Abstract
The perivascular niche for neurogenesis was first reported as the co-association of newly generated neurons and their progenitors with both dividing and mitotically quiescent endothelial cells in restricted regions of the brain in adult birds and mammals alike. This review attempts to summarize our present understanding of the interaction of blood vessels with neural stem and progenitor cells, addressing both glial and neuronal progenitor cell interactions in the perivascular niche. We review the molecular interactions that are most critical to the endothelial control of stem and progenitor cell mobilization and differentiation. The focus throughout will be on defining those perivascular ligand-receptor interactions shared among these systems, as well as those that clearly differ as a function of cell type and setting, by which specificity may be achieved in the development of targeted therapeutics.
Neural stem cells (NSCs) and more restricted neuronal and glial progenitor cells are dispersed widely throughout the adult vertebrate brain1–3. Long after fetal development, multipotent NSCs continue to reside in the forebrain ventricles of experimental animals and humans alike4,5, whereas committed neuronal progenitor cells also remain in both the subependyma of the lateral ventricles6–9 and the subgranular zone of the hippocampal dentate gyrus10,11. In adult mammals, active neurogenesis persists in each of these regions, an extensive subject that has recently been reviewed elsewhere12. In addition, a larger pool of glial progenitor cells pervades adult tissue parenchyma (reviewed in ref. 13). All of these progenitor populations persist in adult humans and, as such, all are potential therapeutic targets (reviewed in ref. 14).
As stem cell progeny depart their localized niches of stem cell maintenance, they commit to more restricted lineages, at which point they are still mitotic, but subject to senescence15. Accordingly, lineage-restricted neuronal progenitor cells of the forebrain subependyma and hippocampus serve as transit-amplifying cells16,17, as do glial progenitor cells of the white matter, which are similarly able to divide and yield phenotypically restricted daughters, and yet are incapable of sustained self-renewal18. Many recent studies have investigated those features that both define and distinguish the stem cell microenvironment from that of transit-amplifying progenitors (reviewed in ref. 19). These studies have revealed that, in both the mammalian ventricular wall20,21 and hippocampus22, as well as in the neurogenic regions of the avian brain23, the local microvascular bed is important for providing a permissive environment for NSC expansion, neuronal differentiation and parenchymal migration. This review will focus on the anatomy and molecular constituents of the perivascular niche for adult neurogenesis, as a means of defining those processes by which addition of new neurons to adult brain tissue occurs naturally, and by which it might be enabled heterotopically.
The perivascular niche for neurogenesis
The normal perivascular environment of the brain includes endothelial cells, smooth muscle pericytes and fibroblasts, as well as microglia, glial progenitors and astrocytic endfeet. The combinatorial interactions among these cells and their region-specific responses to local signaling cues provide a rich set of perivascular microenvironments by which newly generated cells may be serially engaged by both endothelial and non-endothelial cytokines at distinct stages during their mitogenesis, differentiation and parenchymal recruitment23–27.
The perivascular niche for neurogenesis was first described in the adult mammalian hippocampus as the anatomically contiguous co-association of newly generated neurons with dividing endothelial cells22. A direct role for endothelial mitogens in driving this process was then demonstrated in vitro, providing initial evidence that the co-association of endothelial cells with dividing NSCs might be causal28. These studies followed earlier reports that angiogenesis preceded neuronal recruitment in an avian model of hormonally modulated neuronal addition, the songbird vocal control nucleus HVC29. In this model of noncontiguous angiogenesis and neurogenesis, endothelial expansion was found to result in the production of vascular neurotrophins that both directed the recruitment of newly generated neurons to the angiogenic vascular bed and supported their differentiation and survival23. Together, these studies led to the concept of a perivascular niche for neurogenesis, in which neural stem and progenitor cells serially receive instructive and permissive cues from the local microvasculature, enabling both their proliferative expansion and tissue invasion. This concept has proven to be applicable to the development of multiple organ systems, where tissue-defined vascular beds have been found to direct cell fate, proliferative expansion30 and tumor invasion31.
Perivascular agents serially support neuronal development
In the adult mammalian brain, neuronal production is typically restricted to a few discrete regions of subependymally derived granule cell neurogenesis3. In each venue, stem cell mobilization and progenitor cell expansion appear to occur in tight co-association with the local capillary microvasculature. In the hippocampus, Palmer and Gage first reported the contiguous co-association of angiogenesis and neurogenesis in the subgranular zone of the adult dentate gyrus22, suggesting that endothelial cells and neural progenitor cells were either responding to a common mitogenic signal or that the dividing cells of one phenotype generated mitogens that were able to trigger the synchronous mitotic expansion of the other. Several groups subsequently reported that that the proliferative expansion of hippocampal endothelial and neuronal progenitor cells is co-regulated by vascular endothelial growth factor (VEGF), such that hippocampal neurogenesis might, in effect, be co-regulated with angiogenesis22,32–34 (Fig. 1). Notably, another possibility is that newly generated neurons and endothelial cells both derive from a common stem cell with both neural and hemangioblastic lineage competence35, a still-controversial concept that was recently revived by demonstrations of endothelial generation from malignant glioma cells36.
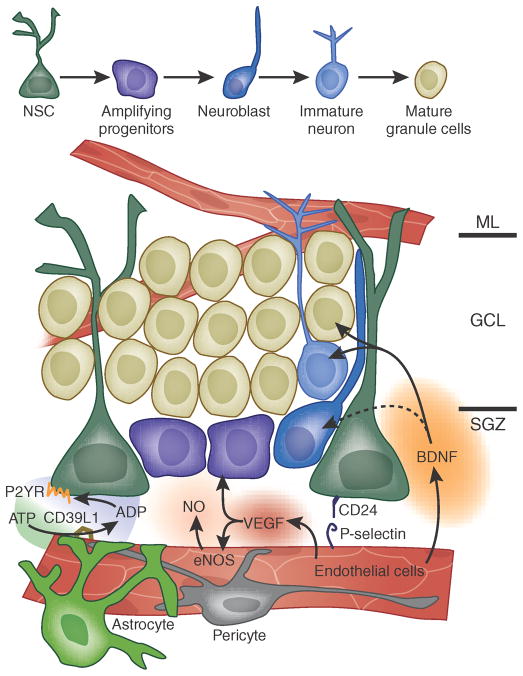
Figure 1.
Perivascular interactions within the subgranular zone of the adult mammalian hippocampus. This cartoon illustrates the architecture of the subgranular zone and dentate granule layer of the adult hippocampus and shows the relationships of hippocampal NSCs to transit-amplifying progeny and their neuronally restricted progeny. Only those interactions identified as being specifically between the microvascular bed and hippocampal stem and progenitor cells are noted; many mitogens and differentiation agents of nonvascular origin have otherwise been defined in the hippocampus. The subgranular zone of the dentate gyrus manifests a cellular hierarchy that is similar to that of the ventricular subependyma. Major molecular interactions, as cited in the text, include the provision of ATP to vascular NTPDase-2/CD39L1, with generation of ADP that interacts with P2YRs expressed by the stem cell pool, vascular VEGF, which interacts with both endothelial and neural precursor receptors, CD24 and its endothelial P-selectin receptors, both endothelial NOS (eNOS) and precursor-derived neuronal NOS (nNOS), with their derived NO and targets thereof, and endothelial BDNF and its neuronal receptors. GCL, granule cell layer; ML, molecular layer; SGZ, subgranular zone.
As in the hippocampus, subependymal capillaries in the wall of the lateral ventricles may serve to regulate both the mitotic activity of neural stem and progenitor cells, and the differentiation of their neuronal progeny20,21,24. Brain microvascular endothelial cells support the differentiation and survival of newly generated neuroblasts in explants of the adult striatal ventricular wall, by serving as rich sources of secreted brain-derived neurotrophic factor (BDNF)24. The mitotic expansion of cultured NSCs can also be supported by endothelial cells28, and subsequent neuronal parenchymal invasion and progenitor expansion can be supported by this subependymal capillary network20 (Fig. 2). Similarly, the potentially causal relationship between angiogenesis and neuronal addition in the adult songbird brain has also been examined, as testosterone treatment triggers both angiogenesis and neuronal addition to the principal song control nucleus, HVC23. Testosterone induces the local production of VEGF in HVC, and the resultant VEGF-mediated induction of local angiogenesis is followed by the recruitment of newly generated neurons from the overlying ventricular zone to the mitotically active capillary bed.
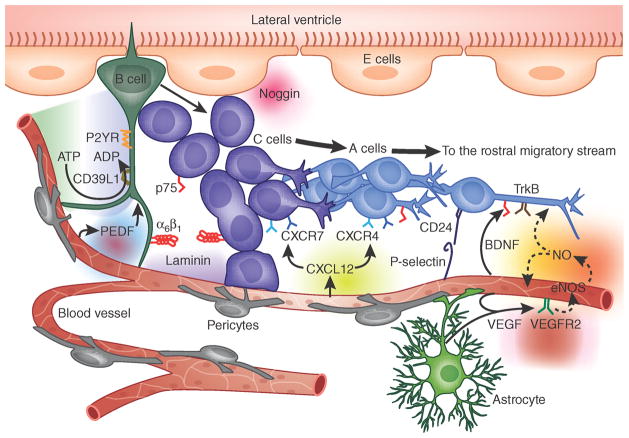
Figure 2.
Perivascular interactions with the adult mammalian subependyma. This cartoon illustrates the architecture of the striatal ventricular wall and shows the relationships of subependymal NSCs (B cells, according to the nomenclature of Alvarez-Buylla3,19,75) to transit-amplifying progeny (C cells) and their neuronally restricted progeny (A cells). Major molecular interactions, as citevd in the text, include the provision of ATP to vascular NTPDase-2/CD39L1, with generation of ADP that interacts with B and C cell P2YRs, vascular VEGF and PEDF, which interact with cognate B cell receptors, B cell CD15 and C and A cell CD24 with their endothelial P-selectin receptors, endothelial SDF1/CXCL12 with its A cell receptors CXCR4 and CXCR7, endothelial BDNF with both its B and C cell p75 and A cell trkB receptors, endothelial laminin with its C and A cell α6β1 integrin receptor, and both endothelial eNOS and neural precursor-derived nNOS, and their derived NO with cellular targets thereof.
Adult neurogenic regions can therefore exhibit contiguous mitotic angiogenesis and neurogenesis, as in the hippocampus, contiguous but mitotically quiescent endothelial support of neuronal progenitor cell expansion, as in the ventricular subependyma, or noncontiguous regulation of neuronal recruitment via endothelial chemotactic and neurotrophic cytokines, as in the HVC and in mammalian neurogenic regions. In broad terms, each of these model systems of adult neurogenesis exhibits a critical need for perivascular elements in providing instructive cues for neuronal production and migration. Chief among these perivascular signals for stem and progenitor cell expansion are activators of several important neural mitogenic pathways that include purinergic signaling, nitric oxide signaling, and both VEGF-dependent and pigment epithelium–derived factor (PEDF)-dependent signaling, each of which appear to act in concert with non-perivascular cytokines to coordinate NSC division and self-renewal. In addition, after initial asymmetric division to yield transit-amplifying progenitors, a different set of agents that promote neuronal differentiation and survival then become more germane. These include BDNF, acting in concert with matrix-derived signals encountered by newly generated neurons on their transit from the subependyma to the perivascular niche (Fig. 2). We will discuss each of these regulatory steps in turn.
Perivascular purinergic signaling in the neurogenic niche
Signaling by the P2Y class of purine receptors (P2YRs) contributes to NSC self-renewal37. In particular, ADP can signal through P2YR1, P2YR2 and P2YR4 to drive the self-renewing expansion of both murine and human NSCs. Several groups have identified perivascular astrocytes and possibly pericytes in neurogenic regions as critical sources of the CD39L1 nucleotide trisphosphate dinucleotide phosphorylase (NTPDase-2), an ectonucleotidase that degrades ATP to ADP, thereby making ADP available to activate P2YR signaling. In particular, high concentrations of CD39L1 in neurogenic regions of the adult rodent brain have been identified38, and ectonucleotidase activity is present in high concentrations in the vascular beds subserving both the sub-ependymal and subgranular zones, the two major neurogenic niches of the adult forebrain37. P2YR1 in particular is expressed by NSCs, which release ATP. On the basis of these observations, we can postulate that ATP release by neural stem and progenitor cells, coupled with regionally restricted expression of the CD39L1 NTPDase-2 by the subependymal and subgranular zone vascular beds, may provide purine nucleotides to purine receptive progenitors at levels sufficient to establish an autonomous paracrine network favoring perivascular expansion.
These data suggest that perivascular purinergic signaling may be critical for the self-renewing expansion of subependymal neural progenitor cells. Indeed, purinergic signals may dictate the proliferative expansion not only of neurogenic NSCs, but of glial progenitor cells, which may be similarly responsive to extracellular purine nucleotides39,40.
Perivascular nitric oxide signaling regulates cell genesis
Nitric oxide (NO) signaling is a critical regulator of both angiogenesis and neurogenesis. In light of the frequency of NO-producing cells in stem cell niches, the role of NO in regulating angiogenesis in a number of systems and the concurrence of neuronal mitogenesis in some NO-producing regions of the adult brain, NO has been proposed to be an important regulator of cell genesis in the perivascular niche41,42. A series of initial reports highlighted the role of NO as a tonic repressor of adult neurogenesis by showing that the nitric oxide synthase (NOS) inhibitors L-NAME and 7-NI increased neurogenesis in both the rodent dentate gyrus and subventricular zone41,43–45. Similarly, it was noted that NO donors suppressed the proliferation of cultured NSCs, and were identified as doing so by the inhibition of the epidermal growth factor receptor (EGFR)-dependent PI3K/Akt signaling46. Interestingly, although NO acts to suppress NSC expansion, NSCs themselves release NO, which may in turn feed back on the vascular bed to induce the production of both BDNF and VEGF47. This observation introduces the possibility of reciprocal interactions by which mobilized NSCs can modulate their own niche for neurogenesis, thereby potentiating BDNF-mediated neuronal differentiation and postmitotic neurotrophic support (Fig. 2).
In addition to acting directly on receptive G protein–coupled receptors in both NSCs and non-neural vascular components, NO can also modify signaling through receptor tyrosine kinases, which are critical modulators of NSC expansion. As a case in point, EGF has been identified as a potent mitogen for NSCs; it appears to be largely of glial origin in vivo. Yet NO can induce the reversible S-nitrosylation of the EGFR, and may thereby suppress EGF-dependent NSC expansion48. Notably, such nitrosylation-based receptor inactivation is a redox state–dependent event and, as such, it may serve to link the fate decisions of NSCs to tissue oxidation state. These observations may have especially broad implications for the influence of ischemia on the role of the perivascular niche in neural stem mobilization.
In that regard, although most in vivo pharmacological studies have indicated that NO acts to tonically suppress neurogenesis in the normal adult brain, its effects may be vastly different under conditions of hypoxic ischemic stress, especially given the number of concurrently active signaling systems modulated by NO. Thus, although most earlier studies emphasized that NO serves to inhibit both NSC expansion and neuronal production, a number of recent studies have stressed the cell type, dose and context dependence of NO’s effects41,42,49–52. For instance, several studies have indicated that, in the unperturbed CNS, neuronal NOS, and hence neuronally derived NO, may suppress NSC expansion by potentiating their neuronal differentiation in a BDNF-dependent manner through the NO-triggered release of BDNF by NO-receptive NSCs42. On the other hand, a recent report noted that under conditions of hypoxic stress, inducible NOS (iNOS)-generated NO can potentiate stem cell proliferation in a p21Ras/MAPK-dependent manner51. Interestingly, this report implicated iNOS in the excessive and heterotopic hippocampal neurogenesis that has been noted in response to seizure activity. Taken together with independent observations that NOS activity promotes stem cell expansion in hypoxia50 and gliomas53,54, these findings might lead one to postulate that the ambient oxygen tension might modulate the cellular effects of NO such that its role in the normal adult perivascular environment may differ from that in the more hypoxic environments of ischemic tissue and tumors. Clearly, further dissection of the relative roles of NO as released by the different constituents of the perivascular niche, considering the distinct thresholds for NOS-mediated NO production by each of the three NOS isoforms, NOS1–3, and the relative effects on hypoxic ischemia and other pathological redox states, is needed before we can fully describe NO’s actions in the perivascular niche for neurogenesis.
Endothelial support of NSC expansion and differentiation
The secreted glycoprotein PEDF (also known as serpin-F1), previously recognized as having anti-angiogenic actions in the retina55, was further identified as a subependymal vascular signal for NSC expansion56. A series of subsequent studies demonstrated that PEDF acts to promote stem cell self-renewal via potentiating Notch-dependent transcription, at least in part by relieving the nuclear receptor co-receptor–mediated repression of Notch-responsive promoters57. The critical role of Notch signals in maintaining adult NSC pools has been well described58 and may be further potentiated in the perivascular niche by the endothelial expression of Dll4, a notch ligand that is selective to vascular beds59,60. As such, PEDF defines a niche signal biasing the symmetric division of NSCs, thus potentiating their undifferentiated expansion to the detriment of neuronal differentiation. In so doing, PEDF’s actions may prove to be complementary to those of purinergic signals, which appear to drive symmetric stem cell expansion while concurrently suppressing neuronal differentiation37.
In contrast with perivascular mitogens, such as the purines and PEDF, endothelial BDNF appears to be critical for the induction of neuronal differentiation by stem cell progeny following their asymmetric division to give rise to transit-amplifying daughters24. As such, it is important for regions of NSC expansion to exclude BDNF, and to therefore limit its availability to areas of desired neuronal differentiation and maturation. In the normal adult ventricular wall, parenchymal striatal BDNF is excluded from the subependymal stem cell population by a dense layer of subependymal astrocytes expressing truncated trkB61, which may act as a scavenger receptor to prevent BDNF’s reception by periventricular NSCs. However, on their transit from the ventricular wall, stem cell progeny quickly encounter perivascular BDNF, which in turn potentiates their neuronal differentiation and maturation27. This sequence is also suggested by the pattern of receptor expression by NSCs and their transit-amplifying progeny (type B and C cells, respectively)3; although both express the low-affinity neurotrophin receptor p75 (ref. 62), only lineage-restricted migratory neuroblasts (type A cells) appear to express the high-affinity BDNF receptor trkB63,64. Indeed, p75 expression may define the potentially neurogenic fraction of subependymal cells62. The absence of trkB expression in NSCs and early transit-amplifying neuronal progenitors and its subsequent appearance only on later-stage neuroblasts suggests that BDNF’s actions in vivo may be principally directed toward neuronal differentiation and postmitotic survival. Similarly, analysis of nestin-GFP transgenic mice has shown that trkB expression by newly generated hippocampal granule neurons develops with their postmitotic maturation65; cycling stem and progenitor cells in the subgranular zone of the dentate gyrus express little trkB. Although the largely postmitotic effects of BDNF on neuronal maturation and survival was predicted by earlier studies in vitro9,66,67, only recently has the critical role of endothelial cells in providing that BDNF become appreciated.
Vascular chemokines as directional modulators of migration
Although subependymally situated capillary vessels appear to strongly potentiate NSC expansion and initial neuronal mitogenesis, once daughter cells depart the subependymal niche, the parenchymal perivascular bed becomes a source of both trophic support and migratory cues for the newly generated neuroblasts. These perivascular signals for neuroblast immigration are both contact dependent and humoral. In regard to the former, a host of matrix constituents that include tenascin, laminin and its family members, the heparan sulfates and the chondroitin sulfate proteoglycans have all been identified as perivascular substrates for neuronal migration. Several groups have focused on laminin and have reported that the transit-amplifying daughter cell progeny of NSCs express α6β1 integrin, which serves as a laminin receptor, and may permit neuronal progenitors to migrate into the brain parenchyma20,68.
In the rostral migratory stream, the rostral extension of the ventricular subependyma, this association of newly generated neuroblasts with the adluminal surfaces of capillaries is maintained; neuroblasts migrate along a physical scaffold of longitudinally oriented capillary endothelial cells26,69. In this microvascular network, humoral signals for neuronal differentiation and survival become an integral part of the perivascular niche. Endothelial BDNF in particular appears to act as both a chemoattractant and survival factor for new neuronal migrants27, recruiting them to the local microvascular bed and supporting their survival as they transit to the BDNF-rich internal granular layer of the olfactory bulb. Notably, the serial attraction and support of newly generated neurons by microvascular endothelial-derived BDNF proceeds in a manner akin to that by which local endothelial BDNF attracts newly generated neurons to the adult HVC of canaries. As such, endothelial BDNF–directed neuronal immigration may prove be a conserved mechanism of neuronal addition to neurogenic regions of both the adult mammalian and avian brain. Indeed, the location of microvascular beds rich in endothelial-derived BDNF may predict that those regions are permissive for neuronal addition in adulthood.
In addition to BDNF, a broad set of endothelial and pericytic cytokines have been associated with the direction and support of adluminal perivascular migration by newly generated neuroblasts. CXCR4 and CXCR7 have been especial targets of interest, as these SDF1/CXCL12 receptors are expressed on newly generated neuroblasts, both in development and in the adult. In a recent study, Temple and colleagues70 demonstrated that the subependymal capillary bed expresses high levels of SDF1/CXCL12, which may attract CXCR4-expressing neuroblasts to leave the subependyma, while concurrently triggering their upregulation of α6β1 integrin. This integrin isoform mediates the migration of these neuroblasts along the laminin-rich adluminal walls of the parenchymal microvasculature, whereupon the new neuronal migrants achieve access to endothelial BDNF and its attendant differentiative and trophic support (Fig. 2).
Niche interactions regionally specify neurogenesis
Neuronal addition to the adult brain entails serial events that are orchestrated by both spatially and temporally distinct signals to which neuroblasts are exposed as they traverse distinct compartments that together comprise the neurogenic niche. Thus, in both the avian and mammalian adult ventricular wall, the persistence of neurogenesis reflects the unique interaction of the periventricular environment and its constituent phenotypes with the subventricular microvascular bed, which together may provide the combinatorially specific array of signals that both permit and potentiate neurogenesis. The ventricular wall itself is comprised of both ependymal cells and subependymal astrocytes, each of which may provide unique neurogenic signals that act together to provide a permissive environment for neuronal production. These signals include the regionally delimited provision by ependymal cells of the BMP inhibitor noggin, which permits NSC daughters to escape a BMP-driven glial fate and instead differentiate as neurons71. Such ependymal cell–derived neurogenic signals may be induced by the interactions of these cells with the ventricular cerebrospinal fluid (CSF) with which they are in contact; CSF contains a panoply of humoral signals, such as Sonic Hedgehog and insulin-like growth factor 2, that may directly activate subependymal cells through their primary cilia, which traverse the ependymal layer to contact the ventricular CSF72–74.
Yet the periventricular provision of a permissive environment for NSC expansion and neurogenesis may also be the product of unique combinatorial interactions between ependymal and subependymal cells and the subventricular microvasculature. For instance, the provision by ependymal cells of a neurogenic environment might be triggered by the subependymal cells as a product of their concurrent interactions with the CSF apically, and the local subependymal vasculature basally. Indeed, it seems likely that the combinatorial integration of signals from both CSF and the perivascular niche, in tandem with a reciprocal interaction with adjacent ependymal cells, might dictate the geographic restriction of adult neurogenesis to discrete regions of the adult ventricular wall. Surprisingly, despite many years of elegant studies of the anatomy of the adult mammalian subependyma75,76, relatively few studies have investigated the paracrine interactions among cells in the adult ventricular wall, the combinatorial nature of which defines the regional specificity of neurogenesis and gliogenesis.
Neuronal addition in the adult songbird brain
In the adult songbird brain, neurogenesis persists in several regions of the forebrain responsive to gonadal steroids, including the forebrain vocal control nucleus HVC1,29. In adult canaries, testosterone-induced neurogenesis in HVC was uniformly preceded by a marked increase in endothelial cell division and angiogenesis29. BDNF is known to be generated by microvascular endothelial cells24 and to have a role in directing the neuronal differentiation of subventricular zone progeny66,67,77. Testosterone has been shown to trigger the production of VEGF by large neurons in HVC; VEGF then acts through VEGF receptor 2 to stimulate the mitotic expansion of HVC capillaries23. The expanded endothelial cell population then serves as a source of secreted BDNF, which acts to support the recruitment of newly generated neurons from the overlying ventricular zone (Fig. 3). Pharmacological inhibition of VEGF receptor 2 blocks both HVC angiogenesis and neuronal addition23, whereas local overexpression of BDNF without either androgen treatment or antecedent angiogenesis is sufficient to restore both HVC neuronal addition and song78. Thus, testosterone-induced neuronal addition in the HVC appears to require antecedent androgen-stimulated angiogenesis, with the release of BDNF by the expanded HVC microvascular bed. As such, the songbird HVC exemplifies the paracrine support of neuronal recruitment by a noncontiguous microvascular bed, which then attracts new neuronal migrants to the perivascular niche in which those neurons can mature and integrate.
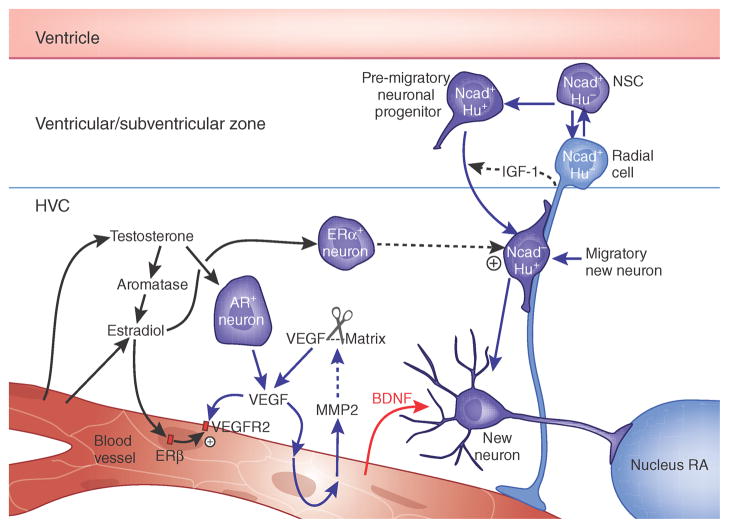
Figure 3.
Angiogenesis and neurogenesis in the adult songbird brain. Testosterone-induced neuronal addition to the adult songbird vocal control center, HVC, requires the androgenic induction of VEGF, followed by VEGF-stimulated matrix metalloproteinase release and angiogenesis. The expanded vasculature acts as a source of BDNF, which supports the immigration of new neurons from the overlying ventricular zone23,77,78,80. AR, androgen receptor; ER, estrogen receptor; Nucleus RA, the HVC target nucleus robustus archistriatalis.
A paradoxical feature of androgen-induced HVC angiogenesis has been its rapid rate of onset; in testosterone-treated females, endothelial proliferation begins within 2–3 days of treatment, even though VEGF mRNA levels are not detectably elevated until 4 days after treatment23. The rapidity of the capillary response to androgen treatment suggested that HVC angiogenesis might be subject to additional mechanisms of androgen-linked control, which might in turn regulate HVC neuronal addition. In that regard, during both development and tumor invasion, angiogenesis is facilitated by the activity of matrix metalloproteases (MMPs), particularly the gelatinases MMP2 and MMP9, which act to release matrix-bound angiogenic cytokines79. Testosterone has been shown to induce perivascular production of MMPs in HVC and potentiate neuronal addition80 by rapidly and specifically inducing perivascular gelatinase activity in HVC. Notably, testosterone-treated birds given a gelatinase inhibitor exhibited marked reductions in endothelial cell division, accompanied by substantially diminished HVC neuronal recruitment80. These experiments revealed endothelial MMP2 to be a critical regulator of cytokine access in the perivascular niche; its upregulation appeared to be necessary for the progression of both angiogenesis and neuronal addition, likely through the release of matrix-bound cytokines critical to both processes. In adult songbirds, the early androgenic induction of endothelial MMP2 thus comprises an important regulatory checkpoint for neuronal addition to HVC. In adult mammals, an analogous process of erythropoietin-triggered release of MMP2 and MMP9 appears to be necessary for the perivascular immigration of neuroblasts into the neostriatum after hypoxic ischemia81. As such, the endothelial production of matrix metalloproteinases may be critical for the perivascular provision of a permissive environment for neuronal immigration in avian and mammalian brain alike.
Coordination of angiogenesis and neurogenesis in disease
The perivascular space may provide a critical niche for the mobilized expansion and differentiation of new cells recruited in response to injury or disease, as well as a conduit for their directed migration. A number of studies have documented both the mobilization of NSCs after ischemia and the subsequent migration of newly generated neurons into affected regions82–84. Notably, the neuronal migrants typically follow adluminal migratory paths and appear to require the perivascular space for both migratory and neurotrophic support25,85. Similarly, the striatal capillary bed appears to provide the principal migratory route by which new neuroblasts invade the adult striatum in response to BDNF overexpression86.
Yet despite this robust phenomenology documenting the use of perivascular routes for neuronal penetration into, and transit through, adult brain parenchyma, little is known of the specific signals that mediate this process. In a similar vein, the molecular signals that trigger the initial expansion of NSCs in response to injury remain unclear. In both cases, however, many of the ligands and pathways implicated in homeostatic NSC turnover are likely to be involved. For instance, in response to injury or ischemia, damaged astrocytes may cooperate with the local vascular endothelium to mobilize the resident progenitor pool: astrocytes release substantial bursts of ATP in response to injury that initiate inter-glial calcium waves, which in turn trigger further ATP release in the astrocytic syncytium87. In the perivascular niche of the adult subependyma, this advancing wave of interstitial ATP release might be expected to encounter a subependymal capillary bed unique for its high-level expression of CD39L1, which would in turn be expected to catalyze the rapid local production of ADP37,38. As a P2YR1 agonist, the resultant interstitial burst of ADP would be expected to potentiate NSC expansion. Similarly, a number of potent angiogenic factors able to co-regulate NSC expansion, such as VEGF, EGF, FGF2 and NO, have been found to be locally upregulated in response to both ischemia and injury, and might be expected to link angiogenesis with the neurogenic responses to these insults88.
Neuroimmune modulation of the perivascular niche
A number of studies have focused on the specific effects of both stress and inflammation on hippocampal neurogenesis, and have emphasized the role of inflammatory mediators in modulating both NSC turnover and neuronal mitogenesis. Local inflammation within brain parenchyma is often angiocentric, and the effects of inflammation on the perivascular niches for both neurogenesis and gliogenesis may be profound.
The effects of inflammation on both progenitor mobilization and the perivascular modulation thereof have been well-studied in radiation injury, which is associated with both diminished stem and progenitor cell turnover and substantial local inflammation. In particular, radiation-induced decrements in hippocampal neurogenesis have been shown to be products of post-radiation inflammation, which is largely perivascular89. Post-radiation dentate granule neurogenesis could thus be restored in part by treatment with the nonsteroidal anti-inflammatory medication indomethacin90. As such, the cellular mechanisms by which perivascular inflammation can influence neuronal and glial cell genesis in the CNS comprise highly attractive targets for future investigation.
The perivascular niche for gliogenesis
Normal glial progenitor cells of the adult forebrain parenchyma, as well as their neoplastic derivatives, may also reside and selectively expand in a perivascular niche. Indeed, the endothelial provision of a perivascular niche for gliogenesis has become of great interest in regard to glial turnover and fate in the adult white matter, in which a number of potential ligand-receptor systems have been identified that may comprise targets for modulating astrocytic and oligodendrocytic recruitment. Sim and colleagues91 first established the gene expression repertoire of adult glial progenitor cells and identified a number of ligand-receptor relationships that suggested specific vascular ligands of potential importance to the homeostatic maintenance of glial and oligodendrocyte progenitors. Foremost among these was the pleiotrophin–receptor tyrosine phosphatase-β/ζ (RTPZ) system. PTPRZ1, the gene encoding receptor tyrosine phosphatase-β/ζ, is selectively expressed by adult glial progenitors91, whereas its primary ligand, pleiotrophin, may be generated both autogenously and by endothelial cells92. Pleiotrophin has been shown to stimulate stem cell expansion in a number of tissues, including tumors, in whose genesis and anaplastic progression it has been implicated93. Thus, endothelial pleiotrophin, and its consequent suppression of glial progenitor cell–borne RTPZ, might be expected to result in the perivascular mobilization of glial progenitor cells, providing a route for the induction of glial progenitor cell expansion by activated vascular beds.
Endothelial cells actively support the maintenance of oligodendrocyte precursor cells94, and perivascular endothelin has been shown to directly modulate gliogenesis and glial progenitor fate, acting directly through endothelin B receptors expressed by parenchymal GPCs95. Notably, the perivascular niche for gliogenesis may be a critical target of ischemic disease and hypoxic stress. The exposure of endothelial cells to sublethal levels of oxidative stress abrogates their support of oligodendrocyte precursor viability94. This intriguing result provides a potential mechanistic basis for the delayed loss of oligodendrocytes and their precursors following insults ranging from transient hypoxia to radiation injury, which share brief, but profound, oxidative stress as a feature and that have long been co-associated with delayed vascular injury.
Coordinate regulation of angiogenesis and gliomagenesis
The perivascular niche for gliogenesis is also of particular interest in that it may share molecular homologies with the vascular microenvironment in which gliomagenesis and tumor invasion proceed31. Indeed, a number of recent studies investigating the molecular basis for the vascular support of glioma progression have expanded our understanding of endothelial-progenitor interactions in normal adult tissue; the respective fields of neuro-oncology and adult neurogenesis are now ripe for cross-fertilization. Many of the pathways that are important for the perivascular support of neurogenesis and gliogenesis are similarly critical for the perivascular support of gliomagenesis. For instance, endothelial NOS–derived NO has recently been reported to regulate the expansion of platelet-derived growth factor–dependent glioma cells via the potentiation of Notch signaling53. Similarly, purinergic signaling, which may provide important mitogenic signals in the perivascular niche for neurogenesis, may similarly regulate the perivascular expansion of glioma cells96. Locally derived perivascular VEGF signaling, as a coordinate regulator of both endothelial and glioma expansion97, is yet another example of a mitogenic mechanism that is used by NSCs and glioma cells alike. In this regard, the use of anti-angiogenic agents such as bevacizumab in malignant glioma highlights the value of targeting these perivascular niche–selective pathways: although bevacizumab was originally developed as a means to slow the provision of VEGF by tumor cells to the local vasculature, it is now clear that its effects on tumor expansion may derive as much from impeding reciprocal endothelial signals to tumor cells and their progenitors98.
In broad terms, by understanding the role of the perivascular niche in supporting neuronal production and migration in adult brain tissue, we may hope to better define strategies for inducing heterotopic neuronal production and directing recruitment to sites of need. Indeed, recent studies have raised the possibility of inducing neuronal addition to otherwise non-neurogenic regions of the adult brain by using viral gene transfer to overexpress BDNF and noggin, key components of the perivascular and subependymal niches, broadly throughout the adult ventricular wall99,100. Thus, by more precisely defining the cell-cell interactions and molecular determinants thereof among the constituents of the perivascular niches for neurogenesis and gliogenesis, we may hope to better control the generation, destination and phenotypic instruction of new cells from endogenous stem and progenitor cells in the adult CNS.
Acknowledgments
We thank M. Nedergaard for her comments on the manuscript and A. Benraiss and C. McClain for designing the schematics. Work discussed in the Goldman laboratory was supported by the National Institute of Neurological Disorders and Stroke (grants R37NS29813, R01NS75345 and R01NS39559) and by grants from the National Multiple Sclerosis Society, the G. Harold and Leila Y. Mathers Charitable Foundation, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the CHDI Foundation, and the New York State Stem Cell Research Program.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Goldman SA. Adult neurogenesis: From canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- 2.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin A. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 4.Morshead CM, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 5.Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells, but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 6.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 7.Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschenbaum B, et al. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 9.Pincus DW, et al. Fibroblast growth factor-2/brain-derived neurotrophic factor– associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 11.Roy NS, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 12.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 14.Goldman SA. Stem and progenitor cell–based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 15.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg G, et al. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- 18.Nunes MC, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 19.Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 24.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 25.Ohab JJ, Fleming S, Blesch A, Carmichael S. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bovetti S, et al. Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci. 2007;27:5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snapyan M, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 29.Goldman SA, Nottebohm F. Neuronal production, migration and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleaver O, Melton D. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 32.Cao L, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 33.Schänzer A, et al. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wurmser AE, et al. Cell fusion–independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430:350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, et al. Glioblastoma stem-like cells give rise to tumor endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 37.Lin JH, et al. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 2007;302:356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braun N, et al. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur J Neurosci. 2003;17:1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- 39.Agresti C, et al. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Packer MA, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci USA. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park C, et al. Inhibition of neuronal nitric oxide synthase enhances cell proliferation in the dentate gyrus of the adrenalectomized rat. Neurosci Lett. 2001;309:9–12. doi: 10.1016/s0304-3940(01)02003-1. [DOI] [PubMed] [Google Scholar]
- 44.Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 45.Moreno-López B, et al. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torroglosa A, et al. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells. 2007;25:88–97. doi: 10.1634/stemcells.2006-0131. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 48.Murillo-Carretero M, Torroglosa A, Castro C, Villalobo A, Estrada C. S-nitrosylation of the epidermal growth factor receptor: a regulatory mechanism of receptor tyrosine kinase activity. Free Radic Biol Med. 2009;46:471–479. doi: 10.1016/j.freeradbiomed.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 49.Reif A, et al. Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behavior. Eur J Neurosci. 2004;20:885–895. doi: 10.1111/j.1460-9568.2004.03559.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carreira BP, et al. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells. 2010;28:1219–1230. doi: 10.1002/stem.444. [DOI] [PubMed] [Google Scholar]
- 52.Luo CX, et al. Bidirectional regulation of neurogenesis by neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells. 2010;28:2041–2052. doi: 10.1002/stem.522. [DOI] [PubMed] [Google Scholar]
- 53.Charles N, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eyler CE, et al. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson DW, et al. Pigment epithelium–derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 56.Ramírez-Castillejo C, et al. Pigment epithelium–derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 57.Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 58.Ables JL, Breunig J, Eisch AJ, Rakic P. Not(ch) just development: Notch signaling in the adult brain. Nat Rev Neurosci. 2011;12:269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noguera-Troise I, et al. Blockade of Dll4 inhibits tumor growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 60.Ridgway J, et al. Inhibition of Dll4 signaling inhibits tumor growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 61.Klein R, Conway D, Parada LF, Barbacid M. The trkB tyrosine protein kinase gene codes for a second neurogenic receptor that lacks the catalytic kinase domain. Cell. 1990;61:647–656. doi: 10.1016/0092-8674(90)90476-u. [DOI] [PubMed] [Google Scholar]
- 62.Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiaramello S, et al. BDNF/TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signaling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- 65.Donovan MH, Yamaguchi M, Eisch AJ. Dynamic expression of TrkB receptor protein on proliferating and maturing cells in the adult mouse dentate gyrus. Hippocampus. 2008;18:435–439. doi: 10.1002/hipo.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation, but not the survival, of CNS stem cell–derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emsley JG, Hagg T. α6β1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 69.Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kokovay E, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lim DA, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 72.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 74.Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quiñones-Hinojosa A, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 77.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 78.Hartog TE, et al. Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries. J Neurosci. 2009;29:15511–15519. doi: 10.1523/JNEUROSCI.2564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim DH, et al. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronal addition to the adult songbird brain. J Neurosci. 2008;28:208–216. doi: 10.1523/JNEUROSCI.3674-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, et al. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin K, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 84.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 85.Yamashita T, et al. Subventricular zone–derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 88.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 89.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 90.Monje ML, Toda H, Palmer T. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 91.Sim FJ, et al. Complementary patterns of gene expression by adult human oligodendrocyte progenitor cells and their white matter environment. Ann Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 92.Poimenidi E, Hatziapostolou M, Papadimitriou E. Serum stimulates Pleiotrophin gene expression in an AP-1–dependent manner in human endothelial and glioblastoma cells. Anticancer Res. 2009;29:349–354. [PubMed] [Google Scholar]
- 93.Chang Y, et al. Secretion of pleiotrophin stimulates breast cancer progression through remodeling of the tumor microenvironment. Proc Natl Acad Sci USA. 2007;104:10888– 10893. doi: 10.1073/pnas.0704366104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351– 4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J Neurosci. 2009;29:10047–10062. doi: 10.1523/JNEUROSCI.0822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aerts I, et al. The expression of ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (E-NPP1) is correlated with astrocytic tumor grade. Clin Neurol Neurosurg. 2011;113:224–229. doi: 10.1016/j.clineuro.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 97.Bao S, et al. Stem cell–like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 98.Gilbertson RJ, Rich JN. Making a tumor’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 99.Cho SR, et al. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Im SH, et al. Induction of striatal neurogenesis enhances functional recovery in an adult animal model of neonatal hypoxic-ischemic brain injury. Neuroscience. 2010;169:259–268. doi: 10.1016/j.neuroscience.2010.04.038. [DOI] [PubMed] [Google Scholar]