Abstract
Intraventricular ependymal infection by adenoviruses expressing brain-derived neurotrophic factor (BDNF) and noggin is sufficient to induce the heterotopic recruitment of new medium spiny neurons to the adult neostriatum, from endogenous subependymal neural progenitor cells. This approach was found to slow disease progression and extend survival in an R6/2 mouse model of Huntington’s disease (HD). However, the practical therapeutic value of this strategy is limited by the transient expression and immunogenicity of adenoviral vectors. In addition, it has been unclear whether sustained overexpression of BDNF and noggin would yield similarly sustained neuronal production and striatal recruitment, or whether progenitor depletion or tachyphylaxis might supervene to limit the therapeutic potential of this approach. To address these issues, we used adeno-associated virus serotype 4 (AAV4), an ependymotrophic vector that is neither immunogenic nor neurotoxic, to achieve sustained BDNF and noggin expression. Using AAV4, we found that BDNF and noggin achieved levels sufficient to initiate and maintain, for at least 4 months, ongoing neuronal addition to the neostriatum and olfactory bulb. Over this period, we noted no diminution of treatment-associated neuronal recruitment from resident progenitors. AAV4:BDNF and noggin-induced neuronal addition may thus provide a means to provide longlasting and persistent striatal neuronal replacement in conditions of striatal neuronal loss, such as HD.
Keywords: neurogenesis, AAV, SVZ, Huntington’s disease
INTRODUCTION
The adult brain can generate new neurons from endogenous neural stem and progenitor cells, which persist in the ventricular subependyma.1–3 In rodents, neurons generated within the subependyma migrate to the olfactory bulb (OB) along the rostral migratory stream, differentiating as olfactory interneurons.3–5 Although similar olfactory neuronal recruitment has been reported in monkeys,6 its persistence in humans remains controversial.7,8 Nonetheless, progenitors able to give rise to new neurons are widespread in the adult human subependyma.9–11
The normal adult brain does not exhibit significant neuronal recruitment from subependymal progenitor cells to either the neostriatum or neocortex. Yet neurons can be recruited to non-neurogenic areas by a variety of pathogenic stimuli, including seizures, ischemia and neurodegeneration.12–16 Neostriatal neuronal addition can also be induced by systemic fibroblast growth factor 2,17 or by intraventricular delivery of brain-derived neurotrophic factor (BDNF), by either protein infusion or viral overexpression.5,18–20 This BDNF-associated striatal neuronal addition can be enhanced by the addition of noggin21 to suppress pro-gliogenic signaling by the bone morphogenetic proteins.22,23 Importantly, BDNF and noggin-induced striatal neurons differentiate into medium spiny neurons (MSNs), which extend fibers to the globus pallidus, their normal target.21
As the new neurons integrate as MSNs, a major phenotype lost in Huntington’s disease (HD),24 we asked whether they might provide benefit in the R6/2 mouse, a transgenic model of HD.25 We found that BDNF/noggin-induced neuronal addition indeed slowed disease progression and significantly improved survival in R6/2 mice.26 Importantly, therapeutic benefit depended upon striatal neuronal recruitment, in that cytosine arabinoside-induced blockade of striatal neurogenesis abolished the survival benefit of AdBDNF/AdNoggin treatment.26
Despite the promise of this regenerative approach to treating HD, it is limited by the transient nature of adenoviral gene expression. To maintain striatal neuronal addition over longer periods of time, we thus needed to provide a means of sustaining BDNF and noggin expression. To this end, we established an adeno-associated virus (AAV)-based strategy to sustain the high-level expression of both BDNF and noggin in rat ventricular cerebrospinal fluid (CSF). AAVs are small single-stranded DNA viruses with linear genomes, that have a broad host and cell type infectivity range, transduce dividing as well as non-dividing cells and are relatively nontoxic.27,28 rAAVs can genomically integrate, but more generally appear to persist in host cells as stable extra-chromosomal episomes.29 As such, they allow sustained transgene expression after intracerebral injection, providing stable gene expression for up to 2 years in rodents30 and over 6 years in primates.31 Importantly, different AAV serotypes exhibit different tropisms in the central nervous system.32,33 One serotype in particular, AAV4, can selectively transduce ependymal cells of the ventricular wall, allowing access to the subependymal precursor cell pool.34,35 On this basis, we used intraventricular injection of the ependymotrophic serotype AAV4 in this study, to stably and selectively transduce the adult rat ventricular wall to tonically overexpress BDNF and noggin. We found that this approach was effective and, remarkably, was accompanied by the continuous addition of new neurons to the adult neostriatum, which proved undiminished throughout at least 4 months of sustained BDNF and noggin overexpression.
RESULTS
AAV4-directed sustained transgene expression in the adult rat ventricular wall
To assess the level of transgene expression that might be afforded by AAV4-delivered BDNF and noggin, as well as to confirm the ependymal selectivity of this vector, we constructed recombinant AAV4 recombinant vectors for BDNF, noggin, and an enhanced green fluorescent protein (EGFP) control, all established in AAV2 backbones. The vectors were then injected into the lateral ventricles of 3-month-old rats, at 3 μl per ventricle. The treated animals were killed either 2 (short term) or 4 months (long term) after viral injection, with CSF withdrawal for enzyme-linked immunosorbent assay estimation of BDNF and noggin concentrations. Both green fluorescent protein fluorescence and immunolabeling revealed transgene expression that was limited to the ventricular wall, and still readily apparent at both 2 and 4 months after AAV4:EGFP injection (Supplementary Figure 1). Enzyme-linked immunosorbent assay revealed that in the treated rats both BDNF and noggin achieved the concentration >2 ng ml−1 (Figure 1 and Supplementary Tables 1a and 2a); these levels were significantly higher in treated animals relative to both their AAV4:EGFP and saline controls. Two-way analysis of variance (ANOVA) followed by F test was used to assess the overall effects of both viral treatment and timepoint. This revealed a significant treatment effect on the concentrations, in ng ml−1 CSF, of both BDNF (F(3,17) = 83.24, P<0.0001) and noggin (F(3,24) = 200.17, P<0.0001; Supplementary Tables 1b and 2b). Importantly, the levels of both BDNF and noggin were maintained without significant fall between 2 and 4 months; two-way ANOVA revealed no effect of timepoint on the CSF concentrations of either BDNF (F[1,17] = 0.63, P>0.05) or noggin (F[1,24] = 0.55, P>0.05). Analogous results were obtained when the achieved levels of BDNF and noggin were expressed as ng μg−1 CSF protein (BDNF treatment effect: F(3,17) = 102.13, P<0.0001; timepoint effect F(1,17) = 0.35, P>0.05; noggin: F(3,24) = 139.38 P<0.0001; timepoint effect F(1,24)) = 0.30, P>0.05). Thus, intraventricular delivery of AAV4 achieved the sustained and phenotype-specific expression of BDNF and noggin by ependymal cells of the adult rat brain, with continuous and stable transgene expression over the 4-month period of observation.
Figure 1.
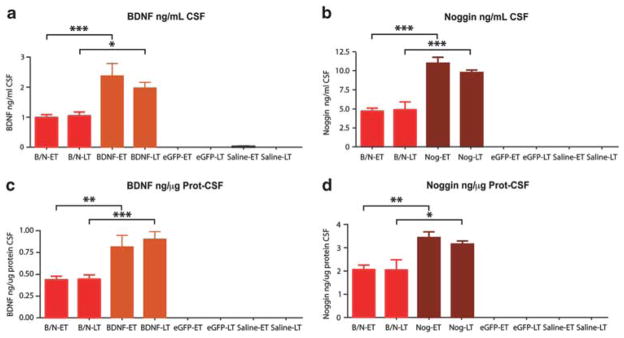
AAV4:BDNF and AAV4:noggin achieve sustained CSF BDNF and noggin delivery. Enzyme-linked immunosorbent assay quantification of CSF BDNF (a, c) or noggin (b, d) proteins was performed either 2 months (early timepoint; ET) or 4 months (late timepoint; LT) after intraventricular injection of AAV4:BDNF and AAV4:noggin (AAV4:B/N), AAV4:BDNF, AAV4:noggin, AAV4:EGFP(null), or saline. *P<0.05; **P<0.01; ***P<0.001, by 2-way ANOVA with post hoc Bonferroni t-tests.
AAV4-mediated BDNF and noggin delivery was both dose-dependent and non-inflammatory
We noted a dose–response relationship of viral dose to CSF concentrations achieved. The CSF of animals injected with an AAV4:BDNF and AAV4:noggin mixture comprising 1.5 μl of each virus per ventricle had 1.00±0.12 ng ml−1 BDNF and 4.76±0.51 ng ml−1 noggin at 2 months, and 1.07±0.15 ng ml−1 BDNF and 4.97±1.21 ng ml−1 noggin at 4 months. In contrast, animals injected with twice the dose (3 μl per ventricle) of a single virus manifested twice the amount of BDNF or noggin (2.42±0.51 ng ml−1 BDNF and 11.30±0.92 ng ml−1 noggin at 2 months; 2.01±0.23 ng ml−1 BDNF and 10.03±0.41 ng ml−1 noggin at 4 months). Bonferroni post hoc analysis confirmed these dose-dependent differences to be significant (Figure 1).
Besides investigating the dose–response relationship of AAV4 viral dose to transgene expression, we also verified the non-inflammatory nature of the AAV4 vector system. To this end, sections from rats killed at both 2 and 4 months after AAV4:BDNF/noggin injection were immunostained for markers of inflammation that included CD4 (T helper cells), CD8b (cytotoxic T cells), CD68 (activated microglia and macrophages), CD3 (mature T lymphocytes) and CD20 (B lymphocytes). We found no evidence of any accumulation of these immune cells in either the ependymal/subependymal zone or adjacent periventricular parenchyma, indicating the lack of any detectable cellular immune response to either the virus or its expressed transgenes (Supplementary Figure 2).
AAV4:BDNF/noggin treatment potentiated neuronal recruitment to the OB
To investigate whether AAV4-driven BDNF and noggin might not only enhance, but also sustain OB neurogenesis, adult rats were injected with AAV4:BDNF/noggin (AAV4:B/N), AAV4:BDNF, AAV4:noggin, AAV4:EGFP or saline. Two matched cohorts were assessed, which were injected with AAV4 at the same time, then killed either 2 or 4 months thereafter, following a 1-month preterminal series of daily 5-bromo-2′-deoxyuridine (BrdU) injections. Group 1 (short timepoint) received 30 daily injections of BrdU that began 1 month after AAV injection, while group 2 (late timepoint) received 30 daily BrdU injections, beginning 3 months after AAV injection. After each group completed 1 month of BrdU injections, its rats were killed a day after the last BrdU injection, at either 2 months (8 weeks) for group 1, or 4 months (16 weeks) for group 2 (Figure 2a). Each treatment × timepoint-defined group included 4 rats, making a total of 40 animals.
Figure 2.
AAV4:BDNF potentiated and sustained neuronal addition to the OB. (a) Experimental design: adult rats were injected with AAV4:BDNF/noggin, AAV4:BDNF, AAV4:noggin, AAV4:EGFP or saline. Two matched cohorts were treated and killed either 2 or 4 months thereafter. The rats received a 1-month series of daily BrdU injections before killing. (b) Brain sections immunostained for BrdU and βIII-tubulin confirmed the neuronal phenotype of the newly generated neurons in the OB. White arrows indicate neurons double-labeled with both BrdU and βIII-tubulin. (c) BrdU indices in the OBs of adult rats were counted 2 or 4 months after treatment with either AAV4:BDNF/noggin (AAV4:B/N; n = 6/timepoint), AAV4:BDNF (n = 4/timepoint), AAV4:noggin (n = 5 and 4 at 2 and 4 months, respectively), AAV4:EGFP (null; n = 4/timepoint) or saline (n = 4/timepoint). At each time point, AAV4:BDNF, whether alone or with AAV4:noggin, yielded significantly more new neuronal addition to the OB than that exhibited by either saline or AAV4:null-treated controls (cells per mm3±s.e.m.). *P<0.05; **P<0.01; ***P<0.001, by 2-way ANOVA with post hoc Bonferroni t-tests.
Serial sagittal brain sections spanning the entire OB were then stained for BrdU (Figure 2b). BrdU+ cells were scored in the entire OB, once in every 16 sections. BDNF overexpression, either alone or together with noggin, significantly increased the number of BrdU+ cells in the OB (Figures 2c and d). At the 2 month timepoint, the OBs of group 1 rats treated with either AAV4:BDNF alone or AAV4:BDNF/noggin together manifested significantly more BrdU+ cells (12100.2±1485.4 and 10 305±1067, respectively), than did their AAV4:EGFP (5085±1300) and saline (6108±1378) controls (P<0.001 and P<0.01, respectively, by 2-way ANOVA with post hoc Bonferroni t-tests; Supplementary Table 3). Similarly, in the group 2 rats killed 16 weeks after viral injection, substantially more BrdU+ cells were noted in the OBs of the AAV4:BDNF (11142±1451) and AAV4:BDNF/noggin (10191±734)-treated rats, relative to their controls (AAV4:EGFP: 3195±1070; saline: 2877±199; P<0.001 by 2-way ANOVA with Bonferroni post hoc comparisons). Of note, the BrdU indices of rats given AAV4:noggin only, without AAV:BDNF, did not differ from those of control rats treated with AAV4:EGFP or saline (P>0.05; Supplementary Table 3).
Confocal analysis confirmed that >80% of newly generated BrdU+ cells co-expressed the neuronal protein βIII-tubulin, and no difference in this proportion was noted as a function of treatment. Together, these data suggest that AAV4-mediated overexpression of BDNF potentiated neuronal addition to the adult OB in a sustained fashion (Supplementary Figure 3).
AAV4:BDNF/noggin increased OB neuronal turnover
Given the substantial increment in the recruitment of new neurons to the OBs of AAV4:BDNF/noggin-treated rats, and the sustained nature of this addition, we next asked whether the total number of neurons per OB differed in the treated rats and their controls. Stereological counts of total OB neurons in AAV4:BDNF/noggin- or AAV4: EGFP-treated rats assessed histologically 4 months after AAV injection revealed no significant differences either in the OB volume (33.6±1.5 mm3 for AAV4:BDNF/noggin-treated rats vs 32.1±0.8 mm3 for AAV4:EGFP-treated), or in the number of resident OB neurons (15.70±0.28 × 106, AAV4:BDNF/noggin-treated, vs 15.37±0.74 × 106, AAV4:EGFP controls; Supplementary Figure 4). Thus, we found no evidence of treated OBs either harboring more neurons, or otherwise differing from their controls in their cell densities or gross tissue volumes.
On that basis, we asked whether the enhanced addition of new neurons to the OB might be compensated by increased turnover, and reflected in the death of either new or resident olfactory interneurons. In this regard, the thousands of new neurons that are recruited into the normal OB on a daily basis are subject to massive cell death upon arrival;36,37 their survival ultimately depends upon their functional integration.38,39 Indeed, rats subjected to an olfactory discrimination paradigm manifested enhanced turnover of newly generated neurons, despite unaltered subventricular zone (SVZ) proliferation and neuroblast migration.39,40 On that basis, we postulated that the existing mechanisms for delimiting OB neuronal number might similarly serve to regulate the addition of new neurons to the OBs of AAV4:BDNF/noggin-treated rats.
To test this possibility, the OBs of AAV4:BDNF/noggin or AAV4:EGFP-treated animals in group 2, those killed 4 months after AAV injection and treated for 1 month antemortem with BrdU, were labeled by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) to identify apoptotic cells (Figure 3a). The AAV4:BDNF/noggin-treated rats—which exhibited over threefold the density of BrdU-tagged newly generated neurons per mm3 than did their controls—similarly manifested many more TUNEL+ cells (96±16 cells mm−3) than did their matched AAV4:EGFP controls (55±4 mm−3; P<0.05 by t-test, t(6) = 2.892; Figure 3b). Interestingly, the percentage of TUNEL+ cells among the BrdU+ cells was similar in both groups (28.1±1.5% and 31.7±3.3%, respectively; P>0.05, t(6) = 1.141; Figure 3c), suggesting that new OB neurons generated in the BDNF/noggin-overexpressing ventricular wall exhibited no preferential post-migratory survival relative to their control counterparts. Thus, the number and density of dying neurons were significantly higher in AAV4:BDNF/noggin-treated OBs than in their AAV4:EGFP controls, in apparent compensation for the increased numbers of newly generated neurons recruited to the OBs of AAV4:BDNF/noggin-treated animals.
Figure 3.
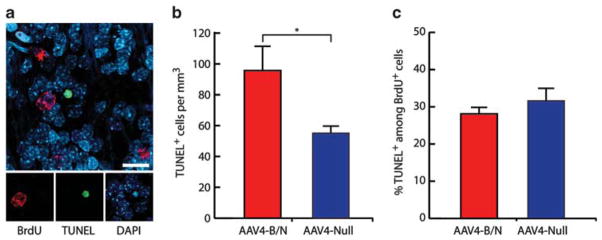
AAV4:BDNF and AAV4:noggin treatment did not result in increased cell death. Serial sections from AAV4:BDNF/noggin or AAV4:EGFP (n = 4 each) were subjected to TUNEL staining in combination with BrdU. (a) DNA degradation as identified by TUNEL was noted in cells with typical pyknotic morphology. (b) TUNEL+ cells were estimated by stereological cell counts throughout the OB, revealing more dying neurons in the AAV4:BDNF/noggin treated OBs than in their controls. (c) However, the fraction of TUNEL+ cells among all BrdU+ cells did not differ between the treated and control groups. Although the density of TUNEL+ cells was higher in AAV4:BDNF/noggin-treated rats (b, the fraction of TUNEL+ cells among all BrdU+ neurons was no different in the BDNF/noggin-treated rats than in their controls (c), suggesting that while increased neuronal recruitment to the OB was compensated for by increased death, the latter was unaffected by treatment. Scale: 20 μm; cells per mm3±s.e.m.
AAV4:BDNF/noggin treatment induced and sustained neuronal addition to the striatum
To address whether intraventricular injection of AAV4:BDNF/noggin resulted in recruitment of newly generated neurons to the neostriatal parenchyma, serial sagittal sections from treated rat brains were co-immunostained for BrdU and a variety of neuronal markers, which included doublecortin (DCX),41 βIII-tubulin,42 NeuN43 and dopamine- and cyclic-AMP-regulated phosphoprotein of molecular weight 32 000 Da (DARPP-32).44 Confocal imaging was used to confirm the colocalization of nuclear BrdU with each neuronal marker. We found that AAV4-delivered BDNF and noggin stimulated the striatal recruitment of new neurons expressing antigenic markers of both migrating neuroblasts and mature neurons, the latter including MSNs (Figure 4). Immunostaining revealed frequent DCX+/BrdU+ neuronal migrants in the striata of AAV4:BDNF/noggin-injected rats at both 2 and 4 months after viral injection (Figures 4c and g and Supplementary Figure 5). Stereological analysis revealed that AAV4:BDNF/noggin-treated striata harbored significantly more BrdU+/Dcx+cells than did their AAV4:EGFP-treated counterparts, when assessed 2 months after viral injection (161±32 and 29±8 BrdU+/Dcx+ cells per mm3, respectively; Bonferroni post hoc P<0.001; Figure 5a). At 4 months after AAV4:BDNF/noggin injection, BrdU+/Dcx+ cells were still abundant, as much as when scored at 2 months (2-way ANOVA with post hoc Bonferroni t-tests, F(1,12) = 0.58, P>0.05; Figure 5a and Supplementary Table 4).
Figure 4.
AAV4:BDNF and AAV4:noggin treatment yielded significant neostriatal neuronal addition. BrdU+ cells per mm3 in the striata of adult rats, at either 8 weeks (early timepoint; ET) or 16 weeks (late timepoint; LT) after treatment with either AAV4:BDNF and AAV4:noggin together, or with AAV4:EGFP (AAV4:null). Newly generated striatal cells expressed the neuroblast marker doublecortin (a), as well as the more mature neuronal markers βIII-tubulin (b) and NeuN (c), and the MSN protein DARPP-32 (d). Most importantly, AAV4:BDNF/noggin-induced striatal neuronal recruitment was maintained for at least 4 months after viral injection, with no measurable diminution between 2 and 4 months. *P<0.05; **P<0.01; ***P<0.001, by 2-way ANOVA with post hoc Boneferroni t-tests.
Figure 5.
AAV4:BDNF/noggin induced MSN addition to the adult striatum. Striatal sections of AAV4:BDNF/noggin-treated rats at early (a–d) and late timepoints (e–f) after AAV injection (2 and 4 months, respectively). Each was double-immunostained for BrdU (green) and neuronal markers (red). Some BrdU+ cells expressed the migratory neuroblast protein DCX (a, e), while others co-expressed BrdU and βIII-tubulin/TuJ1 (b, f), NeuN (c, g) or DARPP-32, a medium spiny neuron specific marker (d, h). Newly generated neurons were confirmed as such by confocal z axis analysis, with orthogonal views in the xz and yz planes (insets). Scale: 10 μm.
To address whether AAV4:BDNF/noggin-induced striatal neuroblasts matured in the striatal parenchyma, serial sagittal sections from treated rat brains were next co-immunostained for BrdU and the neuronal marker βIII-tubulin. Confocal imaging analysis confirmed the three-dimensional colocalization of both markers (Figures 4a and e). Indeed, AAV4:BDNF/noggin-treated rats exhibited abundant newly generated neurons in the neostriatum (Figure 4b). The AAV4:BDNF/noggin-treated rats of group 1, which received BrdU injections throughout weeks 4–8, harbored 123±18 BrdU+/βIII-tubulin+ cells per mm3 when killed 2 months after viral injection. Importantly, this induced neurogenesis was durably maintained at least 4 months, as the AAV4:BDNF/noggin-treated rats of group 2, killed at 16 weeks after receiving BrdU only during weeks 13–16, had 116±24 BrdU+/βIII-tubulin+ cells per mm3. In contrast, newly generated neurons were undetectable in the striata of animals treated with AAV4:EGFP. Statistical analysis confirmed a significant difference between the treatment and control groups in their numbers of newly added neurons per mm3 (2-way ANOVA: F(1;12) = 82.5, P<0.0001 by Bonferroni post hoc t-tests, at each timepoint). Statistical testing confirmed that this effect was maintained over time, in that no difference was noted in the number of new striatal neurons added in month 2 compared with that added in month 4, that is, between the BDNF/noggin-treated rats of groups 1 and 2 (F(1;12) = 0.11, P>0.05; also Supplementary Table 5).
To verify that newly generated AAV4:BDNF/noggin-induced neurons manifested phenotypic maturation, serial sagittal sections from treated rat brains were double-stained for BrdU and the mature neuronal marker NeuN. Whereas no BrdU-tagged NeuN+ cells were detected in the striata of AAV4:EGFP-treated controls, BrdU+/NeuN+ were observed in the striata of AAV4:BDNF/noggin-treated rats at both early and late timepoints (Figures 4b and f). Stereological counts revealed that BrdU+/NeuN+ cells were generated exclusively in AAV4:BDNF/noggin-treated rats, and were recruited in similar numbers 2 or 4 months after viral injection (44.8±15.1 and 36.4±10.9 BrdU+/NeuN+ cells per mm3, respectively; Figure 5c). Statistical analysis revealed that the number of newly recruited NeuN-defined neurons was maintained over time, as no significant difference was noted between the two timepoints (P>0.05 by t-test, Supplementary Table 6a). Together, these data showed that AAV4:BDNF/noggin-induced striatal neurogenesis, and that this process was sustained without decrement for at least 4 months after AAV4:BDNF/noggin injection.
AAV4:BDNF/noggin-associated new striatal neurons are mature MSNs
We next sought to assess the phenotype of those new neurons added to the striata of AAV4:BDNF/noggin-treated rats. We found that a substantial cohort of BrdU+ striatal neurons expressed the prototypic MSN protein DARPP-32, at both early and late timepoints, suggesting their maturation as MSNs (Figures 4d and h). Stereological cell counts using unbiased sampling revealed no significant difference in the numbers or densities of BrdU+/DARPP-32+ neurons in AAV4:BDNF/noggin-treated rats at 2 vs 4 months after AAV injection (56±14 and 42.8±12.8 BrdU+/DARPP-32+ cells per mm3, respectively; P>0.05; Figure 5d). In contrast to the AAV4:BDNF/noggin-treated rats, their AAV4:EGFP-treated controls had no identifiable BrdU+/DARPP-32+ striatal neurons, indicating the restriction of neostriatal neuronal addition to AAV4:BDNF/noggin-treated animals (Supplementary Table 7).
To confirm the mature neuronal phenotype of the BrdU+/DARPP-32+ cells in the AAV4:BDNF/noggin-treated rats, we next used triple immunolabeling for DARPP-32, NeuN and BrdU to determine that the majority of newly generated DARPP-32+/BrdU+ cells indeed co-expressed neuronal NeuN. This analysis revealed no significant difference in the incidence of BrdU+/DARPP-32+ and BrdU+/DARPP-32+/NeuN+ cells: When assessed 4 months after AAV4:BDNF/noggin injection, 43±13 BrdU+/DARPP-32+ and 39±10 BrdU+/DARPP-32+/NeuN+ cells per mm3 were noted. In addition, whereas the AAV4:BDNF/noggin-treated rats had 38.5±9.6 BrdU+/NeuN+/DARPP-32+ neostriatal neurons per mm3, their AAV4:EGFP-treated controls had none (P<0.05). Together, these data showed that AAV4:BDNF/noggin induced and sustained the recruitment of neurons with a mature medium spiny phenotype to the adult neostriatum.
Substance P and enkephalin are expressed in newly recruited striatal neurons
We next asked whether newly generated MSNs recruited in response to AAV4:BDNF and noggin adopted appropriate neurotransmitter-defined anatomic phenotypes. Striatal MSNs include several sub-populations of GABAergic striatopallidal projection neurons. These include sub-populations of MSNs that project to the globus pallidus pars interna and substantia nigra, or to the globus pallidus pars externa and subthalamus, which express the peptide transmitters Substance P and enkephalin, respectively. These MSN phenotypes comprise the major striatal components of the direct and indirect striatofugal pathways, respectively, both of which are required for striatal functional integrity.45 As such, any therapeutic strategy predicated on striatal neuronal regeneration is dependent upon the ability to replace both Substance P-expressing and enkephalinergic MSNs.46 We have previously noted that each of these phenotypes is added to the adult neostriatum in response to transient BDNF and noggin overexpression, in mice treated with adenoviral BDNF and noggin. To assess whether a more sustained delivery of BDNF and noggin afforded by AAV is similarly associated with the striatal incorporation of both Substance P and enkephalin-expressing MSNs, we assessed the incidence of these phenotypes among newly generated neurons.
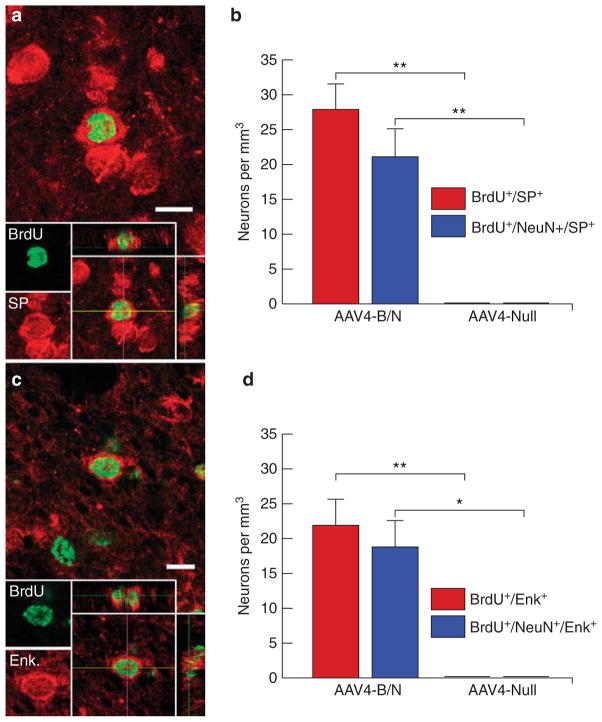
To this end, serial sections from AAV4:BDNF/noggin- or AAV4-EGFP-treated rats, killed 4 months after viral injection, were co-immunostained for either Substance P or met-enkephalin together with BrdU. Both BrdU+/Substance P+ and BrdU+/enkephalin+ cells were identified in the striata of AAV4:BDNF/noggin-injected rats (Figures 6a and c). Stereological counts revealed that the AAV4:BDNF/noggin-treated striata harbored 27.9±3.6 immuno-identifiable Substance P+/BrdU+ cells per mm3 and 21.1±4.0 Substance P+/NeuN+/BrdU+ cells per mm3 (Figure 6b). Similarly, the AAV4:BDNF/noggin-treated striata exhibited 21.9±3.8 enkephalin+/BrdU+ and 18.8±3.7 enkephalin+/NeuN+/BrdU+ cells per mm3 (Figure 6d). Matched AAV4:EGFP controls had no identifiable BrdU+ cells whatsoever that expressed either Substance P or enkephalin, highlighting the significant treatment-associated difference in new neuronal number for each phenotype (P<0.01 for each, by Bonneferroni-adjusted t-test). Thus, roughly equivalent proportions of Substance P and enkephalin-defined MSNs of the direct and indirect striatopallidal pathways, respectively, were recruited in response to sustained BDNF and noggin delivery.
Figure 6.
AAV4:BDNF-noggin treatment resulted in striatal recruitment of Substance P and enkephalinergic MSNs. Striatal sections from AAV4:BDNF/noggin-or AAV4:null-treated rats, injected for a month with BrdU and killed 4 months after viral injection (late timepoint), were co-immunostained for BrdU and either Substance P (a) or enkephalin (c). BrdU+ neurons were confirmed as such by confocal imaging, with orthogonal views in the xz and yz planes (insets). Stereological counts of striatal BrdU+ or BrdU+/NeuN+ cells, co-expressing either Substance P (b) or enkephalin (d), in AAV4:BDNF/noggin or AAV4:EGFP-treated rats. Cells per mm3±s.e.m. *P<0.05; **P<0.01, by t-test. Scale: 10 μm.
AAV4:BDNF/noggin-associated new striatal neurons project to the globus pallidus
The neostriatum, which includes the caudate and putamen, displays a complex mosaic organization of neurochemical systems that are related to its neuroanatomical connections. MSNs, the dominant neostriatal neuronal population, normally project to the globus pallidus. In order to test whether newly generated neostriatal neurons extend projections to their natural target, the globus pallidus, AAV4:BDNF/noggin-treated rats and controls were subjected to a second surgery, in which they received intra-pallidal injection of a retrograde tracer, fluorescent microspheres (FMSs; see Figures 7a and b for experimental design). Following retrograde axonal transport, FMSs are incorporated into cell bodies located within the neostriatum, allowing striatopallidal projection neurons to be identified.47 By this means, we identified newly generated striatopallidal neurons in coronal slices, by virtue of their co-incorporation of BrdU and FMSs (Figure 7c). We found an abundance of BrdU+/FMS+ neurons in the AAV4:BDNF/noggin-treated rats, but not in their controls, indicating the successful extension of axons from newly generated MSNs to their pallidal targets. Importantly, we confirmed that the incorporation of the FMSs occurred only by retrograde transport and not by passive diffusion of the tracer; in this regard, no incorporation of pallidal-injected FMSs whatsoever was noted by intrinsic striatal interneurons (Figure 7e). Using stereological cell counts with unbiased sampling, we noted 38.2±11.7 BrdU+/FMS+ neurons per mm3 in AAV4:BDNF/noggin-treated rats when assessed 4 months after AAV injection, the sole timepoint sampled; this contrasted to the complete absence (0±0 BrdU+/FMS+ cells per mm3) of new neurons, FMS-tagged or otherwise, in the AAV4:EGFP-treated control striata (P<0.05 by t-test; Figure 7d). These observations confirmed that newly recruited neurons, generated in the adult brain in response to AAV4:BDNF/noggin treatment, can project axons to their normal developmental target, the globus pallidus.
Figure 7.
AAV4:BDNF and AAV4:noggin co-treatment yielded significant neostriatal neuronal addition. Pallidal injection of retrogradely transportable FMS identified adult-generated MSNs that extended projections to the globus pallidus. (a) A schematic of the pallidal area injected, and the striatal area (caudate-putamen, CP) sampled, (b) outlines the experimental protocol. (c) The triple-labeled FMS-incorporating (red), NeuN+ (green) and BrdU+ (blue) cells (white arrows) define newly generated MSNs that extend processes to the region of pallidal microsphere injection. (d) Distribution of BrdU+/FMS+ cells in AAV4: B/N- and AAV4:null-treated rats, 20 weeks after viral injection. Cells per mm3±s.e.m. (e) Immunostaining for choline acetyltransferase (ChAT, white arrowheads) revealed that intrinsic cholinergic neurons of the striatum (green) did not incorporate GP-delivered microspheres (red), which were instead taken up by, and transported to, local MSNs. *P<0.05 by t-test. Scale: 10 μm.
DISCUSSION
We have previously shown that intraventricular delivery of adenoviral BDNF is sufficient to both enhance neuronal recruitment to the OB, and to induce heterotopic recruitment of MSNs to the striatum. Striatal neuronal addition was further improved when noggin, a potent BMP inhibitor, was concomitantly delivered into the ventricle. This strategy proved effective to slow the disease progression in R6/2, a well-characterized HD mouse model. However, the potential of this strategy was limited by the transient nature of gene expression by the adenoviral vector used. In addition, adenoviruses are immunogenic and cannot be repeatedly injected.48 To address these limitations of adenoviral delivery, we have developed an alternative strategy for ensuring sustained delivery of BDNF and noggin to the adult ventricular wall, using AAV. In particular, we used AAV serotype 4 because of its tropism for ependymal cells and high, yet restricted, infectivity to the ventricular wall.35 As such, AAV4 permits the subrogation of the ependyma as a secretory source for recombinant proteins, which may then diffuse both locally to the subependymal cell population, as well as distantly through CSF dispersal to access remote parts of the central nervous system49 (Supplementary Figure 1). On that basis, we constructed two AAV4 vectors separately expressing BDNF and noggin, and administered these together into the lateral ventricles of adult rats, so as to assess the efficacy of AAV4 in ensuring the sustained expression and CSF secretion of delivered transgenes. We found that AAV4 indeed effected ependymal transduction in vivo, and yielded sustained overexpression of BDNF and noggin throughout a 4-month period of observation. AAV4-mediated gene expression was sufficiently high enough to support the recruitment of new neurons to both the OB and striata of adult rats, and to an extent similar to that which we previously reported using adenoviral BDNF and noggin delivery.21,26 Importantly though, AAV4’s effects were maintained with stable transgene expression for at least 4 months. Remarkably, this was accompanied by sustained neuronal addition to both the olfactory bulb and striatum, which was continuous and undiminished over a 4-month period of observation. Thus, the tonic exposure of the subependymal progenitor pool to BDNF and noggin, and in particular the tonic inhibition of local BMP activity by noggin, did not exhaust or otherwise deplete the progenitor pool within the SVZ.
Of note, the number of BrdU+-labeled neurons in the OBs of AAV4:BDNF and AAV4:BDNF/noggin-treated rats did not significantly differ, suggesting that neuronal addition to the OB was maximally potentiated by AAV4:BDNF alone. The lack of any noggin-associated increment in OB neuronal addition was in marked contrast to noggin’s robust potentiation of BDNF-associated neuronal addition to the neostriatum. Yet this apparent lack of effect of exogenous noggin upon OB neuronal addition may have reflected the high levels of endogenous noggin already present in the OB.21
In contrast to previous studies using either protein infusion5 or adenoviral delivery,19 in the present study we used a vector capable of long-term transgene expression to achieve the sustained production of BDNF and noggin. The levels of transgene achieved proved sufficient to trigger and sustain neuronal recruitment from the striatal SVZ progenitor cell population. These AAV4:BDNF/noggin-treated animals showed abundant BrdU+/DCX+ cells in the SVZ, subjacent neostriatum, rostral migratory stream and OB at both 2 and 4 months after the viral injection, with no diminution in the number of neurons incorporating BrdU between the second and fourth months after AAV4 injection (Supplementary Figure 3). Indeed, given the ongoing neuronal addition afforded by AAV4:BDNF and noggin, we asked whether the new neurons would result in the monotonic expansion of the extant olfactory neuronal pool, or whether instead homeostatic regulatory control of the OB neuronal population might supervene to delimit OB neuronal number. The latter proved to be the case, in that the increased neuronal addition to the OBs of AAV4:BDNF/noggin-treated rats was fully offset by increased cell death. Apoptotic TUNEL+ cells were significantly more abundant in AAV4:BDNF/noggin-treated OBs than in their AAV4:EGFP controls, even while the percentage of TUNEL+ cells among BrdU+ cells remained the same between the treated and control rats.
In this regard, it is important to note that intraventricularly delivered AAV4 did not transduce SVZ neural progenitor cells or their lineal descendants, but rather the overlying ependymal wall; thus, once generated, the newly migratory neurons could no longer depend upon periventricular BDNF overexpression for their survival. As such, the increased rate of neuronal death in the AAV4:BDNF/noggin-treated OBs—in apparent compensation for the increased OB neuronal addition exhibited by these treated rats—likely reflected competitive constraints upon the number of functional neurons that could be accommodated within the adult OB. Accordingly, the total neuronal counts as well as the volume of the OBs were similar in the AAV4:BDNF/noggin-treated and control rats (Supplementary Figure 4).
Of note, several groups have failed to observe increased olfactory neuronal addition in response to either parenchymally or SVZ-delivered BDNF, despite increased rostral migratory stream neuronal recruitment.50–52 Yet, whereas we used intraventricular delivery of ependymal-restricted expression vectors, other groups have used either protein delivery or viral vectors introduced directly into the SVZ parenchyma. One might envisage that differences in delivery mode, and hence of BDNF access, availability, processed form and effective dose, might all influence both the initial specification and ultimate competitive success of newly generated neurons, in ways that we have yet to define. Together though, these observations suggest an ongoing, dynamic competition among newly generated neurons within the adult OB, whether generated by virtue of exogenous treatment or not, as well as between these new cells and already extant neuronal pools.
From the standpoint of therapeutic design, BDNF and noggin concentrations achieved by AAV4 ependymal delivery proved sufficiently high to trigger and maintain the recruitment of new NeuN+/βIII-tubulin+ neurons into the subjacent neostriatum, a large proportion of which developed as DARPP-32+ MSNs and expressed either enkephalin or Substance P. AAV4:BDNF/noggin infection maintained ependymal expression of BDNF and noggin for at least 4 months after viral delivery, and this in turn was associated with sustained increments in neuronal addition throughout. Indeed, neuronal addition to the striatum was undiminished between the 2 and 4-month timepoints assessed, this suggested a lack of progenitor cell exhaustion in the SVZ over the time frame examined. Thus, AAV4:BDNF/noggin treatment elicited a continuous and stable release of BDNF and noggin by the ventricular system, which was associated with sustained neuronal addition to the adult neostriatum, without measurable evidence of progenitor depletion. Such sustained progenitor responsiveness is a critical prerequisite for using induced neuronal replacement as a treatment strategy for HD, as the slow neuronal loss of HD mandates compensation by a similarly sustained process of functional neuronal addition. Moreover, the robust and sustained response to BDNF and noggin that we noted might be further amplified in the HD environment, in which compensatory expansion of the subependymal progenitor pool may occur.53,54
Our present data suggests both the biological and technical feasibility of an AAV4-based strategy for sustaining robust neuronal addition to the adult mammalian neostriatum, using the ependymal overexpression of BDNF and noggin as a means of initiating and maintaining neuronal recruitment from endogenous subependymal progenitor cells. Remarkably, this process appears to be sufficiently sustainable, with no evidence of progenitor depletion or tachyphylaxis, to suggest its potential utility in the management and treatment of striatal neurodegenerations such as in HD.
MATERIALS AND METHODS
AAV construction and production
PCR-generated endonuclease sites were used to amplify full-length cDNA of the rat BDNF and human noggin ΔB2 (hereafter referred to as noggin; Regeneron Pharmaceuticals, Tarrytown, NY, USA). The ΔB2 mutein of noggin,55 with its heparin binding site deleted, was used so as to improve the tissue penetration of the expressed protein.21 PCR product was subcloned into pENTER/D-Topo (Invitrogen, Carlsbad, CA, USA). BDNF or noggin coding sequences were cloned into pIRES2-EGFP (Clontech, Mountain View, CA, USA) in order to generate the expression cassettes CMV:BDNF:IRES:EGFP and CMV:noggin:IR-ES:EGFP, respectively. These expression cassettes were then subcloned into the AAV2 shuttle vector pFBGR. All the final plasmids were sequenced for the integrity of the transgenes using primers spanning the full length of the expression cassette. AAV2-CMV:BDNF:IRES:EGFP and AAV2-CMV:noggin: IRES:EGFP were used to generate the AAV particles in collaboration with The Gene Transfer Vector Core at the University of Iowa. The Baculovirus System was used to cross-package the AAV4 capsid, and allowed production of high-titer helper-free AAV particles.56 The resulting viruses, AAV2/4:CMV:BDNF:IRES:EGFP, AAV2/4:CMV:noggin:IRES:EGFP or AAV2/4:CMV: EGFP (hereafter referred to as AAV4:BDNF, AAV4:noggin and AAV4:EGFP, respectively), were made and their titers were determined by quantitative PCR, then adjusted to 2 × 1013 viral genomes ml−1.
Viral injections
In all, 12-week-old Sprague Dawley rats (250–280 g) received bilateral 3 μl intraventricular injections of AAV4:BDNF alone or together with AAV4:noggin, AAV4:noggin alone, AAV4:EGFP, or saline (n = 8 for each group). Stereotaxic injections were injected into the ventricle according to the rat brain atlas (anteroposterior, −0.3 mm; mediolateral, ±1.2 mm; dorsoventral, −3.6 mm). One month following viral injection, 4 animals from each group received BrdU for 4 weeks and were killed 1 day after the last BrdU injection (2 months after the viral injection; early timepoint group). The other animals (n = 4 per group) received BrdU starting the fourth month and were also killed 1 day after the last injection (4 months after the viral injection; late timepoint group).
All procedures were performed using protocols approved by the University of Rochester University Committee on Animal Resources, and in compliance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals.
Enzyme-linked immunosorbent assay
Quantification of noggin levels in the CSF was performed by sandwich enzyme-linked immunosorbent assay as previously described.21 BDNF levels in the CSF were also determined using BDNF Emax ImmunoAssay System (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Immunochemistry and quantification
All brains were cut as 14 μm sagittal sections that included the OB, these were stained for BrdU using immunoperoxidase detection when staining for BrdU alone, or using double-immunofluorescence when staining for both BrdU and neuronal markers, as described previously.19,21 Briefly, every 16th section was stained for one of the following: βIII-tubulin (mouse monoclonal TuJ1; Chemicon, Temecula, CA, USA, 1:100), NeuN (mouse anti-NeuN; 1:400; Chemicon), DARPP-32 (rabbit 1:500; Chemicon), DCX (goat anti-DCX; 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), Substance P (rabbit antisera, 1:100; Chemicon), met-enkephalin (rabbit 1:100; Chemicon), green fluorescent protein (rabbit 1:200; Chemicon). Sections co-labeled for BrdU were then washed, treated briefly with 2N HCl at 37 °C and exposed to rat anti-BrdU IgG2AmAb (1:200; Serotec, Raleigh, NC, USA). All secondary antibodies (Invitrogen) were cross-absorbed to avoid nonspecific staining. All immunolabels were compared with appropriate negative controls (omission of primary antibodies, and substitution with equally diluted normal serum or mouse IgG, as appropriate).
Both olfactory and striatal BrdU+ cells counts were done on 8–10 sagittal sections (14 μm) per animal; every 16th section was analyzed at 224 μm intervals, as described previously.19 The striatal region sampled began with the first appearance of striatal fascicles and proceeded laterally. In the OB, every BrdU+ nucleus was counted; the results were reported as the mean number of BrdU+ cells per section and then converted into BrdU+ cells per cubic millimeter after determining the surface areas and hence volumes. Section volumes were measured using Stereo Investigator (MicroBrightField, Inc., Williston, VT, USA). The number of striatal BrdU+/βIII-tubulin+ cells per mm3 was determined as described previously.19,21 Statistical analyses were performed using one-way or two-way ANOVA, followed by post hoc Bonferroni t-tests. For two-way ANOVA, F tests were carried out to analyze the global effects of treatment and timepoint. Otherwise, pairwise comparisons were performed using two-sample Student’s t-tests, as noted. All statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Confocal imaging
In sections double-stained for BrdU and βIII-tubulin, Dcx, NeuN, or DARPP 32 striatal BrdU+ cells were imaged using a Fluoview confocal microscope (Olympus, Lake Success, NY, USA), images were acquired using an argon–krypton laser and analyzed as described.19,21
Retrograde tracing of MSNs
A separate cohort of rats were given either intraventricular AAV4:BDNF/noggin or AAV4:null, followed by 30 daily injections of BrdU, and were then injected 3 months later, in week 18, with 1 μl of FMSs (0.04 μm red fluorospheres, in suspension; Invitrogen). The FMS retrograde tracer was injected bilaterally into the globus pallidus (anteroposterior, −2.4 mm; mediolateral, ±4 mm; dorsoventral, −6.8 mm), according to Paxinos. The injected animals were killed 2 weeks later, 20 weeks after viral injection, and perfused with 4% paraformaldehyde; their brains were sectioned and processed for BrdU immunolabeling, followed by confocal identification of BrdU+/FMS+-tagged striatal neurons.
Assessment of cell death
TUNEL was used to detect apoptotic cells among the newly recruited OB neurons, using digoxigenin-coupled dUTP and an anti-digoxigenin fluorescein-conjugated antibody. Eight sagittal cryosections (14 μm) per animal, representing every 16th section, were stained with an ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (S7111, Chemicon), following the manufacturer’s protocol. Sections were further stained with BrdU as described above.
Acknowledgments
Supported by NINDS R01NS53546 and R37/R01NS29813. We thank Eric Robbins and Michael Toner for technical support, and the Gene Therapy Core Facility of the University of Iowa for producing the AAV2/4-based viruses.
ABBREVIATIONS
- AAV4
adeno-associated virus serotype 4
- BDNF
brain-derived neurotrophic factor
- IRES
internal ribosome entry site
- DARPP-32
dopamine- and cyclic-AMP-regulated phosphoprotein of molecular weight 32 000 Da
- EGFP
enhanced green fluorescent protein
- BrdU
5-bromo-2′-deoxyuridine
- DCX
doublecortin
- MSN
medium spiny neuron
- OB
olfactory bulb
- CNS
central nervous system
- FMS
fluorescent microsphere
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling
- ANOVA
analysis of variance
Footnotes
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 2.Goldman SA, Luskin MB. Strategies utilized by migrating neurons of the postnatal vertebrate forebrain. Trends Neurosci. 1998;21:107–114. doi: 10.1016/s0166-2236(97)01191-0. [DOI] [PubMed] [Google Scholar]
- 3.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 4.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 5.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]
- 6.Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci USA. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on ‘Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension’. Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. author reply 393. [DOI] [PubMed] [Google Scholar]
- 8.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 9.Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, et al. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- 10.Roy NS, Benraiss A, Wang S, Fraser RA, Goodman R, Couldwell WT, et al. Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J Neurosci Res. 2000;59:321–331. doi: 10.1002/(sici)1097-4547(20000201)59:3<321::aid-jnr5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 12.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 13.Magavi S, Leavitt B, Macklis J. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 14.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 15.Tattersfield AS, Croon RJ, Liu YW, Kells AP, Faull RL, Connor B. Neurogenesis in the striatum of the quinolinic acid lesion model of Huntington’s disease. Neuroscience. 2004;127:319–332. doi: 10.1016/j.neuroscience.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 16.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 17.Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, et al. FGF2 promotes neurogenesis and neuroprotection in a transgenic mouse model of Huntington’s disease. Proc Natl Acad Sci USA. 2005;102:18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- 21.Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes WA, Mehler MF, Kessler JA. Transgenic overexpression of BMP4 increases astroglial and decreases oligodendroglial lineage commitment. Dev Biol. 2003;255:164–177. doi: 10.1016/s0012-1606(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 23.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 24.Ross CA, Becher MW, Colomer V, Engelender S, Wood JD, Sharp AH. Huntington’s disease and dentatorubral-pallidoluysian atrophy: proteins, pathogenesis and pathology. Brain Pathol. 1997;7:1003–1016. doi: 10.1111/j.1750-3639.1997.tb00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bates GP, Mangiarini L, Mahal A, Davies SW. Transgenic models of Huntington’s disease. Hum Mol Genet. 1997;6:1633–1637. doi: 10.1093/hmg/6.10.1633. [DOI] [PubMed] [Google Scholar]
- 26.Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 30.Sevin C, Benraiss A, Van Dam D, Bonnin D, Nagels G, Verot L, et al. Intracerebral adeno-associated virus-mediated gene transfer in rapidly progressive forms of metachromatic leukodystrophy. Hum Mol Genet. 2006;15:53–64. doi: 10.1093/hmg/ddi425. [DOI] [PubMed] [Google Scholar]
- 31.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, et al. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther. 2006;14:564–570. doi: 10.1016/j.ymthe.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6(Suppl 1):S212–S222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Chiorini JA, Yang L, Liu Y, Safer B, Kotin RM. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J Virol. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Therapy. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 36.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 37.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi M, Mori K. Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci USA. 2005;102:9697–9702. doi: 10.1073/pnas.0406082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28:11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 42.Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, et al. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci USA. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, et al. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44:1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 44.Gustafson EL, Ehrlich ME, Trivedi P, Greengard P. Developmental regulation of phosphoprotein gene expression in the caudate-putamen of rat: an in situ hybridization study. Neuroscience. 1992;51:65–75. doi: 10.1016/0306-4522(92)90471-d. [DOI] [PubMed] [Google Scholar]
- 45.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 46.Peschanski M, Cesaro P, Hantraye P. Rationale for intrastriatal grafting of striatal neuroblasts in patients with Huntington’s disease. Neuroscience. 1995;68:273–285. doi: 10.1016/0306-4522(95)00162-c. [DOI] [PubMed] [Google Scholar]
- 47.Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- 48.Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu G, Martins I, Wemmie JA, Chiorini JA, Davidson BL. Functional correction of CNS phenotypes in a lysosomal storage disease model using adeno-associated virus type 4 vectors. J Neurosci. 2005;25:9321–9327. doi: 10.1523/JNEUROSCI.2936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galvão RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–13383. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogen-esis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–3525. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- 52.Reumers V, Deroose CM, Krylyshkina O, Nuyts J, Geraerts M, Mortelmans L, et al. Noninvasive and quantitative monitoring of adult neuronal stem cell migration in mouse brain using bioluminescence imaging. Stem Cells. 2008;26:2382–2390. doi: 10.1634/stemcells.2007-1062. [DOI] [PubMed] [Google Scholar]
- 53.Batista CM, Kippin TE, Willaime-Morawek S, Shimabukuro MK, Akamatsu W, van der Kooy D. A progressive and cell non-autonomous increase in striatal neural stem cells in the Huntington’s disease R6/2 mouse. J Neurosci. 2006;26:10452–10460. doi: 10.1523/JNEUROSCI.2850-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips W, Morton AJ, Barker RA. Abnormalities of neurogenesis in the R6/2 mouse model of Huntington’s disease are attributable to the in vivo microenvironment. J Neurosci. 2005;25:11564–11576. doi: 10.1523/JNEUROSCI.3796-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277:2089–2096. doi: 10.1074/jbc.M109151200. [DOI] [PubMed] [Google Scholar]
- 56.Urabe M, Ding C, Kotin R. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]