Abstract
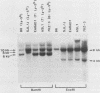
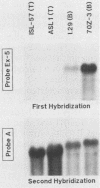
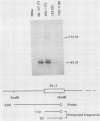
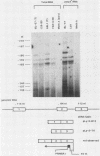
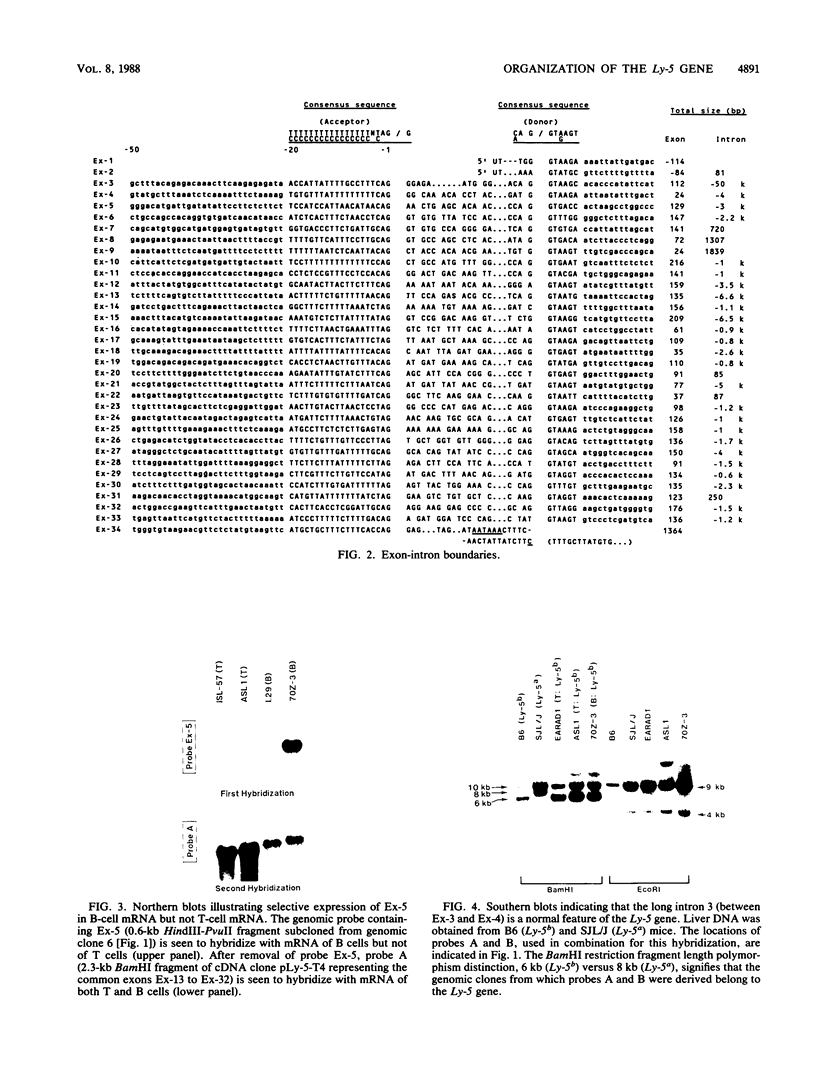
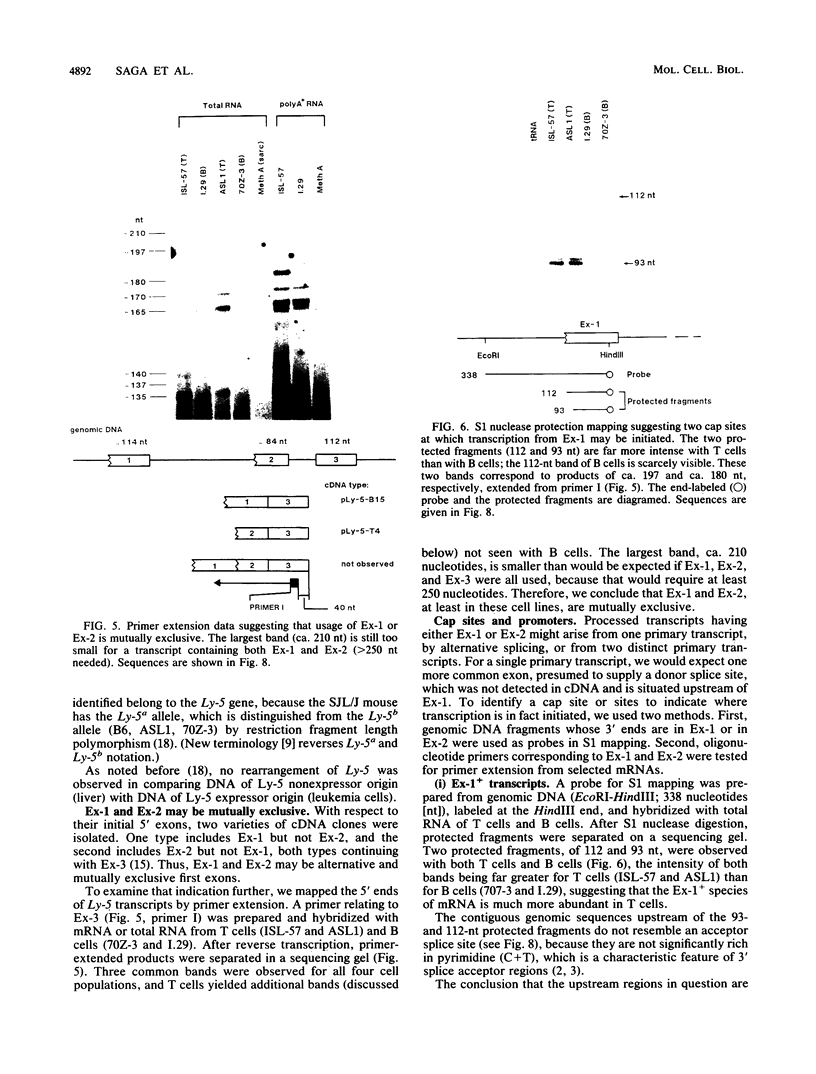
A single Ly-5 gene is known to generate a variety of transmembrane glycoprotein isoforms that distinguish various cell lineages and stages of differentiation within the hematopoietic developmental compartment of the mouse. Systems homologous to Ly-5 are known in rats and in humans. The complete exon-intron organization of the Ly-5 gene is described in this report. The Ly-5 gene occupies about 120 kilobases of chromosome 1 and comprises 34 exons, of which 32 (Ex-3 to Ex-34) are protein coding. Ex-1, Ex-2, and parts of Ex-3 and Ex-34 are untranslated. In all cDNA clones examined, either Ex-1 or Ex-2 was represented, but not both, implying that Ex-1 and Ex-2 in Ly-5 mRNA may be mutually exclusive. Primer extension and S1 nuclease protection mapping were used to identify initiation (cap) sites for transcription. The finding of putative cap sites for Ex-1 and Ex-2, and of corresponding TATA-like sequences, suggests the presence of two promoters. In both Ex-1+ and Ex-2+ cDNA clones the next exon is Ex-3, which has a translation-initiating codon. The intron between Ex-3 and Ex-4 is unusually long, about 50 kilobases. Evidence is given that Ex-5, like Ex-6 and Ex-7 (studied previously), is another alternative exon that is selectively programmed, alone or together with Ex-6 or Ex-7 or both, to generate actual or potential Ly-5 isoforms by alternative splicing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Jackson D. I., Willis A. C., Williams A. F. Lymphocyte specific heterogeneity in the rat leucocyte common antigen (T200) is due to differences in polypeptide sequences near the NH2-terminus. EMBO J. 1987 May;6(5):1259–1264. doi: 10.1002/j.1460-2075.1987.tb02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Puddington L., Machamer C. E., Bevan M. J. Acquisition of cytotoxic T lymphocyte-specific carbohydrate differentiation antigens. J Exp Med. 1985 Oct 1;162(4):1275–1293. doi: 10.1084/jem.162.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois L., Bevan M. J. Functional modifications of cytotoxic T-lymphocyte T200 glycoprotein recognized by monoclonal antibodies. Nature. 1985 Apr 4;314(6010):449–452. doi: 10.1038/314449a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Shen F. W., Hämmerling U. Genetic nomenclature for loci controlling mouse lymphocyte antigens. Immunogenetics. 1987;25(2):71–78. doi: 10.1007/BF00364270. [DOI] [PubMed] [Google Scholar]
- Newman W. Selective blockade of human natural killer cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3858–3862. doi: 10.1073/pnas.79.12.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Battifora H. A. Human homologue of murine T200 glycoprotein. J Exp Med. 1980 Oct 1;152(4):842–852. doi: 10.1084/jem.152.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Scheid M. P. T200 cell surface glycoprotein of the mouse. Polymorphism defined by the Ly-5 system of alloantigens. J Exp Med. 1980 May 1;151(5):1311–1316. doi: 10.1084/jem.151.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S. J., Thomas M. L., Morton C. C., Trowbridge I. S. Structural variants of human T200 glycoprotein (leukocyte-common antigen). EMBO J. 1987 May;6(5):1251–1257. doi: 10.1002/j.1460-2075.1987.tb02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Alternative use of 5' exons in the specification of Ly-5 isoforms distinguishing hematopoietic cell lineages. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5364–5368. doi: 10.1073/pnas.84.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Sequences of Ly-5 cDNA: isoform-related diversity of Ly-5 mRNA. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6940–6944. doi: 10.1073/pnas.83.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. W., Saga Y., Litman G., Freeman G., Tung J. S., Cantor H., Boyse E. A. Cloning of Ly-5 cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7360–7363. doi: 10.1073/pnas.82.21.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F. W., Tung J. S., Boyse E. A. Further definition of the Ly-5 system. Immunogenetics. 1986;24(3):146–149. doi: 10.1007/BF00364741. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Hall L. R., Saga Y., Schlossman S. F., Saito H. Differential usage of three exons generates at least five different mRNAs encoding human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1548–1566. doi: 10.1084/jem.166.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Matsuyama T., Morimoto C., Schlossman S. F., Saito H. Identification of the sequence required for expression of the 2H4 epitope on the human leukocyte common antigens. J Exp Med. 1987 Nov 1;166(5):1567–1572. doi: 10.1084/jem.166.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. L., Barclay A. N., Gagnon J., Williams A. F. Evidence from cDNA clones that the rat leukocyte-common antigen (T200) spans the lipid bilayer and contains a cytoplasmic domain of 80,000 Mr. Cell. 1985 May;41(1):83–93. doi: 10.1016/0092-8674(85)90063-7. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Reynolds P. J., Chain A., Ben-Neriah Y., Trowbridge I. S. B-cell variant of mouse T200 (Ly-5): evidence for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5360–5363. doi: 10.1073/pnas.84.15.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Deere M. C., Boyse E. A. Evidence that Ly-5 product of T and B cells differ in protein structure. Immunogenetics. 1984;19(2):149–154. doi: 10.1007/BF00387858. [DOI] [PubMed] [Google Scholar]
- Tung J. S., Scheid M. P., Pierotti M. A., Hämmerling U., Boyse E. A. Structural features and selective expression of three Ly-5+ cell-surface molecules. Immunogenetics. 1981;14(1-2):101–106. doi: 10.1007/BF00344303. [DOI] [PubMed] [Google Scholar]
- Upholt W. B., Sandell L. J. Exon/intron organization of the chicken type II procollagen gene: intron size distribution suggests a minimal intron size. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2325–2329. doi: 10.1073/pnas.83.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Yakura H., Shen F. W., Bourcet E., Boyse E. A. On the function of Ly-5 in the regulation of antigen-driven B cell differentiation. Comparison and contrast with Lyb-2. J Exp Med. 1983 Apr 1;157(4):1077–1088. doi: 10.1084/jem.157.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]