 2B, right). It should be noted, however, that the “pan-CD44” antibody used here preferentially immunoprecipitates standard CD44 (Fig. 2B, right), although it clearly recognizes variants of CD44 in immunoblots (Fig. 2A, left). In separate experiments, we immunoblotted with antibody raised against the CD44v3 exon product, as well as with the “pan-CD44” antibody. We found v3 to be present within the same region of the gel as the ~130-kDa form of CD44 (Fig. 2C), thus confirming that this band does contain variant CD44; a preference for standard CD44 was again observed with the “pan-CD44” antibody. These experiments indicate that CD44 forms complexes directly or indirectly with MCT1 and MCT4 in breast carcinoma cells. However, because only a small proportion of total CD44 seems to immunoprecipitate with the MCTs (Fig. 2B, left and middle), it is likely that the MCTs interact with a subfraction of CD44, most likely a variant form.
As expected from previous studies (31, 32), both cell types express high and low molecular weight forms of emmprin that correspond to high and low glycosylated forms, respectively (MB231, Fig. 2C, inset; MCF-7, not shown). Previous evidence indicates that emmprin interacts directly with MCT1 and MCT4, and our data above suggest that CD44 also interacts in some way with these MCTs. Accordingly, we found here that emmprin and CD44 coimmunoprecipitate from lysates of MB231 cells, whether using antibody against CD44 (Fig. 2C) or emmprin (Fig. 2D). In the former case, the emmprin in the immunoprecipitate is mainly the high molecular weight, glycosylated form; in the latter case, CD44 in the immunoprecipitate is mainly variant CD44.
Previous work in our laboratory has shown that signaling complexes containing receptor tyrosine kinases and CD44 are dissociated by treatment of cells with antagonists of hyaluronan- CD44 interaction, such as hyaluronan oligomers (23, 33). Thus, we treated MB231 cells with hyaluronan oligomers to determine whether interactions between CD44 and MCT1 or MCT4 are also influenced constitutively by hyaluronan. We found that immunoprecipitates of MCT1 or MCT4 from lysates of cells that had been pretreated with hyaluronan oligomers for 1 hour contained less CD44 than immunoprecipitates from lysates of untreated cells (Fig. 2B, left and middle). It also seemed that somewhat lower amounts of MCT1 and MCT4 were present in immunoprecipitates obtained with antibody against CD44 from lysates of oligomer-treated cells than untreated cells (Fig. 2B, right), but interference from cross-reaction with the CD44 antibody prevented clear-cut interpretation of these gels. However, immunoprecipitation with emmprin antibody clearly yielded lower amounts of MCTs from lysates of oligomer-treated cells than controls, although coimmunoprecipitation of CD44 with emmprin was not significantly affected (Fig. 2D). These results support the possibility that the hyaluronan oligomers interfere with CD44-MCT interactions in some manner.
It is well known that CD44 interacts with the actin cytoskeleton (19). Therefore, we also examined the interaction of β-actin with the MCTs and the effects of treatment with hyaluronan oligomers. We found that β-actin is present in immunoprecipitates of MCT1 and MCT4 but pretreatment of the cells with hyaluronan oligomers reduced this interaction (Fig. 2B, left and middle). However, oligomer treatment of the cells did not inhibit interaction of CD44 with β-actin (Fig. 2B, right). These experiments indicate that the interaction of MCT1 and MCT4 with β-actin is most likely mediated by CD44-actin interaction, but the latter interaction is not affected by hyaluronan-CD44 interaction.
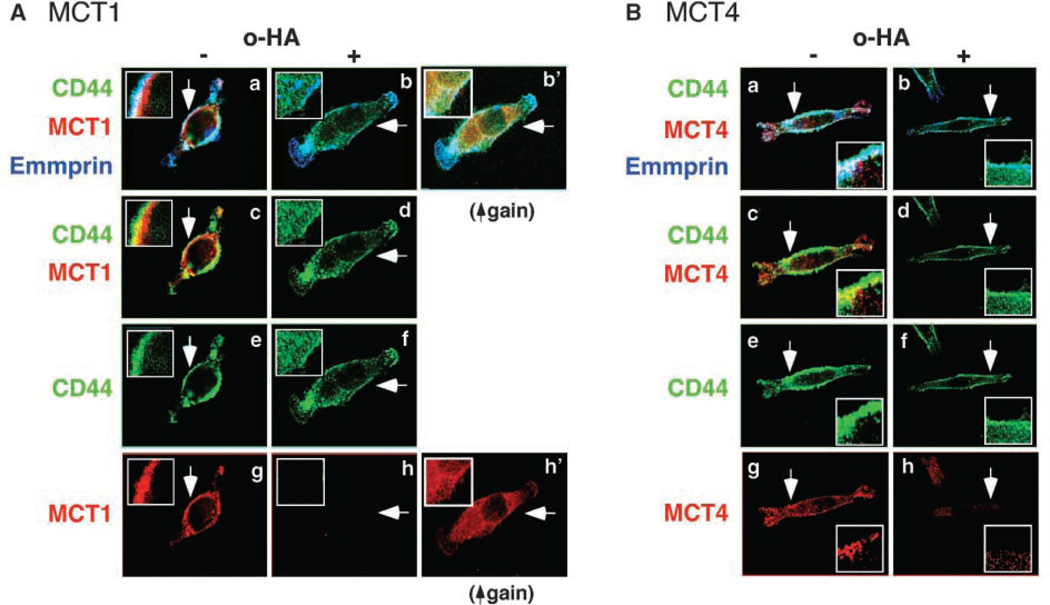
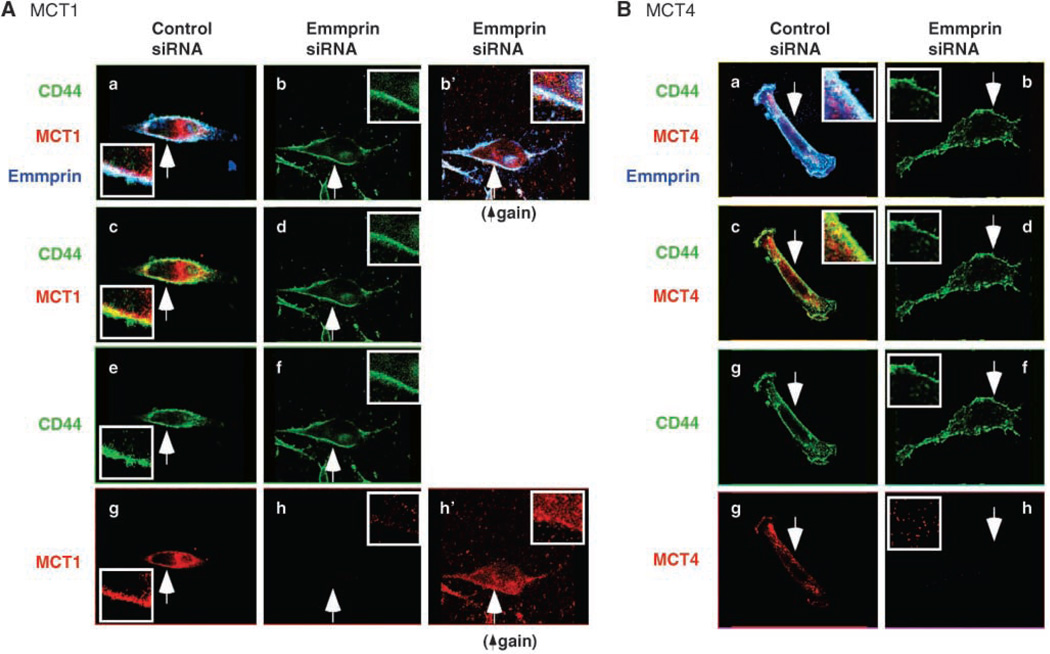
To further explore the associations between CD44, emmprin, and the MCTs, we examined the localization of each protein by confocal microscopy. Colocalization of CD44 (green), MCT1 (red), and emmprin (blue) was observed at the membrane of MDA-MB231 cells (Fig. 3A, a and c) and MCF-7 cells (data not shown). Similar colocalization of CD44 and emmprin with MCT4 was also observed in MB231 cells (Fig. 3B, a and c). These results confirm that interactions between CD44, emmprin, and the MCTs occur at the surface of breast carcinoma cells.
Figure 3.
CD44 and emmprin colocalize with MCT1 and MCT4 at the plasma membrane in a hyaluronan-dependent manner. A, MCT1; B, MCT4. MB231 cells were treated with and without 100 µg/mL hyaluronan oligomers for 1 h. After fixation, permeabilization, and blocking of nonspecific binding, the cells were immunolabeled for CD44 (green), MCT1 or MCT4 (red), and emmprin (blue) and visualized by confocal microscopy at a Z plane corresponding to the approximate center of the cell. Arrows, areas shown at higher magnification in the insets. a, b, and b’, triple labeling for all three components; colocalization appears as a white signal. Triple colocalization is clearly seen in the plasma membrane of the untreated cells but not in the membrane of oligomer-treated cells. Increased intracellular staining for CD44 is seen in the oligomer-treated cells in b. b’ in A is the same as in b, but is shown at higher gain than in a and b, to illustrate internalization of MCT1, as well as CD44; increased gain is necessary for clear visualization because the MCT becomes highly dispersed in the cytoplasm after oligomer treatment. Similar oligomer-induced internalization was observed for MCT4 (not shown). Emmprin seems to remain at the cell surface after hyaluronan oligomer treatment. c and d, double staining for CD44 and MCT1 (A) or MCT4 (B), further illustrating their colocalization (yellow signal in c) and hyaluronan oligomer-induced internalization of CD44 (d). e and f, CD44 only; g and h, the MCTs only. Increased gain revealed intracellular dispersion of MCT1 (A, h’) and MCT4 (not shown). The figure is representative of three or more independent experiments.
Internalization of CD44 and MCTs by treatment of cells with hyaluronan oligomers
Although confocal microscopy showed that CD44 colocalizes with MCT1 and MCT4 (Fig. 3) and treatment with hyaluronan oligomers had a rapid and strong effect on MCT function (Fig. 1), the effects of the oligomers on coimmunoprecipitation of CD44 and the MCTs were not always definitive (e.g., Fig. 2B, right). Nevertheless, these immunoprecipitation experiments did suggest that treatment with hyaluronan oligomers may decrease interactions between CD44 and the MCTs within a subpopulation associated with emmprin (Fig. 2D). Therefore we examined the cellular distribution of CD44, emmprin, and the MCTs after treatment of MB231 cells with hyaluronan oligomers. We found that this treatment induces dissociation of the MCTs from cell surface CD44 and emmprin, as well as dispersion of the MCTs and CD44 throughout the cytoplasm (Fig. 3A and B, b, d, f, and h); because of this dispersion, increased gain is necessary to facilitate visualization of the MCTs (Fig. 3A, b’ and h’). The intracellular CD44 and the MCTs seem to be within vesicles and exhibit decreased colocalization.
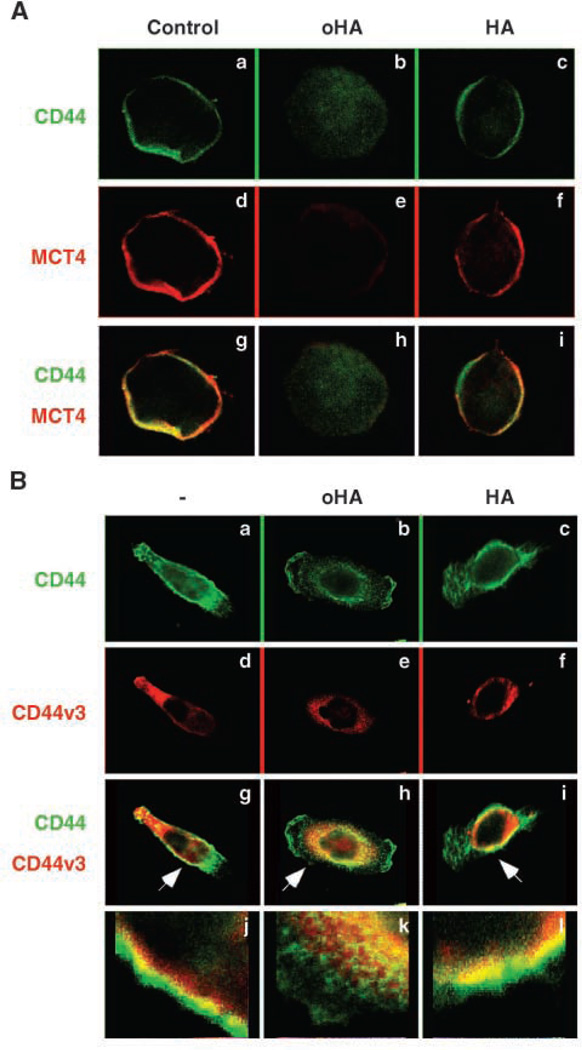
As a control, we compared the effect of hyaluronan oligomers with that of the hyaluronan polymer from which the oligomers were prepared. Whereas treatment with the oligomers induced internalization of CD44 and MCT4, the hyaluronan polymer had no discernible effects (Fig. 4A). In addition, we analyzed the effects of hyaluronan oligomers and polymer on v3-containing variant CD44 and found that the oligomers, but not the polymer, induced internalization (Fig. 4B). Double staining suggested that the v3-containing variant may be preferentially internalized (Fig. 4B, h and k).
Figure 4.
Hyaluronan oligomers, but not hyaluronan polymer, induce internalization of CD44, CD44v3, and MCT4. A, MB231 cells were treated for 1 h with and without 100 µg/mL hyaluronan oligomer (oHA) or polymer (HA). After fixation, permeabilization, and blocking of nonspecific binding, the cells were immunolabeled for CD44 (green) and MCT4 (red) and visualized by confocal microscopy at a Z plane corresponding to the center of the cell. Treatment with hyaluronan oligomer, but not polymer, results in internalization of CD44 and MCT4 from the plasma membrane. B, MB231 cells were treated and processed as in A but were immunolabeled for CD44 (green; a, b, c) and CD44v3 (red; d, e, f). g, h, i, double labeling. Arrows, areas shown at higher magnification in j, k, and l . CD44, including CD44v3, is present at the plasma membrane in control and hyaluronan polymer-treated cells. CD44v3 seems to be internalized preferentially in response to hyaluronan oligomer treatment (h, k, and b versus e). Representative of three or more independent experiments.
As opposed to CD44 and the MCTs, emmprin was not lost from the cell membrane to a significant degree in response to oligomer treatment (Fig. 3A and B, b). Thus we examined the effects of transfection with emmprin siRNA on localization of CD44 and the MCTs. As previously reported, knockdown of emmprin expression prevents trafficking of the MCTs to the plasma membrane and leads to their degradation (16); we confirmed this decrease in MCT1 and MCT4 at the plasma membrane in our study (Fig. 5A and B). Interestingly, however, emmprin knockdown did not cause significant change in localization of CD44 (Fig. 5A and B, b, d, and f).
Figure 5.
Emmprin siRNA inhibits membrane localization of MCT1 and MCT4 but not CD44. A, MCT1; B, MCT4. MB231 cells were transfected with emmprin siRNA and then processed in the same way as in Fig. 3. A and B, a and b (and b’) are triple labeled for CD44 (green), MCT1 or MCT4 (red), and emmprin (blue); c and d are double labeled for CD44 and MCT. Arrows, areas shown at higher magnification in the insets. Note that the levels of emmprin are highly reduced after treatment with emmprin siRNA (b) compared with control siRNA (a). a and c show colocalization in a similar fashion to that shown in Fig. 3. e and f show CD44 only; g and h show the MCTs only. b and d show that CD44 is not significantly internalized after knockdown of emmprin, in contrast to the effect of hyaluronan oligomers shown in Fig. 3. As explained in Fig. 3, increased gain (A, b’ and h’) is necessary to show dispersion of MCT1 in the cytoplasm; a similar result was obtained for MCT4 (not shown). The increased gain used in b’ also shows that emmprin was not completely eliminated by siRNA treatment. The figure is representative of three or more independent experiments.
To confirm that internalization of CD44 in response to treatment with hyaluronan oligomers was an active cellular process, we examined this effect in live cells by adding Alexa 488–conjugated CD44 antibody to the medium an hour before fixation. After fixation, actin filaments were stained with phalloidin. As in the previous experiments, wherein antibody staining was performed after fixation, we observed that CD44 became localized within the cell after oligomer treatment (Supplementary Fig. S1b versus a). Furthermore, we observed colocalization of CD44 with actin in the oligomer-treated cells, as well as in the untreated cells (Supplementary Fig. S1c and d). This agrees with our immunoprecipitation results showing that β-actin remains attached to CD44 after treatment of cells with hyaluronan oligomers (Fig. 2B, right). Consistent with a role for actin in these events, we found that treatment with latrunculin, an inhibitor of actin polymerization (34), causes clustering of CD44 at the membrane (Supplementary Fig. S1e) and decreases oligomer-induced internalization of CD44 (Supplementary Fig. S1f). In separate experiments, wherein cells were fixed and stained in routine fashion, we found that MCT1 (Supplementary Fig. S1) and MCT4 (data now shown) also remained clumped at the cell surface, along with CD44, after latrunculin treatment (Supplementary Fig. S1g) or latrunculin plus oligomer treatment (Supplementary Fig. S1h).
Discussion
An almost universal property of malignant cancers is increased glycolysis, which is detected clinically with positron emission tomography by increased uptake of the glucose analogue tracer 18fluorodeoxyglucose in many cancers (8). Fluctuations in hypoxia most likely lead to increased glycolysis during early stages of tumor progression, and subsequent adaptations may provide survival advantages to cancer cells (8). Hypoxia, acidosis, and mitochondrial defects that are associated with increased glycolysis result in enhanced malignancy and drug resistance (8–13). An important outcome of increased glycolysis is lactate production, and lactate efflux via MCTs is essential to survival of glycolytic cells.
Trafficking of commonly expressed MCTs to the cell surface and their lactate efflux function at the cell surface are dependent on interaction with emmprin (4, 16). Conversely, emmprin may require interaction with MCTs for its stable expression at the cell surface and, therefore, its activity as an inducer of matrix metalloproteinases and invasiveness (16). We have found that emmprin stimulates anchorage-independent growth (17), drug resistance (22), and ErbB2 activation (23) in a hyaluronan-dependent manner. Thus, we have examined relationships of hyaluronan-CD44 interaction, as well as emmprin to MCT1 and MCT4 localization and function.
We find that CD44 forms complexes with MCT1, MCT4, and emmprin at the surface of human breast carcinoma cells and that these interactions most likely involve a variant form of CD44. However, further work is required to clarify the specific involvement of CD44 variants. Perturbation of hyaluronan interactions by treating the cells with small hyaluronan oligomers inhibits lactate secretion. Oligomer treatment also leads to internalization of the hyaluronan receptor, CD44, implying that the oligomers act via disruption of constitutive hyaluronan polymer-CD44 interaction (24). Likewise, the oligomers cause MCT1 and MCT4 to become dissociated from cell surface CD44 and emmprin and to be localized intracellularly. These results suggest that constitutive hyaluronan-CD44 interaction stabilizes complexes containing CD44, emmprin, and the MCTs at the plasma membrane. It is also possible that the oligomers prevent initial assembly of these complexes and that the loss of MCTs from the cell surface is due to turnover. However, the latter alternative seems less likely because the effects of the oligomers on function take place within 10 to 20 minutes and the effects on internalization within an hour. Previously, we found a similar role for hyaluronan-CD44 interaction in assembly or stabilization of complexes containing CD44, receptor tyrosine kinases, and other signaling components (23, 33). Other investigators have also documented analogous relationships between hyaluronan-CD44 interaction and receptor tyrosine kinases (35–38), the Na(+)-H(+) exchanger 1 (39) and the ATP-binding cassette family drug transporter, P-glycoprotein (40, 41). It seems then that hyaluronan- CD44 interaction has a wide-ranging effect on numerous membrane-bound complexes. It remains to be determined whether this is due to unique interactions with specific subfractions of CD44, such as different CD44 variants, more general interactions with membrane compartments, such as lipid rafts, or global effects of a hyaluronan-based pericellular matrix that influences mechanocellular signaling interactions.
It should be emphasized that constitutive cell surface hyaluronan not only tethers the pericellular matrix to the cell surface but also forms complexes with numerous other factors that may play a role in stabilizing membrane-bound complexes (42). Of interest also is the presence of thin plasma membrane protrusions within hyaluronan-dependent pericellular matrices and their stabilization by one another (43). These microvilli-like protrusions are enhanced by overexpression of hyaluronan and destroyed by treatment with hyaluronidase or disruption of actin filaments. They contain lipid rafts and, thus, may represent sites of assembly of signaling complexes. However, although these structures incorporate CD44, their structure is not dependent on CD44 and is not perturbed by treatment with hyaluronan oligomers (43). Nevertheless, perturbation of pericellular matrices, either with hyaluronan oligomers or by other means, may lead to destabilization and internalization of matrix components themselves, as well as associated membrane complexes. For example, protease-generated fragments of aggrecan that are complexed with hyaluronan, but not complexes of intact aggrecan with hyaluronan, are internalized via hyaluronan-CD44 interaction in chondrocytes (44). Further investigation of the relationships of pericellular matrix composition and perturbation to plasma membrane–bound complex formation and stability is needed to clarify these issues.
In addition to the role of hyaluronan-CD44 interactions discussed above, it is apparent that emmprin cooperates in regulating the localization, interactions, and activities of the MCTs and other cell surface complexes. In this study, we found that treatment with siRNA against emmprin had similar effects on lactate secretion as the hyaluronan oligomers. In addition, we showed that emmprin and CD44 coimmunoprecipitate and are colocalized with one another and with the MCTs. However, although knockdown of emmprin led to loss of MCTs from the membrane, it did not cause a corresponding loss of CD44. This and other observations imply that emmprin knockdown and treatment with hyaluronan oligomers act via different pathways. Emmprin knockdown has been shown in previous studies to inhibit trafficking of MCTs to the plasma membrane (16), whereas the oligomers most likely induce internalization of membrane-bound complexes by perturbing CD44 interactions with the MCTs. CD44 is directly linked to the actin cytoskeleton via factors such as ezrin and ankyrin (19), and this relationship may underlie the failure of hyaluronan oligomer-induced internalization of CD44 after treatment with latrunculin. However, perturbing the actin cytoskeleton disturbs many cellular pathways, including endocytic mechanisms (45). Also, endocytosis of CD44 seems to occur by an unusual pathway that does not involve clathrin-coated pits or caveolae (46) and is regulated by palmitoylation-dependent association with lipid rafts (47). Further work is clearly necessary to establish the relationship of actin to internalization of CD44 and associated macromolecules.
In summary, we have shown here that both CD44 and emmprin interact with MCT1 and MCT4 at the plasma membrane of malignant breast carcinoma cells. Perturbation of hyaluronan- CD44 interaction or down-regulation of emmprin destabilize or inhibit assembly of MCT1 and MCT4 complexes in the plasma membrane and, consequently, suppress lactate efflux. These data indicate that interactions among hyaluronan, CD44, and emmprin contribute to the glycolytic phenotype of cancer cells, as well as to other malignant properties described previously (18, 19).
Supplementary Material
Acknowledgments
Grant support: NIH grants R01 CA073839 and R01 CA082867, Charlotte Geyer Foundation award (B.P. Toole), and NIH Clinical and Translational Sciences award (B.L. Maria, B.P. Toole, and C. Beeson).
We thank Gyda Beeson for expert technical advice.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
B.P. Toole is an inventor on a patent related to this article. The other authors disclosed no potential conflicts of interest.
References
- 1.Biswas C, Zhang Y, DeCastro R, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- 2.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost. 2005;93:199–204. doi: 10.1160/TH04-08-0536. [DOI] [PubMed] [Google Scholar]
- 3.Zucker S, Hymowitz M, Rollo EE, et al. Tumorigenic potential of extracellular matrix metalloproteinase inducer (EMMPRIN) Am J Pathol. 2001;158:1921–1928. doi: 10.1016/S0002-9440(10)64660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 5.Kirk P, Wilson MC, Heddle C, et al. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 2003;44:1305–1311. doi: 10.1167/iovs.02-0552. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MC, Meredith D, Fox JE, et al. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: the ancillary protein for the insensitive MCT2 is EMBIGIN (gp70) J Biol Chem. 2005;280:27213–27221. doi: 10.1074/jbc.M411950200. [DOI] [PubMed] [Google Scholar]
- 8.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 9.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 10.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 11.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Zaguilan R, Seftor EA, Seftor RE, et al. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 13.Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang J, Quinones QJ, Holman TL, et al. The H+-linked monocarboxylate transporter (MCT1/SLC16A1): a potential therapeutic target for high-risk neuroblastoma. Mol Pharmacol. 2006;70:2108–2115. doi: 10.1124/mol.106.026245. [DOI] [PubMed] [Google Scholar]
- 15.Froberg MK, Gerhart DZ, Enerson BE, et al. Expression of monocarboxylate transporter MCT1 in normal and neoplastic human CNS tissues. Neuroreport. 2001;12:761–765. doi: 10.1097/00001756-200103260-00030. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 17.Marieb EA, Zoltan-Jones A, Li R, et al. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004;64:1229–1232. doi: 10.1158/0008-5472.can-03-2832. [DOI] [PubMed] [Google Scholar]
- 18.Toole BP, Slomiany MG. Hyaluronan, CD44 and Emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–121. doi: 10.1016/j.drup.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 21.Hill A, McFarlane S, Johnston PG, Waugh DJ. The emerging role of CD44 in regulating skeletal micrometastasis. Cancer Lett. 2006;237:1–9. doi: 10.1016/j.canlet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multi-drug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278:25285–25288. doi: 10.1074/jbc.C300173200. [DOI] [PubMed] [Google Scholar]
- 23.Ghatak S, Misra S, Toole BP. Hyaluronan regulates constitutive ErbB2 phosphorylation and signal complex formation in carcinoma cells. J Biol Chem. 2005;280:8875–8883. doi: 10.1074/jbc.M410882200. [DOI] [PubMed] [Google Scholar]
- 24.Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–26975. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 25.Misra S, Obeid LM, Hannun YA, et al. Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem. 2008;283:14335–14344. doi: 10.1074/jbc.M703811200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 27.Rudrabhatla SR, Mahaffey CL, Mummert ME. Tumor microenvironment modulates hyaluronan expression: the lactate effect. J Invest Dermatol. 2006;126:1378–1387. doi: 10.1038/sj.jid.5700255. [DOI] [PubMed] [Google Scholar]
- 28.Formby B, Stern R. Lactate-sensitive response elements in genes involved in hyaluronan catabolism. Biochem Biophys Res Commun. 2003;305:203–208. doi: 10.1016/s0006-291x(03)00723-x. [DOI] [PubMed] [Google Scholar]
- 29.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Ghatak S, Misra S, Toole BP. Hyaluronan oligosaccharides inhibit anchorage-independent growth of tumor cells by suppressing the phosphoinositide 3-kinase/Akt cell survival pathway. J Biol Chem. 2002;277:38013–38020. doi: 10.1074/jbc.M202404200. [DOI] [PubMed] [Google Scholar]
- 31.Guo H, Zucker S, Gordon MK, Toole BP, Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24–27. [PubMed] [Google Scholar]
- 32.Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15:4043–4050. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- 34.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin-A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 35.Bourguignon LY, Zhu H, Zhou B, et al. Hyaluronan promotes CD44v3-2 interaction with Grb2-185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- 36.Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci. 2002;9:282–288. doi: 10.1054/jocn.2001.1063. [DOI] [PubMed] [Google Scholar]
- 37.Wobus M, Rangwala R, Sheyn I, et al. CD44 associates with EGFR and erbB2 in metastasizing mammary carcinoma cells. Appl Immunohistochem Mol Morphol. 2002;10:34–39. doi: 10.1097/00129039-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Asteriou T, Bernert B, Heldin CH, Heldin P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem J. 2007;404:327–336. doi: 10.1042/BJ20061757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 40.Miletti-Gonzalez KE, Chen S, Muthukumaran N, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–6667. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 41.Colone M, Calcabrini A, Toccacieli L, et al. The multidrug transporter P-glycoprotein: a mediator of melanoma invasion? J Invest Dermatol. 2008;128:957–971. doi: 10.1038/sj.jid.5701082. [DOI] [PubMed] [Google Scholar]
- 42.Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kultti A, Rilla K, Tiihonen R, et al. Hyaluronan synthesis induces microvillus-like cell surface protrusions. J Biol Chem. 2006;281:15821–15828. doi: 10.1074/jbc.M512840200. [DOI] [PubMed] [Google Scholar]
- 44.Embry Flory JJ, Fosang AJ, Knudson W. The accumulation of intracellular ITEGE and DIPEN neoepitopes in bovine articular chondrocytes is mediated by CD44 internalization of hyaluronan. Arthritis Rheum. 2006;54:443–454. doi: 10.1002/art.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- 46.Tammi R, Rilla K, Pienimaki JP, et al. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J Biol Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 47.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281:34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.