Abstract
Proteinase inhibitor 9 (PI-9, SerpinB9) is the only known human intracellular granzyme B inhibitor. Whether expression of PI-9 is sufficient to block cytolysis induced by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells remains controversial. To evaluate the roles of PI-9, we isolated and tested 3 lines of stably transfected HeLa cells expressing wild-type PI-9 and one line expressing an inactive mutant PI-9. Expression of wild-type PI-9, but not the inactive mutant PI-9, inhibited cytolysis induced by human NK92 and NKL natural killer cells. Expression of high levels of PI-9 is therefore sufficient to protect human cells against NK cell-mediated cell death. Using two assays, we show that expressing wild-type PI-9, but not the inactive mutant PI-9, blocks Fas/Fas ligand (Fas/FasL)-mediated apoptosis. PI-9 expression has no effect on etoposide-induced apoptosis. HeLa cells exhibiting substantial resistance to Fas/FasL-mediated apoptosis contain 2–3 fold higher PI-9 levels than HCT116 human colon cancer cells and 2–3 fold lower PI-9 levels than MCF7/ERHA breast cancer cells, in which PI-9 is strongly induced by estrogens, and by tamoxifen. Expression of increasing levels of PI-9 in target cells may progressively inhibit immune surveillance by blocking NK and CTL-induced cytotoxicity through the perforin/granzyme pathway and then through the Fas/FasL pathway.
Keywords: CL, PI-9, proteinase inhibitor 9, SerpinB9, perforin/granzyme, NK cells, Fas/FasL
1. Introduction
Cytolytic lymphocytes (CLs) identify and induce cytolysis of neoplastic cells and cells infected with viruses and other intracellular pathogens. CLs include both natural killer (NK) cells and CD8+ cytotoxic T lymphocytes (CTLs). CLs use the perforin/granzyme and Fas/Fas ligand (Fas/FasL) pathways to induce cytotoxicity of target cells. The perforin/granzyme pathway is often thought to play the predominant role (1–3).
In perforin/granzyme-mediated apoptosis, recognition of a target cell triggers the release of proteases stored in granules, or granzymes, from their complex with serglycin (4). Granzyme B (GrB), and other granzymes, then enter the target cell by endocytosis. Perforin facilitates release of GrB and other granzyme proteases from the endosome. In target cells, GrB induces apoptosis by direct cleavage of caspase and non-caspase substrates. For example, GrB cleavage of BH3, leads to activation of the mitochondrial apoptosis pathway (1, 2). Proteinase inhibitor 9 (PI-9, SerpinB9) is the only known human intracellular inhibitor of GrB (5). Granzyme A (GrA) induces rapid cell death by a pathway distinct from the GrB pathway. Granzyme A can induce cytolysis without granzyme B, or act synergistically with granzyme B (6). The function of human granzymes K, L and M is less clear and they are thought to activate redundant signaling pathways, culminating in cell stress or apoptosis. There have been no in vitro studies evaluating PI-9’s ability to inhibit granzyme A, K and L.
The intensively-studied Fas/FasL pathway is important in many cellular pathways including the induction of apoptotic cell death and inflammation (7). Fas/APO-1/CD95, is a member of the tumor necrosis factor (TNF) receptor superfamily of transmembrane receptors. In a simple model, binding of Fas ligand induces trimerization of the membrane Fas receptor (8). The activated Fas receptor recruits adaptor molecules such as Fas-associating protein with death domain (FADD), which recruits procaspase 8, and often procaspase 10, to the receptor complex, where they undergo autocatalytic activation. Activated caspase 8 cleaves Bcl-2 interacting protein (Bid) which then translocates to the mitochondria where it activates the mitochondrial apoptosis pathway leading to activation of caspase 3. Fas-induced apoptosis can be blocked at several stages by FLICE-inhibitory protein (FLIP), Bcl-2, or by the cytokine response modifier A (CrmA) (9).
We, and others, have investigated the regulation of PI-9 gene expression. Interferon γ (IFN-γ) and Hypoxia Inducible Factor 2 (HIF2) induce PI-9 through as yet unidentified sites (10, 11). Modulators of inflammation including lipopolysaccaharide (LPS) and interleukin-1β (IL-1β) acting at an AP-1 site and 2 NF-κB sites induce PI-9 (12–14). Estrogens also induce PI-9 (15). Since chromatin immunoprecipitations show binding of estrogen-estrogen receptor complex to an estrogen responsive unit (ERU) in the PI-9 promoter region, PI-9 is a primary estrogen-inducible gene (16, 17).
Several types of data are consistent with the idea that expression of the granzyme B inhibitor, PI-9, or its closest mouse homologue, serine protease inhibitor 6 (SPI-6), plays a role in modulating immune responses. PI-9 levels tend to be high in immune-privileged sites (18). Overexpression of the related mouse granzyme B inhibitor, SPI-6, prolongs the life span of CD8+ memory lymphocytes (19). Knockout of SPI-6 causes severe damage to CTLs and reduces their ability to induce apoptosis of target cells (20).
Whether expression of PI-9, or SPI-6, is sufficient to protect target cells against CL-induced cytolysis remains controversial. Expression of high levels of PI-9 in stably transfected MCF-7, human breast cancer cells, increased their resistance to apoptosis induced by long-term activated NK cells (21). In cultured hepatoma cells, induction of PI-9 by IFN-γ, or by estrogen, partially blocks CTL and NK cell-induced cytolysis (10, 22). We recently showed that induction of increasing levels of endogenous PI-9 by estrogen in MCF-7 cells resulted in a progressive increase in resistance to NK cell-induced perforin/granzyme-mediated cell death (23). Earlier studies suggested that overexpression of SPI-6 reduced CTL-induced apoptosis of target cells (24) and that high levels of PI-9 are associated with a poor therapeutic response and prognosis in lymphomas and melanomas (25, 26). However, the sensitivity of lymphomas to CL-induced cytotoxicity did not correlate with PI-9 level (27). Doubts have been expressed about this report (28). An in vivo study using knockout mice suggests mouse granzymes A and B function primarily in viral defense and perforin plays a key role in tumor rejection (29). Since recent studies indicate that mouse and human granzymes are quite different (30), and that human granzyme B is 30 times more cytotoxic than mouse granzyme B (31), it is likely that granzyme B, and its inhibitor PI-9, play a larger role in humans than in mice.
Mouse SPI-6 reportedly does not inhibit CTL-induced membranolysis of target cells without another serpin, SPI-CI, which presently has no human homologue (32). We showed that RNAi knockdown of human PI-9 abolished estrogens ability to inhibit CL-induced cytolysis in hepatoma cells and in MCF-7, human breast cancer cells (22, 23). While the RNAi data indicates that PI-9 is necessary to block CL-induced cytolysis, they do not establish whether or not PI-9 expression is sufficient.
PI-9 has not been reported to inhibit Fas/FasL-mediated apoptosis. Although estrogen strongly induces PI-9 in MCF-7 cells stably transfected to express high levels of estrogen receptor (MCF7/ERHA cells), MCF-7 cells could not be used for these studies because they lack Fas receptor and are not killed by Fas/FasL (22, 23, 33).
To determine whether expression of PI-9 is sufficient to block CL-induced cytotoxicity and whether expressing PI-9 inhibits Fas/FasL-mediated apoptosis, we produced three stably transfected HeLa cell lines expressing a wild-type PI-9 and one cell line expressing a mutant PI-9 containing two inactivating mutations in and around its C-terminal reactive center loop (RCL) (21). PI-9 was expressed using a tetracycline-regulated system in which doxycycline was used to repress expression of PI-9.
We analyzed the effects of expressing wild-type and mutant PI-9 on cytotoxicity induced by two lines of human NK cells. NK92 cells express predominantly granzyme B while NKL cells express primarily granzyme A (34, 35). We also tested the ability of cell lines expressing different levels of PI-9 to undergo Fas/FasL-mediated apoptosis. Our data shows that expression of wild-type PI-9 is sufficient to inhibit both the perforin/granzyme and Fas/FasL-mediated death pathways. Comparison of levels of PI-9 expressed in the stably transfected HeLa cell lines with the PI-9 levels in a human colon cancer cell line, HCT116, and in fully induced MCF7/ERHA cells showed that the HeLa cell lines expressed levels of PI-9 2–3 fold higher than the HCT116 cells and 2–3 fold lower than the MCF7/ERHA cells.
2. Materials and methods
2.1. Cell Lines and cell culture
HeLa, human cervical cancer cells, were maintained in DMEM supplemented with 10% FBS. PI-9 expressing clones were maintained in 1.0 mg/ml of the antibiotic, G418 (Invitrogen, Carlsbad, CA). Human NK92 cells (provided by Dr. J. Miller) were cultured in alpha-MEM plus 15% FBS, 15% horse serum, supplemented with 200 U/ml IL-2 (PeproTech, Rocky Hill, NJ) and 100 U/ml IL-15 (PeproTech). Human NKL cells (provided by Dr. M. Robertson) were cultured in RPMI 1640 supplemented with 200 U/ml IL-2, 10% FBS. All cells were maintained at 37°C in 5% CO2. Tet-inducible MCF-7 cells stably transfected to express additional ERα, MCF7/ERHA cells (36), were grown as described (23, 37). All media included 50,000 IU penicillin and 50 mg streptomycin per liter. All use of anti-human Fas antibody (Upstate, Charlottesville, VA) was at 100 ng/ml for 2 days. All use of Etoposide (Sigma, St. Louis, MO) was at 12.5 μM for 2 days.
2.2. Production of stably transfected cell lines expressing PI-9
The full length human wild-type PI-9 was ligated into the ptetCMV-FO(AS) vector (38). This plasmid was used as a template for two rounds of site-directed mutagenesis using the Quick-Change mutagenesis kit (Stratagene, La Jolla, CA), as described in the manufacturer’s protocol. The following set of primers containing the desired single mutation was used: (F: 5′-GGTGAATGAAGAAGGCAGAGAGGCAGCGGCAGC-3′, R: 5′-GCTGCCGCTGCCTCTCTGCCTTCTTCATTCACC-3′); (F: 5′-GCTGCTTTGTAGTTGCAGCATGCTGCATGGAATCTGG-3′, R: 5′-CCAGATTCCATGCAGCATGCTGCAACTACAAAGCAGC-3′). This yields a double mutant of PI-9; 320VEVNEEGREAAAASSCFVVAA=CCMESGPRFCADHPFL356, where “=” represents the bond between the P1 and P1′ residues in the C-terminal RCL and the underlined letters represent the mutated amino acids. The presence of the double mutation was verified by DNA sequencing. pIE-tTA (derived from pUHD15-1) (39) and ptetOFF-FLAG-wtPI-9 or ptetOFF-FLAG-mutPI-9 were cotransfected into our HeLa cells by electroporation at 190 V and 1180 μF for ~30 msec using a BioRad electorporator (BioRad, Hercules, CA). HeLa cells were then plated in 10 cm2 dishes and grown in the absence of doxycycline (Sigma) for ~4–5 weeks in 1.0 mg/ml of G418 (Invitrogen). Colonies were trypsinized, transferred to a 96 well plate, grown until they reached confluence, harvested and transferred to a 12 well plate and finally to a T75 flask. Screening for PI-9 expression was performed by western blot.
2.3. Western blotting
Whole cell extracts were prepared in 1X RIPA buffer (0.05 M Tris-HCl pH7.4, 0.15 M NaCl, 0.25% deoxycholic acid, 1.0% NP-40, 1.0 mM EDTA; Upstate) containing a 1:100 dilution of the mammalian protease inhibitor cocktail (104 mM AEBSF, 80 μM aprotinin, 2 mM leupeptin, 4 mM bestatin, 1.5 mM pepstatin A, 1.4 mM E-64; Sigma). For western blotting, cell lysates were run on 10% SDS/PAGE gels and transferred to nitrocellulose membranes. β-actin was used as the loading control. PI-9 was detected using our affinity purified polyclonal antibody to PI-9 (12). Blots were incubated with the appropriate secondary antibodies (Invitrogen), and bands visualized with ECL Plus (Amersham, Piscataway, NJ) and detected using a STORM PhosphorImager (Amersham). When blots were to be reprobed, they were first stripped for ~10 minutes in Re-Blot Plus Solution (Chemicon International, Temecula, CA). Quantitation of band intensities was by PhosphorImager scanning and analysis using Molecular Dynamics ImageQuant software.
2.4. Cell-mediated cytotoxicity assay using time resolved fluorescence
Time-resolved fluorescence assays for cell death were carried out with the modifications of the supplier’s instructions we recently described (23). Briefly, target HeLa cells were incubated at 106 cells/ml in DMEM plus 10% FBS with BATDA (PerkinElmer, Wellesley, MA) for 10 min at room temperature, followed by 4 washes with culture medium before incubating with the effector NK cells at the indicated ratios of effector cells/target cells (E/T). Cytotoxicity assays were carried out in 96-well round bottom plates containing 10,000 target cells in a total volume of 200 μl/well. The plates were subjected to centrifugation for 30 seconds at 1,000 rpm to facilitate contact between target and effector cells. After incubation for 2 hours at 37°C, 20 μl of supernatant from each reaction was added to 200 μl of the europium (Eu3+) solution. Following agitation of the plates for 5 min, time-resolved fluorescence was measured by using an excitation wavelength of 315 nm and an emission wavelength of 623 nm using a PHERAstar microplate fluorometer (BMG Labtech, Durham, NC). Maximum release and spontaneous releases were determined as described in the manufacturer’s protocol. Percent specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
2.5. Assays for cell survival assay and apoptosis
Cell survival was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) based cell growth determination assay (40)(Sigma). Briefly, 20,000 cells were plated in a 24 well plate in 1 ml of medium. On day 1, the medium was changed and the cells were maintained for 2 days in medium containing anti-human Fas antibody at 100 ng/ml. MTT stock solution, 10% (volume/volume) dissolved in medium at a concentration of 5 mg/ml was added to the medium directly, and the cells were incubated for 3 hr at 37°C. MTT solvent (0.1 N HCl in anhydrous isopropanol) was added to solubilize the MTT formazan crystals. Absorbance was measured at 570 and 650 nm using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
Apoptosis was measured by flow cytometry using the Vybrant Apoptosis Assay Kit # 3 (Invitrogen). Cells were double stained with recombinant annexin V antibody conjugated to fluorescein (annexin V-FITC), as well as the red-fluorescent propidium iodide (PI) nucleic acid binding dye. Different cell populations were identified using a BD FACSCanto flow cytometer (BD Biosciences, San Jose, CA) with the 488 nm line of an argon-ion laser for excitation.
3. Results
3.1. Stably transfected cell lines expressing PI-9
Upon binding to a serine protease, a serpin, such as PI-9 is cleaved within a protruding reactive center loop (RCL) that resembles the natural substrates of the protease. This triggers a conformational change that results in inhibition of the protease. The mutant PI-9 we constructed contains two amino acid changes (T327R and E340A) in and around the RCL. Each of these changes abolishes the ability of PI-9 to inhibit granzyme B in vitro (21).
We used HeLa cells because wild-type HeLa cells express negligible levels of endogenous PI-9 (data not shown, and Fig. 1A, wtPI-9, OFF). We identified and characterized stably transfected lines of HeLa cells expressing wild-type PI-9 and mutant PI-9. Expression of PI-9 was tightly regulated by doxycycline. Maintaining the cells in 2.0 μg/ml doxycycline for 7 days largely abolished expression of wild-type PI-9 (Fig. 1A, wtPI-9, OFF). The HeLa cell line expressing the mutant PI-9 expressed a very high level of the protein and even in the presence of doxycycline a trace level of PI-9 was seen (Fig. 1A, mutPI-9, OFF). Since this cell line expressed 5–10 times more of the protein than the cells expressing wild-type PI-9 (Fig. 1A, wtPI-9, ON), we identified a concentration of doxycycline (12.5 ng/ml) that resulted in nearly equivalent expression of wild-type and mutant PI-9 (Fig. 1B, wt and mt*).
Fig. 1. Doxycycline-regulated expression of wild type and mutant PI-9 in stably transfected HeLa cells.
A, Western blot of cell extracts from stably transfected lines of HeLa cells expressing either wild-type PI-9 (wtPI-9) or PI-9 mutated to change two critical amino acids in and around its reactive center loop (mutPI-9). Cells were maintained in 2.0 μg/ml doxycycline for 1 week to shut off PI-9 expression and allow PI-9 to decay (OFF), or in the absence of doxycycline to allow full expression of PI-9 (ON). PI-9 was detected using our affinity purified polyclonal antibody to PI-9 (12). B, Western blot of expression of similar levels of wild-type PI-9 and mutant PI-9. In the absence of doxycycline, the level of mutant PI-9 was several-fold higher than the level of wild-type PI-9. To compare effects in cells expressing similar levels of the two proteins, the mutPI-9 cell line was maintained in 12.5 ng/ml doxycycline for 1 week to decrease the level of mutPI-9 expression (mt*) so that it was similar to the level of PI-9 in the fully expressing wtPI-9 (wt) cell line. C, Determination of the rate at which PI-9 disappears from cells. The stably transfected cell line expressing wild-type PI-9 was allowed to express maximum levels of PI-9 (day 0) and then put in doxycycline to turn off PI-9 expression. Extracts were prepared from cells maintained in doxycycline for the indicated times, analyzed by western blot analysis and band intensity quantitated by PhosphorImager analysis.
The half-life of PI-9 protein has not been reported. To determine the time we needed to maintain cells in doxycycline in order for the PI-9 expressed from the doxycycline-regulated promoter to largely disappear from the cells, we maintained the cell line expressing wild-type PI-9 in medium containing 2.0 μg/ml doxycycline for several days. Extracts were prepared daily and analyzed for PI-9 content by western blotting and quantitated using a PhosphorImager. Although the doxycycline results in repression of new PI-9 gene transcription, at early times after addition of doxycycline, the rate of disappearance of PI-9 is slow because there is significant synthesis of new PI-9 protein from pre-existing PI-9 mRNA that has not yet decayed. Since our regulatory studies suggest that PI-9 mRNA has a relatively short half-life (15, 23), at later times, when the pre-existing PI-9 mRNA has largely disappeared, the western blot data primarily represents the rate at which PI-9 protein is degraded (Fig. 1C). While continued synthesis of PI-9 protein from pre-existing PI-9 mRNA precludes a precise determination of the half-life of PI-9 protein, during days 2–4, when most pre-existing PI-9 mRNA has decayed, PI-9 levels fall from ~65% to ~15% of the day 0 value. In all of our experiments, to allow sufficient time for intracellular PI-9 to be largely degraded after the gene is turned off, we maintained the cells in medium containing doxycycline for at least 7 days.
3.2. Expression levels of PI-9 in the stably transfected HeLa cells and in cells expressing endogenous PI-9
We isolated and characterized three lines of stably transfected HeLa cells expressing wild-type PI-9 (wtPI-9, wt14 and wt18). We evaluated the levels of PI-9 expressed in the transfected cell lines and compared it to the levels of endogenous PI-9 in cell lines. HCT116 cells are a well-studied human colon cancer cell line that is resistant to Fas/FasL-mediated apoptosis and retain Fas receptor (41). Some human breast cancers express high levels of ERα. The widely used breast cancer therapeutic, tamoxifen, and its active metabolite, 4-hydroxytamoxifen (OHT), which normally act as estrogen antagonists often act as estrogens in this class of tumors (42, 43). A model for this class of tumor has been developed in which widely used MCF-7 human breast cancer cells are stably transfected to express additional ERα in the presence of doxycycline (MCF7/ERHA cells) (36, 37). In these cells, the classic estrogen, 17β-estradiol (E2) or OHT plus the mitogen, epidermal growth factor (EGF), each induce a high level of PI-9 (23).
To compare the levels of PI-9 in the stably transfected HeLa cells and the HCT116 and MCF7/ERHA cells, we performed western blot analysis and quantitation by PhosphorImager analysis. We analyzed two concentrations of protein for each sample (2.5 μg and 5.0 μg) and showed that band intensities were in the linear range. A representative western blot with quantitation is shown in Fig. 2A and quantitative data averaged from 3 western blots is presented in Fig. 2B. The stably transfected cell lines expressed 2–3 fold more PI-9 than the HCT116 cells and 2–3 fold less PI-9 than the MCF7/ERHA cells. Thus, the relatively high levels of PI-9 expressed in the stably transfected cell lines are in the range of PI-9 expressed in the HCT116 and MCF7/ERHA cells. Because the stably transfected cells express FLAG epitope-tagged PI-9, as expected, the PI-9 band shows slightly lower mobility than PI-9 expressed from the endogenous cellular PI-9 gene in the HCT116 and MCF7/ERHA cells.
Fig. 2. Comparison of PI-9 protein levels in different cell lines.


A, Western blot comparing the level of PI-9 protein in cell extracts from five different cell lines. Electrophoresis used samples containing 2.5 and 5.0 μg of protein from each cell line. The intensity of the PI-9 band from the three stably transfected cell lines expressing wild-type PI-9 (wtPI-9, wt14, wt18), HCT116, a human colon cancer cell line, and MCF7/ERHA cells were quantitated by PhosphorImager analysis. Relative PI-9 levels across different cell lines are represented by a ratio of PI-9 to β-actin band intensity and have been normalized to the wtPI-9 cell line at 2.5 and 5.0 μg of total cell lystate, which were each set equal to 1. Band intensities were in the linear range and 5 μg of protein yielded PI-9 bands whose intensity was approximately twice the intensity of the 2.5 μg samples. Band intensity at 2.5 μg of protein is denoted by an asterisk (*). B, Quantitation of PI-9 levels. The ratio of PI-9/β-actin in the 2.5 μg samples from the 5 cell lines was quantitated by PhoshorImager analysis, and is the average ± SEM of data from the western blot shown in panel A and 2 additional blots.
3.3. Expression of wild-type PI-9, but not mutant PI-9, blocks perforin/granzyme-mediated cytolysis
The HeLa wtPI-9 cells stably expressing wild-type PI-9 and the same cell line in which PI-9 expression is turned off with doxycycline differ only in the presence of doxycycline. Similarly, the HeLa wtPI-9 and the HeLa mutPI-9 cells differ only in the expression of a mutant form of PI-9 containing two critical amino acid changes.
We recently showed that the human NK cell lines NK92, which contain primarily granzyme B (GrB) and some granzyme A (GrA), and NKL, which primarily contain GrA (34, 35) and traces of GrB, both predominantly use the perforin/granzyme pathway to kill target cells (23). Others have also shown that this pathway is the predominant pathway in these short-term cytotoxicity assays (1). The combination of our stably transfected cell lines as target (T) cells and the NK cell lines as effector (E) cells was therefore well-suited to determining whether PI-9 expression is sufficient to block perforin/granzyme-mediated cytolysis mediated primarily by human GrB, or by GrA.
To measure cytotoxicity of the target cells, we used a time resolved fluorescence assay that is based on the idea that cells that lose membrane integrity release a fluorescent substrate into the medium. We, and others, showed that the time resolved fluorescence assay produces results similar to those obtained using the classical chromium release assay (23, 34, 44). When the transgene was turned off and the cells contained no detectable wild-type PI-9, the NK92 and NKL cells induced cytotoxicity (Fig. 3A, wtPI-9-OFF, open bars). At both ratios of effector cells to target cells, expression of wild-type PI-9 was sufficient to completely block cytotoxicity induced by NK92 cells and nearly completely blocked cytotoxicity induced by NKL cells (Fig. 3A, wtPI-9, black bars). Expression of the inactive mutant PI-9 did not inhibit cytotoxicity induced by NK92 or NKL cells (Fig. 3B, mutPI-9, black bars). These data show that the protease inhibitor activity of PI-9 is required for it to block NK cell-induced cell death.
Fig. 3. Expression of wild type PI-9, but not mutant PI-9, blocks NK cell-induced cytolysis.
A and B, Target (T) HeLa cells expressing wild-type PI-9 (panel A, wtPI-9, black bars) or mutant PI-9 (panel B, mutPI-9, black bars) or cells in which PI-9 expression was turned off with doxycycline (panels A and B, wtPI-9-OFF and mutPI-9-OFF, open bars, respectively) were incubated with the indicated ratios of NK92 or NKL effector (E) cells as described in Materials and Methods. Cytotoxicity was measured using time resolved fluorescence as described in Materials and Methods. The data in panels A-B represent the mean ± SEM for at least 3 separate experiments.
3.4. Increasing levels of PI-9 expression elicits increased resistance to granzyme-mediated cytotoxicity
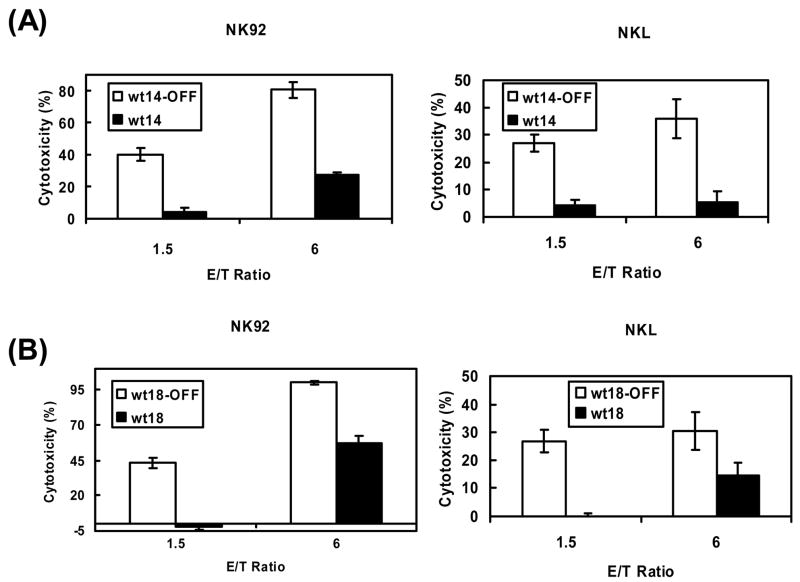
The experiments shown in figure 3 indicate that expression of high levels of PI-9 is sufficient to block granzyme/perforin-induced apoptosis mediated by granzyme B and by granzyme A. Since these experiments were performed with a single stably transfected cell line it was important to test the generality of our observation using additional clones of stably transfected HeLa cells. When PI-9 expression in these cell lines (wt14 and wt18) was turned off with doxycycline, the cells were highly sensitive to granzyme-mediated cytotoxicity (Fig. 4, open bars). wt14 and wt18 express ~80% and ~60% as much PI-9 as wtPI-9 cells, respectively (Fig. 2). These levels of PI-9 expression effectively inhibit cytotoxicity caused by the granzyme B-containing NK92 cells (Fig. 4, NK92) and by the granzyme A-containing NKL cells (Fig. 4, NKL). Since the wt14 and wt18 cells, which express somewhat lower levels of PI-9 than the wtPI-9 cells are somewhat less effective in blocking NK92 and NKL mediated cytotoxicity (Fig. 4), expression of increasing levels of PI-9 in the wtPI-9 cells (Fig. 3) is associated with increased resistance to granzyme-mediated cytotoxicity.
Fig. 4. Expression of lower levels of PI-9 still results in protection against NK cell-induced cytolysis.
A and B, Target (T) HeLa cells expressing lower levels of PI-9 (panel A, wt14, black bars) or (panel B, wt18, black bars) or cells in which PI-9 expression is turned off (panel A, wt14-OFF, open bars) or (panel B, wt18-OFF, open bars) were incubated with the indicated ratios of NK92 or NKL effector (E) cells as described in Materials and Methods. Cytotoxicity was measured using time resolved fluorescence as described in Materials and Methods. The data in panels A-B represent the mean ± SEM for at least 3 separate experiments.
3.5. Expression of PI-9 inhibits of Fas/FasL-mediated apoptosis
Since our stably transfected cell lines expressing PI-9 were highly resistant to perforin/granzyme-mediated cytotoxicity, we decided to test whether these cells were resistant to the other major apoptosis pathway used by CLs, Fas/FasL-mediated apoptosis. We used two different assays to determine sensitivity to Fas/FasL-mediated killing. The MTT assay provides a measure of overall cell metabolism. Flow cytometry using annexin V antibody (annexin V-FITC) and propidium iodide (PI) is widely used to evaluate Fas/FasL-mediated apoptosis. We carried out a standard flow cytometry assay in which annexin V-FITC was used to measure the membrane inversion that often accompanies apoptosis and PI fluorescence was used to measure loss of membrane integrity that occurs in cell death.
Consistent with other studies (45), in both untransfected HeLa cells and the stably transfected wtPI-9-OFF cells in which PI-9 expression was turned off, exposure to Fas antibody resulted in loss of ~50% of the cells as measured by the MTT assay (Fig. 5A, HeLa and wtPI-9-OFF; open bars). Expression of wild-type PI-9 completely abolished the effect of Fas antibody (Fig. 5A, wtPI-9, black bar). Expressing the mutant PI-9 had no effect on the sensitivity of the cells to Fas antibody (Fig. 5B). Since our finding that expression of wild-type PI-9 blocks Fas/FasL-induced apoptosis was unexpected, we also carried out flow cytometry.
Fig. 5. Expression of PI-9 blocks Fas/FasL-mediated apoptosis.
A–D, The MTT assay was used to determine the percentage of dead cells (A and B) and flow cytometry was used to determine the percentage of cells undergoing Fas/FasL-mediated apoptosis (C and D). Cells in which neither wild-type nor mutant PI-9 is expressed are represented as open bars (A-D, HeLa, wtPI-9-OFF and mutPI-9-OFF). Black bars are used to represent cells in which wild type PI-9 (A and C, wtPI-9), mutant PI-9 (B and D, mutPI-9) or mutant PI-9 expressed at a level similar to that seen in wtPI-9 (D, mt*) are expressed. A and B, wild-type PI-9, but not mutant PI-9, blocks cell death induced by Fas antibody. The cells were maintained in the presence of Fas antibody and assayed using the MTT assay as described in Materials and Methods. The percentage of dead cells was obtained by subtracting the absorbance of cells treated with Fas antibody from the absorbance of the corresponding set of cells in the absence of Fas antibody, as described (47). C, Expression of wild-type PI-9 blocks Fas/FasL, but not etoposide-induced apoptosis. The wtPI-9 cells were maintained in the presence of 100 ng/ml Fas antibody (Fas) or 12.5 μM etoposide (Etop) and apoptosis was assayed by flow cytometry as described in Materials and Methods. D, Expressing mutant PI-9 does not inhibit Fas/FasL-induced apoptosis. The mutPI-9 cells were maintained in the presence of Fas antibody and apoptosis was assayed by flow cytometry. Data shown in panels C and D is corrected for the low basal level of apoptosis of control cells (control was subtracted from Fas and Etop and mutPI-9). The data represents the mean ± SEM for at least 3 separate experiments.
The flow cytometry assay demonstrated that activating the Fas/FasL pathway dramatically increased apoptosis in the stably transfected cells in which expression of wild-type PI-9 had been turned off (Fig. 5C, Fas, wtPI-9-OFF, open bar). Expression of high levels of wild-type PI-9 completely blocked Fas/FasL-induced apoptosis (Fig. 5C, Fas, wtPI-9, black bar). Etoposide is a widely used activator of the classical mitochondrial apoptosis pathway. In contrast to the ability of PI-9 to block Fas/FasL-mediated apoptosis, apoptosis induced by etoposide, which occurs via caspase 9 and the mitochondrial pathway was completely unaffected by expression of PI-9 (Fig. 5C, Etop). This shows that the stably transfected cells expressing PI-9 are not simply resistant to all forms of apoptosis. In the stably transfected cell line expressing the mutant PI-9, Fas/FasL effectively induced apoptosis when no mutant PI-9 was expressed (Fig. 5D, mutPI-9-OFF, open bar), when the mutant PI-9 was expressed at a level similar to wild-type PI-9 panel C (Fig. 5D, mt*, black bar) and when the unrepressed, extremely high level of mutant PI-9 was expressed (Fig. 5D, mutPI-9, black bar). These data demonstrate that expression of high levels of wild type PI-9 blocks Fas/FasL-induced apoptosis.
These data showed that wtPI-9 cells which express high levels of PI-9, both granzyme mediated cytotoxicity and Fas/FasL-mediated apoptosis are nearly completely inhibited (Figs. 3 and 5). Since the wt14 and wt18 cells that express somewhat lower levels of PI-9 than the wtPI-9 cells, showed strong, but less complete inhibition of granzyme-mediated cytotoxicity, it was of interest to test the effect of these levels of PI-9 expression on Fas/FasL-mediated apoptosis.
Using flow cytometry, the wt14 and wt18 cells were sensitive to Fas/FasL-induced apoptosis when expression of PI-9 was off (Fig. 6, OFF, open bars). Expression of these lower levels of PI-9 produced a statistically significant (P<0.05) partial resistance to Fas/FasL-induced apoptosis (Fig. 6, ON, black bars). These data suggest that the level of PI-9 in a cell targeted by CLs determines its sensitivity to Fas/FasL-mediated apoptosis.
Fig. 6. Expressing intermediate levels of PI-9 results in partial protection against Fas/FasL-mediated apoptosis.

Flow cytometry analysis of the effect of expressing intermediate levels of PI-9 on Fas/FasL-induced apoptosis. wt14 and wt18 cells were maintained in the fully off state (wt14-OFF and wt18-OFF, open bars) or left on (wt14 and wt18, black bars) and incubated in the absence or presence of Fas antibody and apoptosis assayed by flow cytometry. The data represents the mean ± SEM for at least 3 separate experiments. The difference between the Fas antibody-treated wt14-OFF and wt14 cells and wt18-OFF and wt18 cells was evaluated by Student’s T test and found to be significant (wt14-OFF vs. wt14, P=0.01; wt18-OFF vs. wt18, P=0.04).
4. Discussion
We used FLAG epitope-tagged PI-9 to produce our stably transfected HeLa cell lines because the presence of the FLAG epitope-tag might facilitate isolation of PI-9 complexed to intracellular proteases whose identity is currently unknown. Use of the tetracycline-regulated promoter system in our stably transfected cell lines combined with the parallel production of a cell line expressing inactive PI-9 allowed us to clearly show that expressing PI-9 is sufficient to inhibit perforin/granzyme-mediated cytotoxicity induced by two established lines of human NK cells. PI-9’s ability to block cytolysis induced by CLs is eliminated by mutations that abolish its ability to inhibit granzyme B in vitro (21). Our earlier studies used three different assays (thymidine release, chromium release and time resolved fluorescence) to show that estrogen induction of PI-9 in hepatoma cells (HepG2ER7) and in MCF-7 breast cancer cells inhibits perforin/granzyme-mediated apoptosis and cytolysis induced by CTLs and NK cells (22, 23). Since RNAi knockdown of PI-9 abolished estrogens’ ability to protect the cells against CTL and NK cell-induced cytolysis, estrogen induction of PI-9 was clearly necessary for estrogens’ ability to block CL-induced cell death. The present data extends these studies, and together with research by others (21), demonstrates that expression of human PI-9 is sufficient to block perforin/granzyme-mediated cytolysis mediated by human CLs.
The extent to which granyzme-mediated apoptosis involves redundant pathways mediated by multiple granzymes is the subject of vigorous research (29, 31, 46). A recent study indicates that mouse and human granzyme B and granzyme A are quite different and that human granzyme B is 30 times more effective in inducing apoptosis than mouse granzyme B (31). We find that all three stable cell lines expressing PI-9 show strong ability to inhibit apoptosis induced by both human NK92 cells which contain primarily granzyme B and by human NKL cells, which contain primarily granzyme A (34, 35). While the extent to which human granzyme A can independently induce cytotoxicity is unclear (31), whether or not human granzyme A in NKL cells acts independently, together with the low level of granzyme B in these cells, or with other proteins, the expression of functional PI-9 clearly interferes with NKL cells ability to induce apoptosis. Whatever the precise role human granzyme A is in NKL cell-mediated cytotoxicity, our data suggest that PI-9 has at least some ability to inhibit different activators of granzyme-mediated cytotoxicity.
Previously, there was no indication that PI-9 could inhibit Fas/FasL-mediated apoptosis. We use two assays to show expression of high levels of wild type PI-9 completely blocks Fas/FasL-induced apoptosis (Fig. 5). Expression of somewhat lower levels of PI-9 partially blocks Fas/FasL-mediated apoptosis (Fig. 6).
Our surprising finding that expression of PI-9 inhibits Fas/FasL-induced apoptosis raises the question of whether the levels of PI-9 that inhibits Fas/FasL-mediated apoptosis can actually be reached by human cells expressing the endogenous PI-9 gene. We therefore compared the level of wild type PI-9 expressed in the three stably transfected HeLa cells to the level of PI-9 in a readily available human colon cancer cell line, HCT116, that is resistant to Fas/FasL-mediated apoptosis, and in MCF7/ERHA cells in which we recently showed that estrogen or 4-hydroxytamoxifen + EGF strongly induce PI-9. Quantitation of band intensities from multiple western blots showed that the stably transfected cell lines expressed 2–3 fold more PI-9 than the HCT116 cells and 2–3 fold less PI-9 than the MCF7/ERHA cells (Fig. 2). This data suggests that PI-9 levels in the range of those expressed from the endogenous PI-9 promoter in different cell lines have at least some ability to inhibit Fas/FasL-mediated apoptosis.
Consistent with our view that inhibition of Fas/FasL-mediated apoptosis is a specific effect of PI-9 and not a relatively non-specific effect due to expression of high levels of PI-9, etoposide-induced apoptosis is completely unaffected in the cell line that expresses the highest level of wild-type PI-9.
Although we did not carry out detailed studies of the mechanism by which PI-9 inhibits Fas/FasL-mediated apoptosis, our data is consistent with a simple mechanism based on inhibiting the activity of caspases specific to the Fas/FasL pathway. A stably transfected HeLa cell line expressing PI-9 with two inactivating mutations in and around its reactive center loop remained fully sensitive to Fas/FasL-induced apoptosis, even when the mutant PI-9 was expressed at levels several fold higher than the levels at which wild-type PI-9 completely blocks Fas/FasL-mediated apoptosis. Thus, the ability of PI-9 to act as a protease inhibitor is critical to its ability to inhibit Fas/FasL-induced apoptosis. The same level of expression of wild-type PI-9 that completely blocks Fas/FasL-induced apoptosis has no effect on etoposide-induced apoptosis (Fig. 5C, Etop). Therefore, PI-9 must inhibit Fas/FasL-mediated apoptosis at a site unique to the Fas/FasL pathway and not shared with the etoposide pathway. Etoposide-induced apoptosis is based on activation of pro-caspase 9 leading to activation of the mitochondrial apoptosis pathway and activation of pro-caspase 3. In Fas/FasL-mediated apoptosis, activation of procaspase 8, and often procaspase 10, leads to activation of the mitochondrial apoptosis pathway and activation of pro-caspase 3 (8). It therefore seems likely that PI-9, acting as a protease inhibitor, inhbits Fas/FasL-mediated apoptosis at caspase 8 and caspase 10.
Recently, we used studies in which breast cancer cells induced different levels of PI-9 to suggest there was a graded response in which increasing levels of PI-9 confer increasing levels of resistance to CL-mediated cytolysis of target cells. We suggest that as levels of PI-9 increase, there is a progressive increase in resistance to perforin/granzyme-mediated cell death (23), and at higher levels of PI-9, progressive resistance to Fas/FasL-mediated apoptosis. Expression of increasing levels of PI-9 allows for a graduated increase in the resistance of target cells to cell death induced by both of the major pathways used by immune system cells and may provide a novel mechanism by which tumor cells and virally infected cells escape killing by immune cells.
Acknowledgments
This research was supported by National Institutes of Health grants DK071909 and HD16720 and by an NICHD Pre-doctoral Traineeship in Reproductive Biology to T.D.C. We are grateful to Dr. J. Miller, Dr. M. Robertson and Dr. E. Alarid who generously provided the NK92, NKL and MCF7/ERHA cell lines, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lieberman J. Nat Rev Immunol. 2003;3:361–70. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 2.Russell JH, Ley TJ. Annu Rev Immunol. 2002;20:323–70. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 3.Barry M, Bleackley RC. Nat Rev Immunol. 2002;2:401–9. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 4.Raja SM, Metkar SS, Froelich CJ. Curr Opin Immunol. 2003;15:528–32. doi: 10.1016/s0952-7915(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, Trapani JA, Bird PI. J Biol Chem. 1996;271:27802–9. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman J, Fan Z. Curr Opin Immunol. 2003;15:553–9. doi: 10.1016/s0952-7915(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 7.Peng SL. Rheumatology (Oxford) 2006;45:26–30. doi: 10.1093/rheumatology/kei113. [DOI] [PubMed] [Google Scholar]
- 8.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Annu Rev Immunol. 1999;17:331–67. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi C, Schmitz I, Krammer PH, Peter ME. J Biol Chem. 1999;274:1541–8. doi: 10.1074/jbc.274.3.1541. [DOI] [PubMed] [Google Scholar]
- 10.Barrie MB, Stout HW, Abougergi MS, Miller BC, Thiele DL. J Immunol. 2004;172:6453–9. doi: 10.4049/jimmunol.172.10.6453. [DOI] [PubMed] [Google Scholar]
- 11.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, Poellinger L, Pahlman S. Cancer Cell. 2006;10:413–23. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Kannan-Thulasiraman P, Shapiro DJ. J Biol Chem. 2002;277:41230–9. doi: 10.1074/jbc.M200379200. [DOI] [PubMed] [Google Scholar]
- 13.Medema JP, de Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP, Melief CJ, Offringa R. Proc Natl Acad Sci U S A. 2001;98:11515–20. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buzza MS, Hirst CE, Bird CH, Hosking P, McKendrick J, Bird PI. Cell Immunol. 2001;210:21–9. doi: 10.1006/cimm.2001.1806. [DOI] [PubMed] [Google Scholar]
- 15.Kanamori H, Krieg S, Mao C, Di Pippo VA, Wang S, Zajchowski DA, Shapiro DJ. J Biol Chem. 2000;275:5867–73. doi: 10.1074/jbc.275.8.5867. [DOI] [PubMed] [Google Scholar]
- 16.Krieg SA, Krieg AJ, Shapiro DJ. Mol Endocrinol. 2001;15:1971–82. doi: 10.1210/mend.15.11.0719. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AJ, Krieg SA, Ahn BS, Shapiro DJ. J Biol Chem. 2004;279:5025–34. doi: 10.1074/jbc.M307076200. [DOI] [PubMed] [Google Scholar]
- 18.Bladergroen BA, Strik MC, Bovenschen N, van Berkum O, Scheffer GL, Meijer CJ, Hack CE, Kummer JA. J Immunol. 2001;166:3218–25. doi: 10.4049/jimmunol.166.5.3218. [DOI] [PubMed] [Google Scholar]
- 19.Phillips T, Opferman JT, Shah R, Liu N, Froelich CJ, Ashton-Rickardt PG. J Immunol. 2004;173:3801–9. doi: 10.4049/jimmunol.173.6.3801. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Immunity. 2006;24:451–61. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Bird CH, Sutton VR, Sun J, Hirst CE, Novak A, Kumar S, Trapani JA, Bird PI. Mol Cell Biol. 1998;18:6387–98. doi: 10.1128/mcb.18.11.6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X, Orr BA, Kranz DM, Shapiro DJ. Endocrinology. 2006;147:1419–26. doi: 10.1210/en.2005-0996. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Ellison SJ, Alarid ET, Shapiro DJ. Oncogene. 2007 doi: 10.1038/sj.onc.1210197. in press. [DOI] [PubMed] [Google Scholar]
- 24.Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA, Laban S, Toes RE, Toebes M, Schumacher TN, Bladergroen BA, Ossendorp F, Kummer JA, Melief CJ, Offringa R. J Exp Med. 2001;194:657–67. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ten Berge RL, Oudejans JJ, Ossenkoppele GJ, Meijer CJ. J Pathol. 2003;200:4–15. doi: 10.1002/path.1331. [DOI] [PubMed] [Google Scholar]
- 26.van Houdt IS, Oudejans JJ, van den Eertwegh AJ, Baars A, Vos W, Bladergroen BA, Rimoldi D, Muris JJ, Hooijberg E, Gundy CM, Meijer CJ, Kummer JA. Clin Cancer Res. 2005;11:6400–7. doi: 10.1158/1078-0432.CCR-05-0306. [DOI] [PubMed] [Google Scholar]
- 27.Godal R, Keilholz U, Uharek L, Letsch A, Asemissen AM, Busse A, Na IK, Thiel E, Scheibenbogen C. Blood. 2006;107:3205–11. doi: 10.1182/blood-2005-07-2880. [DOI] [PubMed] [Google Scholar]
- 28.Bots M, Offringa R, Medema JP. Blood. 107:4974–5. doi: 10.1182/blood-2006-01-0291. author reply 4975, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Smyth MJ, Street SE, Trapani JA. J Immunol. 2003;171:515–8. doi: 10.4049/jimmunol.171.2.515. [DOI] [PubMed] [Google Scholar]
- 30.Bots ML, VANB, Rademaker MT, Offringa R, Medema JP. Immunol Cell Biol. 2006;84:79–86. doi: 10.1111/j.1440-1711.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaiserman D, Bird CH, Sun J, Matthews A, Ung K, Whisstock JC, Thompson PE, Trapani JA, Bird PI. J Cell Biol. 2006;175:619–30. doi: 10.1083/jcb.200606073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bots M, Kolfschoten IG, Bres SA, Rademaker MT, de Roo GM, Kruse M, Franken KL, Hahne M, Froelich CJ, Melief CJ, Offringa R, Medema JP. Blood. 2005;105:1153–61. doi: 10.1182/blood-2004-03-0791. [DOI] [PubMed] [Google Scholar]
- 33.Lamboley C, Bringuier AF, Feldmann G. Cell Mol Biol (Noisy-le-grand) 2000;46:13–28. [PubMed] [Google Scholar]
- 34.Mahrus S, Craik CS. Chem Biol. 2005;12:567–77. doi: 10.1016/j.chembiol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Ko YH, Park S, Jin H, Woo H, Lee H, Park C, Kim K. Lab Invest. 2007;87:241–50. doi: 10.1038/labinvest.3700517. [DOI] [PubMed] [Google Scholar]
- 36.Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET. Faseb J. 2004;18:81–93. doi: 10.1096/fj.03-0038com. [DOI] [PubMed] [Google Scholar]
- 37.Fowler AM, Solodin NM, Valley CC, Alarid ET. Mol Endocrinol. 2006;20:291–301. doi: 10.1210/me.2005-0288. [DOI] [PubMed] [Google Scholar]
- 38.Wu SY, Chiang CM. Biotechniques 21. 1996;718–22:724–5. doi: 10.2144/96214rr05. [DOI] [PubMed] [Google Scholar]
- 39.Gossen M, Bujard H. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 41.Houghton JA, Harwood FG, Gibson AA, Tillman DM. Clin Cancer Res. 1997;3:2205–9. [PubMed] [Google Scholar]
- 42.Lewis JS, Jordan VC. Mutat Res. 2005;591:247–63. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 43.Yager JD, Davidson NE. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 44.Blomberg K, Hautala R, Lovgren J, Mukkala VM, Lindqvist C, Akerman K. J Immunol Methods. 1996;193:199–206. doi: 10.1016/0022-1759(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 45.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Shankar P, Xu Z, Harnisch B, Chen G, Lange C, Lee SJ, Valdez H, Lederman MM, Lieberman J. Blood. 2003;101:226–35. doi: 10.1182/blood-2002-03-0791. [DOI] [PubMed] [Google Scholar]
- 47.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. J Cell Biol. 2004;165:347–56. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]