Abstract
In this review, we summarized data on the formation and structure of the long and highly adhesive membrane tubulovesicular extensions (TVEs, membrane tethers or cytonemes) observed in human neutrophils and other mammalian cells, protozoan parasites and bacteria. We determined that TVEs are membrane protrusions characterized by a uniform diameter (130–250 nm for eukaryotic cells and 60–90 nm for bacteria) along the entire length, an outstanding length and high rate of development and a high degree of flexibility and capacity for shedding from the cells. This review represents TVEs as protrusions of the cellular secretory process, serving as intercellular adhesive organelles in eukaryotic cells and bacteria. An analysis of the physical and chemical approaches to induce TVEs formation revealed that disrupting the actin cytoskeleton and inhibiting glucose metabolism or vacuolar-type ATPase induces TVE formation in eukaryotic cells. Nitric oxide is represented as a physiological regulator of TVE formation.
Keywords: bacteria, cell secretion, cell-cell communications, cytonemes, eukaryotic cells, exocytotic carriers, host-pathogen interactions, membrane tethers, membrane tubulovesicular extensions (TVEs), protozoan parasites
Introduction
Cell-cell communication through highly dynamic and long tubular filopodia or cytonemes was described 40 years ago for sea urchin and later for insect embryonic cells.1-4 The formation of similar tubular structures, called membrane tethers, membrane tubulovesicular or thread-like extensions was also observed in human neutrophils and primary splenic lymphocytes.5-13 Later, many studies concerning cell-cell communication through “tunneling nanotubes” or “membrane nanotubules” were initiated after thin intercellular connections were detected in rat pheochromocytoma cells and a variety of mammalian immune cells.14,15
Many nanotubule studies have focused on the “detection” of nanotubules in different cell types and have not employed experimental approaches to initiate the formation of these structures in cells. Thus, the mechanism underlying nanotubule formation remains undetermined. The size of nanotubules is near the threshold of resolution for optic microscopy. However, nanotubules are primarily examined using phase contrast and fluorescent microscopy, as fine membrane structures are easily destroyed during preparation for electron microscopy. The intrinsic difficulties in the studies of thin membrane structures have broadened our understanding of the term “nanotubules” and nanotubule functions. Currently, tubular, taper and branched filopodia varying in diameter from 50 to 2,000 nm are all typically described using the terms cytonemes or nanotubules.14-17 Nanotubules are presented as F-actin-driven intercellular connections for the cell-cell transport of ions, cytoplasmic and membrane proteins, intracellular organelles, bacteria or viruses.18-20
In this review, we summarize the data concerning the origin, structure and function of a special type of membrane tubulovesicular extensions (TVEs, membrane tethers or cytonemes). TVEs are characterized by a strictly uniform diameter along the entire length in the range of 130–250 nm for eukaryotic cells and 60–90 nm for bacteria, which varies depending on the conditions. TVEs are also characterized by an outstanding length and high rate of development and a high degree of flexibility and capacity for shedding from the cells. We have only reviewed studies that clearly described methods, which were utilized to initiate TVE formation in the experiments.
In addition, we have focused on studies performed on human neutrophils. As polymorphonuclear leukocytes, neutrophils play an important role in host defense due to their capacity to penetrate blood vessel walls, migrate to infected tissues and phagocytose and kill bacteria. Membrane tethers can be extracted from the human neutrophils in vitro through micropipette manipulation or using laser tweezers.5,6,9,12,21,22 The formation and shedding of membrane tethers or TVEs are involved in main aspects of neutrophil physiology. In the bloodstream, the pulling and shedding of membrane tethers from neutrophil cell bodies under shear stress controls the rolling velocity of neutrophils.7,10,13,23-25 The increased nitric oxide (NO) concentration in infected lesions induces formation of TVEs in neutrophils. Extracellular binding of bacteria by neutrophil TVEs represents an alternative phagocytotic mechanism to bind and kill pathogens.26-29
Notably, there are principle differences between TVEs and so-called “neutrophil extracellular traps,” or NETs. According to Zychlinsky and colleagues, neutrophils exposed to uncoated coverslips in the presence of phorbol ether for 4–6 h “extrude” chromatin and granule proteins that form NETs for binding and killing pathogens.30 NETs were described as fibers of 15–17 nm in diameter aggregated in 50-nm threads that are not surrounded by membranes.30 Subsequent studies have shown that the release of DNA and NET formation resulted from neutrophil cell death and plasma membrane rupture.31 Whether the formation of NETs occurs in vivo remains unknown.32 The release of highly aggressive neutrophil bactericides upon neutrophil break can significantly injure surrounding tissues, thus devaluating the contribution of NETs in the host defense.
TVEs or cytonemes are 200 nm-wide membrane tubulovesicular extensions of living cells with intact nuclei that develop during 20 min. According to our hypothesis, TVEs represent protrusions of the neutrophil secretory process, which establish direct contacts with other cells and bacteria.
Protozoan parasites and bacteria also form membrane tethers and tubulovesicular extensions to adhere to each other and to eukaryotic cells.33,34 Cell-cell communications via long, thin secretory membrane tubulovesicular protrusions appear to be common in prokaryotic and eukaryotic cells.
Pulling and Shedding of Membrane Tethers from the Neutrophil Bodies
Thin membrane tethers can be pulled from neutrophils using applied forces through physiological flow.7,10,13,23-25 The pulling of long membrane tethers from human neutrophil bodies was observed through high speed, high resolution video microscopy of neutrophils flowing over spread platelets or P-selectin-coated surfaces.7 Thin membrane tethers with an average length of 6 µm were pulled from the neutrophil bodies at an average rate of 6–40 µm/s at a wall shear rate of 100–250 sec−1. Tether formation was blocked using antibodies against P-selectin or PSGL-1, but not with anti-CD18 (common β subunit of β2 integrin) antibodies. Thus, the formation of membrane tethers could result from the binding of PSGL-1 located on neutrophil microvillus tips to P-selectin and following microvillus elongation under shear stress. In the bloodstream, neutrophils stabilize the rolling velocity through rapid adjustments in the tether number in response to changes in wall shear stress.7,10,13 The characteristic jerking motion of neutrophils over P-selectin coated surfaces has been observed with tether growth, whereas tether breakage resulted in an acute increase in the rolling velocity. The tether number rapidly increased with increasing wall shear stress and diminished as wall shear stress declined.13
The membrane tethers formed during the rolling process on a ligand-bearing substrate under physiological flow are susceptible to shedding from the cells. The detached tethers were 100–200 nm in diameter reaching 100 µm in length.7 The pretreatment of neutrophils with actin depolymerizing agents, such as latrunculin A or cytochalasin D, increased the average projected membrane tether length and prolonged the average tether lifetime.23
Using micropipette manipulation or optical trapping (tweezers) and antibody-coated beads, Shao and Hochmuth and others demonstrated that under a pulling force, a neutrophil microvillus of ≈0.2 µm in diameter and ≈0.3 µm in length can form a long, thin membrane cylinder (the pulling force ≤ 60 pN).5,6,21,22 The threshold force required to pull a membrane tether did not depend on the coating bead antibodies, medium osmolality or temperature increase from 22°C to 37°C. The disruption of the actin cytoskeleton using latrunculin A or cytochalasin D relieved the pulling of membrane tethers from human neutrophils.9,12,21,35
Structure and Functions of TVEs Formed in Human Neutrophils upon Adhesion to Fibronectin
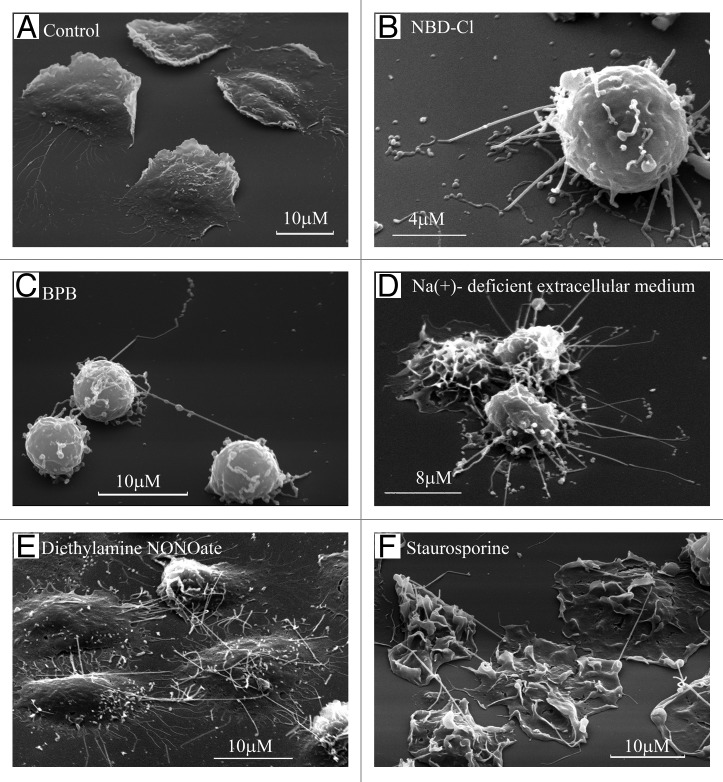
TVEs, resembling neutrophil membrane tethers in size and behavior, develop on the surface of neutrophils during adhesion to fibronectin.8 Resting neutrophils adhere and spread (flatten) onto the fibronectin-coated substrata (Fig. 1A) in a β2-integrin-dependent manner. Neutrophils plated onto fibronectin in Na+-deficient extracellular medium or in the presence of chemical agents [inhibitor of vacuolar-type ATPase 7-chloro-4-nitrobenz-2-oxa-1,3-diazole (NBD-Cl), alkylating agent 4-bromophenacyl bromide (BPB), NO donor diethylamine NONOate, microbial alkaloid staurosporine, and agents that induce actin cytoskeleton depolymerization, such as cytochalasins B and D] develop multiple surface tubulovesicular extensions. These extensions interconnected neutrophils and attached cells to (Figs. 1B–F and 2A). Neutrophils plated onto fibronectin in the presence of NBD-Cl or BPB are anchored to fibronectin-coated substrata through TVEs (Figs. 1C and 2B). Monoclonal anti-L-selectin antibodies inhibited BPB-treated neutrophil adhesions in this case, and monoclonal anti-β1-integrin and anti-CD18 (common β subunit of all β2-integrins) antibodies, separately or in combination, did not reduce neutrophil attachment. These data demonstrated that TVEs attach cells to the substrata in a selectin-dependent, but β2- and β1-integrin-independent, manner.8,26
Figure 1. Formation of TVE in human neutrophils upon adhesion to the fibronectin-coated substrata. Scanning electron microscopy images of human neutrophils plated onto fibronectin-coated substrata for 20 min at 37°C: (A) in control conditions; (B) in the presence of inhibitor of V-ATPase NBD-Cl (100 µm); (C) in the presence of BPB (10 μM); (D) in the extracellular medium, where Na+ ions were substituted with K+ ions; (E) in the presence of the NO donor diethylamine NONOate (1 mM) or (F) microbial alkaloid staurosporine (200 nM).8,26-28
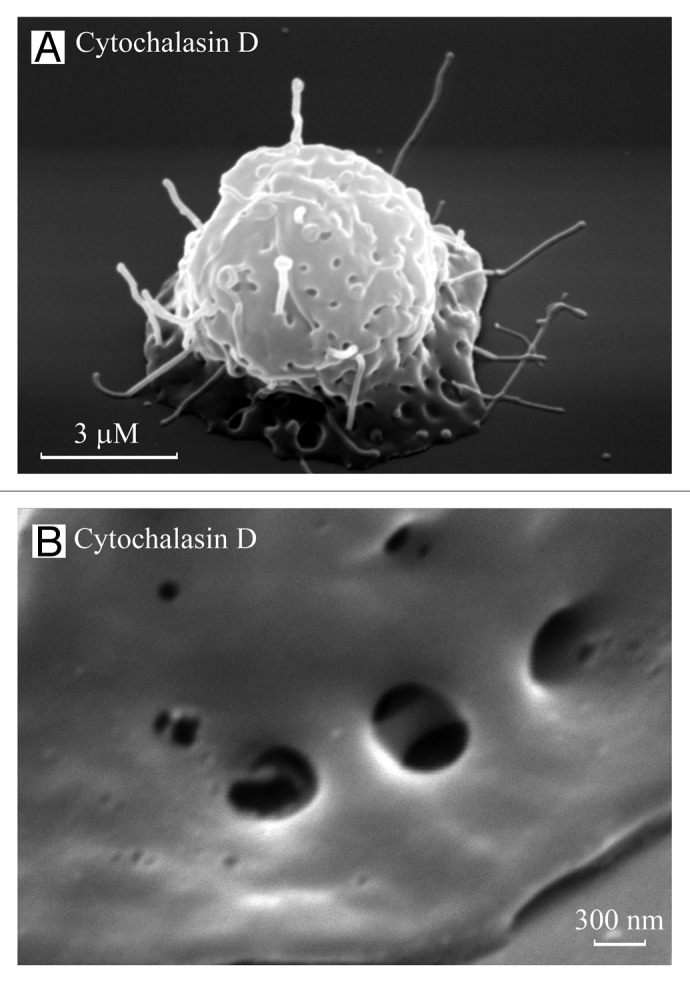
Figure 2. TVEs and invaginations on neutrophil bodies. Scanning electron images of neutrophils plated onto fibronectin in the presence of the actin-disrupting agent cytochalasin D (10 µg/ml) for 20 (A) and 40 (B) min. TVEs and specific invaginations were observed on the cell body. (B) The invaginations on the neutrophil bodies resemble “porosomes” of exocrine and neuroendocrine cells, with two depressions.
TVEs with unattached tips were flexible and had a tendency to shed from the cells. The extensions interconnecting neutrophils were either flexible or straight. The formation of straight connections could reflect the binding and entering of the TVE from one neutrophil into another neutrophil. TVE tips are either endocytosed by neighboring cells or fused with the plasma membrane. In addition, we cannot exclude that straight membrane tethers could be pulled from neutrophil bodies when cells are dispersed after contact.
Scanning electron microscopy revealed that TVEs had a uniform diameter along the entire length and comprised interconnected tubular and vesicular fragments. The uniform TVE diameter varied in the range of 160–240 nm, depending on the conditions.8,27,28 TVEs can obtain lengths of greater than 80 nm in 20 min. Fluorescent microscopy using cytoplasmic and lipid dyes demonstrated that TVEs are covered with membrane and contain neutrophil cytoplasm.27,28
In neutrophils, TVEs grow unhindered in the presence of the microtubule-destabilizing agent colchicine or in the presence of agents that disrupt the actin cytoskeleton, such as cytochalasin B or D (Fig. 2).8,28 These data indicate that the development of TVEs occurs independently of the cytoskeleton, i.e., neutrophil TVEs are not F-actin- or microtubule-driven protrusions.
Whether membrane tethers pulled from neutrophil bodies are identical to neutrophil TVEs developed after neutrophil adhesion in the presence of chemical agents remains unknown. TVEs and tethers are similar in size and behavior. However, TVEs also have a strictly uniform diameter along the entire length, with an ability to reach extraordinary lengths and a capacity for shedding from the cells. Actin depolymerization stimulates the development of both membrane tethers and TVEs.
Metabolic Regulation of TVE Formation in Human Neutrophils upon Adhesion to Fibronectin
The analysis of the chemical drugs that induce TVE formation in neutrophils facilitated the discrimination of these agents into the groups: (1) inhibitors of glucose uptake and glycolysis, (2) inhibitors of vacuolar-type ATPase (V-ATPase), (3) inhibitors of Cl- channels and Na+-deficient extracellular medium, (4) agents disrupting actin cytoskeleton and (5) nitric oxide donor diethylamine NONOate.8,27,28,36
Similar to malignant cells, leukocytes possess a high rate of glycolysis, even in the presence of oxygen.37 The extrusion of TVEs from the cells occurred upon neutrophil adhesion to the fibronectin-coated substrata in the presence of iodoacetic acid, an inhibitor of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or phloretin, an inhibitor of facilitative glucose transporters. The inhibition of oxidative phosphorylation did not induce the formation of membrane extensions.36 Experiments inhibiting the three main classes of proton-translocating ATPases, i.e., P-, V- and F-type ATPases,38 revealed that tubulovesicular extensions developed in neutrophils in the presence of inhibitors of V-ATPase, such as NBD-Cl (Fig. 1B), NEM or bafilomycin A. Inhibitors of P-or F-type ATPases did not induce cytoneme formation.36
V-ATPase couples the hydrolysis of cytosolic ATP to proton transport out of the cytosol. This enzyme comprises a peripheral domain (V1) that contains the ATP biding sites and an integral membrane domain (V0), which form the proton pore. V1 and V0 can exist separately, but they must combine to pump protons.39 The V-ATPase proton-pumping activity is tightly regulated through glucose metabolism.40 V-ATPase and glycolytic enzymes, such as phosphofructokinase-1 and aldolase, interact at the protein level, providing a basis for coupling the ATP-generating glycolytic pathway directly to the ATP-hydrolyzing proton pump.41-43 The glycolytic enzyme GAPDH was also physically associated with the aldolase-V-ATPase complexes.42
We propose that in neutrophils, TVEs represent secretory protrusions as membrane tubular or vesicular exocytotic carriers. These carriers fuse with the neutrophil plasma membrane upon neutrophil spreading on the substrata (Fig. 1A) or extend from the cell surface as TVEs, when fusion is impaired (Fig. 1B–F). Complex interactions between V-ATPase and glycolytic enzymes control TVE formation via membrane fusion events. This hypothesis is based on the data demonstrating the involvement of V-ATPase and the glycolytic enzymes GAPDH and enolase in membrane fusion events.44-51 It has been suggested that the V0 domains of V-ATPase from two opposing membranes undergoing fusion form a channel. The radial expansion of this channel leads to membrane fusion.44,45 The involvement of V-ATPase in membrane fusion and fission events was demonstrated for yeast vacuoles,44,45 synaptic vesicles exocytosis in fly neurons,46,51 the release of exosomes from multivesicular bodies and exocytosis of morphogenes in worms,47,48 and for insulin secretion from pancreatic islets in mice.49 A growing amount of evidence has shown that the glycolytic enzymes GAPDH and enolase also play a role in membrane fusion.52-56
The capacity of NO and agents that disrupt the actin cytoskeleton to induce TVE formation could be realized via the modulation of glucose metabolism and V-ATPase activity. NO inhibits both V-ATPase and glycolytic enzyme GAPDH.57-59 Filamentous actin directly binds the B and C subunits of the V1 domain of V-ATPase as well as glycolytic enzymes thus regulating the assembly of V-ATPase and V-ATPase/glycolytic enzyme complexes.60-63 The depolymerization of actin filaments impairs interactions between V-ATPase and glycolytic enzymes upon the fusion of exocytotic carriers with the neutrophil plasma membrane.
V-ATPase is an electrogenic enzyme.39 The Cl- efflux through chloride channels perform charge compensation for protons pumped out of the cells through V-ATPase to regulate V-ATPase activity.8,36 Blocking chloride channels thus can initiate TVE formation via the inhibition of V-ATPase activity. How the extracellular Na+ ion deficit induces TVE formation in neutrophils remains unknown. The role of the extracellular Na+ ions concentration and Cl– ion efflux in the development of tubulovesicular extensions seems to be strongly associated with water influx, as electrolytes and water transport affect membrane tension and provide the driving force for cytoneme formation.
Cytoneme Formation in Embryonic, Blood and Other Eukaryotic Cells
During gastrulation, sea urchin embryo cells migrate from the vegetal pole to a site below the equator of the embryo. As these cells migrate, they extend dynamic thin filopodia that interact with the basal lamina, which lines the blastocoel and underlying ectoderm. These filopodia serve as sensory organelles, anchoring appendages and intercellular connections that join cell bodies and execute long distance cell-cell communications associated with signaling and patterning during gastrulation. Isolated primary mesenchyme cells also develop similar filopodia upon adhesion to the extracellular matrix or fibronectin in the presence of extracellular material from mesenchyme blastula. Filopodia extend at a rate of 1 µm/min from cells migrating in vitro or as rapidly as 10–25 µm/min in vivo and reach 30–80 µm in length. The filopodia diameter varies from 0.2 to 0.4 µm.1-3,64
In primary splenic lymphocytes and Bal 17 cells, the engagement of B-cell antigen receptor by IgM, which is a surrogate for antigen, can induce the formation of thread-like projections or cytonemes.11 IgM-induced cytonemes that are 0.2–0.4 µm in diameter and reach 80 µm in length after 30 min may participate in long-distance communication between the antigen-stimulated B cells and the other immune cells. The time course for cytoneme appearance is consistent with a potential role in the presentation of antigen uptake from the BCR to helper T cells.
The transfection of the B144/LST1 gene in a variety of eukaryotic cells induces the development of flexible and dynamic cytoneme-like filopodia reaching 300 µm in length.65 The B144/LST1 gene encoded within the human major histocompatibility complex is highly expressed in dendritic cells and professional antigen-presenting cells. The occurrence of dynamic long cellular extensions is a potential mechanism for occasionally obtaining a T cell receptor to recognize antigens presented on the dendritic cell.
The binary actin-ADP-ribosylating toxins CDT, Clostridium botulinum C2 toxin and Clostridium perfringens iota toxin induce the rearrangement of microtubules and the formation of long (up to 150 µm) microtubule-based protrusions with a diameter ranging from 0.05 to 0.5 µm at the surface of human colon carcinoma cells (Caco-2 cells).66,67 Scanning electron microscopy demonstrated that CDT-induced protrusions form a dense meshwork at the cell surface, which wraps and embeds bacterial cells, thus increasing the adherence of Clostridia. The formation of protrusions seemed to be a consequence of the ability of bacterial toxins to affect the cellular actin cytoskeleton. Cytochalasin D and latrunculin A induced the formation of similar protrusions in intestinal epithelial cells.
The suspension of breast tumor cells (MCF10A human or EpH4 mouse mammary epithelial cells) over ultra-low-attachment plates or 2% agarose coated plates produced long and dynamic protrusions of the plasma membrane.68 The protrusions were enhanced through actin depolymerization with cytochalasin D or latrunculin A to promote efficient cell-cell attachment and homotypic aggregation. These protrusions were described as microtubule-driven (the protrusions were partially clocked using colchicine) and enriched in detyrosinated α-tubulin. Because blood-borne metastasis depends on both cell-cell and cell-matrix attachments, protrusions in detached transformed mammary epithelial cells provide a novel mechanism that influences the metastatic spread of breast tumors.
The Role of Actin Cytoskeleton in TVE Formation in Neutrophils and Other Cells
The analysis of drugs that induce cytoneme formation revealed that many of these agents initiate actin depolymerization. Cell permeable and potent inhibitors of actin polymerization cytochalasins D and B (mycotoxins produced by Helminthosporium and other molds) and latrunculin A (a natural toxin produced by certain sponges, including genus Latrunculia) stimulated cytoneme formation in neutrophils,8,28 colon and breast carcinoma cells,66,68 and relieve the pulling of membrane tethers from the cell bodies with a physiological flow23 or upon the pulling of membrane tethers through micropipette manipulation9,12,35
The ability of staurosporine (STS), a natural product originally isolated from the bacterium Streptomyces staurosporeus, and Ro-31-8220, a structural analog of STS, to induce TVE formation in neutrophils was also coupled to the depolymerization of the actin cytoskeleton. Central cytoskeletal regulators also include actin-depolymerizing factor (ADF)/cofilin, which depolymerizes actin filaments. The phosphorylation of cofilin blocks this activity. The neutrophil serine 3-cofilin kinase is constitutively active and insensitive to a variety of selective antagonists of protein kinases. STS specifically inhibits the neutrophil serine 3 cofilin protein kinase, thus maintaining actin in a depolymerized state.69
Another target of STS is the leukocyte-specific actin-bundling protein L-plastin, which is phosphorylated in response to adhesion or phagocytosis.70,71 STS72 and Ro-31-8220,73 but not other protein kinases inhibitors, inhibit L-plastin phosphorylation. L-plastin is a single cytosolic protein in neutrophils that binds BPB, an inducer of TVE formation.74 In murine macrophages, L-plastin is one of the major S-nitrosylation targets for NO, a natural inducer of TVE formation.75,76
NO also promotes actin depolymerization through the ADP ribosylation of β/γ however, according to our hypothesis, the disruption of actin filaments impairs the fusion of the carriers with the plasma membrane. As a result, tubulovesicular extensions protrude from the cell bodies.
Cytochalasin-induced TVEs are characterized by rapid development and simultaneous rapid destruction.28,66 Scanning electron microscopy studies revealed the presence of TVEs and specific invaginations on the cytochalasin D-treated neutrophils formed during first 10–20 min of experiment. After 40–60 min of neutrophil adhesion in the presence of cytochalasin D TVEs were practically disrupted (Fig. 2).28 Similar invaginations were observed on the neutrophil bodies after shedding of TVEs as a result of interactions with bacteria.27 The diameter of the invaginations was similar to that of TVEs, at 200–230 nm.27 The invaginations resemble porosomes of exocrine and neuroendocrine cells.81 The porosomes resembled circular pits of 0.4–1.2 µm in diameter containing 3–4 depressions of 100–150 nm in diameter. The depressions served as fusion pores, where membrane-bound secretory vesicles transiently dock and fuse to expel vesicular contents.81 The neutrophil invaginations resembled porosomes with two depressions, potentially fulfilling the same role in cell secretion as porosomes (Fig. 2). However, the invaginations could also represent the sites of compensatory endocytosis, which accompanies neutrophil secretion via the shedding of vesicles and tubules from the TVE tips. Both invaginations and TVEs are vulnerable structures, and the mechanism of TVE extrusion from the neutrophil remains to be elucidated.
Cytochalasin D-induced cytonemes are short-lived structures. Many investigators consider that nanotubules or cytonemes are F-actin- or actin-polymerization-driven protrusions, as nanotubules were not detected after pre-treatment with cytochalasin D. Thus, we propose that the formation and destruction of cytonemes is completed after the cells are incubated with cytochalasin D for 30–60 min.
Proteome Analysis Revealed Neutrophil TVEs as Protrusions of Secretory Bactericide Trafficking
Neutrophils are phagocytic cells that constitute the first line of host defense against bacterial infections due to the ability of neutrophils to engulf and kill microorganisms. A microbicidal function has been ascribed to the abundant cytoplasmic granules that are discharged into phagocytic vacuole containing microbes. Neutrophil bactericides are packaged into four types of intracellular secretory granules: azurophil (primary), specific (secondary) and gelatinase (tertiary) granules and secretory vesicles.82,83 The bactericide content of granules overlaps. The specific granules contain more lactoferrin and lipocalin, and the azurophil granules contain more myeloperoxidase, cathepsin G and defensins HNP 1–3.
The proteome analysis of the neutrophil TVE content was performed to confirm the secretory origin of these structures. The TVEs developed in neutrophils plated on fibronectin in the presence of nitric oxide donor diethylamine NONOate, BPB and cytochalasin D were disrupted following the removal of the inducers. High-performance liquid chromatography and mass spectrometry investigations indicated that TVE disruption released (a) the granular bactericides lactoferrin, lipocalin, myeloperoxidase, cathepsin G and defensins HNP 1–3, (b) energy metabolism enzymes, such as transketolase, glucose-6-phosphate dehydrogenase and glycolytic enzymes phosphoglucose isomerase, enolase, GAPDH and hexokinase II, (c) the actin cytoskeleton proteins moesin, L-plastin, β- and γ-actin, (d) S100A8, S100A9, S100A8/A9, S100A12 proteins and (e) other proteins, such as glutathione transferase, annexin 1 and ov-serpin.29
The presence of specific and primary granular bactericides including lactoferrin, lipocalin, mieloperoxidase, cathepsin G and defensins HNP 1–3 in neutrophil TVEs confirmed the hypothesis that TVEs represent protrusions of neutrophil secretory bactericide trafficking.29 The abundant occurrence of glycolytic enzymes, actin cytoskeletal proteins, annexin1 and S100 proteins in TVEs might be required to execute TVEs fusion with the neutrophil plasma membrane. The role of glycolytic enzymes and the actin cytoskeleton in membrane fusion and TVE formation was discussed above. Annexins attach and bring different membranes into close proximity to facilitate fusion.84 Annexin 1 and GAPDH appear to be the major fusion proteins of neutrophils shown to promote aggregation and fusion neutrophil granules.53,85,86 The S100A8/A9 protein binds and transports arachidonic acid,87 an essential component of annexin-mediated membrane fusion.88
Neutrophil TVEs Capture Bacteria and Yeast: A Novel Pathogen Scavenging Mechanism
Under the control conditions, neutrophils spread on fibronectin-coated substrata and phagocyte (engulf) bacteria (Fig. 3A) or yeast (Fig. 4A). During phagocytosis, tubular and vesicular bactericide carriers fused with the membrane of phagosome containing microbes and expelled their contents into phagosomes. The spreading of neutrophils on substrata might also represent unsealed phagocytosis, in which the neutrophil engulfs a huge particle (Fig. 1A). Subsequently, exocytotic carriers expel their contents from the unsealed phagosomes into the extracellular medium.
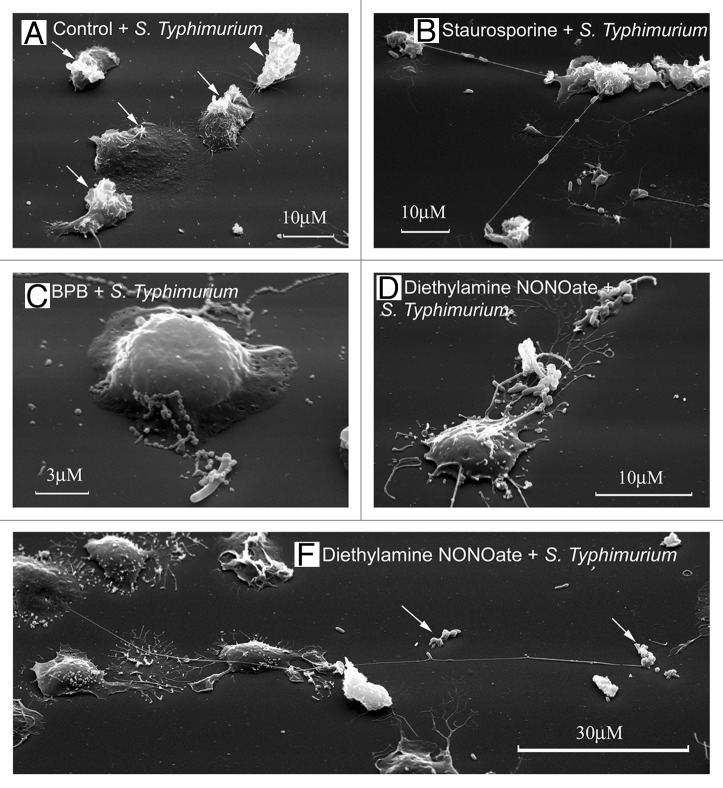
Figure 3. Neutrophil TVEs bind and hold pathogenic bacteria Salmonella enterica serovar Typhimurium over long distances. Scanning electron microscopy images of human neutrophils plated onto fibronectin-coated substrata for 20 min at 37°C: (A) in the control conditions, (B) in the presence of staurosporine (200 nM), (C) in the presence of BPB (10 μM) or (E) in the presence of the NO donor diethylamine NONOate (1 mM). At the end of incubation, serum-opsonized S. Typhimurium bacteria (bacteria/cells ratio 20:1) were added to neutrophils for 5 min. The neutrophils plated at control conditions phagocytosed the bacteria. “Ruffles” formed on the cell surface where bacteria entered the cell [(A), arrows]. The neutrophils plated onto fibronectin in the presence of inducers of TVE formation (B–E) bound and held bacteria through TVEs.27,28
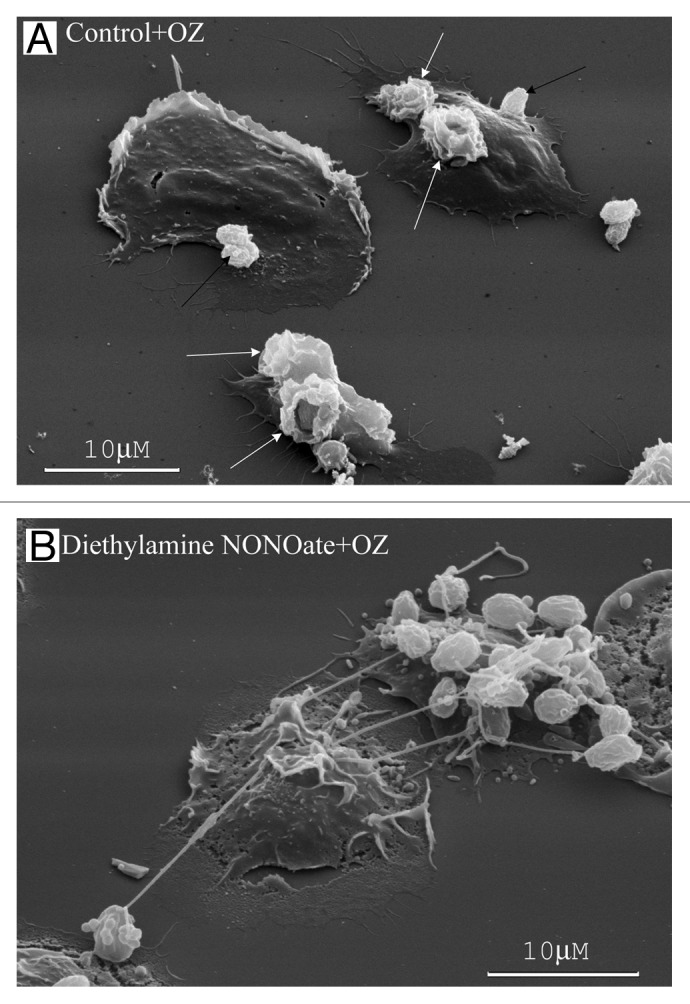
Figure 4. NO alters human neutrophil interactions with yeast. (A) The neutrophils plated onto fibronectin under the control conditions phagocytosed opsonized zymosan particles (dried yeast coated with serum) forming specific “cups” (white arrows) on the cell surface or bound yeast particles onto the cell surface (black arrows). (B) The neutrophils plated onto fibronectin in the presence of the NO donor diethylamine NONOate (1 mM) bound opsonized zymosan particles through TVEs.26
In the presence of inducers of cytoneme formation, bactericide tubulovesicular trafficking extends from the cells as TVEs and binds and holds Salmonella Typhimurium or opsonized zymosan particles (dried yeast particles coated with serum) over a long distance (Figs. 3B–E and 4B).8,26-28,36 The binding of bacteria or yeast via TVEs did not represent an initial stage of phagocytosis, but it resulted in the shedding, swelling and lysis of TVEs together with bound microbes.27 The extracellular binding of bacteria via TVEs containing primary and specific granule bactericides and the subsequent killing of bound bacteria through bactericides released from TVEs upon lysis represents a novel mechanism for eliminating bacteria via neutrophils.29 This mechanism could share some of the advantages of phagocytosis. The micrometer-long TVEs greatly widen the area, where bacteria are subjected to neutrophilic attack. Bacteria cannot enter the cells, where they can survive and multiply. The membrane-packed content and outstanding length of TVEs might allow targeted neutrophil secretion of aggressive bactericides over a long distance without dilution and injury to the surrounding tissues.
A similar mechanism for binding Clostridia via CDT toxin-induced protrusions was described for the human carcinoma cell line Caco-2.66,67 The protrusions strongly increased the colonization of intestinal epithelium with Clostridium difficile.
Protozoan Parasites Initiate the Development of Finger-Like Filopodia in Human Neutrophils
High-resolution scanning and transmission electron microscopy studies of rat neutrophil interactions with infectious tachyzoites form of the parasitic protozoan Toxoplasma gondii have demonstrated that after a 30 min incubation with parasites at rates of 5:1 (zoites:neutrophil), the surface of the neutrophils was full of finger-like projections.89 These projections resembled TVEs in size and engulfed the parasites. Another protozoan parasite Trichomonas vaginalis also induced the formation of similar finger-like filopodia on the neutrophil surface. The filopodia were involved in parasites binding, as revealed through scanning electron microscopy.90
Protozoan parasites can induce finger-like filopodia by stimulating production of NO, an effective inducer of cytoneme formation in neutrophils. Neutrophils exposed to Trichomonas vaginalis produced nitric oxide and interleukin-8.90,91 Interleukin-8 along with the other cytokines can, in turn, stimulate NO synthesis.92
Nitric Oxide as a Physiological Regulator of TVE Formation in Neutrophils
Nitric oxide, endothelium-derived relaxing factor, plays an important role in regulation of neutrophils adhesion to endothelium lining the vessel walls. In the blood stream, neutrophils roll along vessel walls, temporarily adhering to the endothelium via microvillus-like membrane tethers in a selectin-dependent manner. Metabolic disturbances, such as hypoxia or ischemia and reperfusion, stimulate firm β2-integrin-mediated leukocyte adhesion to vessel walls.93 The attached neutrophils injure the endothelium through the secretion of lytic enzymes and reactive oxygen species. The firm adhesion of neutrophils to the endothelium is responsible for capillary closure and the development of diabetic angiopathies.94,95
In the circulation, shear stress and a network of mediators, such as prostanoids and nitric oxide (NO) prevent firm leukocyte adhesion to the endothelium.96 Both endothelial cells and neutrophils express constitutively active NO synthase isoforms, such as eNOS in endothelial cells and nNOS (neuronal) and iNOS (inducible) in neutrophils, for the production of NO.97-99 NO derived from both nNOS and eNOS is critical for the regulation of leukocyte-endothelial cell interactions.100 NO reduces firm leukocyte adhesion to the endothelium and relieves the endothelial damage induced through adherent leukocytes.100-102 The inhibition of NO synthesis via Nω-nitro-l-arginine methyl ester (L-NAME) induced an increase in leukocyte adhesion via CD18, a common β-chain of β2-integrins.102,103 In contrast, NO or NO donors inhibited the adherence of β2 integrin-mediated neutrophils to endothelial cells or fibrinogen-coated plates, but did not affect P-selectin-dependent neutrophil rolling.104-106 No direct effect of NO on the expression of CD18 was observed.102,104,106
Our data revealed NO as an inducer of TVE formation in neutrophils.26,27,29 We propose that NO regulates neutrophil adhesive interactions via the formation of long and dynamic TVEs, which attach neutrophils to the endothelium in a long-range selectin-dependent manner.26,36In this case the role of integrins, which are located on the cell bodies, in neutrophil adhesion is reduced. The inhibition of TVE formation as a result of blocking NO synthesis facilitates neutrophil adhesion via β2-intergins.
NO play an important role in host defense against multiple bacterial infections.107-111 To resist the antimicrobial action of the NO generated in host cells, some bacteria, such as Salmonella, possesses NO-metabolizing enzymes, such as the flavohemoglobin Hmt, which is required for the Salmonella virulence in mice.112,113 One of the targets of NO in host defense is the enhancement of the bactericidal activity of human phagocytes (macrophages and neutrophils) against Salmonella species.109-111,114 The induction and activation of NO synthases and excessive production of NO are common features of inflammation and infection lesions.92
Our data demonstrated that NO initiated TVE formation in human neutrophils and potentiated long-range binding of bacterial or yeast pathogens by TVE, while the inhibition of NO synthesis stimulated phagocytosis of bacteria. Extracellular binding of pathogens by TVE and following killing of bound pathogens by bactericides released from TVEs upon lysis represents a novel mechanism of elimination of bacteria by neutrophils, which might be more effective than phagocytosis. We propose that NO-induced the transition of neutrophil-bacteria (Fig. 3D and E) and neutrophil-yeast (Fig. 4B) interactions from phagocytosis to the long-range extracellular binding of pathogens through TVE plays an important role in NO contribution to the host defense against bacterial infections.26,27
According to our hypothesis, NO induces TVE formation via inhibition of the fusion of exocytotic carriers with the plasma membrane, thus blocking the emptying of tubular and vesicular exocytotic carriers. This hypothesis was based on data demonstrating the capacity of NO to block a late step of exocytosis (granule emptying) in chromaffin cells, and inhibit the exocytosis of Weibel-Palade bodies in endothelial cells, dense, lysosomal and α-granules from human platelets and cytolytic granules from lymphokine-activated killer cells.115,116 In contrast to the inhibitory effects on exocytosis, NO accelerates endocytosis.116
Cytonemes of Protozoan Parasites
Highly adhesive, thread-like filopodia arising from the surface of trypanosomes were described 40 years ago.33 Recently, the development of dynamic membrane tubulovesicular filaments was observed on the surface of the gametes of malaria pathogen Plasmodium falciparum.33 The sexual phase of the malaria parasite development occurs in the mosquito midgut. Plasmodium gametocytes enveloped within human erythrocytes are activated in the mosquito midgut through a reduction in temperature and egress from erythrocytes. Filaments actively form within minutes after gametocyte activation in both the emerging macrogametes (female) and exflagellating microgametocytes (male). The length of the protrusions varied between 2 and 180 μm, with an average length of 10–20 μm. Adhesion proteins, such as Pfs230, Pfs48/45 or Pfs25, which are typically found in gametes, were expressed on the surface of these filaments. Electron microscopy revealed that the filaments represented 200 nm-wide membranous protrusions of the gamete plasma membrane, with a beaded structure containing cytoplasm. Immunofluorescence studies revealed that the parasite filaments contained the actin isoform Pfact2, but did not contain tubulin. The filaments produced by parasite gametes exhibited a pronounced similarity, in structure and behavior, to neutrophil TVEs. The malaria parasite can utilize these extraordinary long cellular connectives to facilitate intracellular contacts between female and male gametes during the sexual reproduction stage in the mosquito midgut.
Cytonemes of Bacteria
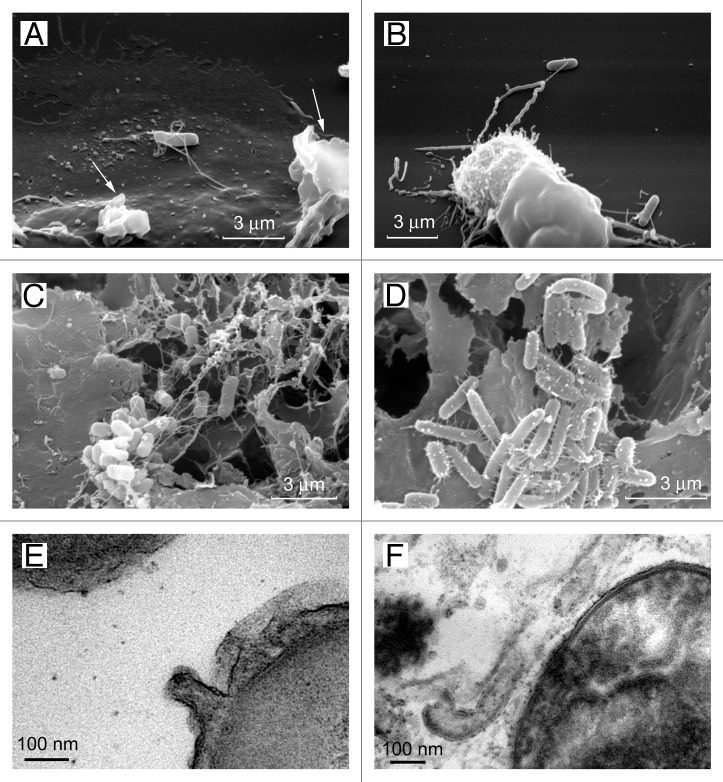
Scanning electron microscopy revealed that contact with eukaryotic cells results in the formation of unusually wide (60 nm in diameter) tubular appendages of S. Typhimurium interconnecting bacteria and attaching bacteria to the eukaryotic cells (Fig. 5).27,34,117,118 These appendages strongly exceeded bacterial flagella (15–20 nm in diameter) or pili (6–7 nm in diameter). Further studies estimated that tubular appendages interconnecting S. Typhimurium in biofilms varied in diameter between 60 and 90 nm.34 Transmission electron microscopy revealed that tubular bacteria appendages represent membrane tubules, which are formed as extensions of the outer membrane.34
Figure 5. Cytonemes of bacteria. The scanning electron microscopy images show Salmonella enterica serovar Typhimurium attached to the surface of the control neutrophils (A) or to the TVEs of BPB-treated neutrophils (B) through cytonemes. Salmonella of the virulent C53 strain (C) and the non-flagellated SJW880 strain (D) were interconnected through cytonemes in biofilms grown on the surface of gallstones. Transmission electron microscopy images of 60-nm membrane tubules derived from the outer membrane of the bacteria (E and F).34
Bacterial membrane tubules, in their size, structure and behavior, correspond to the so-called “membrane sheaths” of bacteria. Early studies of flagella of Vibrio metchnikovii, Bdellovibrio bacteriovorus or Helicobacter pylori using electron microscopy reveled an internal electron-dense filament and a surrounding flagellar sheath exhibiting the typical bilayer structure of a membrane.119-121 The tubular membrane structures were 3 to 4 times thicker than typical bacterial flagella. Moreover, formations of tubular membrane structures that do not contain flagella filament have been observed in some strains of Beneckea. These tubular membrane evaginations of the outer membrane of the cell wall are often beaded to a variable degree.122 Although these structures were observed many years ago, the function of membrane sheaths of flagella remains unknown.123,124
The membrane tethers can be extracted from bacteria Escherichia coli using optical tweezer manipulation.125 Similar to tethers pulled from eukaryotic cells, the bacterial tethers are extremely long and are primarily composed of the asymmetric lipopolysaccharide-containing bilayer of the outer membrane. Recently, bacterial dynamin-like protein was prepared from cyanobacteria, suggesting that bacterial dynamin-like proteins play a role in membrane tubulation/vesiculation identical to that of eukaryotic dynamins.126,127
Bacterial tubular appendages, such as the cytonemes of neutrophils and other eukaryotic cells, have a uniform diameter along the extraordinary length and demonstrate a high rate of development, flexibility and capacity for shedding from the cell surface.
Membrane Tubulovesicular Structures Created by Bacterial and Protozoan Pathogens Inside the Host Cells
Notably, intracellular bacterial or protozoan pathogens induce the formation of membrane tubulovesicular structures in the host cells, which serve as secretory organelles. The malaria protozoan parasite Plasmodium falciparum establishes membrane tubulovesicular structures or “Maurer clefts” within host erythrocytes to direct parasitic proteins across the host cell cytoplasm to the erythrocyte surface.128 A unique feature of Salmonella-infected cells is the presence of tubular structures originated from Salmonella-containing vacuole. The Salmonella-induced tubular network comprises different types of tubules, including tubules containing the secretory carrier membrane protein 3 (SCAMP3), a post-Golgi secretory pathway protein.129
Conclusion and Future Perspectives
In summary, eukaryotic cells, protozoan parasites and bacteria develop long membrane tubules or tubulovesicular membrane extensions. Long-range communications via membrane tubulovesicular extensions are a new adhesive and communicative mechanism common to eukaryotic cells and bacteria. TVEs have been described as cellular secretory protrusions that establish direct contact between cells and bacteria over long distances. The membrane-packed content and outstanding length of TVEs might facilitate targeted cellular secretion over a long distance without dilution or injury to surrounding tissues.
The mechanism of TVE extrusion and membrane reservoir for TVE formation remains unknown. TVEs or cytonemes, as thin membrane structures, have an enormous surface square/volume ratio and a high rate of development. To build these numerous and long membrane structures, additional membrane must be quickly delivered to the plasma membrane. It has been shown that fusing the endoplasmic reticulum with the macrophage plasmalemma, underneath phagocytic cups, is a source of additional membrane for phagosome formation in macrophages.130 In neutrophils, phagosome maturation includes the incorporation of multiple subcellular compartments, such as the endoplasmic reticulum, plasma membrane, specific and azurophilic granules and mitochondria.131 We speculate that the reorganization of membrane trafficking and the contribution of multiple intracellular compartments provide membrane for cytoneme building.
Another important question for further studies is the driving force for TVE formation. Cytoneme growth is an actin cytoskeleton-independent process, as actin depolymerization initiates TVE formation. Moreover, the development of cytonemes occurs in the presence of inhibitors of microtubule formation. Thus, it is our opinion that water and ion fluxes play a crucial role in the extrusion, swelling and lysis of fine lipid structures.
The mechanism responsible for tubule and vesicle interconnections within a TVE unit and TVE shedding from the cells also requires further examination. In human neutrophils, TVE shedding occurred upon interactions with bacteria or opsonized zymosan particles.27,28 Whether neutrophils or bacteria initiate shedding of TVEs from neutrophils and whether bacteria or neutrophils benefit from this process is still unknown.
How do cytonemes facilitate cell-cell communication? We suggest that cytonemes move as a unit from one cell to another, as cytoneme movement results from the secretion of tubular and vesicular exocytotic carriers from one neutrophil and from the simultaneous endocytic uptake of carriers by the connected neutrophil. Cytoneme movement could execute the transport of membrane and cytoplasm molecules between interconnected cells. Investigations in this field might further our understanding of fundamental cellular processes, such as membrane trafficking during endocytosis, phagocytosis and exocytosis and degranulation.
Acknowledgments
This work was supported through grants from the Russian Foundation of Basic Research 09-04-00367-а and 10-04-01479-a.
Glossary
Abbreviations:
- TVEs
membrane tubulovesicular extensions (cytonemes, membrane tethers)
- NO
nitric oxide
- NOS
nitric oxide synthase
- V-ATPase
vacuolar-type ATPase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- P-selectin
platelet selectin
- PSGL-1
P-selectin glycoprotein ligand-1
- BPB
4-bromophenacyl bromide
- NBD-Cl
7-chloro-4-nitrobenz-2-oxa-1,3-diazole
- CDT
Clostridium difficile transferase
- G-actin
globular (monomeric) actin
- F-actin
filamentous actin
- STS
staurosporine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/23130
References
- 1.Gustafson T, Wolpert L. Cellular movement and contact in sea urchin morphogenesis. Biol Rev Camb Philos Soc. 1967;42:442–98. doi: 10.1111/j.1469-185X.1967.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 2.Solursh M, Lane MC. Extracellular matrix triggers a directed cell migratory response in sea urchin primary mesenchyme cells. Dev Biol. 1988;130:397–401. doi: 10.1016/0012-1606(88)90445-9. [DOI] [PubMed] [Google Scholar]
- 3.Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–11. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- 4.Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 5.Shao JY, Hochmuth RM. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys J. 1996;71:2892–901. doi: 10.1016/S0006-3495(96)79486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao JY, Ting-Beall HP, Hochmuth RM. Static and dynamic lengths of neutrophil microvilli. Proc Natl Acad Sci U S A. 1998;95:6797–802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidtke DW, Diamond SL. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J Cell Biol. 2000;149:719–30. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galkina SI, Sud’ina GF, Ullrich V. Inhibition of neutrophil spreading during adhesion to fibronectin reveals formation of long tubulovesicular cell extensions (cytonemes) Exp Cell Res. 2001;266:222–8. doi: 10.1006/excr.2001.5227. [DOI] [PubMed] [Google Scholar]
- 9.Marcus WD, Hochmuth RM. Experimental studies of membrane tethers formed from human neutrophils. Ann Biomed Eng. 2002;30:1273–80. doi: 10.1114/1.1528614. [DOI] [PubMed] [Google Scholar]
- 10.Park EY, Smith MJ, Stropp ES, Snapp KR, DiVietro JA, Walker WF, et al. Comparison of PSGL-1 microbead and neutrophil rolling: microvillus elongation stabilizes P-selectin bond clusters. Biophys J. 2002;82:1835–47. doi: 10.1016/S0006-3495(02)75534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol Biol Cell. 2003;14:432–44. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus WD, McEver RP, Zhu C. Forces required to initiate membrane tether extrusion from cell surface depend on cell type but not on the surface molecule. Mech Chem Biosyst. 2004;1:245–51. [PubMed] [Google Scholar]
- 13.Ramachandran V, Williams M, Yago T, Schmidtke DW, McEver RP. Dynamic alterations of membrane tethers stabilize leukocyte rolling on P-selectin. Proc Natl Acad Sci U S A. 2004;101:13519–24. doi: 10.1073/pnas.0403608101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 15.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–3. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 16.Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, et al. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–83. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- 17.Wittig D, Wang X, Walter C, Gerdes HH, Funk RH, Roehlecke C. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS One. 2012;7:e33195. doi: 10.1371/journal.pone.0033195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–18. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Veranic P, Lokar M, Schütz GJ, Weghuber J, Wieser S, Hägerstrand H, et al. Different types of cell-to-cell connections mediated by nanotubular structures. Biophys J. 2008;95:4416–25. doi: 10.1529/biophysj.108.131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurtig J, Chiu DT, Onfelt B. Intercellular nanotubes: insights from imaging studies and beyond. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:260–76. doi: 10.1002/wnan.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu G, Shao JY. Human neutrophil surface protrusion under a point load: location independence and viscoelasticity. Am J Physiol Cell Physiol. 2008;295:C1434–44. doi: 10.1152/ajpcell.00136.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Goergen CJ, Shao JY. Effect of temperature on tether extraction, surface protrusion, and cortical tension of human neutrophils. Biophys J. 2007;93:2923–33. doi: 10.1529/biophysj.107.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmondson KE, Denney WS, Diamond SL. Neutrophil-bead collision assay: pharmacologically induced changes in membrane mechanics regulate the PSGL-1/P-selectin adhesion lifetime. Biophys J. 2005;89:3603–14. doi: 10.1529/biophysj.105.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh H, Diamond SL. Ethanol enhances neutrophil membrane tether growth and slows rolling on P-selectin but reduces capture from flow and firm arrest on IL-1-treated endothelium. J Immunol. 2008;181:2472–82. doi: 10.4049/jimmunol.181.4.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh H, Mohler ER, 3rd, Tian A, Baumgart T, Diamond SL. Membrane cholesterol is a biomechanical regulator of neutrophil adhesion. Arterioscler Thromb Vasc Biol. 2009;29:1290–7. doi: 10.1161/ATVBAHA.109.189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galkina SI, Molotkovsky JG, Ullrich V, Sud’ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp Cell Res. 2005;304:620–9. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Galkina SI, Romanova JM, Stadnichuk VI, Molotkovsky JG, Sud’ina GF, Klein T. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Microbiol. 2009;56:162–71. doi: 10.1111/j.1574-695X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 28.Galkina SI, Stadnichuk VI, Molotkovsky JG, Romanova JM, Sud’ina GF, Klein T. Microbial alkaloid staurosporine induces formation of nanometer-wide membrane tubular extensions (cytonemes, membrane tethers) in human neutrophils. Cell Adh Migr. 2010;4:32–8. doi: 10.4161/cam.4.1.10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galkina SI, Fedorova NV, Serebryakova MV, Romanova JM, Golyshev SA, Stadnichuk VI, et al. Proteome analysis identified human neutrophil membrane tubulovesicular extensions (cytonemes, membrane tethers) as bactericide trafficking. Biochim Biophys Acta. 2012;1820:1705–14. doi: 10.1016/j.bbagen.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nauseef WM. Editorial: Nyet to NETs? A pause for healthy skepticism. J Leukoc Biol. 2012;91:353–5. doi: 10.1189/jlb.1011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupp I, Sologub L, Williamson KC, Scheuermayer M, Reininger L, Doerig C, et al. Malaria parasites form filamentous cell-to-cell connections during reproduction in the mosquito midgut. Cell Res. 2011;21:683–96. doi: 10.1038/cr.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galkina SI, Romanova JM, Bragina EE, Tiganova IG, Stadnichuk VI, Alekseeva NV, et al. Membrane tubules attach Salmonella Typhimurium to eukaryotic cells and bacteria. FEMS Immunol Med Microbiol. 2011;61:114–24. doi: 10.1111/j.1574-695X.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 35.Evans E, Heinrich V, Leung A, Kinoshita K. Nano- to microscale dynamics of P-selectin detachment from leukocyte interfaces. I. Membrane separation from the cytoskeleton. Biophys J. 2005;88:2288–98. doi: 10.1529/biophysj.104.051698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galkina SI, Sud’ina GF, Klein T. Metabolic regulation of neutrophil spreading, membrane tubulovesicular extensions (cytonemes) formation and intracellular pH upon adhesion to fibronectin. Exp Cell Res. 2006;312:2568–79. doi: 10.1016/j.yexcr.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Biswas S, Ray M, Misra S, Dutta DP, Ray S. Is absence of pyruvate dehydrogenase complex in mitochondria a possible explanation of significant aerobic glycolysis by normal human leukocytes? FEBS Lett. 1998;425:411–4. doi: 10.1016/S0014-5793(98)00273-7. [DOI] [PubMed] [Google Scholar]
- 38.Nelson N. Structure and pharmacology of the proton-ATPases. Trends Pharmacol Sci. 1991;12:71–5. doi: 10.1016/0165-6147(91)90501-I. [DOI] [PubMed] [Google Scholar]
- 39.Nishi T, Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 40.Parra KJ, Kane PM. Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol Cell Biol. 1998;18:7064–74. doi: 10.1128/mcb.18.12.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su Y, Zhou A, Al-Lamki RS, Karet FE. The a-subunit of the V-type H+-ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem. 2003;278:20013–8. doi: 10.1074/jbc.M210077200. [DOI] [PubMed] [Google Scholar]
- 42.Lu M, Holliday LS, Zhang L, Dunn WA, Jr., Gluck SL. Interaction between aldolase and vacuolar H+-ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J Biol Chem. 2001;276:30407–13. doi: 10.1074/jbc.M008768200. [DOI] [PubMed] [Google Scholar]
- 43.Lu M, Sautin YY, Holliday LS, Gluck SL. The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J Biol Chem. 2004;279:8732–9. doi: 10.1074/jbc.M303871200. [DOI] [PubMed] [Google Scholar]
- 44.Peters C, Bayer MJ, Bühler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–8. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- 45.Bayer MJ, Reese C, Buhler S, Peters C, Mayer A. Vacuole membrane fusion: V0 functions after trans-SNARE pairing and is coupled to the Ca2+-releasing channel. J Cell Biol. 2003;162:211–22. doi: 10.1083/jcb.200212004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, et al. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell. 2005;121:607–20. doi: 10.1016/j.cell.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liégeois S, Benedetto A, Garnier JM, Schwab Y, Labouesse M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J Cell Biol. 2006;173:949–61. doi: 10.1083/jcb.200511072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liégeois S, Benedetto A, Michaux G, Belliard G, Labouesse M. Genes required for osmoregulation and apical secretion in Caenorhabditis elegans. Genetics. 2007;175:709–24. doi: 10.1534/genetics.106.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci. 2006;119:4531–40. doi: 10.1242/jcs.03234. [DOI] [PubMed] [Google Scholar]
- 50.Baars TL, Petri S, Peters C, Mayer A. Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol Biol Cell. 2007;18:3873–82. doi: 10.1091/mbc.E07-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang W, Wang D, Volk E, Bellen HJ, Hiesinger PR, Quiocho FA. V-ATPase V0 sector subunit a1 in neurons is a target of calmodulin. J Biol Chem. 2008;283:294–300. doi: 10.1074/jbc.M708058200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glaser PE, Gross RW. Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehyde-3-phosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry. 1995;34:12193–203. doi: 10.1021/bi00038a013. [DOI] [PubMed] [Google Scholar]
- 53.Hessler RJ, Blackwood RA, Brock TG, Francis JW, Harsh DM, Smolen JE. Identification of glyceraldehyde-3-phosphate dehydrogenase as a Ca2+-dependent fusogen in human neutrophil cytosol. J Leukoc Biol. 1998;63:331–6. doi: 10.1002/jlb.63.3.331. [DOI] [PubMed] [Google Scholar]
- 54.Glaser PE, Han X, Gross RW. Tubulin is the endogenous inhibitor of the glyceraldehyde 3-phosphate dehydrogenase isoform that catalyzes membrane fusion: Implications for the coordinated regulation of glycolysis and membrane fusion. Proc Natl Acad Sci U S A. 2002;99:14104–9. doi: 10.1073/pnas.222542999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagawa T, Hirano Y, Inomata A, Yokota S, Miyachi K, Kaneda M, et al. Participation of a fusogenic protein, glyceraldehyde-3-phosphate dehydrogenase, in nuclear membrane assembly. J Biol Chem. 2003;278:20395–404. doi: 10.1074/jbc.M210824200. [DOI] [PubMed] [Google Scholar]
- 56.Decker BL, Wickner WT. Enolase activates homotypic vacuole fusion and protein transport to the vacuole in yeast. J Biol Chem. 2006;281:14523–8. doi: 10.1074/jbc.M600911200. [DOI] [PubMed] [Google Scholar]
- 57.Forgac M. The vacuolar H+-ATPase of clathrin-coated vesicles is reversibly inhibited by S-nitrosoglutathione. J Biol Chem. 1999;274:1301–5. doi: 10.1074/jbc.274.3.1301. [DOI] [PubMed] [Google Scholar]
- 58.Wu K, Aoki C, Elste A, Rogalski-Wilk AA, Siekevitz P. The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc Natl Acad Sci U S A. 1997;94:13273–8. doi: 10.1073/pnas.94.24.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohr S, Hallak H, de Boitte A, Lapetina EG, Brüne B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1999;274:9427–30. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 60.Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, et al. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem. 2000;275:32331–7. doi: 10.1074/jbc.M004795200. [DOI] [PubMed] [Google Scholar]
- 61.Chen SH, Bubb MR, Yarmola EG, Zuo J, Jiang J, Lee BS, et al. Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem. 2004;279:7988–98. doi: 10.1074/jbc.M305351200. [DOI] [PubMed] [Google Scholar]
- 62.Vitavska O, Merzendorfer H, Wieczorek H. The V-ATPase subunit C binds to polymeric F-actin as well as to monomeric G-actin and induces cross-linking of actin filaments. J Biol Chem. 2005;280:1070–6. doi: 10.1074/jbc.M406797200. [DOI] [PubMed] [Google Scholar]
- 63.Arnold H, Pette D. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem. 1970;15:360–6. doi: 10.1111/j.1432-1033.1970.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 64.Karp GC, Solursh M. Dynamic activity of the filopodia of sea urchin embryonic cells and their role in directed migration of the primary mesenchyme in vitro. Dev Biol. 1985;112:276–83. doi: 10.1016/0012-1606(85)90398-7. [DOI] [PubMed] [Google Scholar]
- 65.Raghunathan A, Sivakamasundari R, Wolenski J, Poddar R, Weissman SM. Functional analysis of B144/LST1: a gene in the tumor necrosis factor cluster that induces formation of long filopodia in eukaryotic cells. Exp Cell Res. 2001;268:230–44. doi: 10.1006/excr.2001.5290. [DOI] [PubMed] [Google Scholar]
- 66.Schwan C, Stecher B, Tzivelekidis T, van Ham M, Rohde M, Hardt WD, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aktories K, Lang AE, Schwan C, Mannherz HG. Actin as target for modification by bacterial protein toxins. FEBS J. 2011;278:4526–43. doi: 10.1111/j.1742-4658.2011.08113.x. [DOI] [PubMed] [Google Scholar]
- 68.Whipple RA, Cheung AM, Martin SS. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp Cell Res. 2007;313:1326–36. doi: 10.1016/j.yexcr.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lian JP, Marks PG, Wang JY, Falls DL, Badwey JA. A protein kinase from neutrophils that specifically recognizes Ser-3 in cofilin. J Biol Chem. 2000;275:2869–76. doi: 10.1074/jbc.275.4.2869. [DOI] [PubMed] [Google Scholar]
- 70.Messier JM, Shaw LM, Chafel M, Matsudaira P, Mercurio AM. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil Cytoskeleton. 1993;25:223–33. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- 71.Jones SL, Brown EJ. FcgammaRII-mediated adhesion and phagocytosis induce L-plastin phosphorylation in human neutrophils. J Biol Chem. 1996;271:14623–30. doi: 10.1074/jbc.271.24.14623. [DOI] [PubMed] [Google Scholar]
- 72.Shibata M, Ohoka T, Mizuno S, Suzuki K. Characterization of a 64-kd protein phosphorylated during chemotactic activation with IL-8 and fMLP of human polymorphonuclear leukocytes. I. Phosphorylation of a 64-kd protein and other proteins. J Leukoc Biol. 1993;54:1–9. doi: 10.1002/jlb.54.1.1. [DOI] [PubMed] [Google Scholar]
- 73.Paclet MH, Davis C, Kotsonis P, Godovac-Zimmermann J, Segal AW, Dekker LV. N-Formyl peptide receptor subtypes in human neutrophils activate L-plastin phosphorylation through different signal transduction intermediates. Biochem J. 2004;377:469–77. doi: 10.1042/BJ20031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosales C, Jones SL, McCourt D, Brown EJ. Bromophenacyl bromide binding to the actin-bundling protein l-plastin inhibits inositol trisphosphate-independent increase in Ca2+ in human neutrophils. Proc Natl Acad Sci U S A. 1994;91:3534–8. doi: 10.1073/pnas.91.9.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao C, Guo H, Wei J, Mi Z, Wai PY, Kuo PC. Identification of S-nitrosylated proteins in endotoxin-stimulated RAW264.7 murine macrophages. Nitric Oxide. 2005;12:121–6. doi: 10.1016/j.niox.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Keszler A, Broniowska KA, Hogg N. Characterization and application of the biotin-switch assay for the identification of S-nitrosated proteins. Free Radic Biol Med. 2005;38:874–81. doi: 10.1016/j.freeradbiomed.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Clancy R, Leszczynska J, Amin A, Levartovsky D, Abramson SB. Nitric oxide stimulates ADP ribosylation of actin in association with the inhibition of actin polymerization in human neutrophils. J Leukoc Biol. 1995;58:196–202. doi: 10.1002/jlb.58.2.196. [DOI] [PubMed] [Google Scholar]
- 78.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:C1690–700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 79.Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–98. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitchell T, Lo A, Logan MR, Lacy P, Eitzen G. Primary granule exocytosis in human neutrophils is regulated by Rac-dependent actin remodeling. Am J Physiol Cell Physiol. 2008;295:C1354–65. doi: 10.1152/ajpcell.00239.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jena BP. Discovery of the Porosome: revealing the molecular mechanism of secretion and membrane fusion in cells. J Cell Mol Med. 2004;8:1–21. doi: 10.1111/j.1582-4934.2004.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Lominadze G, Powell DW, Luerman GC, Link AJ, Ward RA, McLeish KR. Proteomic analysis of human neutrophil granules. Mol Cell Proteomics. 2005;4:1503–21. doi: 10.1074/mcp.M500143-MCP200. [DOI] [PubMed] [Google Scholar]
- 84.Creutz CE. The annexins and exocytosis. Science. 1992;258:924–31. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- 85.Francis JW, Balazovich KJ, Smolen JE, Margolis DI, Boxer LA. Human neutrophil annexin I promotes granule aggregation and modulates Ca(2+)-dependent membrane fusion. J Clin Invest. 1992;90:537–44. doi: 10.1172/JCI115892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meers P, Mealy T, Tauber AI. Annexin I interactions with human neutrophil specific granules: fusogenicity and coaggregation with plasma membrane vesicles. Biochim Biophys Acta. 1993;1147:177–84. doi: 10.1016/0005-2736(93)90002-H. [DOI] [PubMed] [Google Scholar]
- 87.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–9. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 88.Creutz CE. cis-Unsaturated fatty acids induce the fusion of chromaffin granules aggregated by synexin. J Cell Biol. 1981;91:247–56. doi: 10.1083/jcb.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.MacLaren A, Attias M, de Souza W. Aspects of the early moments of interaction between tachyzoites of Toxoplasma gondii with neutrophils. Vet Parasitol. 2004;125:301–12. doi: 10.1016/j.vetpar.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 90.Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, et al. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–32. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frasson AP, De Carli GA, Bonan CD, Tasca T. Involvement of purinergic signaling on nitric oxide production by neutrophils stimulated with Trichomonas vaginalis. Purinergic Signal. 2012;8:1–9. doi: 10.1007/s11302-011-9254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–78. doi: 10.1016/S0008-6363(99)00187-X. [DOI] [PubMed] [Google Scholar]
- 94.Gilcrease MZ, Hoover RL. Neutrophil adhesion to endothelium following hyperosmolar insult. Diabetes Res. 1991;16:149–57. [PubMed] [Google Scholar]
- 95.Kim SY, Johnson MA, McLeod DS, Alexander T, Hansen BC, Lutty GA. Neutrophils are associated with capillary closure in spontaneously diabetic monkey retinas. Diabetes. 2005;54:1534–42. doi: 10.2337/diabetes.54.5.1534. [DOI] [PubMed] [Google Scholar]
- 96.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 97.Sessa WC. The nitric oxide synthase family of proteins. J Vasc Res. 1994;31:131–43. doi: 10.1159/000159039. [DOI] [PubMed] [Google Scholar]
- 98.Wallerath T, Gath I, Aulitzky WE, Pollock JS, Kleinert H, Förstermann U. Identification of the NO synthase isoforms expressed in human neutrophil granulocytes, megakaryocytes and platelets. Thromb Haemost. 1997;77:163–7. [PubMed] [Google Scholar]
- 99.Greenberg SS, Ouyang J, Zhao X, Giles TD. Human and rat neutrophils constitutively express neural nitric oxide synthase mRNA. Nitric Oxide. 1998;2:203–12. doi: 10.1006/niox.1998.0176. [DOI] [PubMed] [Google Scholar]
- 100.Lefer DJ, Jones SP, Girod WG, Baines A, Grisham MB, Cockrell AS, et al. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–50. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 101.Carey C, Siegfried MR, Ma XL, Weyrich AS, Lefer AM. Antishock and endothelial protective actions of a NO donor in mesenteric ischemia and reperfusion. Circ Shock. 1992;38:209–16. [PubMed] [Google Scholar]
- 102.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitchell DJ, Yu J, Tyml K. Local L-NAME decreases blood flow and increases leukocyte adhesion via CD18. Am J Physiol. 1998;274:H1264–8. doi: 10.1152/ajpheart.1998.274.4.H1264. [DOI] [PubMed] [Google Scholar]
- 104.Banick PD, Chen Q, Xu YA, Thom SR. Nitric oxide inhibits neutrophil beta 2 integrin function by inhibiting membrane-associated cyclic GMP synthesis. J Cell Physiol. 1997;172:12–24. doi: 10.1002/(SICI)1097-4652(199707)172:1<12::AID-JCP2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 105.Kubes P, Kurose I, Granger DN. NO donors prevent integrin-induced leukocyte adhesion but not P-selectin-dependent rolling in postischemic venules. Am J Physiol. 1994;267:H931–7. doi: 10.1152/ajpheart.1994.267.3.H931. [DOI] [PubMed] [Google Scholar]
- 106.Kosonen O, Kankaanranta H, Malo-Ranta U, Moilanen E. Nitric oxide-releasing compounds inhibit neutrophil adhesion to endothelial cells. Eur J Pharmacol. 1999;382:111–7. doi: 10.1016/S0014-2999(99)00581-6. [DOI] [PubMed] [Google Scholar]
- 107.Alam MS, Akaike T, Okamoto S, Kubota T, Yoshitake J, Sawa T, et al. Role of nitric oxide in host defense in murine salmonellosis as a function of its antibacterial and antiapoptotic activities. Infect Immun. 2002;70:3130–42. doi: 10.1128/IAI.70.6.3130-3142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.MacFarlane AS, Schwacha MG, Eisenstein TK. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun. 1999;67:891–8. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–36. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klink M, Cedzyński M, St Swierzko A, Tchórzewski H, Sulowska Z. Involvement of nitric oxide donor compounds in the bactericidal activity of human neutrophils in vitro. J Med Microbiol. 2003;52:303–8. doi: 10.1099/jmm.0.04974-0. [DOI] [PubMed] [Google Scholar]
- 111.McCollister BD, Bourret TJ, Gill R, Jones-Carson J, Vázquez-Torres A. Repression of SPI2 transcription by nitric oxide-producing, IFNgamma-activated macrophages promotes maturation of Salmonella phagosomes. J Exp Med. 2005;202:625–35. doi: 10.1084/jem.20050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stevanin TM, Poole RK, Demoncheaux EA, Read RC. Flavohemoglobin Hmp protects Salmonella enterica serovar typhimurium from nitric oxide-related killing by human macrophages. Infect Immun. 2002;70:4399–405. doi: 10.1128/IAI.70.8.4399-4405.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the salmonella flavohemoglobin hmp. J Biol Chem. 2006;281:28039–47. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- 114.Klink M, Bednarska K, Jastrzembska K, Banasik M, Sulowska Z. Signal transduction pathways affected by nitric oxide donors during neutrophil functional response in vitro. Inflamm Res. 2007;56:282–90. doi: 10.1007/s00011-007-6205-4. [DOI] [PubMed] [Google Scholar]
- 115.Machado JD, Segura F, Brioso MA, Borges R. Nitric oxide modulates a late step of exocytosis. J Biol Chem. 2000;275:20274–9. doi: 10.1074/jbc.M000930200. [DOI] [PubMed] [Google Scholar]
- 116.Lowenstein CJ. Nitric oxide regulation of protein trafficking in the cardiovascular system. Cardiovasc Res. 2007;75:240–6. doi: 10.1016/j.cardiores.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ginocchio CC, Olmsted SB, Wells CL, Galán JE. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–24. doi: 10.1016/0092-8674(94)90510-X. [DOI] [PubMed] [Google Scholar]
- 118.Reed KA, Clark MA, Booth TA, Hueck CJ, Miller SI, Hirst BH, et al. Cell-contact-stimulated formation of filamentous appendages by Salmonella typhimurium does not depend on the type III secretion system encoded by Salmonella pathogenicity island 1. Infect Immun. 1998;66:2007–17. doi: 10.1128/iai.66.5.2007-2017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Follett EA, Gordon J. An Electron Microscope Study of Vibrio Flagella. J Gen Microbiol. 1963;32:235–9. doi: 10.1099/00221287-32-2-235. [DOI] [PubMed] [Google Scholar]
- 120.Seidler RJ, Starr MP. Structure of the flagellum of Bdellovibrio bacteriovorus. J Bacteriol. 1968;95:1952–5. doi: 10.1128/jb.95.5.1952-1955.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Geis G, Suerbaum S, Forsthoff B, Leying H, Opferkuch W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J Med Microbiol. 1993;38:371–7. doi: 10.1099/00222615-38-5-371. [DOI] [PubMed] [Google Scholar]
- 122.Allen RD, Baumann P. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J Bacteriol. 1971;107:295–302. doi: 10.1128/jb.107.1.295-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sjoblad RD, Emala CW, Doetsch RN. Invited review: bacterial flagellar sheaths: structures in search of a function. Cell Motil. 1983;3:93–103. doi: 10.1002/cm.970030108. [DOI] [PubMed] [Google Scholar]
- 124.McCarter LL. Polar flagellar motility of the Vibrionaceae. Microbiol Mol Biol Rev. 2001;65:445–62. doi: 10.1128/MMBR.65.3.445-462.2001. [table of contents.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jauffred L, Callisen TH, Oddershede LB. Visco-elastic membrane tethers extracted from Escherichia coli by optical tweezers. Biophys J. 2007;93:4068–75. doi: 10.1529/biophysj.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Low HH, Löwe J. A bacterial dynamin-like protein. Nature. 2006;444:766–9. doi: 10.1038/nature05312. [DOI] [PubMed] [Google Scholar]
- 127.Low HH, Sachse C, Amos LA, Löwe J. Structure of a bacterial dynamin-like protein lipid tube provides a mechanism for assembly and membrane curving. Cell. 2009;139:1342–52. doi: 10.1016/j.cell.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lanzer M, Wickert H, Krohne G, Vincensini L, Braun Breton C. Maurer’s clefts: a novel multi-functional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. Int J Parasitol. 2006;36:23–36. doi: 10.1016/j.ijpara.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 129.Mota LJ, Ramsden AE, Liu M, Castle JD, Holden DW. SCAMP3 is a component of the Salmonella-induced tubular network and reveals an interaction between bacterial effectors and post-Golgi trafficking. Cell Microbiol. 2009;11:1236–53. doi: 10.1111/j.1462-5822.2009.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–31. doi: 10.1016/S0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 131.Burlak C, Whitney AR, Mead DJ, Hackstadt T, Deleo FR. Maturation of human neutrophil phagosomes includes incorporation of molecular chaperones and endoplasmic reticulum quality control machinery. Mol Cell Proteomics. 2006;5:620–34. doi: 10.1074/mcp.M500336-MCP200. [DOI] [PubMed] [Google Scholar]