Abstract
Trapline foraging (repeated sequential visits to a series of feeding locations) is a taxonomically widespread but poorly understood behavior. Investigating these routing strategies in the field is particularly difficult, as it requires extensive tracking of animal movements to retrace their complete foraging history. In a recent study, we used harmonic radar and motion-triggered video cameras to track bumblebees foraging between artificial flowers in a large open field. We describe how all bees gradually developed a near optimal trapline to link all flowers and have identified a simple learning heuristic capable of replicating this optimisation behavior. Our results provide new perspectives to clarify the sequence of decisions made by pollinating insects during trapline foraging, and explore how spatial memory is organized in their small brains.
“I have always regretted that I did not mark the bees by attaching bits of cotton wool or eiderdown to them with rubber, because this would have made it much easier to follow their paths.” Charles Darwin1
Keywords: Bombus terrestris, trapline foraging, artificial flowers, harmonic radar, navigation, spatial cognition
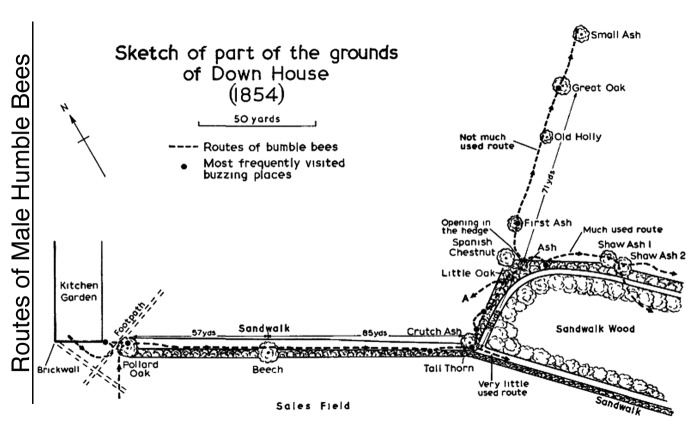
Many animals, from pollinating insects2,3 to frugivorous mammals,4,5 feed on patchy renewable resources and develop routes to visit patches in stable sequences. This behavior is called ‘trapline foraging’ by analogy to fur-trappers checking their traps by following habitual routes.6 Although taxonomically widespread, trapline foraging remains poorly understood due to the difficulty of tracking the movements of individual animals in the field. In his précis on the routes of ‘humble bees’, Darwin1 explained the difficult challenge of reconstructing the paths of bees flying between multiple locations over several hundred meters (see above; Figure 1).
Figure 1. Sketch of the flight paths of male bumblebees (Bombus hortorum) by Charles Darwin. Observations were made between 1854–1861 on the grounds of Darwin’s home in Downe (Kent, UK). For several successive years, bees appeared to follow the same routes (dotted lines) linking plants and several “buzzing places.” Image from,1 with permission of the Natural History Museum of London.
Research on trapline foraging by pollinators has traditionally focused on the adaptive value of this behavior, through optimal foraging models assuming that animals use hardwired movement rules (choices of movement distances or turning angle) as if they were continually exploring new habitats.7-9 More recently, laboratory studies on bees have begun to investigate the behavioral mechanisms underpinning trapline foraging by recording the movements of individually marked foragers exploiting remote controlled artificial flowers10,11 fitted with automated tracking systems12,13 in indoor flight cages. These studies have consistently shown that bees improve their foraging performance as they accumulate experience, by approximating the shortest possible route to visit all flowers,10,11,14,15 prioritizing visits to flowers offering greater rewards,16 and trading off accuracy of route repeatability against flight speed.13 These behaviors are incompatible with hardwired movement rules,11,15 indicating that bees acquire a spatial memory of flower locations and use this information to minimize travel costs.
Tracking Bees with Radar and Motion-Triggered Cameras in the Field
However, no one had determined if this ability to optimise routes is also observed at ecologically-relevant spatial scales in the field. This is a fundamental question as bees typically visit hundreds of flowers and can fly several kilometres during a foraging bout.17 To answer this question, we observed bumblebees (Bombus terrestris) developing traplines between five artificial flowers arranged in a regular pentagon (50 min side) on a mown pasture.18 We fitted each flower with a motion-triggered video camera to record all visits and attached a radar transponder to the back of the bees to monitor their flight paths (Fig. 2). Although harmonic radar has been used to track low-flying insects for over 15 y,19 this is first time this technology has been used to follow the same individual over successive foraging bouts in arrays with multiple feeding stations, and thus to study the processes of route learning and refinement.
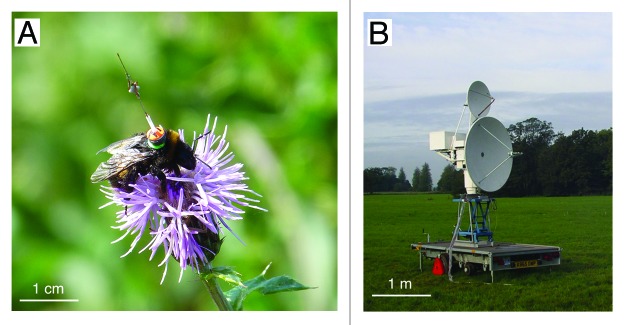
Figure 2. Tracking bees in the field with harmonic radar. (A) A Bumblebee (Bombus terrestris) forager with a radar transponder attached to its back, visiting a thistle flower (Image by Stephan Wolf, with permission). (B) Harmonic radar used to track bees’ flight paths in the field (Image by Oscar Ramos-Rodriguez, with permission). The transponder re-radiates a harmonic of the radar signal which can be detected against a strong ground clutter over a range of about 700 min. Radar tracking of tagged bumblebees visiting artificial flowers arranged in a regular pentagon revealed how bees discover flowers and gradually learn the shortest possible sequence to visit all flowers once and return to the nest (Fig. 3).
This combination of technology enabled us to ‘visualize’ the foraging routes used by each bee and how they were modified with experience (Fig. 3). All bees developed a stable trapline to link all flowers in an optimal sequence after 26 foraging bouts, having tried only 20 of the 120 possible different routes. Their average travel distance decreased dramatically by 80% between their first and last bout, and their final routes were very close to the shortest possible path to visit all flowers. We then investigated how flexible this behavior was by recording the foraging patterns of bees after having removed a familiar flower from the array and introduced a new one in a different location. During a few foraging bouts, all bees kept visiting the empty location (indicating that spatial information stays in memory for a long time) but also engaged in search flights to explore new areas. One bee localized the new flower and developed a new optimal trapline in two bouts, illustrating just how efficient bee’s optimisation behavior is.
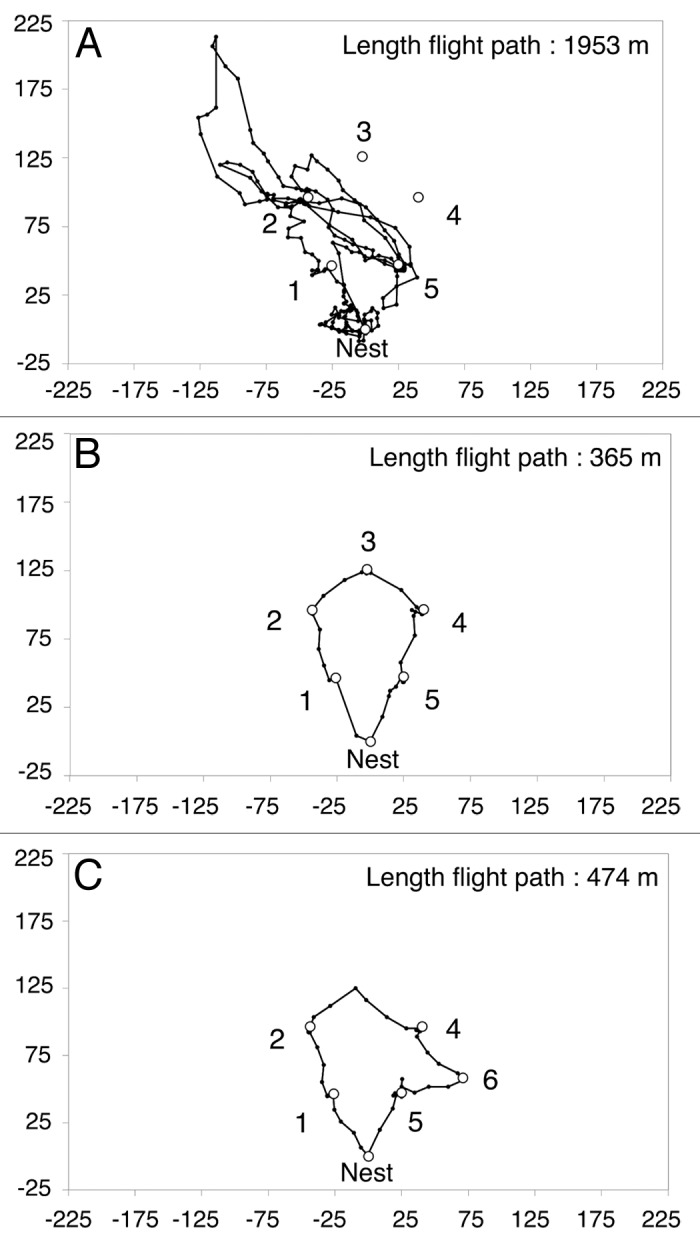
Figure 3. Radar tracks of bumblebees (Bombus terrestris) visiting artificial flowers arranged in a pentagon in the field. Black dots show the position of bees at 3 sec intervals as recorded by the radar. White circles indicate the locations of the artificial flowers (1–6) and the nest-box (Nest). Distances are in meters. (A) Flight path of a naïve bee during its first foraging bout in an array of five flowers (1–5) arranged in a regular pentagon. This initial path is long, doesn’t link all flowers and returns several times at the same (empty) flowers. (B) Flight path of an experienced bee during its 28th foraging bout in the same array as in A. The route was very close to the optimal path to visit all flowers once (312 min). (C) Flight path of an experienced bee during its 8th foraging bout after a familiar flower (flower 3) has been removed and a new flower (flower 6) has been introduced. The bee has discovered the new flower and integrated it into a new optimal sequence, although still visiting the location with the missing flower. Images modified from.18
A Simple Learning Heuristic Accurately Replicates Trapline Optimization
Finding the shortest route between flowers is not a trivial task. In fact, bees solved a problem analogous to the Traveling Salesman Problem: finding the shortest route to visit a set of locations once before returning to the origin.20 This problem is difficult to solve as the number of possible routes increases factorially with the number of locations to visit. Therefore, even in our simple design with five flowers, bees had to choose among 120 possible routes. To try to identify bees’ optimisation strategy, we compared their flower visitation sequences with predictions from heuristic (approximate) solutions to the Traveling Salesman Problem. We identified a simple model, consistent with our current understanding of bees’ navigational toolkit,21 that closely matched our observations: upon returning to the nest, a bee compares the length of the route just traveled to the shortest route previously experienced, and if this new route is shorter the bee will be more likely to repeat it in future foraging bouts. Through a positive feedback, route segments that shorten the overall route are reinforced in memory, while others are abandoned, allowing bees to select an optimal trapline while retaining some ability to adjust their route in response to changes in the spatial configuration of flowers.18
Toward a Mechanistic Understanding of Trapline Foraging
Despite a long history of research on bee navigation, most knowledge has been deduced from the behavior of foragers traveling between their nest and a single feeding site.21,22 Therefore, investigating how bees develop multi-destination routes has the potential to fill an important gap in our understanding of their biology and their impact on pollination. The demonstration that complex routing behavior can emerge from simple learning rules is especially interesting as it suggests that bees can develop optimal routes using only procedural instructions that inform them of the appropriate action for a given place, without requiring a centralized representation of space or a “cognitive map”—the idea that animals build an internal coherent representation of the spatial connectivity between important features of their environments.23 Although our study did not specifically test this hypothesis, our model now provides a useful theoretical platform to generate specific empirically testable predictions about how different organisations of spatial memory might produce different movement patterns and optimisation dynamics by bees in various configurations of flowers.
Another informative approach to address these questions is to directly investigate the neural underpinnings of spatial memories. This approach has long been hampered by the lack of a suitable paradigm to study visual learning on intact restrained bees. However, two recent studies indicate this is now feasible using proboscis extension response conditioning,24,25 a classical associative learning task that emulates the sequence of behavior a bee exhibits during foraging.26 With this paradigm, it is conceivable to use electrophysiological recording and brain-imaging techniques on harnessed subjects to explore the mechanisms underlying visual learning and the suite of decision-making processes involved in routes development. Ultimately, better integration between behavioral and neuroscience approaches to research on insect navigation might help unravel how spatial information is perceived, encoded and stored in the insect brain, and clarify whether this takes the form of a ‘map’ or not.
Acknowledgments
This research was supported by a combined grant from the Wellcome Trust, the Biotechnology and Biological Sciences Research Council, and the Engineering and Physical Sciences Research Council (BB/F52765X/1). We are also grateful to Stephan Wolf and Oscar Ramos-Rodriguez for providing the photos shown in Figure 2.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/22701
References
- 1.Freeman RB. Charles Darwin on the routes of male humble bees. Bull Br Mus Nat Hist. 1968;3:177–89. [Hist Ser] [Google Scholar]
- 2.Ribbands CR. The foraging method of individual honey-bees. J Anim Ecol. 1949;18:47–66. doi: 10.2307/1581. [DOI] [Google Scholar]
- 3.Janzen DH. Euglossine bees as long-distance pollinators of tropical plants. Science. 1971;171:203–5. doi: 10.1126/science.171.3967.203. [DOI] [PubMed] [Google Scholar]
- 4.Janson CH. Experimental evidence for spatial memory in foraging wild capuchin monkeys, Cebus apella. Anim Behav. 1998;55:1229–43. doi: 10.1006/anbe.1997.0688. [DOI] [PubMed] [Google Scholar]
- 5.Lemke TO. Foraging ecology of the long-nosed bat, Glossophaga soricina, with respect to resource availability. Ecology. 1984;65:538–48. doi: 10.2307/1941416. [DOI] [Google Scholar]
- 6.Thomson JD, Slatkin M, Thomson A. Trapline foraging by bumble bees: II. Definition and detection from sequence data. Behav Ecol. 1997;8:199–210. doi: 10.1093/beheco/8.2.199. [DOI] [Google Scholar]
- 7.Possingham HP. The distribution and abundance of resources encountered by a forager. Am Nat. 1989;133:42–62. doi: 10.1086/284900. [DOI] [Google Scholar]
- 8.Ohashi K, Thomson JD. Efficient harvesting of renewing resources. Behav Ecol. 2005;16:592–605. doi: 10.1093/beheco/ari031. [DOI] [Google Scholar]
- 9.Pyke GH. Optimal foraging: movement patterns of bumblebees between inflorescences. Theor Popul Biol. 1978;13:72–98. doi: 10.1016/0040-5809(78)90036-9. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K, Thomson JD, D’Souza D. Trapline foraging by bumble bees: IV. Optimization of route geometry in the absence of competition. Behav Ecol. 2007;18:1–11. doi: 10.1093/beheco/arl053. [DOI] [Google Scholar]
- 11.Lihoreau M, Chittka L, Raine NE. Travel optimization by foraging bumblebees through readjustments of traplines after discovery of new feeding locations. Am Nat. 2010;176:744–57. doi: 10.1086/657042. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi K, D’Souza D, Thomson JD. An automated system for tracking and identifying individual nectar foragers at multiple feeders. Behav Ecol Sociobiol. 2010;64:891–7. doi: 10.1007/s00265-010-0907-2. [DOI] [Google Scholar]
- 13.Ohashi K, Thomson JD. Trapline foraging by bumble bees: VI. Behavioral alterations under speed-accuracy trade-offs. Behav Ecol First Published online; DOI: 10.1093/beheco/ars152.
- 14.Saleh N, Chittka L. Traplining in bumblebees (Bombus impatiens): a foraging strategy’s ontogeny and the importance of spatial reference memory in short-range foraging. Oecologia. 2007;151:719–30. doi: 10.1007/s00442-006-0607-9. [DOI] [PubMed] [Google Scholar]
- 15.Lihoreau M, Chittka L, Le Comber SC, Raine NE. Bees do not use nearest-neighbour rules for optimization of multi-location routes. Biol Lett. 2012;8:13–6. doi: 10.1098/rsbl.2011.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lihoreau M, Chittka L, Raine NE, Kudo G. Trade-off between travel distance and prioritization of high-reward sites in traplining bumblebees. Funct Ecol. 2011;25:1284–92. doi: 10.1111/j.1365-2435.2011.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagen M, Wikelski M, Kissling WD. Space use of bumblebees (Bombus spp.) revealed by radio-tracking. PLoS ONE 2011; 6:e19997. PMID: 21603569; DOI: 10.1371/ journal.pone.0019997. [DOI] [PMC free article] [PubMed]
- 18.Lihoreau M, Raine NE, Reynolds AM, Stelzer RJ, Lim KS, Smith AD, et al. Radar tracking and motion-sensitive cameras on flowers reveal the development of pollinator multi-destination routes of bumblebees over large spatial scales. PLoS Biol. 2012;10:e1001392. doi: 10.1371/journal.pbio.1001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley JR, Smith AD, Reynolds DR, Edwards AS, Osborne JL, Williams IH, et al. Tracking bees with harmonic radar. Nature. 1996;379:29–30. doi: 10.1038/379029b0. [DOI] [Google Scholar]
- 20.Applegate DL, Bixby RE, Chvátal V, Cook WJ. The traveling salesman problem: a computational study. Princeton, NJ: Princeton University Press, 2006. [Google Scholar]
- 21.Srinivasan MV. Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physol Rev 2011; 91:413–460. PMID: 21527730; DOi:10.1152/physrev.00005.2010. [DOI] [PubMed]
- 22.von Frisch K. The dance language and orientation of bees. Cambridge, MA: Harvard University Press, 1967. [Google Scholar]
- 23.Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 24.Dobrin SE, Fahrbach SE. Visual associative learning in restrained honey bees with intact antennae. PLoS One. 2012;7:e37666. doi: 10.1371/journal.pone.0037666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riveros AJ, Gronenberg W. Decision-making and associative color learning in harnessed bumblebees (Bombus impatiens). Anim Cognit ; 15:1183-1193; PMID: 22837045; DOI: 10.1007/s10071-012-0542-6. [DOI] [PubMed]
- 26.Giurfa M, Sandoz JC. Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem. 2012;19:54–66. doi: 10.1101/lm.024711.111. [DOI] [PubMed] [Google Scholar]